Abstract
Thermally responsive elastin like polypeptides (ELPs) can be used to purify proteins from Escherichia coli culture when proteins are expressed as a fusion with an ELP. Nonchromatographic purification of ELP fusion proteins, termed inverse transition cycling (ITC), exploits the reversible soluble–insoluble phase transition behavior imparted by the ELP tag. Here, we quantitatively compare the expression and purification of ELP and oligohistidine fusions of chloramphenicol acetyltransferase (CAT), blue fluorescent protein (BFP), thioredoxin (Trx), and calmodulin (CalM) from both a 4-h culture with chemical induction of the plasmid-borne fusion protein gene and a 24-h culture without chemical induction. The total protein content and functional activity were quantified at each ITC purification step. For CAT, BFP, and Trx, the 24-h noninduction culture of ELP fusion proteins results in a sevenfold increase in the yield of each fusion protein compared to that obtained by the 4-h–induced culture, and the calculated target protein yield is similar to that of their equivalent oligohistidine fusion. For these proteins, ITC purification of fusion proteins also results in ~75% recovery of active fusion protein, similar to affinity chromatography. Compared to chromatographic purification, however, ITC is inexpensive, requires no specialized equipment or reagents, and because ITC is a batch purification process, it is easily scaled up to accommodate larger culture volumes or scaled down and multiplexed for high-throughput, microscale purification; thus, potentially impacting both high-throughput protein expression and purification for proteomics and large scale, cost-effective industrial bioprocessing of pharmaceutically relevant proteins.
Keywords: protein purification, elastin, elastin-like polypeptide, fusion protein, phase transition, solubility tag
Completion of the sequencing of the human genome is beginning to provide a plethora of protein targets for drug discovery. These protein targets need to be expressed in significant (approximately several hundred milligram to gram) quantities with high purity for structure determination and proteomics research, thus creating an urgent need for rapid, high-throughput purification techniques for proteins. Similarly, a large number of protein and peptide drugs are currently under development, and one of the major hurdles in their development pipeline is the ability to express and purify these biopharmaceuticals at a multikilogram scale in a timely and cost-effective manner.
A number of protein expression systems have been developed to simplify protein purification (Maina et al. 1988; Smith and Johnson 1988; Uhlén and Moks 1990; Lavallie et al. 1993; Nilsson et al. 1997) by expression of a recombinant protein as a fusion with a carrier protein or peptide that allows one-step purification by affinity chromatography using an immobilized, moderate affinity ligand, specific to the carrier protein (Sassenfeld 1990; Nilsson et al. 1997). Although useful for laboratory scale purification in a serial format, affinity purification using immobilized ligands has several limitations for high-throughput, multiplexed purification of proteins at the milligram scale typical in a laboratory setting. Although miniaturization and multiplexing of affinity chromatography is possible (e.g., on 96-well plates using SwellGel Discs from Pierce Biotechnology), the yields obtained for a single purification are typically no more than 1 mg because of the limited protein loading capacity of most affinity resins and the limited amount of immobilized affinity ligand that can be used in most multiplexed purification formats. Most affinity chromatography in a multiplexed format is also typically carried out with a step-wise elution of the bound protein, so that the purity of the final product is typically less than 90%. Furthermore, affinity chromatography is typically not used for industrial scale protein purification because of the high cost of the affinity resin and associated problems, such as leaching of bound ligand from the column and difficulties in scale-up. More economical and technically simple methods for purification of soluble proteins, which can be easily multiplexed or scaled up, are therefore clearly needed.
Elastin-like polypeptides (ELPs) (Urry 1988, 1992, 1997) are artificial biopolymers containing repeats of the pentapeptide sequence Val-Pro-Gly-Xaa-Gly (VPGXG), where X can be any naturally occurring amino acid except Pro (Urry 1992). This sequence is derived from the characteristic repeat motif, VPGVG, found in native, mammalian elastin. ELPs undergo an inverse phase transition: Below their inverse transition temperature (Tt) ELPs are structurally disordered, highly solvated, and therefore soluble in aqueous solutions, but as the temperature is raised the polymer gradually collapses to shed bound water resulting in the formation of intramolecular contacts between nonpolar regions of the ELP (Li et al. 2001; Li and Daggett 2003). At a critical temperature—termed the inverse transition temperature (Tt)—the ELP undergoes a phase transition, leading to aggregation of the polypeptide within a narrow temperature range (Urry et al. 1985; 1992). The Tt of an ELP is a function of a number of variables including the identity and stoichiometry of the guest residue (X), the ELP molecular weight (MW), and the ELP concentration, as well as the pH and ionic strength of the aqueous solution.
We have previously shown that the environmental sensitivity and reversible solubility of ELPs are retained when an ELP is fused at the gene level with other proteins, and that the activity of the ELP fusion protein is retained after cycling through the inverse phase transition (Meyer and Chilkoti 1999; Meyer et al. 2001; Trabbic-Carlson et al. 2004). We exploited this finding to demonstrate that the environmentally triggered, reversible solubility of ELP fusion proteins can be used to devise a simple, nonchromato-graphic method for purification of proteins, which we have termed inverse transition cycling (ITC). In ITC, a recombinant ELP fusion protein is selectively separated from other contaminating Escherichia coli biomolecules to high purity by the sequential and repeated steps of aggregation, centrifugation, and resolubilization of the fusion protein (Meyer and Chilkoti 1999; Meyer et al. 2001).
This study has two related objectives. The first objective was motivated by a previous observation of Urry and colleagues, that the expression of free ELPs in E. coli is greatly enhanced by growing cultures for long periods of time (~24 h) without induction (Guda et al. 1995). Using this method, ELP accumulation occurs as a result of the intrinsic leakiness of the T7 promoter (Grossman et al. 1998), allowing for the gradual accumulation of greater amounts of the ELP over longer periods of time in “inclusion body-like” compartments that can be subsequently resolubilized (due to the reversible nature of ELP aggregation) by cell lysis in cold, low ionic strength buffer (Guda et al. 1995). We hypothesized that overexpression of ELP fusion proteins might occur by a similar mechanism, thereby increasing target protein yield under similar conditions of E. coli culture as opposed to the typical chemical induction protocol. Motivated by these findings, in this study we examine the effects of the expression conditions on the yields of four different proteins that were expressed in E. coli as ELP fusions: chloramphenicol acetyltransferase (CAT), blue fluorescent protein (BFP), thioredoxin (Trx), and calmodulin (CalM). These proteins were selected to investigate the utility of ELPs as widely applicable and efficient protein purification tags because they constitute a divergent set of target proteins with respect to their MW, surface hydrophobicity, and surface charge.
The second, and related, objective of this study is to quantitatively compare the expression and purification of ELP fusion proteins with their respective oligohistidine fusion proteins purified by immobilized metal affinity binding chromatography (IMAC). We chose to benchmark the ELP fusion protein technology against IMAC, because it is, at least within the laboratory setting, the most commonly used fusion protein expression and purification system. For proteins whose activity could be easily assayed in the presence of contaminating native E. coli proteins (CAT and BFP), the activity and total protein content were assayed as a function of purification step during ITC to determine the efficiency of the ITC purification process.
The results of this study are significant, because they establish that (1) the ELP fusion protein technology is a generic method to express and purify soluble, recombinant proteins; (2) the yield and purification efficiency (also referred to as recovery) of ELP fusion proteins by ITC at least matches that of IMAC; and (3) the ease, scalability, and high-throughput of protein purification by ITC provide a compelling alternative to conventional methods to purify proteins by multiple stages of chromatography.
Results
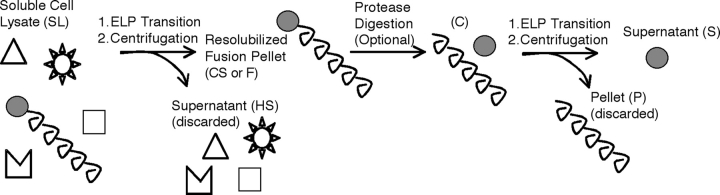
In a typical implementation of ITC, as illustrated in Figure 1 ▶, the ionic strength of the soluble cell lysate (SL) of an E. coli culture is increased such that ELP fusion protein aggregates at a moderate (i.e., 25°C) temperature and thus can be separated from soluble E. coli contaminants by centrifugation; this step is referred to as the “hot spin.” Because the ELP-mediated aggregation of the fusion protein is reversible, the ELP fusion protein (but not denatured and aggregated contaminating proteins) can be resolubilized in fresh, cold PBS buffer, and the denatured and aggregated contaminating biomolecules can be subsequently separated from the resolubilized ELP fusion protein by centrifugation at cold (i.e., 4°C) temperatures; this step is termed the “cold spin.” The soluble protein after each of these centrifugation steps is referred to as the hot spin (HS) or cold spin (CS) fraction. This process of aggregation, separation, and resolubilization is repeated as needed to increase the purity of the ELP-fusion protein; numbers following HS and CS (e.g., HS1, CS2, etc.) in all figures refer to the cycle number during the ITC purification process.
Figure 1.
Inverse transition cycling (ITC) purification scheme. The ELP fusion protein is separated from other contaminating biomolecules in the cell lysate (SL) by triggering the inverse temperature phase transition of the ELP. The inverse phase transition of the ELP fusion is triggered by an increase in temperature and/or ionic strength, and aggregated protein is separated by centrifugation leaving the contaminating biomolecules largely in the soluble fraction (HS). The ELP fusion protein is then resolubilized in fresh buffer by cooling to below its Tt (CS or F). If desired, the target protein can be liberated from the fused ELP tag by cleavage at the thrombin recognition site engineered between the ELP tag and the target protein (C). The cleaved ELP can be removed by another round of ITC. After centrifugation, the purified target protein is obtained in the supernatant (S), while the aggregated ELP is discarded in the pellet (P).
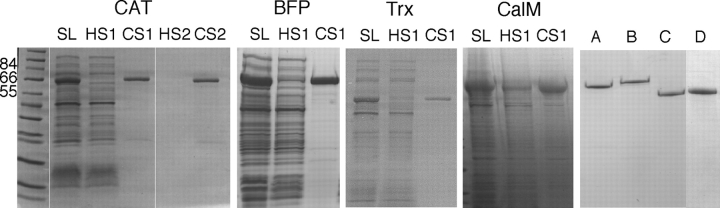
Figure 2 ▶ shows an SDS-PAGE gel of the ITC process, which tracks the protein content through two rounds of ITC purification (as an example) for CAT-ELP, the first round of ITC purification for BFP-ELP, Trx-ELP, and CalM-ELP, and the final purified fusion proteins after three rounds of ITC (six rounds for CalM-ELP only). The SDS-PAGE results qualitatively demonstrate that all four proteins could be purified to reasonable purity after only a few rounds of ITC. The vast majority of contaminating proteins remain in the supernatant of the soluble lysate after the first hot spin during the first round of ITC (HS1). The protein that is aggregated and pelleted at this step largely constitutes ELP fusion protein, as seen by the fact that upon resolubilization of the pelleted fraction in cold buffer, the protein obtained is relatively pure and has a size consistent with that of the ELP fusion protein. Typical results for a second round of ITC are shown for CAT, and visually illustrate the finding that a second round of ITC led to an increase in purity of all four proteins.
Figure 2.
SDS-PAGE gels showing two rounds of CAT-ELP purification by ITC and one round for BFP-ELP, Trx-ELP, and CalM-ELP. Legend: Soluble lysate, SL; the supernatant after the hot spin, HS; the supernatant after cold spin, CS. The two rounds of purification are denoted by 1 and 2, respectively. The fifth gel on the right of the figure shows CAT-ELP (A), BFP-ELP (B), and Trx-ELP (C) after three rounds of purification by ITC, and CalM-ELP (D) after six rounds. In all gels, the amount of protein loaded in each lane corresponds to 0.006% of the 1-L culture volume for all the proteins, so that the intensity of the protein bands in each lane is proportional to the protein content in that sample.
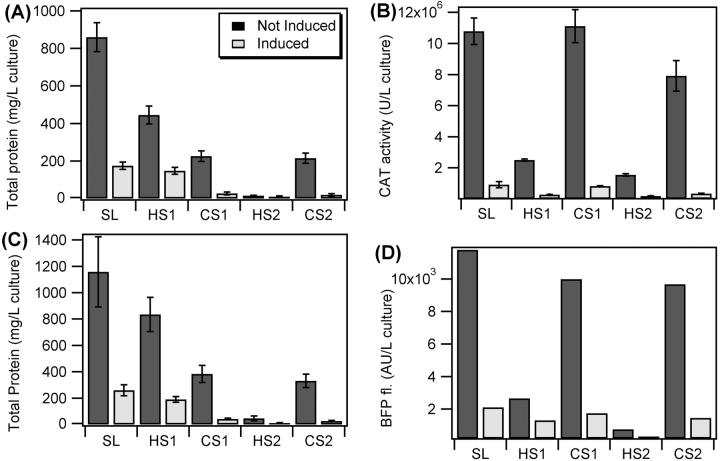
Next, we examined this process in more detail by quantifying the protein content and target protein activity at each step of the ITC purification process for two easily assayed proteins, CAT and BFP. Figure 3 ▶, A and C, shows the total protein content, as determined by a BCA assay, for 1-L cultures of CAT-ELP and BFP-ELP purified through two rounds of ITC, grown using both a conventional expression protocol, in which cultures were induced by 1 mM isopropyl α-thiogalactopyranoside (IPTG) at a precise optical density (typically OD600 = 0.8) and grown for a relatively short period of time (4 h) postinduction, and a noninduction protocol where identically seeded cultures were grown at the same temperature for 24 h without induction, a culture condition that has been previously demonstrated to markedly enhance the expression of ELPs from E. coli culture (Guda et al. 1995).
Figure 3.
Quantitative analysis of CAT-ELP (A,B) and BFP-ELP (C,D) purification by ITC showing total protein content for a 1-L culture as determined by BCA assay (A,C) and target protein activity as determined by the FAST CAT assay for CAT-ELP (B) and quantitative fluorescence emission for BFP-ELP (D) for uninduced (dark bars) and induced (light bars) cultures. Protein content and activity were determined for the soluble E. coli lysate (SL), the supernatant after the first “hot spin” to selectively remove by centrifugation the aggregated ELP fusion protein above its Tt (HS1), the resuspended fusion protein pellet after the first cold centrifugation to remove insoluble debris (CS1), the supernatant after the second “hot spin” (HS2), and the resuspended pellet after the second cold centrifugation (CS2). Error bars represent the first standard deviation of the mean for at least three replicate measurements.
Fusion protein expression levels and purification yields were found to be highly dependent upon the expression protocol. The total protein content in the soluble lysate (SL in Fig. 3 ▶), measured by a BCA assay, was four to five times greater for E. coli cultures grown for 24 h with the noninduction protocol compared to the 4-h IPTG induction protocol. After increasing the ionic strength and increasing the temperature to 25°C, 16% to 48% of the total protein (depending on the construct and culture conditions) underwent the phase transition, aggregated, and was removed from the soluble lysate by centrifugation. The remaining soluble protein (i.e., the supernatant after the hot spin) largely consisted of contaminant proteins (HS1 in Fig. 3A,C ▶), and the relative amount of contaminating proteins was qualitatively consistent with the SDS-PAGE results in Figure 2 ▶. The pelleted fraction obtained during the first “hot spin” was resuspended in cold PBS buffer and centrifuged again to remove any irreversibly aggregated protein. The soluble protein after the first “cold spin” (CS1 in Fig. 3A,C ▶) accounted for the majority of protein that was aggregated and pelleted in the first “hot spin” (HS1 in Fig. 3 ▶). A second round of purification showed a small loss of protein in the second hot spin (HS2), and recovery of the majority of the protein in the second cold spin (CS2) (Fig. 3A,C ▶) was consistent with the SDS-PAGE results of Figure 2 ▶.
Figure 3 ▶, B and D, show the total activity of the CAT-ELP and BFP-ELP, respectively, at each ITC purification step obtained from the same 1-L cultures for both expression protocols. These assays show that a significant fraction of the active fusion protein is efficiently removed from the soluble lysate (SL) and retrieved after its resuspension in fresh buffer both in the first (CS1) and second rounds (CS2) of purification by ITC. While SDS-PAGE gels and BCA assays suggest that nearly all the fusion protein is recovered through multiple rounds of ITC, activity assays, which are far more sensitive to the presence of active target protein, indicate that 5%–20% active protein may be lost during each round of purification depending on the fusion protein and culture conditions. Activity assays also suggest that conditions that promote higher concentrations of ELP fusion protein during purification also promote smaller overall purification losses by ITC. Noninduced cultures exhibit an overall 73%–75% recovery of the ELP fusion protein after two rounds of ITC, while induced cultures exhibit only 37%–55% recovery. We believe that this is because efficient removal of the fusion protein from solution is dependent on the formation of aggregates of sufficient size and number, so that conditions that promote aggregate formation (higher ELP concentration) also promote more efficient separation.
The total amount of CAT-ELP and BFP-ELP purified after three rounds of ITC was ~180 mg/L and 220 mg/L, respectively. In contrast, the yields of purified protein from the conventional IPTG, 4-h induction protocol were merely 25–30 mg/L. These results clearly demonstrate the dramatic increase in yield obtained by choice of the expression conditions for ELP fusion proteins, and can be attributed to the synergistic effect of three interrelated factors. First, uninduced, 24-h cultures exhibit total protein contents in the soluble lysate that is approximately four to five times greater than the protein content in 4-h induced cultures (Fig. 3A,C ▶). Second, ELP fusion proteins comprise approximately 25%–28% of the protein mass when grown without induction, while they represent only 10%–11% of the protein content in the induced cultures (Fig. 3 ▶; Table 1). Third, protein losses at each stage of purification scale inversely with the concentration of the ELP fusion protein. Combined, all three factors result in yields of purified ELP fusion protein that are six to seven times greater for 24-h noninduced cultures compared to the 4-h IPTG-induced cultures.
Table 1.
Yield of purified protein in mg per liter of E. coli culture as determined from absorbance at 280 nm after IMAC purification of oligohistidine fusions or three rounds of ITC purification of ELP fusion proteins
| Oligohistidine fusiona | ELP fusion protein | Target proteinb | ||||
| Not induced | Induced | Not induced | Induced | Not induced | Induced | |
| CAT | 140 | 130 | 180 | 27 | 80 | 13 |
| BFP | 110 | 100 | 220 | 30 | 100 | 14 |
| Trx | 90 | 63 | 200 | 29 | 50 | 7 |
| CalM | 99 | 46 | 120 | 120 | 44 | 44 |
a Yields of oligohistidine fused constructs are estimated from purification of approximately 1/10 of the 1-L culture volume.
b Target Protein Yields are calculated from their mass fraction in the ELP fusion protein.
Table 1 extends these results by summarizing the yield of all four purified proteins with ELP and oligohistidine tags grown with and without induction in this study, as well as the calculated target protein yield from the ELP fusion proteins. The 29-mg/L yield for Trx-ELP with 4-h growth, post-IPTG induction is qualitatively consistent with the results of a previous study (Meyer et al. 2001). In contrast, growth for 24 h without IPTG induction resulted in a sevenfold increase in the yield of purified Trx-ELP to 200 mg from a single liter of E. coli culture. We note, parenthetically, that these yields of the ELP fusion proteins are extraordinary by any measure, in that they are to our knowledge, rarely observed, even for model proteins in shaker flask cultures of E. coli. This amplification in yield with the noninduction protocol is clearly unique to the ELP purification tag, because oligohistidine fusions of the same proteins grown under identical culture conditions show at the most only a twofold increase in protein yield from the noninduction protocol, and in the cases of CAT and BFP show no measurable increase in yield.
Purification of CalM-ELP shows slightly different results than the other three ELP fusion proteins (Table 1). First, after three rounds of ITC, CalM-ELP exhibits similar yields regardless of culture condition. Furthermore, CalM-ELP exhibits much higher contamination from native E. coli proteins at each ITC round than any of the other three fusion proteins (Fig. 2 ▶), and CalM-ELP exhibits significant losses during each round of ITC (note significant CalM-ELP band in HS1 lane of CalM-ELP purification in Fig. 2 ▶). Because of the significant E. coli contamination, CalM must be subjected to as many as six rounds of ITC to obtain fusion protein of purity similar to the other three fusion proteins. As a result, only 12–24 mg of CalM-ELP can be purified from a single liter of E. coli culture.
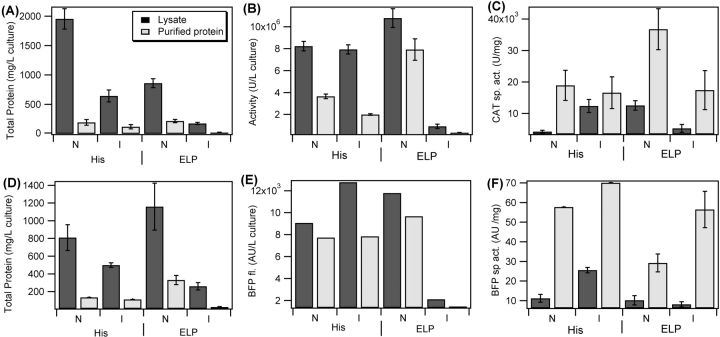
Comparison of the initial (soluble lysate) and end points of protein purification by IMAC and ITC for oligohistidine and ELP fusion proteins, respectively, shows the advantages of ELP fusion proteins as protein purification tools. Figure 4 ▶, A and D, shows the total protein contents and measures of total protein activity for 1-L cultures (Fig. 4B,E ▶) of CAT (upper row) and BFP (lower row) cultured with both fusion tags and using both culture conditions, as well as the specific activity associated with each of the cultures (Fig. 4C,F ▶). Culturing 24 h without induction results in two- to fourfold increase in total protein content in the soluble lysate over a 4-h IPTG-induced culture, regardless of purification tag; however, only the ELP fusion proteins exhibit substantial (10–13-fold) increases in purified fusion protein content by culturing without induction. Although oligohistidine fusion proteins exhibit similar increases in soluble lysate protein content, no significant increase in purified protein content is observed. These trends are corroborated by measured target protein activity assays; only ELP fusion proteins exhibit dramatic differences in total activity as a function of culture condition. Oligohistidine fusion proteins exhibit nearly the same target protein activity before and after purification regardless of culture condition. Thus, the amplification of purified ELP fusion protein yield by culturing without induction results in the production of total target protein activity from a l-L culture, which nearly equals that produced from the oligohistidine fusion proteins cultured under either condition.
Figure 4.
Quantitative analysis of total protein content determined by BCA assay (A,D), total target protein activity determined by commercial CAT activity assay and BFP arbitrary fluorescence units (B,E), and specific activity (C,F) as a function of purification tag and growth protocol measured from 1-L E. coli cultures of CAT (upper row) and BFP (lower row) showing the beginning and ending points of purification (respectively, lysate by dark bars and purified protein by light bars) by either two rounds of ITC for ELP fusion proteins and IMAC for oligohistidine fusion proteins. Bars labeled “I” represent cultures grown according to the induction protocol. Bars labeled “N” represent cultures grown without induction for 24 h. Error bars reflect the first standard deviation of the mean for at least three replicate measurements.
Target protein activity measurements also highlight the challenges associated with the purification of oligohistidine fusion proteins by IMAC. CAT-His6 exhibits significant losses during purification that are not observed for CAT- ELP. Purified fractions CAT-His6 exhibit only 25%–50% of the activity of the soluble lysate from which they were obtained. Only slight losses were observed for BFP-His6 during IMAC purification; however, this likely reflects the gentler buffer used to wash the BFP-His6. Once bound to the His•Bind column, BFP-His6 was washed with buffer containing only 5 mM imidazole rather than the wash buffer recommended by the resin manufacturer (Novagen), which contains 60 mM imidazole, because the use of the recommended wash buffer resulted in complete elution of the BFP-His6 during the wash step. In contrast, activity measurements suggest that nearly 80% of the active CAT-ELP is captured and purified after two rounds of ITC. Specific activity measurements also highlight these purification challenges (Fig. 4C,F ▶). Smaller increases in specific activity measurements for CAT-His6 between soluble lysate and purified protein, compared to BFP-His6, are indicative of increased CAT-His6 losses during purification compared to BFP-His6. Culture condition has no measurable effect on the specific activity of both purified oligohistidine fusion proteins.
Changes in the specific activity during ITC purification and the specific activity of the purified ELP fusion proteins cultured under the two different conditions vary to a greater degree than their oligohistidine analogs. The exact cause of this variation is unknown; however, the reduced specific activity of BFP-ELP purified from the noninduced culture is likely indicative of a greater degree of impurities associated with this fusion protein. After two rounds of purification (when these specific activity measurements were taken) uninduced BFP-ELP solutions were significantly more colored than BFP-ELP purified from the induced culture, indicating a higher level of contamination. If contaminating proteins are carried along during ITC purification by association with BFP-ELP, then it is not surprising that the uninduced culture, which has an expression level that is sevenfold greater than the 4-h IPTG culture, would be more likely to carry along a greater degree of contamination and require a greater number of rounds of ITC to achieve the same degree of purity. It should be noted that to get an accurate measure of BFP-ELP yield for Table 1, a final round of ITC was performed after raising the temperature to 65°C for 15 min, which resulted in the denaturation and aggregation of the contaminating proteins with no detectable change in the BFP fluorescence. It is unclear why CAT-ELP does not exhibit similar contamination problems with increases in fusion protein yield; however, it is not especially surprising that different target proteins with different surface properties would attract contaminating proteins to different degrees.
Although fusion proteins maintain their activity with the ELP purification tag still attached as evident in Figures 3 ▶ and 4 ▶, for many applications it is necessary to obtain the target protein free of its ELP purification tag. To this end we incorporated a thrombin cleavage site between the target protein and ELP domains of the expressed protein. Thus, thrombin can be used to cleave the ELP from the target protein, and ITC can be used to separate the thrombin cleavage products. Figure 5 ▶ shows cleavage of CAT-ELP (Fig. 5A,C ▶), and BFP-ELP (Fig. 5B,D ▶) by thrombin, and the effect of salt concentration on the separation of the cleavage products for the two different fusion proteins by ITC. Upper gels (Fig. 5A,B ▶) show the presence of both ELP and target protein fragments by copper staining, and the lower gels (Fig. 5C,D ▶) show only the staining of the target protein with Coomassie blue staining, as ELP does not stain by this method. While both fusion proteins (lane F) show complete cleavage (lane C) after overnight digestion with thrombin at 4°C, BFP and CAT show different separation characteristics as a function of the salt concentration used in the “hot spin.” BFP remains entirely in the supernatant (lane S) following the hot spin at low (150 mM), medium (1.65 M), and high (3.15 M) NaCl concentrations used to induce ELP aggregation. This is evident by both the SDS-PAGE gels and the location of the fluorescence in the supernatant (S) following the “hot spin.” These results show that the cleaved ELP can be entirely separated into the pellet (lane P) from the BFP protein, which remains in the supernatant using only a moderate concentration of NaCl to trigger the inverse transition of the ELP.
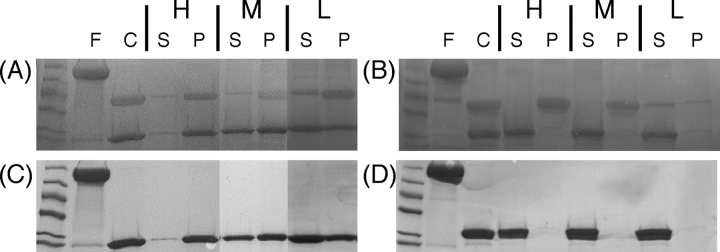
Figure 5.
SDS-PAGE showing the effect of salt on the separation of CAT (A,C) and BFP (B,D) from ELP after cleavage of the fusion protein by thrombin. Copper-stained gels (A,B) show bands for ELP (36 kDa) and target proteins (~29 kDa) while Coomassie-stained gels (C,D) allow selective visualization of only the target proteins. Lanes for undigested fusion protein, F; the cleavage products of digestion with thrombin, C; the supernatant after separation of aggregated ELP by centrifugation, S; and the resuspended ELP pellet, P. Lanes marked H, M, and L indicate that ELP aggregation induced with high (2.65 M NaCL for BFP and 3.15 M NaCl for CAT), medium (1.65 M NaCl for both CAT and BFP), and low (0.15 M NaCl BFP and 0.90 M NaCl for CAT) salt concentrations. Gels show that BFP is completely separated from the cleaved ELP under all three salt conditions. CAT is only partially separated from the cleaved ELP under any of these conditions and the amount of cleaved CAT remaining in the supernatant decreases with ionic strength. Molecular weight standards shown are 66, 55, 45, 36, 29, and 24 kDa.
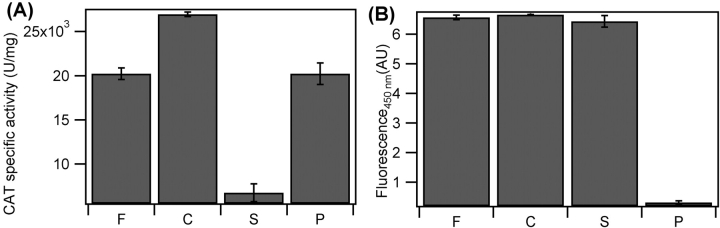
In contrast, CAT is only partially separated from the cleaved ELP, and the degree to which CAT remains in the supernatant is dependent upon the salt concentration used to aggregate the cleaved ELP. As the salt concentration is increased, a greater fraction of free CAT is captured by the aggregating cleaved ELP. When high salt concentrations are used to aggregate the cleaved ELP, nearly 100% of the cleaved CAT is captured by the aggregating ELP. This captured, but still properly folded, and active CAT can be resuspended in fresh PBS buffer along with the cleaved ELP without significant change in the CAT activity, as shown in Figure 6A ▶. The amount of free CAT successfully separated from the cleaved and aggregated ELP increases as the amount of salt used in the “hot spin” is decreased. At relatively low NaCl concentrations (900 mM), little more than half of the free CAT remains in the supernatant after the “hot spin.” Additionally, CAT could not be successfully completely separated from the cleaved ELP in PBS buffer only using heat to aggregate the cleaved ELP because the temperature needed to aggregate the ELP (~50°C) resulted in the thermal denaturation of the cleaved CAT, as evident by its irreversible aggregation. Cleavage of CAT from the fused ELP also results in a modest increase in its activity (Fig. 6A ▶), likely due to the removal of any steric hindrance of ELP around the active site of the enzyme. Similar changes in enzyme activity have been observed when synthetic, thermally sensitive polymers of N,N-dimethylacrylamide (DMA)/N-4-phenylazo-phenylacrylamide (AZAAm) copolymers (DMAAm) have been covalently attached to enzymes. Depending on the size of the enzyme and the location of the conjugated polymer relative to the enzyme binding pocket, the enzyme can exhibit reduced activity even in the expanded state of the polymer (Shimoboji et al. 2002, 2003). Cleavage of BFP, in contrast, results in no measurable change in BFP fluorescence (Fig. 6B ▶). This is consistent with the fact that BFP activity is intrinsic to the protein itself, and is not dependent upon diffusion of reagents to an active site as with an enzyme, which are subject to diffusional perturbations caused by the presence of the ELP polymer.
Figure 6.
Quantitative analysis of specific activity for CAT (A) and BFP (B) in the ELP fusion protein (F), after (C) cleavage from the ELP fusion partner by thrombin, in supernatant (S) after centrifugation above the cleaved ELP inverse phase transition, and in the resuspended pellet (P) following centrifugation. Specific activity for CAT is expressed in units activity per milligram of total fusion protein, and in arbitrary fluorescence units for 5-μM solutions of BFP-ELP fusion protein. Error bars represent the first standard deviation of three replicate measurements.
Discussion
Protein purification using the environmentally triggered, reversible solubility of fused ELPs has several advantages over traditional protein purification techniques such as metal affinity binding. First, the expression conditions conducive to high ELP fusion protein yields are easier and cheaper to implement than more traditional E. coli culture protocols that utilize IPTG to induce expression of a recombinant protein. For efficient expression of ELP fusion proteins, longer culture times of 24 h, which are easily implemented in the laboratory, are desirable. These cultures require no monitoring of the optical density and do not require the addition of IPTG, thereby reducing the cost of the culture.
Second, ITC is a nonchromatographic separation method. By circumventing chromatography, the expense and challenges associated with chromatographic resins and equipment are eliminated. ITC requires no specialized technical equipment or reagents, as fusion protein capture is mediated only by simple changes in ionic strength and temperature with separation by centrifugation, and as much as 75% of the total expressed fusion protein can be successfully isolated with high purity through three rounds of purification. Third, because inverse transition cycling is a nonchromatographic method, scale-up from μg-gram quantities is facile and the technique may be multiplexed for high-throughput purification.
Although purification of BFP would suggest that the percent yield of purified protein is higher for IMAC purification, these numbers are extremely deceptive. CAT-His6 exhibits significantly greater losses during purification, indicating that losses in target protein are highly dependent on the protein itself. Also, in contrast to purification of the ELP fusion proteins, for which the entire 1 L of culture was purified, only a small fraction (1/40 to 1/10) of a 1-L culture of oligohistidine fused ELP was purified at a time, because of the large volume of resin required, which would make the cost of purifying the entire 1-L culture for the different constructs prohibitive in this context. The total purified yield was calculated based upon 100% efficiency in the scale-up to purify an entire liter of culture at one time. This assumption is not completely realistic, and it is likely that protein losses in IMAC will not scale linearly, so that these numbers represent an upper bound of the protein yield with IMAC. Thus, ITC purification of ELP fusion proteins is not only technologically simpler, faster, and cheaper, producing target protein yields similar to their oligohistidine fusion protein counterparts, but it ultimately has the potential for far more efficient purification with minimal protein losses in comparison to chromatographic techniques.
Finally, ITC is fast and technically simple, with only a few short centrifugation or filtration steps followed by resolubilization of the purified protein in a low ionic strength buffer. Because of the rapid and simple nature of ITC, an ELP fusion protein from as much as 8 L of E. coli culture can be purified by one individual in approximately 8 h. For highly expressed proteins (such as Trx, BFP, or CAT), based on the yields observed in this study, purification from an 8-L culture would yield as much as 400–800 mg of the target protein (~1600 mg of ELP fusion protein) from a single day’s work.
Furthermore, ELP tags stabilize fused target proteins against irreversible aggregation and denaturation at high concentrations. All the investigated oligohistidine fusion proteins were less stable than their corresponding ELP fusion proteins at high concentrations in PBS. Dialysis to remove the imidazole used to elute the proteins from the His-Bind resin, resulted in irreversible denaturation and aggregation of the proteins if not first diluted by approximately 10-fold in PBS buffer prior to dialysis against PBS buffer. ELP fusion proteins showed no such irreversible aggregation and denaturation at high concentrations. It is remarkable that ELP fusion proteins can be concentrated in the coacervate up to 300 mg/mL (Betre et al. 2002) during the “hot spin” step of ITC purification. Furthermore, after completion of their purification by inverse transition cycling, we observed that the soluble, purified ELP fusion proteins could be frozen in PBS buffer at concentrations on the order of 100–150 mg/mL without the need for protein stabilizers such as glycerol, and showed no freeze/thaw induced aggregation and no experimentally significant change in protein activity with storage. The ability of ELP tags to stabilize proteins in aqueous solution at high concentration was further confirmed by the observation that proteolytic cleavage of target proteins from their ELP fusion partners must be performed at relatively low concentrations (~6 mg/mL for BFP-ELP and CAT-ELP) to maintain the solubility of the target protein. Concentrations greater than 6 mg/mL BFP-ELP or CAT-ELP resulted in the irreversible aggregation and denaturation of the target protein. In contrast, both BFP-ELP and CAT-ELP are soluble in aqueous buffer up to concentrations of at least 200 mg/mL.
Although proteins maintain their activity with ELP tags still attached, cleavage can result in a slight increase in the activity of enzymes whose active site could be partially sterically hindered by the presence of a sizable ELP tag. After enzymatic cleavage of the target protein from its ELP fusion partner, the two can be at least partially separated by another round of ITC. BFP and Trx can be completely separated from the ELP fusion partner, while CAT can only be partially separated. Active CAT protein is captured by the aggregating ELP, and the degree to which this occurs is proportional to the amount of NaCl used to induce the inverse phase transition of the free ELP. Clues as to why only certain proteins are captured by aggregating cleaved ELP may lie in the molecular properties presented on the surface of the fused target proteins. In previous studies we have shown that the inverse transition behavior of an ELP is dictated by the hydrophobicity of surfaces within close molecular proximity to an ELP (Trabbic-Carlson et al. 2004). Specifically, we showed the temperature at which an adsorbed ELP transitioned on the surface of a chemically modified gold colloid was dependent on the hydrophobicity of that surface and the reversibility of the ELP-mediated aggregation of the gold colloids is dependent on the hydrophobicity of the colloid surface, with only the hydrophilic surface showing reversibility. We also showed that the Tt of an ELP fusion protein was negatively correlated with the fraction of hydrophobic area presented on the surface of the fused folded protein. Proteins with a relatively high hydrophobic solvent-accessible surface area (SAShydrophobic), such as CAT (SAShydrophobic = 0.35), depressed the inverse transition temperature of the fused ELP, whereas proteins with more hydrophilic surfaces, such as BFP (SAShyrophobic = 0.198), slightly elevated the transition temperature of the ELP fusion protein relative to that of the ELP.
Because the inverse phase transition of ELPs has been shown to involve the collapse of ELP molecules and loss of their waters of hydrophobic hydration, thereby increasing the contact between hydrophobic residues (Li et al. 2001), we hypothesized that hydrophobic surfaces presented on the surface of folded proteins provided hydrophobic surfaces with which ELPs could interact during this hydrophobic collapse that precedes the inverse phase transition, thereby releasing water not only from the fused ELP but also from these hydrophobic moieties on the protein surface. The release of these extra waters of hydrophobic hydration increases the entropic contribution to the phase transition, leading to a lowered transition temperature of the ELP fusion protein, compared to the same ELP.
If hydrophobic surfaces in close molecular proximity can affect the phase transition of ELPs through ELP–protein interactions to maximize the release of waters of hydrophobic hydration, we believe that the ELP tag might similarly have a propensity to associate with hydrophobic proteins even after their cleavage from the ELP by thrombin. Solution conditions that alter the structure of water (i.e., high dissolved salt concentrations) and perturb the ability of water to form more ordered structures around hydrophobic surface residues of the folded protein would likely promote the formation of contacts between hydrophobic residues on the cleaved ELP and hydrophobic patches on the surface of folded proteins. It then stands to reason that proteins exhibiting greater amounts of surface hydrophobicity (i.e., CAT) would be more susceptible to this hydrophobic interaction than proteins with more hydrophilic surfaces (i.e., BFP). A potential solution to this problem is to use more salt-sensitive ELPs (through the incorporation of charged residues such as lysine in the guest residue position of the ELP [Trabbic-Carlson et al. 2003]) as purification tags for hydrophobic proteins to prevent their association with the ELP after cleavage.
Although this study clearly illustrates the potential advantages of ELPs as protein purification tags, the tag used in this investigation is not optimized. For example, Trx is a small protein (11.7 kDa) relative to the 36 kDa ELP purification tag used in this study. As such, only approximately 25% of the overexpressed fusion protein biomass is due to the target protein mass. Previous studies showed that truncation of the ELP fusion tag to 9kDa resulted in a 70% increase in fusion protein yield and because of the higher mass fraction of Trx in the fusion protein, this increased yield reflects a nearly fourfold increase in the yield of Trx. Thus, with the smaller ELP tag and uninduced culture conditions we would anticipate yields as much as 200 mg of Trx/L of culture, more than twice that of Trx expressed with an oligohistidine tag using traditional culture conditions.
Similarly, the molecular basis for the challenges in the purification of CalM-ELP lie in its highly charged nature. CalM has a pI of 4.15 (Walker et al. 1984), has a charge of approximately −24 at pH 7.0 (DNAStar calculations using Protean), and has a molecular weight similar to that of the ELP tag. Previous studies have shown that highly charged target proteins with ELP tags having similar molecular weight can form micellar phases that will not efficiently separate during centrifugation (Meyer et al. 2001). In this previous study, increasing the molecular weight of the ELP tag allowed more efficient separation of the fusion protein. Thus, we might anticipate better recovery of CalM-ELP with fewer purification steps by engineering a new CalM-ELP fusion protein having an ELP tag of higher molecular weight.
In conclusion, we have clearly demonstrated in this study that ELP tags can be used to efficiently purify CAT, BFP, Trx, and CalM, with target protein yields similar to conventional oligohistidine fusion proteins purified by IMAC with equal to, and in some cases better, retention of functional activity, vastly improved solubility, and significantly easier scale-up. Although ELP fusion protein technology may or may not ultimately prove to be applicable to all varieties of target proteins, these examples highlight the great potential of this technology.
Materials and methods
Gene synthesis
A synthetic gene with SfiI-generated, compatible sticky ends encoding for a 90-pentapeptide ELP was synthesized by recursive directional ligation in pUC-19 (Meyer and Chilkoti 2002). The characteristic ELP repeat sequence of VPGXG contained 50% Val, 30% Gly, and 20% Ala at the X position in this particular ELP. The DNA sequence of this synthetic gene has been published previously (Meyer and Chilkoti 1999). ELP fusions with blue fluorescent protein (BFP, Qbiogene), chloramphenicol acetyltransferase (CAT, donated by Invitrogen), and calmodulin (CalM, donated by Invitrogen) were synthesized by inserting the target gene 5′ to the ELP gene in a modified pET25b vector. The modified pET25b vector was produced by replacement of the NotI to AvaI segment of pET25b (Novagen) with an oligonucleotide cassette encoding for an oligohistidine tag, thrombin cleavage site, and an SfiI restriction site (Trabbic-Carlson et al. 2004). The ELP gene was inserted into the SfiI restriction site as previously described (Meyer and Chilkoti 1999). Details regarding gene cloning for BFP and CAT have been published previously (Trabbic-Carlson et al. 2004). The ELP fusion to thioredoxin (Trx, Novagen) was created by modification of pET32a (Novagen) in which the thioredoxin gene was already present. Details regarding Trx-ELP cloning have been published previously (Meyer and Chilkoti 1999).
The CalM gene was retrieved from its plasmid (provided by Invitrogen) by PCR and TA cloning (Invitrogen), in which NdeI and SalI restriction sites were also incorporated 5′ and 3′ to the gene, respectively. The gene was excised from the TA vector using NdeI and SalI, separated from the linearized vector by gel electrophoresis and isolated from the agarose gel after electrophoresis. The modified pET25b vector, previously described, was digested with NdeI and SalI, and the fusion protein was assembled by ligation of the CalM gene with the restricted pET25b vector containing the ELP gene. The DNA sequence of each fusion protein was confirmed by DNA sequencing using dye terminator chemistry. Genes for BFP, Trx, CAT, and CalM were also inserted between the NdeI and SalI sites in an unmodified pET25b vector, which contains an oligohistidine tag (His6) 3′ to the gene of the inserted protein for purification by IMAC. Oligohistidine fused Trx was expressed from the modified pET32a vector lacking the ELP gene (Meyer and Chilkoti 1999).
Protein expression
BFP, Trx, CAT, and CalM were expressed as His6 or ELP fusion proteins from BLR(DE3) E. coli (Novagen). All proteins were expressed as 1-L cultures from CircleGrow media (Qbiogene), supplemented with 100 μg/mL ampicillin. One-liter cultures were inoculated with cells from 20 mL of a starter culture (250 mL flask containing 50 mL of medium supplemented with 100 μg/mL ampicillin) that was inoculated from frozen (−80°C) DMSO stocks and grown overnight. Two growth protocols were investigated for the expression of both ELP and His6 fusion proteins. In the first, chemical induction protocol, 1-L cultures of E. coli harboring the expression plasmid for an ELP fusion protein or a His6 fusion protein were grown at 37°C with shaking (~250 rpm), and were induced with IPTG at an optical density at 600 nm (OD600) of 0.8. After induction, the E. coli were cultured for an additional 4 h, and were then harvested by centrifugation. In the second expression protocol, identical E. coli were grown under the same conditions for 24 h without induction with IPTG.
ELP fusion protein purification
E. coli cultures were harvested by centrifugation for 15 min at ~7000 rcf at 4°C, were resuspended in 35 mL of PBS buffer supplemented with a protease inhibitor cocktail (Roche Catalog number 1697498), and were stored at −80°C until purification. The frozen cultures were thawed, were lysed by ultrasonic disruption on ice, and the lysate was centrifuged at ~20,000 rcf at 4°C for 15 min to remove insoluble debris. Polyethyleneimine was added to the soluble lysate (0.5% w/v) to precipitate nucleic acids, which were removed by centrifugation (~20,000 rcf for 15 min). Each ELP fusion protein was purified from soluble E. coli lysate using inverse transition cycling (Meyer and Chilkoti 1999). The ionic strength of the soluble lysate was increased to cause aggregation of the ELP fusion protein in the cell lysate, and the aggregated ELP fusion proteins were separated from soluble E. coli proteins by centrifugation at ~20,000 rcf for 15 min. CAT, BFP, and Trx fusions were purified by the addition of NaCl (1.25–2 M, depending on construct and expression level), and centrifugation to separate the fusion protein was performed at ~25°C. CalM-ELP was aggregated by the addition of ammonium sulfate (2 M) with centrifugation at 17°C. The pellet containing the ELP fusion protein coacervate was resuspended in cold PBS and centrifuged at 4°C to remove insoluble contaminants. This technique of thermal cycling and centrifugation was repeated (usually three times) until the ELP fusion protein was determined to be approximately 95% pure of E. coli contamination by visualization of Coomassie and/or copper stained SDS-PAGE gels. Target proteins were liberated from their ELP fusion partner by enzymatic cleavage using thrombin. All proteins were cleaved overnight at 4°C from solutions containing approximately 100 μM fusion protein using 10 units of thrombin per μmole of fusion protein. The cleavage products were purified by another round of inverse transition cycling.
IMAC purification
His6 fused protein cultures were harvested by centrifugation at 4°C, were resuspended in 35 mL of binding buffer (20 mM Tris [pH 7.9], 0.5 M NaCl, 5 mM imidazole) supplemented with an EDTA-free protease inhibitor cocktail (Roche, catalog number 1873580), were aliquoted into volumes representing 1/5 of a culture, and were stored at −80°C until purification. Resuspended cultures were lysed by ultrasonic disruption at 4°C and centrifuged at ~20,000g rcf for 15 min to remove insoluble matter. IMAC resin (His•Bind Resin, Novagen) was charged with 50 mM NiSO4, and equilibrated with IMAC binding buffer (2 mL/min on an ISCO low-pressure liquid chromatography (LPLC) instrument. A fraction of the soluble cell lysate (typically 1/40 to 1/10 of the total lysate) was diluted to 100 mL with binding buffer and loaded onto His•Bind resin in a column (10 mL bed volume) coupled to a Tris (ISCO) low-pressure chromatographic system. For CAT, CalM, and Trx, the column was equilibrated with wash buffer (20 mM Tris [pH 7.9], 1.0 M NaCl, 60 mM imidazole), and the OD280 was reequilibrated to provide a flat baseline. For BFP, the binding buffer was used, because wash buffer eluted the oligohistidine fused BFP from the column with the contaminant proteins. All four proteins were eluted with a linear gradient of imidazole (20 mM Tris [pH 7.9], 0.5 M NaCl, 60–1000 mM imidazole), and 3-mL fractions were collected. Fractions exhibiting a peak absorption at 280 nm were combined. One milliliter of this solution was diluted by 10-fold in PBS and dialyzed against PBS overnight at 4°C. Failure to dilute the oligohistidine fusion proteins prior to dialysis resulted in the irreversible aggregation of the target protein. The protein concentration following dialysis of this small fraction of total yield (typically only 1/300–1/400 of the total culture) was used to estimate the anticipated yield from the whole 1-L culture.
Assays
Target protein concentrations were determined from their absorption at 280 nm using extinction coefficients at 280 nm calculated from the primary amino acid sequence with the software program Protean (DNA Star). UV-visible spectra were collected on a Shimadzu UV-1601 UV-visible spectrometer. Protein content during stages of ITC and IMAC purification was determined by a BCA protein assay kit (Pierce) using BSA as a calibration standard.
Protein activity for easily assayed proteins was assessed at various stages of the purification process as well as before and after cleavage to liberate the fused protein from its ELP fusion partner. BFP activity was assayed by its fluorescence emission intensity at 450 nm. Samples were diluted until they fell within the dynamic range of the detector. Fluorescence spectra were collected between 390 and 600 nm with 387 nm excitation on an Aminco-Bowman, Series 2, Luminescence Spectrometer. Arbitrary fluorescence units reported as a measure of BFP activity per liter of E. coli culture were scaled by the sample dilution factor for fluorescence measurement.
CAT activity was assayed by a FAST CAT Green assay kit (Molecular Probes). The protocol published by Molecular Probes was used to determine the quantitative CAT activity from the relative fluorescence of the substrate and product at 525 nm after 30 min of CAT-mediated acetylation of BODIPY FL 1-deoxychloramphenicol substrate at 37°C. Purification samples taken from cultures grown with and without induction were diluted by 32,500- and 325,000-fold, respectively, prior to reaction with the fluorescent substrate. The reaction was carried out according to the kit protocol with a 30-min incubation at 37°C. The substrate and product of the reaction were separated by thin-layer chromatography using a chloroform:methanol (85:15 v/v) mixture, were visualized with UV transilluminator, were carefully scraped into individual microcentrifuge tubes, and were extracted with 1 mL of methanol. The fluorescence of the methanol extracts was measured to determine the percentage of substrate conversion and therefore the units of CAT activity in each reaction. TLC samples were run in triplicate from each reaction.
Acknowledgments
The gene for CAT was graciously donated by Dr. Jon Chesnut at Invitrogen. We thank the NIH (GM-061232) for their financial support of this research.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04931604.
References
- Betre, H., Setton, L.A., Meyer, D.E., and Chilkoti, A. 2002. Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules 3 910–916. [DOI] [PubMed] [Google Scholar]
- Grossman, T.H., Kawasaki, E.S., Punreddy, S.R., and Osburne, M.S. 1998. Spontaneous cAMP-dependent derepression of gene expression in stationary phase plays a role in recombinant expression instability. Gene 209 95–103. [DOI] [PubMed] [Google Scholar]
- Guda, C., Zhang, X., McPherson, D.T., Xu, J., Cherry, J.H., Urry, D.W., and Daniell, H. 1995. Hyper expression of an environmentally friendly synthetic polymer gene. Biotechnol. Lett. 17 745–750. [Google Scholar]
- Lavallie, E.R., Diblasio, E.A., Kovacic, S., Grant, K.L., Schendel, P.F., and McCoy, J.M. 1993. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the Escherichia-coli cytoplasm. Biotechnology 11 187–193. [DOI] [PubMed] [Google Scholar]
- Li, B. and Daggett, V. 2003. The molecular basis of the temperature- and pH-induced conformational transitions in elastin-based peptides. Biopolymers 68 121–129. [DOI] [PubMed] [Google Scholar]
- Li, B., Alonso, O.V., and Daggett, V. 2001. The molecular basis for the inverse temperature transition of elastin. J. Mol. Biol. 305 581–592. [DOI] [PubMed] [Google Scholar]
- Maina, C.V., Riggs, P.D., Grandea, A.G.I., Slatko, B.E., Moran, L.S., Tagliamonte, J.A., McReynolds, L.A., and diGuan, C. 1988. An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from maltose binding protein. Gene 74 365–373. [DOI] [PubMed] [Google Scholar]
- Meyer, D.E. and Chilkoti, A. 1999. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 17 1112–1115. [DOI] [PubMed] [Google Scholar]
- ———. 2002. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: Examples from the elastin-like polypeptide system. Biomacromolecules 3 357–367. [DOI] [PubMed] [Google Scholar]
- Meyer, D.E., Trabbic-Carlson, K., and Chilkoti, A. 2001. Protein purification by fusion with an environmentally responsive elastin-like polypeptide: Effect of polypeptide length on the purification of thioredoxin. Biotechnol. Prog. 17 720–728. [DOI] [PubMed] [Google Scholar]
- Nilsson, J., Ståhl, S., Lundeberg, J., Uhlén, M., and Nygren, P.Å. 1997. Affinity fusion strategies for detection, purification, and immobilization of recombinant proteins. Protein Expr. Purif. 11 1–16. [DOI] [PubMed] [Google Scholar]
- Sassenfeld, H.M. 1990. Engineering proteins for purification. Trends Biotechnol. 8 88–93. [DOI] [PubMed] [Google Scholar]
- Shimoboji, T., Larenas, E., Fowler, T., Kulkarni, S., Hoffman, A.S., and Stayton, P.S. 2002. Photoresponsive polymer–enzyme switches. Proc. Natl. Acad. Sci. 99 16592–16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoboji, T., Larenas, E., Fowler, T., Hoffman, A.S., and Stayton, P.S. 2003. Temperature-induced switching of enzyme activity with smart polymer–enzyme conjugates. Bioconjug. Chem. 14 517–525. [DOI] [PubMed] [Google Scholar]
- Smith, D.B. and Johnson, K.S. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67 31–40. [DOI] [PubMed] [Google Scholar]
- Trabbic-Carlson, K., Setton, L.A., and Chilkoti, A. 2003. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules 4 572–580. [DOI] [PubMed] [Google Scholar]
- Trabbic-Carlson, K., Meyer, D.E., Liu, L., Piervincenzi, R., Nath, N., LaBean, T., and Chilkoti, A. 2004. Effect of protein fusion on the transition temperature of an environmentally responsive elastin-like polypeptide: A role for surface hydrophobicity? Protein Eng. Des. Sel. 17 57–66. [DOI] [PubMed] [Google Scholar]
- Uhlén, M. and Moks, T. 1990. Gene fusions for purpose of expression: An introduction. Methods Enzymol. 195 129–143. [DOI] [PubMed] [Google Scholar]
- Urry, D.W. 1988. Entropic elastic processes in protein mechanisms. I. Elastic structure due to an inverse temperature transition and elasticity due to internal chain dynamics. J. Protein Chem. 7 1–34. [DOI] [PubMed] [Google Scholar]
- ——— 1992. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog. Biophys. Mol. Biol. 57 23–57. [DOI] [PubMed] [Google Scholar]
- ———. 1997. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. J. Phys. Chem. 101 11007–11028. [Google Scholar]
- Urry, D.W., Trapane, T.L., and Prasad, K.U. 1985. Phase-structure transitions of the elastin polypentapeptide–water system within the framework of composition-temperature studies. Biopolymers 24 2345–2356. [DOI] [PubMed] [Google Scholar]
- Walker, S.W., Wark, J.D., Macneil, S., Mellersh, H., Brown, B.L., and Tomlinson, S. 1984. Isolation, purification and cell-free synthesis of calmodulin from the pig anterior-pituitary gland. Biochem. J. 217 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]