Abstract
Xylokorys chledophilia, a new arthropod with three-dimensionally preserved soft tissues, is described from the Herefordshire (Silurian) Lagerstätte of England. The head and trunk are covered by a relatively featureless ovoid carapace, which comprises a domed central part and a flange-like border. The head bears five pairs of appendages. The first is uniramous, with dorsal and ventral projections distally. Appendages two to four are biramous and each endopod terminates in two projections. Appendage five is possibly biramous. The hypostome is very long and subrectangular in outline. There are approximately 35 pairs of biramous trunk appendages. Each exopod comprises a long slender shaft bearing numerous fine filaments; each endopod comprises a ribbon-like shaft bearing paddle-like endites. Morphological comparisons and cladistic analyses of X. chledophilia indicate affinity with Vachonisia rogeri from the Lower Devonian Hunsrück Slate, within the marrellomorphs, but assignment to Marrellomorpha is provisional pending revision of other members of this clade. Xylokorys is the first ‘marrellomorph’ to be reported from the Silurian. It is interpreted as a benthic particle filter feeder, which may also have consumed prey items.
Keywords: Arthropoda, exceptional preservation, Herefordshire Lagerstätte, Marrellomorpha, Silurian, Wenlock Series
1. Introduction
A new arthropod, Xylokorys chledophilia, is described from the Herefordshire Lagerstätte of the Welsh Borderland (Briggs et al. 1996: see Siveter et al. in press and references therein), in which a wide variety of invertebrates of mid-Silurian, Wenlock Series age (approx. 425 Myr ago) are exceptionally preserved in three dimensions. Arthropods form one of the main components of this fauna, including a stem-group chelicerate (Orr et al. 2000b; Sutton et al. 2002), two myodocopid ostracodes (Siveter et al. 2003, 2007), a phyllocarid (Briggs et al. 2004), a pycnogonid (Siveter et al. 2004), a barnacle (Briggs et al. 2005) and a stem-group crustacean (Siveter et al. 2007). Xylokorys chledophilia is placed provisionally within Marrellomorpha pending revision of other taxa assigned to this inadequately known arthropod group; it provides the most complete three-dimensional data on any ‘marrellomorph’ species. The four previously recorded marrellomorph genera are known from a few localities of Cambrian, Ordovician and Devonian age in, respectively, Canada and China (Marrella), Bohemia and Morocco (Furca) and Germany (Mimetaster and Vachonisia). Furca is known from approximately 55 specimens (Chlupáč 1999; P. Van Roy 2007, personal communication), Mimetaster and Vachonisia from, respectively, approximately 75 and 20 (Stürmer & Bergström 1976; Bartels et al. 1998; G. Oltmann 2007, personal communication), whereas over 24 000 specimens have been recorded for Marrella (García-Bellido & Collins 2006). Xylokorys is the first example reported from the Silurian: just two specimens are known, comprising approximately 0.05% of the recorded number of specimens from the Herefordshire Lagerstätte.
Marrellomorphs have been considered by many authors as basal to trilobites and their ‘arachnomorph’ relatives, the latter comprising many fossil taxa as well as living and fossil chelicerates; however, in some investigations, they have resolved basal to all other schizoramian arthropods, including the crustaceans (see §4).
2. Material and methods
The fossils of the Herefordshire Lagerstätte are preserved in three-dimensional form as calcitic void infills in early diagenetic carbonate concretions within a volcaniclastic horizon (Orr et al. 2000a). They retain fine morphological detail of external surfaces, and some also show the remains of the gut. In order to make them available for study, the specimens are serially ground, digitally photographed and then rendered in the round as ‘virtual fossils’ by computer (Sutton et al. 2001a,b, 2002). One of the Xylokorys specimens (OUMNH C.29604) has been reconstructed following serial grinding at 30 μm intervals, and then reconstruction at 60 μm intervals except for detailed reconstruction of the trunk appendages, which used every 30 μm ‘slice’. Prior to grinding, this specimen was traversed by three parallel cuts with a 300 μm saw. The first traverses the anterior extremity of the specimen, passing through the carapace on the right-hand side as well as the distal part of the first pair of appendages; the second occurs at the junction of the head and trunk appendages; and the third is near the posterior margin. The virtual fossil was studied on-screen using interactive stereo-capable viewing software, supplemented by hard copy stereo-pair images.
3. Systematic palaeontology
Phylum: Arthropoda
? Class: Marrellomorpha Beurlen, 1934
Genus: Xylokorys gen. nov.
Derivation of name. Greek, xylos (pith)+korys (helmet), after the fancied resemblance of the carapace to the favoured tropical headgear of European military personnel during colonial times.
Diagnosis. Carapace covering head and trunk, ovoid in outline, with a domed central part and a flange-like border indented by a narrow anterior notch and a wide posterior embayment. The head bears five pairs of appendages. The first is short, uniramous, with dorsal and ventral projections distally, the dorsal one (at least) ending in an ear-like process. Appendages two to four are large and biramous. Each endopod bears two projections distally and each exopod bears a fringe of setae distally. The exopod of the fourth appendage is very long. Appendage five is possibly biramous. The hypostome is very long and subrectangular in outline. There are approximately 35 pairs of biramous trunk appendages. Each exopod comprises a long slender shaft bearing approximately 25 fine filaments. Each endopod comprises a ribbon-like shaft with one or two endites proximally, four paddle-like endites along its length and a similar terminal process.
Species: Xylokorys chledophilia sp. nov.
Derivation of name. Greek, chledos (mud)+philia (fondness)
Diagnosis. As for the genus.
Holotype. OUMNH C.29603 (figure 2k), a complete specimen; median length approximately 32 mm.
Figure 2.
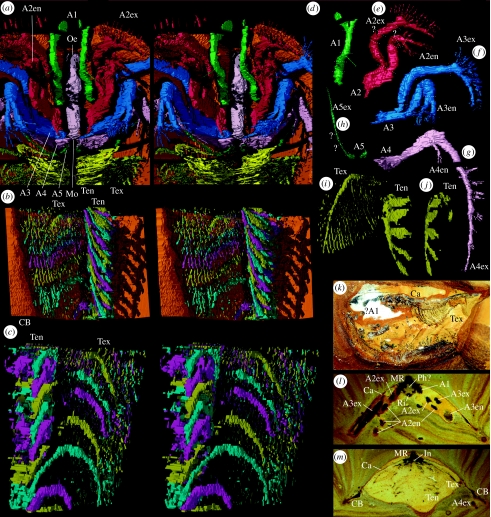
Xylokorys chledophilia. (a–j,l,m) Complete specimen, OUMNH C.29604, (a–j) ‘virtual’ reconstructions. (a) Median cephalic structures, hypostome removed, ventral stereo-pair, ×3.1. (b) Selected trunk appendages (right-hand side), coloured in a repeating sequence of cyan, magenta and yellow, body removed, ventral stereo-pair, ×6.9. (c) Trunk appendages from (b), dorsal stereo-pair, ×10.3. (d–g) Appendages 1–4 (left-hand side), ventrolateral views, ×2.7. The dotted lines in (d–g,h) indicate discrete parts of the appendage defined by flexures that are presumed to be podomere boundaries; some of these parts probably equate to a single podomere, others to more than one podomere (see description). (h) Appendage 5 (right-hand side), ventrolateral view, ×2.7. (i) Trunk appendage (right-hand side) in subventral view, ×7.8. (j) Endopod of trunk appendage from (i), ventrolateral view, ×7.8. (l,m) Serial grinding sections, subtransversely orientated, through posterior part of cephalic region and mid-trunk region, ×3.0. (k) Complete specimen, holotype, OUMNH C.29603, submedian section, ×1.5. Ca, carapace; Ri, ridge; all other abbreviations used are the same as given in figure legend 1.
Other material. OUMNH C.29604 (figures 1a–l and 2a–j,l,m), a complete, serially ground and reconstructed specimen; median length 30.5 mm, maximum transverse width 26.1 mm.
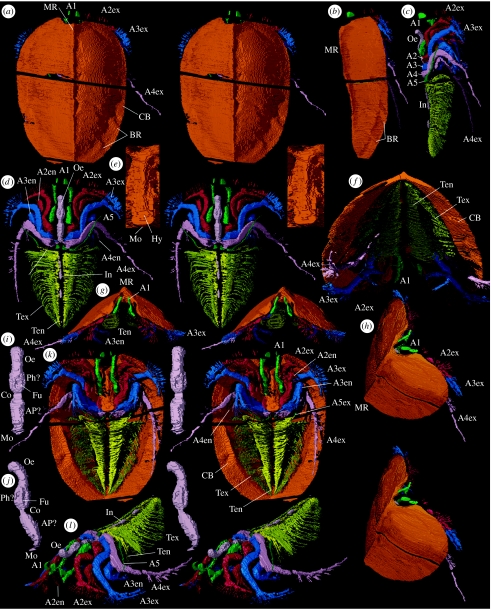
Figure 1.
Xylokorys chledophilia. (a–l) Almost complete specimen, OUMNH C.29604, ‘virtual’ reconstructions. (a,b,g,k) Dorsal stereo-pair, lateral view, anterior and ventral stereo-pairs, ×1.6. (f) Posteroventral view, ×2.4. (h) Anterior oblique stereo-pair, ×1.7. (c,d,l) Carapace removed, lateral view, dorsal and anterior oblique stereo-pairs, ×1.6. (e) Hypostome with surrounding appendages removed, ventral stereo-pair, ×2.7. (i,j) Gut infill, ventral and lateral stereo-pairs, ×3.4. A1, appendage 1; A2, limb base of appendage 2; A2en, appendage 2 endopod; A2ex, appendage 2 exopod; A3, limb base of appendage 3; A3en, appendage 3 endopod; A3ex, appendage 3 exopod; A4, limb base of appendage 4; A4en, appendage 4 endopod; A4ex, appendage 4 exopod; A5, limb base of appendage 5; A5ex, appendage 5, presumed exopod; AP?, anterior section of pharynx ?; BR, border ridge; CB, carapace border; Co, constriction; Fu, furrow; Hy, hypostome; In, intestine; Mo, mouth; MR, median ridge; Oe, oesophagus; Ph?, pharynx ?; Ten, trunk appendage endopod; Tex, trunk appendage exopod.
The datasets from serial grinding are housed in the Oxford University Museum of Natural History (OUMNH). The description is based on OUMNH C.29604.
(a) Carapace
The carapace (figures 1a,b,f–h,k and 2l,m) is ovoid in outline, the length approximately 1.5 times the width. In frontal profile, it is dome-like, the height approximately 0.5 times the width; each side is gently convex dorsally and slopes laterally at approximately 45°. The maximum height is very close to the anterior margin (figure 1b). In lateral view, the median ridge approximates a straight line with a slight concavity posterior of the mid-length. The anterior margin slopes steeply anteroventrally curving abruptly into the convex ventral margin that converges gradually and then more rapidly with the dorsal margin in the posterior third of its length, the two presumably merging in the carapace border. The first appendage emerges through a rounded median notch that is approximately 0.25 times the width of the carapace. Posteriorly, the carapace margin is very broadly embayed laterally (preserved on left-hand side only); its shape medially is unknown. A median ridge runs the length of the carapace; it is very narrow anteriorly, widens slightly almost to the mid-length and narrows slightly to the posterior margin. There is a pronounced carapace border, which is narrowest anteriorly, becoming wider and more distinct posteriorly where the weak border furrow becomes broader. At the mid-length, the border is approximately 0.1 times the width of the carapace. On the posterior part of the border, particularly on the right-hand side, two weak parallel ridges are evident orientated obliquely to the margin. The lateral border is thicker than the main part of the carapace, especially near the two ridges (figure 2m). The border is slightly concave ventrally here and its inner margin is most sharply defined. The carapace inside the border is featureless apart from the median ridge and a slightly flattened ovoid area that flanks the median ridge and at maximum is 25% as wide as the carapace, and is from approximately 10 to 70% of the carapace length from its anterior end. This area appears to reflect the internal attachment area of the body.
(b) Head
Eyes are absent. The first appendage is uniramous; it originates immediately anterior to the lateral shoulder of the hypostome and extends just beyond the carapace margin (figures 1a–d,f–h,k,l and 2a,d,k,l). Five parts are evident, presumably corresponding to podomeres. The most proximal podomere is stout and directed anteriorly. The appendage flexes anteroventrally at the articulation between podomeres one and two and anteriorly again between three and four. The third podomere bears a long, fine seta ventrolaterally. The appendage terminates in a dorsal and a ventral projection; the former comprises a laterally flat, ear-like process, and the latter expands for a short distance proximally but is unknown distally. One of these is presumably a projection of the penultimate podomere (similar to the structure of the great appendage in, for example, Alalcomenaeus (see Briggs & Collins 1999), or the fixed finger of a chela).
The second appendage is biramous (figures 1a–d,f–h,k,l and 2a,e,l). The elongate proximal part of the limb base bears a spine-like extension posteriorly that is directed strongly posteriorly and slightly inwards near the mouth. The more distal part of the limb base is laterally compressed with subrectangular inner and outer faces, the inner of which is weakly divided by a shallow groove into anterior and posterior portions that continue distally into an exopod and endopod, respectively. The proximal part of the endopod (? a single podomere) is directed subanteriorly and is laterally compressed. The second part (? two subequally short podomeres) flexes subventrally and is shorter, and bears several setae distally on its outer margin. In the third part, also subventrally directed, the endopod divides into a long outer and a slightly shorter inner projection that both bear radiating setae distally, and the inner branch has three discrete setae (? corresponding to three podomeres) along its inner margin. One of the projections is presumably that of the penultimate podomere, as in appendage one. The proximal part of the exopod (? a single podomere) is directed anteriorly, the second (? two podomeres) flexes subventrally and the third (? four or five short podomeres) flexes posteriorly to be subparallel to the carapace margin, and it bears two rows of setae that project normal to the axis. All three parts are laterally compressed.
The third appendage (figures 1a–d,f–h,k,l and 2a,f,l) inserts posterodorsal to the second and is very similar to it, except that it is slightly larger and the spine-like extension of the limb base trends more inwards.
The fourth appendage is biramous (figures 1a–d,f–h,k,l and 2a,g,l,m). It inserts at the posterior margin of the hypostome, posterodorsal to the third appendage. In contrast to the spine-like extension in appendages two and three, a relatively large, transversely elongate gnathobase-like structure is present in the same position, comprising a sharp medial flange and a small posteromedial spine. The endopod is similar in structure to that of the second appendage but is much smaller. The first two parts of the exopod are similar to those of the second appendage. The third part extends backwards from the second part with weak geniculation, is twice the length of its second appendage homologue and bears six setal tufts on its outer margin and terminates in one or two setae (probably indicating seven podomeres in total).
The fifth appendage is incompletely preserved and comprises a limb base that inserts immediately posterior of the fourth appendage and a single ramus (figures 1c,d,k,l and 2a,h). The ramus trends sinuously anterolaterally (separate podomeres are unresolved), is antenna-like and thickened slightly proximally. It is laterally compressed in cross section and shows no evidence of setae. The articulation to the limb base is unknown, but this ramus is interpreted as an exopod owing to its general similarity to that of the fourth appendage. A small endopod may have originally been present, and subsequently ‘lost’ within the preservation gap between head and trunk.
There is a long, narrow, subrectangular hypostome (length to width ratio 3.3 : 1) subdivided at a pointed lateral shoulder just posterior to mid-length (figure 1e,k). The anterior part is aligned subparallel to the dorsal ridge on the carapace, and in transverse profile has a pitched, roof-like form with a long, apically rounded median ridge. The anterior border appears to be subrhomboidal in form and is attached to the carapace border as part of the carapace notch, and there is a small subtriangular anterior wing. The posterior part of the hypostome is flexed ventrally at approximately 15°; the median ridge on this part is less pronounced. The posterior margin of the hypostome is gently convex.
Sections of the alimentary canal are preserved as a sedimentary infill (figures 1c,d,i,j,l and 2a,l,m). The presumed mouth lies dorsal to the posterior hypostomal margin. It opens into an anteriorly expanding tube-like section (? buccal cavity/anterior section of pharynx) above the posterior section of the hypostome, followed by a very short constriction that is triangular in cross section (apex dorsal) above the juncture of the anterior and posterior parts of the hypostome (interpretation uncertain). Anteriorly, the canal widens into a short section comprising six longitudinal ridges and furrows, which may represent (? pharyngeal) muscle; alternate ridges (ventral and dorsolateral) are broader. Further anteriorly, it becomes a simple tube again (presumed oesophagus), subcircular in cross section, and bends dorsally and sharply backwards at the front of the head. Three traces of the intestine are present in the trunk region; the most anterior shows constrictions; the tiny, most posterior trace constrains the position of the anus to near the end of the trunk where it was probably terminal.
(c) Trunk
The trunk is narrow, tapers very gradually posteriorly and bears at least 32 biramous appendages; the most posterior are not fully resolved and there are probably approximately 35 in total (figures 1d,f,k,l and 2a–c). The proximal part of each limb is small, the preservation poor and the detailed form is obscured. The endopods are imbricated and collectively form a cone-like basket that in outline flares slightly outwards anteriorly. The shaft of each endopod is broad and ribbon-like. One or two smaller endites occur on the most proximal part of the endopod but details here are not well resolved. More distally, there are four large, flat, paddle-like endites and the shaft terminates in a similar fifth process. The exopods are confined within the domed part of the carapace. Those of the more anterior appendages extend outwards and slightly downwards from their attachment, before curving posteroventrally; in the posterior part of the trunk, they become progressively more tightly flexed. The main shaft of the exopod is narrow and dorsoventrally flattened (podomeres cannot be discerned). On its posterior margin, it bears very fine, straight, discrete filaments that trend posteriorly and overlap those of the preceding exopod. At mid-trunk position, there are approximately 25 such filaments per exopod. They are longest at about one-third from the base of the main shaft; distally from here they gradually shorten to nothing and proximally they shorten to approximately one-quarter their maximum length. The filaments appear as dots at the limit of resolution in section images; they are likely to be subcircular to oval in cross section. A minute section of the trunk extends posteriorly beyond the last appendage pair, but it is insufficiently resolved for discrimination of possible segment boundaries or telson.
4. Discussion
(a) Affinities
A cladistic analysis was performed using the arthropod morphological character matrix of Wills et al. (1995, 1998), with the addition of Xylokorys and the inclusion of a reinterpreted appendage morphology of Vachonisia (see below and electronic supplementary material). Xylokorys falls in Marrellomorpha, forming a basal sister taxon to a (Vachonisia (Marrella+Mimetaster)) clade, in 35% of the most parsimonious trees. The marrellomorphs resolve as stem-group crustaceans in 87% of the most parsimonious trees. Although our analysis supports the association of Xylokorys with Marrellomorpha, we treat the recovered position within the marrellomorphs and the relationship of this clade to other arthropod groups as merely indicative: other studies of the marrellomorphs are pending and a strictly morphological comparison suggests a close relationship between Xylokorys and Vachonisia (see below). An identical stem-group crustacean position for marrellomorphs was recovered in a small subset of the trees obtained by Wills et al. (1998), but marrellomorphs mainly resolved within the schizoramian (arachnomorph and crustacean) stem in their analysis (see also Cotton & Braddy 2004). Many other positions for Marrellomorpha have been argued: an isolated position within Arachnomorpha (Stürmer & Bergström 1976); a basal position within Lamellipedia (Hou & Bergström 1997) or within a resurrected Trilobitomorpha (Bergström & Hou 2003); and as a stem group to other schizoramians (arachnomorphs and parvancorinomorphs, the latter possibly including Vachonisia; Lin et al. 2006).
A rigorous reassessment of the relationships of the marrellomorphs is beyond the scope of this paper. Even though the Wills et al. (1998) study was a landmark in using morphological data from fossils and living taxa, it has been somewhat superseded by recent molecular and total evidence approaches (e.g. Giribet et al. 2001; Mallat & Giribet 2006) and new fossil discoveries. New data on Marrella from the Burgess Shale (García-Bellido & Collins 2006), Vachonisia from the Hunsrück Shale (Oltmann et al. in preparation) and Furca from the Ordovician of Morocco and Bohemia (Van Roy 2006a,b; see also Chlupáč 1999 and Bergström & Hou 2003), will need to be integrated into a new assessment. Marrella and Mimetaster have always been regarded as closely related (e.g. Stürmer & Bergström 1976; Wills et al. 1995, 1998) and the carapace of Furca is very similar to that of Marrella (e.g. Chlupáč 1999). The relationship of Vachonisia to this clade has proved more problematic, and several authors have regarded its marrellomorph affinities as at best uncertain (e.g. Stürmer & Bergström 1976; Hou & Bergström 1997; Lin et al. 2006; Van Roy 2006a,b). Cladistic analyses that placed Vachonisia together with Marrella and Mimetaster (Wills et al. 1995, 1998) used data matrices of morphological characters largely without ordering or weighting. This may place undue emphasis on the large number of trunk appendages that decrease in size posteriorly, a condition that may be plesiomorphic. The cephalic appendages of Xylokorys and Vachonisia, on the other hand, are very different from those of Marrella and Mimetaster. Xylokorys and Vachonisia are also united by the large carapace that covers body and appendages.
Stürmer & Bergström's (1976, figure 10) investigation of the head region of Vachonisia identified a pair of uniramous antennae followed by three pairs of uniramous appendages that lack exopods, the posterior two bearing strong endites with a spinose tip. The morphology of Xylokorys suggests that the three post-antennal uniramous appendages reconstructed by Stürmer & Bergström (1976, figure 10) in Vachonisia are a composite of the exopods and endopods of appendages two to four (Oltmann et al. in preparation). The endites reconstructed by Stürmer & Bergström are distal projections of the endopods of two and three (see also Bartels et al. 1998, figure 99).
Xylokorys differs from Vachonisia in the following ways: in lacking a median rostrum within the anterior carapace notch; in the lack of posterolateral projections of the carapace; in the relatively narrower carapace border; in the longer antenna that is divided distally; in the presence of fewer (from approx. 35 as opposed to approx. 80) trunk appendages (though note that the number of appendages in Vachonisia was estimated in the holotype (see Lehmann 1955; Stürmer & Bergström 1976), which is approximately 1.7 times as long as OUMNH C.29604); and in its subrectangular as opposed to suboval hypostome (see Stürmer & Bergström 1976, plate 10; Bartels et al. 1998).
(b) Mode of life
The depositional setting of the Herefordshire Lagerstätte site is that of the outer shelf of the Anglo-Welsh Basin, under maximum water depths of approximately 200 m (Briggs et al. 1996). The overall form of Xylokorys suggests that it was a soft-bottom dweller. The wide carapace border would have prevented sinking into unconsolidated sediment. Xylokorys was apparently blind, and therefore unsuited for life primarily in the water column. The first appendages were presumably sensory; there is no evidence that their dorsal and ventral distal projections could articulate in a chela-like fashion, but they are imperfectly preserved distally. The distal fringe of setae on the exopods of limbs two to four could have made contact with the sediment surface in order to support the body when at rest (figure 1a–c,g,k); they may also have had a sensory function. The long fourth and short fifth exopod could be accommodated within the carapace dome, and possibly functioned in sweep-cleaning.
The trunk exopods, at least, were presumably respiratory; together with the endopods they may have created currents to draw in fresh drafts of water, and one or both of these branches probably provided propulsion. The strongly geniculate endopods of appendages two and three may have raked the sediment and brought food into suspension. The trunk endopods may have captured edible particles on the endites from suspension and transported them anteriorly to the mouth. The proximal parts of the head appendages are robust, arranged in overlapping fashion, and project towards the mouth, where they could have been involved in the mechanical breakdown of food. The sharply edged, flange-like extension of the fourth appendage may have served as an incisor. Xylokorys does not appear to have ingested large items of food: the lack of space between carapace and oesophagus (figures 1b,c and 2l) precludes an expanded stomach. Thus, Xylokorys is interpreted as essentially a benthic particle filter feeder, which may also have consumed prey items (the feeding habits of the living branchiopod Triops may be analogous; see Brusca & Brusca 2003).
Acknowledgments
The Leverhulme Trust (F/08581/E) and English Nature are thanked for their support; K. Saunders and N. Francis for technical assistance; R. Fenn, T. Hall and J. Sinclair for their general assistance, and G. Oltmann, J. Rust and J. Bergström for discussion of Vachonisia.
Supplementary Material
Coding used herein for Xylokorys and Vachonisia within the character matrix of Wills et al. (1998)
References
- Bartels C, Briggs D.E.G, Brassel G. Cambridge University Press; Cambridge, UK: 1998. The fossils of the Hunsrück Slate. Marine life in the Devonian. [Google Scholar]
- Bergström J, Hou X.-G. Arthropod origins. Bull. Geosci. Czech. Geol. Surv. 2003;78:323–334. [Google Scholar]
- Beurlen K. Die Pygaspiden, eine neue Crustacean—(Entomostracen)—gruppe aus den Mesosaurier führenden Iraty-Schichten Brasiliens. Paläont. Z. 1934;16:122–138. [Google Scholar]
- Briggs D.E.G, Collins D. The arthropod Alalcomenaeus cambricus Simonetta, from the Middle Cambrian Burgess Shale of British Columbia. Palaeontology. 1999;42:953–977. doi:10.1111/1475-4983.00104 [Google Scholar]
- Briggs D.E.G, Siveter David J, Siveter Derek J. Soft-bodied fossils from a Silurian volcaniclastic deposit. Nature. 1996;382:248–250. doi:10.1038/382248a0 [Google Scholar]
- Briggs D.E.G, Sutton M.D, Siveter David J, Siveter Derek J. A new phyllocarid (Crustacea: Phyllocarida) from the Silurian Fossil-Lagerstätte of Herefordshire, England. Proc. R. Soc. B. 2004;271:131–138. doi: 10.1098/rspb.2003.2593. doi:10.1098/rspb.2003.2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs D.E.G, Sutton M.D, Siveter David J, Siveter Derek J. Metamorphosis in a Silurian barnacle. Proc. R. Soc. B. 2005;272:2365–2369. doi: 10.1098/rspb.2005.3224. doi:10.1098/rspb.2005.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusca R.C, Brusca G.J. Sinauer Associates; Sunderland, MA: 2003. Invertebrate. [Google Scholar]
- Chlupáč I. Some problematical arthropods from the Upper Ordovician Letná formation of Bohemia. J. Czech. Geol. Soc. 1999;44:79–92. [Google Scholar]
- Cotton T.J, Braddy S.J. The phylogeny of arachnomorph arthropods and the origin of the Chelicerata. Trans. R. Soc. Edin. 2004;94:169–193. (for 2003) [Google Scholar]
- García-Bellido D.C, Collins D.H. A new study of Marrella splendens (Arthropoda, Marrellomorpha) from the Middle Cambrian Burgess Shale, British Columbia, Canada. Can. J. Earth Sci. 2006;43:721–742. doi:10.1139/E06-012 [Google Scholar]
- Giribet G, Edgcombe G.D, Wheeler W.C. Arthropod phylogeny based on eight molecular loci and morphology. Nature. 2001;413:157–161. doi: 10.1038/35093097. doi:10.1038/35093097 [DOI] [PubMed] [Google Scholar]
- Hou X.-G, Bergström J. Arthropods of the Lower Cambrian Chengjiang fauna, southwest China. Fossils Strata. 1997;45:1–116. [Google Scholar]
- Lehmann W.M. Vachonisia rogeri n. g. n. sp., ein Branchiopod aus dem unterdevonischen Hunsrückscheifer. Paläont. Z. 1955;29:126–130. [Google Scholar]
- Lin J.-P, et al. A Parvancorina-like arthropod from the Cambrian of South China. Hist. Biol. 2006;18:33–45. [Google Scholar]
- Mallatt J, Giribet G. Further use of nearly complete 28S and 18S rRNA genes to classify Ecdysozoa: 37 more arthropods and a kinorhynch. Mol. Phylogenet. Evol. 2006;40:772–794. doi: 10.1016/j.ympev.2006.04.021. doi:10.1016/j.ympev.2006.04.021 [DOI] [PubMed] [Google Scholar]
- Orr P.J, Briggs D.E.G, Siveter David J, Siveter Derek J. Three-dimensional preservation of a non-biomineralized arthropod in concretions in Silurian volcaniclastic rocks from Herefordshire, England. J. Geol. Soc. Lond. 2000a;57:173–186. [Google Scholar]
- Orr P.J, Siveter Derek J, Briggs D.E.G, Siveter David J, Sutton M.D. A new arthropod from the Silurian Konservat-Lagerstätte of Herefordshire, England. Proc. R. Soc. B. 2000b;267:1497–1504. doi: 10.1098/rspb.2000.1170. doi:10.1098/rspb.2000.1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siveter David J, Sutton M.D, Briggs D.E.G, Siveter Derek J. An ostracode crustacean with soft parts from the Lower Silurian. Science. 2003;302:1749–1751. doi: 10.1126/science.1091376. doi:10.1126/science.1091376 [DOI] [PubMed] [Google Scholar]
- Siveter Derek J, Sutton M.D, Briggs D.E.G, Siveter David J. A Silurian sea spider. Nature. 2004;431:978–980. doi: 10.1038/nature02928. doi:10.1038/nature02928 [DOI] [PubMed] [Google Scholar]
- Siveter David J, Siveter Derek J, Sutton M.D, Briggs D.E.G. Brood care in a Silurian ostracod. Proc. R. Soc. B. 2007;274:464–469. doi: 10.1098/rspb.2006.3756. doi:10.1098/rspb.2006.3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siveter Derek J, Sutton M.D, Briggs D.E.G, Siveter David J. A new probable stem lineage crustacean with three-dimensionally preserved soft-parts, from the Herefordshire (Silurian) Lagerstätte, UK. Proc. R. Soc. B. 2007;274:2099–2107. doi: 10.1098/rspb.2007.0429. doi:10.1098/rspb.2007.0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürmer W, Bergström J. The arthropods Mimetaster and Vachonisia from the Devonian Hunsrück Shale. Paläont. Z. 1976;50:78–111. [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter David J, Siveter Derek J. An exceptionally preserved vermiform mollusc from the Silurian of England. Nature. 2001a;410:461–463. doi: 10.1038/35068549. doi:10.1038/35068549 [DOI] [PubMed] [Google Scholar]
- Sutton, M. D., Briggs, D. E. G., Siveter, David J. & Siveter, Derek J. 2001b Methodologies for the visualization and reconstruction of three-dimensional fossils from the Silurian Herefordshire Lagerstätte. Paleont. Elec. 4 (1), art. 2. (http://palaeo-electronica.org/2001_1/s2/issue1_01.htm).
- Sutton M.D, Briggs D.E.G, Siveter David J, Siveter Derek J, Orr P.J. The arthropod Offacolus kingi (Chelicerata) from the Silurian of Herefordshire, England: computer based morphological reconstructions and phylogenetic affinities. Proc. R. Soc. B. 2002;269:1195–1203. doi: 10.1098/rspb.2002.1986. doi:10.1098/rspb.2002.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy, P. 2006a Non-trilobite arthropods from the Ordovician of Morocco. PhD thesis, Ghent University.
- Van Roy, P. 2006b Exceptionally preserved marrellomorph arthropod specimens from the Lower Ordovician of Morocco. In Abstracts, 50th Annual Meeting of the Palaeontological Association
- Wills M.A, Briggs D.E.G, Fortey R.A, Wilkinson M. The significance of fossils in understanding arthropod evolution. Verh. Dtsch. Zool. Ges. 1995;88:203–215. [Google Scholar]
- Wills M.A, Briggs D.E.G, Fortey R.A, Wilkinson M, Sneath P.H.A. An arthropod phylogeny based on fossil and recent taxa. In: Edgecombe G.D, editor. Arthropod fossils and phylogeny. Columbia University Press; New York, NY: 1998. pp. 33–105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coding used herein for Xylokorys and Vachonisia within the character matrix of Wills et al. (1998)