Abstract
Conversion of the cellular prion protein (PrPC) into the pathogenic isoform (PrPSc) is the fundamental event underlying transmission and pathogenesis of prion diseases. To control the expression of PrPC in transgenic (Tg) mice, we used a tetracycline controlled transactivator (tTA) driven by the PrP gene control elements and a tTA-responsive promoter linked to a PrP gene [Gossen, M. and Bujard, H. (1992) Proc. Natl. Acad. Sci. USA 89, 5547–5551]. Adult Tg mice showed no deleterious effects upon repression of PrPC expression (>90%) by oral doxycycline, but the mice developed progressive ataxia at ≈50 days after inoculation with prions unless maintained on doxycycline. Although Tg mice on doxycycline accumulated low levels of PrPSc, they showed no neurologic dysfunction, indicating that low levels of PrPSc can be tolerated. Use of the tTA system to control PrP expression allowed production of Tg mice with high levels of PrP that otherwise cause many embryonic and neonatal deaths. Measurement of PrPSc clearance in Tg mice should be possible, facilitating the development of pharmacotherapeutics.
Prion diseases are fatal neurodegenerative illnesses that can present as genetic, sporadic, or infectious disorders (1). In these diseases, the normal, cellular prion protein (PrPC), which is encoded by a chromosomal gene, undergoes a posttranslational modification to generate the pathogenic isoform PrPSc). Prion diseases include scrapie of sheep and goats, bovine spongiform encephalopathy, and Creutzfeldt–Jakob disease of humans.
The function of PrPC is unknown. In two lines of PrP-deficient (Prnp0/0) mice, no clinical signs of illness have been reported, and the animals seem to develop normally (2, 3). Brain slices from these two Prnp0/0 lines have been reported to show defective neurotransmission at GABAergic synapses and diminished long-term potentiation (4, 5). In two other studies, no detectable electrophysiological defects could be identified in one of the Prnp0/0 lines (6, 7). However, defective sleep–wake cycles and altered circadian rhythms have been reported for both of these Prnp0/0 lines (8). Subsequently, an additional line of Prnp0/0 mice was reported to develop ataxia due to Purkinje cell degeneration at about 70 weeks of age (9). Whether PrP has a role in Cu2+ metabolism is unclear, but studies of Cu2+ in Prnp0/0 mice support such a hypothesis (10). Several studies have suggested that PrP may bind Cu2+ in the octarepeat region (11). Two Cu2+ ions appear to be bound per PrP molecule through a square planar geometry (12).
To challenge the hypothesis that PrPSc is required for transmission and pathogenesis of prion disease (13), Prnp0/0 mice were inoculated with prions. These PrP-deficient mice neither developed disease (14) nor replicated prions (15, 16). Moreover, mice hemizygous for PrP gene ablation showed prolonged incubation times (15, 17, 18).
On this background, we undertook development of a system where the level of PrP expression could be regulated to modulate the rate of prion formation. We chose the tetracycline-responsive gene system that was developed by using the Escherichia coli tetracycline resistance Tn10 operon (19). It makes use of a transactivator (tTA) obtained by fusing the tetracycline repressor with the transactivation domain of the herpes simplex virus VP16 transcription factor. The tTA binds specifically with high affinity to the tetracycline operator (tetO) and activates transcription from a minimal promoter linked to the target gene. Binding of doxycycline, a tetracycline analog, to tTA prevents the protein from binding to the tetO region, thereby preventing target gene expression.
MATERIALS AND METHODS
Constructs.
Target constructs containing the MoPrP-A ORF were obtained by cloning a 5-kbp promoterless Prnpa genomic fragment (XbaI) excised from pPrP-5′HG (20) downstream from the tTA responsive promoter at the XbaI site of the pUHD10-3 vector. This Prnpa minigene contains the first, second, and third exons of MoPrP-A together with the 3′ untranslated region. The transactivator construct was generated with the EcoRI–BamHI tTA fragment from pUHD15-1 (19). Fragments were rendered blunt by using Klenow enzyme and introduced into the SalI-digested blunt-ended CosSHa.tet vector as described (21, 22).
Tg Mice.
Tg mice were obtained by microinjection of FVB. Prnp0/0 N4F1 obtained by crossing Prnp+/0 with FVB animals for four generations before interbreeding to homozygosity. Breeding and screening of Tg mice was performed as described (21). Antibiotics were administered as follows. Doxycycline (2 mg/ml) was added to the drinking water with 5% sucrose to mask the bitter taste. Alternatively, a 21-day-release doxycycline or minocycline pellet of 50 or 200 mg (Innovative Research of America, Sarasota, FL) was surgically implanted subcutaneously. Intravenous injection of doxycycline at 25 mg/kg was carried out in the tail vein once a day for 3 days before animals were terminated on the fourth day.
Immunodetection.
Protein concentration was assessed for cell lysates and brain homogenates by bicinchoninic acid assay (Pierce). Western blot analyses were performed as already described (23) by using an enhanced chemiluminescent detection method (Amersham) with the RO73 polyclonal α-PrP antiserum at 1:5000 dilution. Histoblots were performed on coronal brain sections (10 mm), transferred onto a nitrocellulose membrane, and processed for immunohistochemistry by using the α-PrP polyclonal RO73 antiserum as described (24).
Pathology.
Central nervous system (CNS), sciatic nerve, and muscle histology were assessed by standard procedures as described (21, 25). Brains were taken and immediately immersion-fixed in 10% buffered formalin. Specimens were embedded in paraffin, sectioned, and stained with hematoxylin and eosin for neuropathological evaluation. Evaluation of reactive astrocytic gliosis was performed by immunostaining of glial fibrillary acidic protein (GFAP) by using an α-bovine-GFAP rabbit antiserum (Dako) as described (26).
RESULTS
Establishment of Tg(Prnp-tTA) and Tg(tetO-PrP) Lines.
The tTA gene (19) was introduced into the CosSHa.tet cosmid expression vector (22) and microinjected into Prnp0/0 zygotes to establish three Tg(Prnp-tTA)FVB/Prnp0/0 lines (Table 1). Individual lines were characterized by assessing the level and distribution of mRNA by in situ hybridization by using antisense tTA as well as Prnp probes for wild-type FVB mice. The Tg(Prnp-tTA/F973) mice exhibited the highest expression levels followed by the Tg(Prnp-tTA/F959) and Tg(Prnp-tTA/F966) lines, respectively (Table 1). Expression was detected throughout the brain with the strongest signals in the cortex and hippocampus and lower levels in the thalamic, hypothalamic, and brainstem regions. Interestingly, the cerebellar granular layer of Tg(Prnp-tTA) mice presented a stronger expression signal relative to that of Prnp signals observed in FVB and Tg(MoPrP-A)4053/Prnp0/0 mice (data not shown).
Table 1.
Characteristics of tTA lines
| Line | Expression level, mRNA | Ability to induce target line |
|---|---|---|
| Prnp-tTA/F959 | +++ | ++ |
| Prnp-tTA/F966 | ++ | ++ |
| Prnp-tTA/F973 | +++ | ND |
Target MoPrP-A transgenes were constructed by introducing a promoterless MoPrP-A minigene downstream from the tetO-heptamer linked to a CMV IE minimal promoter (designated tetO-PrP). The construct was introduced into Prnp0/0 zygotes to establish five Tg lines referred to as Tg(tetO-PrP) (Table 2). Brains from Tg(tetO-PrP) lines displayed basal expression, ranging from less than 1% to 50% of that found in FVB mice as determined by immunoblot by using α-PrP RO73 rabbit antiserum (27).
Table 2.
Characteristics of target lines
| Line | Basal PrP-A expression, %* | Induction by tTA
|
|
|---|---|---|---|
| Fibroblasts | Tg mice | ||
| tetO-PrP/E6539 | <1 | ++ | − |
| tetO-PrP/E6550 | 25–50 | ++++ | +++ |
| tetO-PrP/E6725 | 10 | ++ | ND |
| tetO-PrP/E6740 | 10 | ++++ | ++ |
| tetO-PrP/E7655 | 2 | ++ | + |
ND, not determined.
Expression levels reported were standardized by using FVB PrPC levels as 100%
Production of Tg(tTA:PrP) Mice.
The Tg(tetO-PrP) target lines were selected for inducibility prior to breeding with the tTA lines. After primary fibroblast cultures were established and transfected with CMV-tTA, MoPrP expression was measured. The Tg(tetO-PrP/E6740) and Tg(tetO-PrP/E6550) lines exhibited high inducibility, whereas Tg(tetO-PrP/E7655) mice showed moderate induction (Table 2). These mice were bred to the transactivator Tg(Prnp-tTA/F959) and Tg(Prnp-tTA/F966) lines (Table 3). For convenience, animals expressing both the Prnp-tTA and tetO-PrP transgenes are designated Tg(tTA:PrP) mice. Tg(tTA:PrP) mice untreated or treated up to 4 weeks with doxycycline administered orally (2 mg/ml doxycycline) were tested for PrPC expression. Brains processed for Western blots (Fig. 1) displayed high levels of PrP expression that could be repressed by treatment with tetracycline analogs.
Table 3.
Characteristics of regulated Tg lines
| Regulated Tg line | Genotype | Residual expression, %*†
|
Induction level, % | ||
|---|---|---|---|---|---|
| 2 days | 7 days | 30 days | |||
| Tg(tTA:PrP)1 | tTA/F966:PrP/E6740 | 50 | 15 | 10 | 100–200 |
| Tg(tTA:PrP)2 | tTA/F959:PrP/E7655 | ND | 5 | ND | 50–100 |
| Tg(tTA:PrP)3 | tTA/F959:PrP/E6740 | ND | 15 | ND | 200–400 |
| Tg(tTA:PrP)4 | tTA/F959:PrP/E6550 | ND | 50–100 | ND | 400–800 |
Expression levels reported were standardized by using FVB PrPc levels as 100%.
Measurements were made after the mice were orally administered doxycycline (2 mg/ml) for 2, 7, or 30 days, as indicated. ND, not determined.
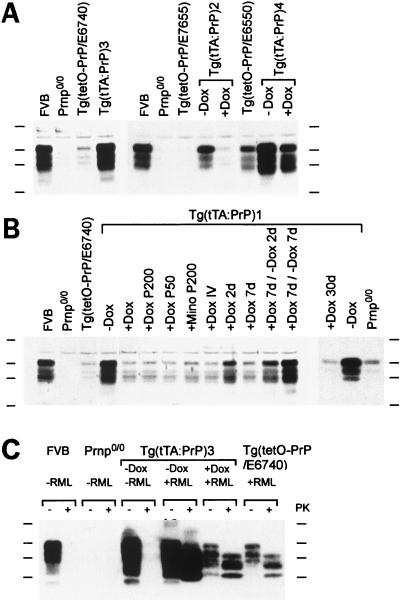
Figure 1.
PrPC and PrPSc in the brains of Tg(tTA:PrP) mice. Four lines of Tg(tTA:PrP) mice (Tables 1–3) were tested for PrP expression by Western blot analysis by using α-PrP polyclonal RO73 antiserum. Levels of expression in FVB, Prnp0/0, and Tg(tetO-PrP) lines are also shown. (A and B) Experimental conditions were as follows: “− Dox” denotes no treatment; “+ Dox” corresponds to doxycycline administered for 7 days in the drinking water at 2 mg/ml unless otherwise specified. “P50” corresponds to a 50 mg pellet (21-day release) of doxycycline placed subcutaneously for 7 days, and “P200” is a 200 mg pellet (21-day release). “IV” corresponds to a daily intravenous dose of 25 mg/kg of doxycycline for 3 days, and “d” is the abbreviation used for day. Induction of PrPC expression was observed in Tg(tTA:PrP) mice. Repression was obtained upon administration of doxycycline or minocycline (Mino) by using various routes and could be reversed upon ceasing antibiotic treatment. (C) Tg(tTA:PrP)3 animals left untreated (− Dox) and inoculated with RML prions (+ RML) were sacrificed at the time they presented neurological deficits consistent with development of scrapie (69 days postinoculation). Tg(tTA:PrP)3 mice treated with doxycycline (+ Dox) 1 week before inoculation as well as uninoculated Tg(tTA:PrP)3 and inoculated Tg(tetO-PrP/E6740) mice remained clinically healthy and were sacrificed at 200 days postinoculation. Brain homogenate consisting of 40 μg (A and B) or 60 μg (C) of protein was loaded per lane. Proteinase K digestion (20 mg/ml) was performed for 60 min at 37°C. Protein molecular weight markers from top to bottom correspond to 48, 35, 28, and 19 kDa.
The genotypic distribution of Tg(Prnp-tTA × tetO-PrP)F1 mice obtained by crossing Tg(Prnp-tTA) with Tg(tetO-PrP) was non-Mendelian. For example, from the 460 pups obtained by using Tg(Prnp-tTA/F959) crossed with Tg(tetO-PrP/E6740), 92 (20.0%) died during the first 2 weeks of life and out of the 368 remaining, only 28 (7.6%) were found to harbor both transgenes (Table 4). Out of 4 randomly selected litters ranging from 2 to 14 days of age (n = 33), 7 (21%) severely runted pups were sacrificed and found to be the only double Tg neonates among these litters. The proportion of double Tg mice (24.0%) that survived through weaning was restored to the expected Mendelian ratio when mating pairs were kept on doxycycline during the entire pregnancy and through weaning of the litters (Table 4). A similar phenomenon was observed when Tg(Prnp-tTA/F966) mice were crossed with Tg(tetO-PrP/E6740) (Table 4). Doxycycline treatment initiated during the last third of the pregnancy appeared sufficient to rescue Tg pups harboring both transgenes (Table 4). These observations implicate the overexpression of wt MoPrPC during the last third of the gestation or during the neonatal period as a cause of neonatal death of Tg(tTA:PrP) mice.
Table 4.
Genotypic frequency distribution of transgenic mice obtained from tetO-MoPrP × tTA crosses
| Crosses
|
Treatment* | No. born | No. neonatal deaths | Deaths, %† | No. screened | Genotypes, %
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| tTA | PrP | TetO-MoPrP | tTA | Non-Tg | tTA:PrP | |||||
| F959 | E6740 | − Dox | 460 | 92 | 20.0 | 368 | 30.7 | 32.3 | 29.3 | 7.6 |
| + Dox | 187 | 14 | 7.5 | 175 | 22.9 | 25.1 | 28.0 | 24.0 | ||
| + Dox E13 or E14‡ | 24 | 4 | 16.7 | 20 | 30.0 | 25.0 | 25.0 | 20.0 | ||
| + Dox E17 or E18‡ | 45 | 3 | 6.7 | 42 | 21.0 | 31.0 | 43.0 | 5.0 | ||
| + Dox P2, P4, or P6§ | 39 | 8 | 20.5 | 31 | 21.0 | 31.0 | 43.0 | 0.0 | ||
| F966 | E6740 | − Dox | 188 | 22 | 11.7 | 166 | 42.2 | 23.5 | 24.1 | 10.2 |
| + Dox | 216 | 8 | 3.7 | 208 | 29.8 | 24.5 | 24.5 | 21.2 | ||
Treatment refers to 2 mg/ml of doxycycline administered orally; − Dox, no treatment; + Dox, doxycyline during embryonic and postnatal development.
Neonatal deaths were observed during the first 3 postnatal weeks.
E refers to the embryonic days at which doxycycline treatment was initiated.
P refers to the postnatal days at which treatments were initiated.
Regulation of PrPC Expression in Vivo.
We examined the ability of tetracycline analogs to repress the expression of MoPrPC mediated by tTA in Tg mice. Crosses of Tg(Prnp-tTA/F959) mice with Tg(tetO-PrP/E7655), Tg(tetO-PrP/E6740), or Tg(tetO-PrP/E6550) mice produced Tg(tTA:PrP)2, Tg(tTA:PrP)3, and Tg(tTA:PrP)4 mice, respectively (Table 3). The levels of inducible MoPrPC expression in these three lines of Tg(tTA:PrP) mice were 0.5- to 8-fold that of wild-type FVB mice (Table 3; Fig. 1 A and B). Although induction of MoPrPC expression was also observed when Tg(Prnp-tTA/F966) mice were crossed with Tg(tetO-PrP/E6740) mice to produce Tg(tTA:PrP)1 mice, crosses using Tg(Prnp-tTA/F959) mice were superior with respect to the higher levels of expression obtained (Fig. 1 A and B).
Of all the Tg mice studied, the best regulated expression of MoPrPC by doxycycline was found in Tg(tTA:PrP)3 mice. Basal expression of MoPrPC after 7 days of doxycycline treatment was ≈15% of that found in FVB mice, whereas expression in the absence of doxycycline was ≈2-fold that of FVB (Table 3, Fig. 1 A and C).
MoPrPC expression was nearly fully repressed by various treatments with tetracycline analogs as demonstrated by using Tg(tTA:PrP)1 mice: 7 days of doxycycline in the drinking water (2 mg/ml), 7 days of a 50 or a 200 mg subcutaneous doxycycline pellet (21-day release period), 7 days of a 200 mg minocycline subcutaneous pellet (21-day release), or 3 days of a daily intravenous injection of doxycycline (25 mg/kg) (Fig. 1B). The residual expression is unlikely because of an insufficient dose of antibiotic because it was observed in all animals treated, irrespective of the route or dose administered. A 30-day oral doxycycline treatment of Tg(tTA:PrP)1 mice was effective in repressing expression to levels equivalent to those seen in target Tg(tetO-PrP/E6740) mice (Fig. 1B).
Repression of MoPrPC Expression in Adult Mice.
A time course of repression and induction was performed by using doxycycline administered orally (2 mg/ml) to Tg(tTA:PrP)1 mice (Fig. 1B). PrPC expression was repressed ≈50% after 2 days of treatment and nearly fully repressed after 7 days; baseline levels were found in Tg(tTA:PrP)1 mice sacrificed after 30 days of treatment. Stopping doxycycline after 7 days produced an ≈50% reinduction of PrPC expression within 2 days, and complete reactivation was observed by 7 days.
Two-month-old adult Tg(tTA:PrP)1 and 3 mice remained well for 30 days with no signs of systemic or CNS dysfunction when PrP expression was repressed ≈90% to basal levels by oral doxycycline. The Tg(tTA:PrP) mice were either treated with doxycycline in the drinking water for 1 month (n = 8) or untreated (n = 7). Groups of age-matched controls consisting of Prnp0/0 (n = 8), FVB (n = 8), Tg(MoPrP-A)4053/Prnp0/0 (n = 10), Tg(Prnp-tTA/F959) (n = 4), and Tg(tetO-PrP/6740) (n = 4) mice were treated similarly or kept untreated. All of the treated and untreated mice remained well over the entire duration of the experiment. At the end of the observation period, animals were sacrificed and their brains taken for measurement of PrPC levels and histologic examination (Figs. 1B and 2).
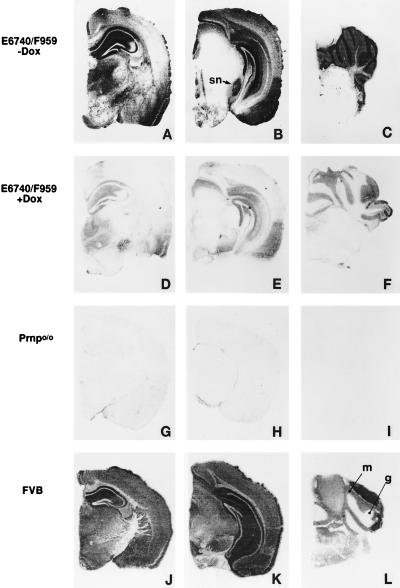
Figure 2.
Distribution of PrPC expression in the brain of Tg(tTA:PrP)3 mice. Distribution of MoPrPC is revealed by histoblots by using α-PrP polyclonal RO73 antiserum on half coronal brain sections of Tg(tTA:PrP)3 mice (A–C) untreated or (D–F) treated with oral doxycycline (2 mg/ml) for 30 days. Control brain sections from untreated (G–I) Prnp0/0 and (J–L) FVB mice. Tg(tTA:PrP)3 mice express PrPC at high levels in the hippocampus, neocortex, entorhinal cortex, substantia nigra (sn), and thalamus, as well as cerebellar granular (g) and molecular (m) layers. Although doxycycline treatment repressed PrPC expression, low levels of residual expression were still detected in the hippocampus, neocortex, entorhinal cortex, and granular layer of the cerebellum.
In related experiments described below, 8- to 10-week-old adult Tg(tTA:PrP)3 mice were given oral doxycycline for over 380 days and have displayed no signs of illness (Table 5). These findings extend those described above for adult Tg(tTA:PrP)1 mice that were studied after 30 days of oral doxycycline treatment.
Table 5.
Inhibition of scrapie disease development by repression of Prnp gene expression in transgenic mice
| Recipient | Treatment | Inoculum | CNS dysfunction | Incubation time (mean days ± SEM) |
|---|---|---|---|---|
| FVB | —* | RML | 11/11 | 122 ± 3 |
| Tg(tTA:PrP)3 | —* | RML | 4/4† | 51 ± 0 |
| Tg(tTA:PrP)3 | − Dox‡ | — | 0/6 | >200 |
| Tg(tTA:PrP)3 | + Dox§ | RML | 0/7 | >380 (n = 5)¶ |
| Non Tg(tTA:PrP)3‖ | —* | RML | 0/10 | >380 (n = 8)¶ |
| Tg(tetO-PrP/E6740) | —* | RML | 0/8 | >380 (n = 6)¶ |
| Prnp0/0 | —* | — | 0/10 | >380 |
Animals from these groups were kept at all times without doxycycline.
Animals in this category first presented with ataxia at 51 days postinoculation and with additional signs of neurologic dysfunction at 69 days. Traditionally, the diagnosis of experimental scrapie in mice requires two signs of neurologic dysfunction as described (32).
Animals were born from parents maintained on doxycycline in the drinking water. Treatment was ceased at 3 weeks of age.
Doxycycline was administered in the drinking water (2 mg/ml) 1 week before inoculation.
Two animals from each of these groups were sacrificed for histopathology at 200 days postinoculation.
Animals in this group did not harbor any transgene.
Immunoblot analysis showed that Tg(tTA:PrP)1 and Tg(tTA:PrP)3 mice treated for 30 days with doxycycline exhibited levels of PrPC equivalent to those of target Tg(tetO-PrP) mice alone (Fig. 1 and data not shown). Histoblots (24) revealed high expression of PrPC in the hippocampus, cingulate gyrus, neocortex, entorhinal cortex, thalamus, substantia nigra, molecular, and granular layers of the cerebellum in untreated Tg(tTA:PrP)3 mice (Fig. 2 A–C).
The expression of PrPC was more restricted in the grey matter of Tg(tTA:PrP)3 mice than in FVB mice (Fig. 2 J–L). For example, PrPC was not detected in a portion of the CA3 region of the hippocampus (Fig. 2A), the parietal neocortex (Fig. 2 A and B), or the brainstem tegmentum of Tg(tTA:PrP)3 mice (Fig. 2 B and C). In contrast, the expression of PrPC was higher in the substantia nigra (Fig. 2 B and K), the entorhinal cortex (Fig. 2 B and K), and the molecular and granule cell layers of the cerebellar cortex (Fig. 2 C and L) of Tg(tTA:PrP)3 mice than that seen in FVB mice. Similar patterns of PrPC expression were found in Tg(tTA:PrP)2 and Tg(tTA:PrP)4 mice (data not shown).
After 30 days of doxycycline treatment, PrPC expression was substantially repressed (Fig. 1B), with residual expression still detectable within the cingulate gyrus, caudate nucleus, hippocampus, thalamus, entorhinal cortex, and cerebellum (Fig. 2 D–F). Microscopic examination revealed no specific pathological feature associated with acute repression of PrPC after 30 days (data not shown).
High PrPC Expression Induces Reactive Astrocytic Gliosis.
A moderate degree of reactive astrocytic gliosis was detected in the thalamus, substantia nigra, and cerebellum granular and molecular layers of Tg(tTA:PrP)3 mice (n = 5) and Tg(tTA:PrP)4 mice (n = 2) expressing high levels of MoPrPC (data not shown). However, the neocortex, entorhinal cortex, hippocampus, and caudate nucleus, which also expressed high levels of PrPC, were normal. Repression of PrPC for 1 month had no effect on the extent of astrocytic gliosis observed, suggesting that this phenomenon may have resulted from the early overexpression of PrPC. Astrocytic gliosis was not observed in doxycycline-treated or untreated age-matched controls consisting of Prnp0/0 (n = 2), FVB (n = 4), Tg(tetO-PrP/E6740) (n = 3), Tg(Prnp-tTA/F959) (n = 4), and Tg(MoPrP-A)4053/Prnp0/0 (n = 6) mice or in randomly selected 10-month-old Prnp0/0 (n = 4), FVB (n = 5), and Tg(MoPrP-A)4053/Prnp0/0 (n = 4) animals.
Oral Doxycycline Prevents Prion Disease in Tg(tTA:PrP) Mice.
When doxycycline was administered orally to adult Tg(tTA:PrP)3 mice, beginning 7 days before inoculation with RML prions, the mice remained free of any signs of CNS dysfunction for more than 380 days. In contrast, mice from the same line not given doxycycline developed a progressive ataxia starting ≈50 days after inoculation with prions (Fig. 3; Table 5). Wild-type FVB mice developed signs of prion disease at ≈120 days after inoculation, whereas Prnp0/0 mice were not susceptible to prions, as previously reported (15, 17, 18).
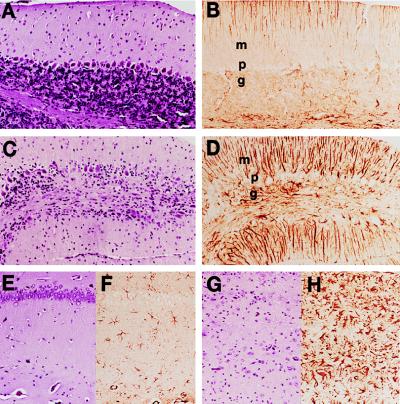
Figure 3.
Doxycycline prevented neuronal loss, vacuolation, and gliosis in Tg(tTA:PrP)3 mice inoculated with RML prions. (A, B, E, and F) Tg(tTA:PrP)3 mice treated with oral doxycycline (2 mg/ml) and sacrificed at 200 days postintracerebral inoculation with RML prions. (C, D, G, and H) Tg(tTA:PrP)3 mice untreated with doxycycline and sacrificed at 70 days postintracerebral inoculation with RML prions when they showed multiple signs of CNS dysfunction; the first sign of neurologic disease was ataxia at 50 days after inoculation. (A, C, E, and G) Hematoxylin and eosin-stained brain sections. (B, D, F, and H) α-GFAP-immunostained brain sections. (A–D) Views of cerebellar molecular (m), Purkinje cell (p), and granular (g) layers. (E–H) Views of the hippocampus CA1 pyramidal cell layer (aligned in the upper part of Insets) with underlying stratum radiatum. Focal loss of Purkinje cells and granule cells was observed in the cerebellum of the ill Tg(tTA:PrP)3 mice and was accompanied by low grade vacuolation. Reactive astrocytic gliosis was demonstrated by the α-GFAP immunostaining that revealed moderate to severe gliosis in the granular and molecular layers (D) (Bergmann’s gliosis). In the hippocampal CA1 region most neuronal cell bodies were lost, and low grade vacuolation was apparent together with severe astrocytic gliosis (H).
Tg(tTA:PrP)3 mice not treated with doxycycline were sacrificed after developing progressive CNS dysfunction. Levels and distribution of PrPC and PrPSc in Tg(tTA:PrP)3 mice were assessed by Western blot analysis (Fig. 1C) and histoblot analysis (data not shown). Both PrPC and PrPSc levels were high in the brains of untreated Tg(tTA:PrP)3 mice that developed scrapie. PrPSc accumulated in the neocortex, hippocampus, corpus callosum, and white matter tracts of the cerebellum and pons. Doxycycline treatment repressed PrPC to low levels similar to those observed in target Tg(tetO-PrP/E6740) (Fig. 1C). This residual expression was nonetheless sufficient to permit the conversion of low levels of PrPSc (Fig. 1C), which accumulated mostly within the forebrain and in particular in the corpus callosum and caudate nucleus (data not shown).
The brains from untreated Tg(tTA:PrP)3 mice exhibited extensive neuronal loss in the hippocampal pyramidal cell layer (Fig. 3G) and dentate gyrus and focal loss of Purkinje cells and granular cells in the cerebellum (Fig. 3C). These changes were accompanied by moderate to severe astrocytic gliosis in all regions examined, including the neocortex, hippocampus, entorhinal cortex, thalamus, caudate nucleus, and substantia nigra, as well as cerebellar granular and molecular layers. As noted above, the doxycycline-treated Tg(tTA:PrP)3 mice did not show signs of CNS dysfunction. When these mice were sacrificed ≈200 days after inoculation with prions, their brains showed no signs of neurodegeneration.
DISCUSSION
To test the hypothesis that developmental compensation in PrP-deficient mice prevented any recognizable dysfunction in adult mice, we used the tTA system in Tg mice to regulate PrP expression. Doxycycline administered to adult Tg(tTA:PrP) mice acutely repressed the expression of PrPC but did not produce any recognizable adverse effects in the mice over a 30-day period. Neither the viability nor the neurological status of the mice was compromised, and histological examination of the brains did not reveal any abnormalities. These results indicate that high levels of PrPC are not essential for short-term neuronal survival, as its expression can be repressed over 20-fold without adverse effects. It is noteworthy that adult Tg(tTA:PrP)3 mice were placed on oral doxycycline to repress their PrPC expression and have remained well for >380 days with continual administration of doxycycline (Table 5).
PrPSc Accumulation Causes Prion Disease.
The accumulation of PrPSc in the brains of animals and humans is a specific hallmark of prion disease. Often, but not always, proteolytic fragments of PrPSc coalesce in the extracellular space to form amyloid plaques. Such extracellular accumulations of PrPSc were thought to be of little consequence in the pathogenesis of prion disease because they are a nonobligatory feature of the disease (28). Moreover, PrPSc either within caveolae or within an intracellular compartment has been implicated in the pathogenesis of prion disease (29, 30), a conclusion supported by neuronal cell grafts producing PrPSc in the CNS of Prnp0/0 mice (31).
With the production of Tg(tTA:PrP) mice, it is possible to examine the effects of low or intermediate levels of PrPSc in the CNS. We found that low levels of PrPSc did not produce any deleterious clinical or histological effects up to 380 days after inoculation of RML prions in Tg(tTA:PrP)3 mice (Fig. 3C). Studies of Prnp+/0 mice with one functional PrP allele show greatly prolonged incubation times (15) but at a higher accumulation of PrPSc than was anticipated (17). Studies with Tg(tTA:PrP) mice where the levels of PrPC expression are held at different levels throughout the incubation time should help to clarify this issue.
The findings reported here clearly show that repression of PrPC expression in young adult Tg(tTA:PrP) mice is not deleterious, whereas accumulation of PrPSc in the same line of animals is lethal (Table 5). Even though Purkinje cell degeneration in 70-week-old Prnp0/0 mice has been found (9), our data continue to argue that the accumulation of PrPSc and not a loss of PrPC function is responsible for the pathogenesis of prion disease.
New Approaches Arising from These Studies.
The use of the tTA-regulated PrP transgene expression revealed that high levels of even wild-type PrPC are often incompatible with neonatal development, as most Tg(tTA:PrP) mice died within the first 3 weeks of life (Table 4). Repression of PrPC expression by beginning doxycycline treatment during embryonic development was sufficient to prevent this mortality. The ability to control PrPC expression should allow the establishment of Tg mice expressing higher levels of wild-type or mutant PrP than was previously possible. Such mice may demonstrate unique sensitivities to prion infection and provide the basis for a truly rapid bioassay.
Reversing the course of prion diseases by blocking the production of PrPSc through repression of PrPC expression will allow us to measure the removal of PrPSc. Such clearance studies, which were not previously possible, are a prelude to the development of effective therapies where drugs that block PrPSc formation are administered at the earliest onset of symptoms to patients with sporadic Creutzfeldt–Jakob disease. At present, we have no understanding of how much PrPSc can be tolerated by the CNS and how rapidly it will disappear once synthesis of its precursor, PrPC, is repressed.
Acknowledgments
We thank F. E. Cohen, D. Groth, A. Serban, S. Freundlieb, and U. Baron for many helpful discussions during the course of these studies. This work was supported by grants from the National Institutes of Health and the American Health Assistance Foundation, as well as by a gift from the Bernard Osher Foundation. Z.M. was supported by a Brody Grant from the Alzheimer’s Association. M.G. is supported by a Human Frontiers of Science Fellowship.
ABBREVIATIONS
- PrP
prion protein
- PrPC
cellular isoform of PrP
- PrPSc
pathogenic isoform of PrP
- Tg
transgenic
- tTA
tetracycline transactivator
- Prnp0/0
PrP-deficient mice
- tetO
tetracycline operator
- CNS
central nervous system
- GFAP
glial fibrillary acidic protein
References
- 1. Prusiner S B. Science. 1997;278:245–251. doi: 10.1126/science.278.5336.245. [DOI] [PubMed] [Google Scholar]
- 2.Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp H-P, DeArmond S J, Prusiner S B, Aguet M, Weissmann C. Nature (London) 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 3.Manson J C, Clarke A R, Hooper M L, Aitchison L, McConnell I, Hope J. Mol Neurobiol. 1994;8:121–127. doi: 10.1007/BF02780662. [DOI] [PubMed] [Google Scholar]
- 4.Collinge J, Whittington M A, Sidle K C, Smith C J, Palmer M S, Clarke A R, Jefferys J G R. Nature (London) 1994;370:295–297. doi: 10.1038/370295a0. [DOI] [PubMed] [Google Scholar]
- 5.Whittington M A, Sidle K C L, Gowland I, Meads J, Hill A F, Palmer M S, Jefferys J G R, Collinge J. Nat Genet. 1995;9:197–201. doi: 10.1038/ng0295-197. [DOI] [PubMed] [Google Scholar]
- 6.Lledo P-M, Tremblay P, DeArmond S J, Prusiner S B, Nicoll R A. Proc Natl Acad Sci USA. 1996;93:2403–2407. doi: 10.1073/pnas.93.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herms J W, Kretzschmar H A, Titz S, Keller B U. Eur J Neurosci. 1995;7:2508–2512. doi: 10.1111/j.1460-9568.1995.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 8.Tobler I, Gaus S E, Deboer T, Achermann P, Fischer M, Rülicke T, Moser M, Oesch B, McBride P A, Manson J C. Nature (London) 1996;380:639–642. doi: 10.1038/380639a0. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Katamine S, Nishida N, Moriuchi R, Shigematsu K, Sugimoto T, Nakatani A, Kataoka Y, Houtani T, Shirabe S, et al. Nature (London) 1996;380:528–531. doi: 10.1038/380528a0. [DOI] [PubMed] [Google Scholar]
- 10.Brown D R, Qin K, Herms J W, Madlung A, Manson J, Strome R, Fraser P E, Kruck T, von Bohlen A, Schulz-Schaeffer W, et al. Nature (London) 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- 11.Hornshaw M P, McDermott J R, Candy J M. Biochem Biophys Res Commun. 1995;207:621–629. doi: 10.1006/bbrc.1995.1233. [DOI] [PubMed] [Google Scholar]
- 12.Stöckel J, Safar J, Wallace A C, Cohen F E, Prusiner S B. Biochemistry. 1998;37:7185–7193. doi: 10.1021/bi972827k. [DOI] [PubMed] [Google Scholar]
- 13.Prusiner S B. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 14.Büeler H, Aguzzi A, Sailer A, Greiner R-A, Autenried P, Aguet M, Weissmann C. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 15.Prusiner S B, Groth D, Serban A, Koehler R, Foster D, Torchia M, Burton D, Yang S-L, DeArmond S J. Proc Natl Acad Sci USA. 1993;90:10608–10612. doi: 10.1073/pnas.90.22.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sailer A, Büeler H, Fischer M, Aguzzi A, Weissmann C. Cell. 1994;77:967–968. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 17.Büeler H, Raeber A, Sailer A, Fischer M, Aguzzi A, Weissmann C. Mol Med. 1994;1:19–30. [PMC free article] [PubMed] [Google Scholar]
- 18.Manson J C, Clarke A R, McBride P A, McConnell I, Hope J. Neurodegeneration. 1994;3:331–340. [PubMed] [Google Scholar]
- 19.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 21.Scott M, Foster D, Mirenda C, Serban D, Coufal F, Wälchli M, Torchia M, Groth D, Carlson G, DeArmond S J, et al. Cell. 1989;59:847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- 22.Scott M, Köhler R, Foster D, Prusiner S B. Protein Sci. 1992;1:986–997. doi: 10.1002/pro.5560010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry R A, Prusiner S B. J Infect Dis. 1986;154:518–521. doi: 10.1093/infdis/154.3.518. [DOI] [PubMed] [Google Scholar]
- 24.Taraboulos A, Jendroska K, Serban D, Yang S-L, DeArmond S J, Prusiner S B. Proc Natl Acad Sci USA. 1992;89:7620–7624. doi: 10.1073/pnas.89.16.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westaway D, DeArmond S J, Cayetano-Canlas J, Groth D, Foster D, Yang S-L, Torchia M, Carlson G A, Prusiner S B. Cell. 1994;76:117–129. doi: 10.1016/0092-8674(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 26.Muramoto T, DeArmond S J, Scott M, Telling G C, Cohen F E, Prusiner S B. Nat Med. 1997;3:750–755. doi: 10.1038/nm0797-750. [DOI] [PubMed] [Google Scholar]
- 27.Serban D, Taraboulos A, DeArmond S J, Prusiner S B. Neurology. 1990;40:110–117. doi: 10.1212/wnl.40.1.110. [DOI] [PubMed] [Google Scholar]
- 28.Prusiner S B, Scott M, Foster D, Pan K-M, Groth D, Mirenda C, Torchia M, Yang S-L, Serban D, Carlson G A, et al. Cell. 1990;63:673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- 29.DeArmond S J, Mobley W C, DeMott D L, Barry R A, Beckstead J H, Prusiner S B. Neurology. 1987;37:1271–1280. doi: 10.1212/wnl.37.8.1271. [DOI] [PubMed] [Google Scholar]
- 30.DeArmond S J, Sánchez H, Yehiely F, Qiu Y, Ninchak-Casey A, Daggett V, Camerino A P, Cayetano J, Rogers M, Groth D, et al. Neuron. 1997;19:1337–1348. doi: 10.1016/s0896-6273(00)80424-9. [DOI] [PubMed] [Google Scholar]
- 31.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Nature (London) 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 32.Carlson G A, Goodman P A, Lovett M, Taylor B A, Marshall S T, Peterson-Torchia M, Westaway D, Prusiner S B. Mol Cell Biol. 1988;8:5528–5540. doi: 10.1128/mcb.8.12.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]