Abstract
During maize (Zea mays) C4 differentiation, mesophyll (M) and bundle sheath (BS) cells accumulate distinct sets of photosynthetic enzymes, with very low photosystem II (PSII) content in BS chloroplasts. Consequently, there is little linear electron transport in the BS and ATP is generated by cyclic electron flow. In contrast, M thylakoids are very similar to those of C3 plants and produce the ATP and NADPH that drive metabolic activities. Regulation of this differentiation process is poorly understood, but involves expression and coordination of nuclear and plastid genomes. Here, we identify a recessive allele of the maize high chlorophyll fluorescence (Hcf136) homolog that in Arabidopsis (Arabidopsis thaliana) functions as a PSII stability or assembly factor located in the thylakoid lumen. Proteome analysis of the thylakoids and electron microscopy reveal that Zmhcf136 lacks PSII complexes and grana thylakoids in M chloroplasts, consistent with the previously defined Arabidopsis function. Interestingly, hcf136 is also defective in processing the full-length psbB-psbT-psbH-petB-petD polycistron specifically in M chloroplasts. To determine whether the loss of PSII in M cells affects C4 differentiation, we performed cell-type-specific transcript analysis of hcf136 and wild-type seedlings. The results indicate that M and BS cells respond uniquely to the loss of PSII, with little overlap in gene expression changes between data sets. These results are discussed in the context of signals that may drive differential gene expression in C4 photosynthesis.
In maize (Zea mays), photosynthetic activities are partitioned between two morphologically and biochemically distinct cell types, mesophyll (M) and bundle sheath (BS; Edwards and Walker, 1983). M and BS cells are organized as concentric files around the vasculature in a classical Kranz anatomy. Functionally, these two cell types cooperate in photosynthesis, carbon fixation (Edwards et al., 2001a; Majeran et al., 2005), nitrogen metabolism (Rathnam and Edwards, 1975, 1976; Harel et al., 1977; Becker et al., 1993), and sulfur assimilation (Burgener et al., 1998). Notably, M plastids contain grana thylakoids, perform linear electron transport, and photoreduce NADP+ (Andersen et al., 1972). In contrast, BS chloroplasts are agranal (Andersen et al., 1972; Kirchanski, 1975; Miller et al., 1977), PSII depleted (Schuster et al., 1985), and perform most of the reactions of the Calvin cycle (Chollet, 1973; Kagawa and Hatch, 1974). Partitioning of photosynthetic activities between M and BS cell types is mediated by cell-specific localization of multiple transcripts (Furumoto et al., 2000; Sawers et al., 2007) and proteins (Majeran et al., 2005). However, little is known about the transcriptional program regulating C4 differentiation.
Previous studies have suggested that a small number of regulatory changes are sufficient to establish the C4 syndrome (Ku et al., 1996). To date, localization of a limited number of transcripts has been shown to be mediated by cis-regulatory elements (Langdale et al., 1991; Schaffner and Sheen, 1991, 1992; Sheen, 1991; Stockhaus et al., 1997). However, the discovery of a master switch that will explain the thousands of genes with cell-specific patterns of expression (Sawers et al., 2007) is unlikely (Edwards et al., 2001b). Rather, the accumulation of many of these transcripts may be mediated by changes that have resulted in novel cellular environments in the C4 leaf that continue to control gene expression through preexisting networks. Factors could include differential sugar concentrations, protein complexes, and gradients of small metabolites that influence M and BS cell identity.
Another factor that may influence the differentiation process is redox poise. In the leaf blade, M cells contain both PSII and PSI activities and perform linear photosynthetic electron transport (PET). In contrast, BS cells lack detectable levels of functional PSII and are believed to be restricted to cyclic electron transport (Gregory et al., 1979; Ghirardi and Melis, 1983; Romanowska et al., 2006). As a result, proper functioning of M and BS cells is dependent on the intercellular transfer of photosynthetically derived reducing equivalents from M to BS cells. Specifically, NADPH generated during linear PET in M cells is exported to BS cells for Calvin cycle activity (Edwards and Walker, 1983). Thus, these differences in photochemistry lead to distinct redox profiles in M and BS cells.
The characterization of mutants that are selectively disrupted in either M or BS cell photosynthetic differentiation may prove useful in understanding the networks that drive this process. For instance, bundle sheath defective2 (bsd2) seedlings do not accumulate Rubisco (Roth et al., 1996; Brutnell et al., 1999) and lack a functional Calvin cycle (Smith et al., 1998). Consequently, the linear PET chain is likely to be more reduced in mutant M cells than in wild type because it is lacking an electron sink. Conversely, M cell-defective mutants that lack PSII are unable to generate electron flow and likely result in overly oxidized linear PET chains. Additionally, both of these mutant classes will fail to accumulate soluble sugars due to the absence of photosynthesis. Thus, mutations that disrupt the cellular environments of M and BS cells may provide useful tools for probing the differentiation process.
Several maize mutants have been reported with defects in PSII function, including high chlorophyll fluorescence3 (hcf3), hcf19G, and hcf19YG (Leto and Miles, 1980). However, the molecular lesions associated with these PSII-defective mutants have yet to be determined. In this study, we identify an Activator (Ac)-induced maize mutant that lacks PSII activity. Cloning and characterization of this gene indicates that it is a homolog of HCF136, which is necessary for PSII assembly or stability (Meurer et al., 1998; Plucken et al., 2002). In wild-type plants, ZmHcf136 transcript accumulation is predominantly confined to M cells, and proteomic analysis of hcf136 total leaf tissue shows that monomeric and dimeric PSII complexes do not accumulate. Interestingly, the plastid-encoded psbB-psbT-psbH-petB-petD polycistron is misprocessed in the mutant specifically in M cells. Microarray analysis reveals that M and BS cell transcript pools are altered by the hcf136 mutation. The loss of PSII leads to a disruption in spatial regulation of typically BS-enriched genes and an increase in the cellular specificity of typically M-enriched genes. Additionally, data from the protein and transcript profiles do not always correspond, suggesting that posttranscriptional/translational controls are also involved in C4 differentiation.
RESULTS
Ac-Tagged Zmhcf136 Is Seedling Lethal
The Zmhcf136 mutant was first identified in sand bench screens of an Ac-mutagenized population as a recessive hcf seedling-lethal mutant (see “Materials and Methods”). DNA-blot analysis identified a 2.5-kb EcoRI fragment containing an Ac insertion that cosegregated with the mutant phenotype. Inverse PCR with primers designed to Ac (Kolkman et al., 2005) was used to amplify 265-bp of DNA flanking the Ac insertion. Initial BLAST searches revealed that this fragment has significant similarity to the Arabidopsis (Arabidopsis thaliana) gene HCF136, suggesting the Ac inserted into an exon of a maize Hcf136 homolog. The hcf136 mutant displays somatic instability consistent with an active transposable element insertion. In Arabidopsis, HCF136 is a lumenal protein that is specifically required for the assembly or stability of PSII (Meurer et al., 1998; Plucken et al., 2002). In maize, PSII accumulation is preferentially localized to mature M chloroplasts (Edwards and Walker, 1983; Schuster et al., 1985; Majeran et al., 2005).
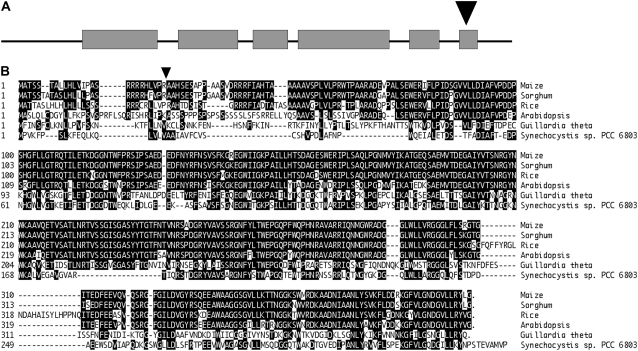
To identify full-length maize coding sequences for Hcf136, the 265 bp flanking the Ac was used to search available genomic and EST databases and a nearly full-length pseudomolecule of Hcf136 transcript was assembled. To confirm the cloning of Hcf136 and recover noncoding sequences associated with the Hcf136 gene, we exploited the somatic instability of an active Ac allele to selectively amplify sequences flanking the Ac insertion in ZmHcf136. By utilizing a genome-walking technique known as Ac casting (Singh et al., 2003), we were able to recover additional sequence upstream and downstream of the original Ac insertion site. PCR reactions performed using gene-specific and Ac end primers resulted in the amplification of 2,530 bp of genomic sequence flanking the Ac insertion, including 278 bp upstream of the start of translation (see “Materials and Methods”). Exon-intron boundaries were defined using reverse-transcription (RT)-PCR as described in “Materials and Methods” and a schematic of the gene is shown in Figure 1A. The Ac element is oriented in the 3′ to 5′ direction relative to the start of transcription of Hcf136 and is inserted in the sixth exon between bp 1,151 and 1,152 of the coding sequence. Rather than leading to a truncated protein, this orientation is predicted to produce a fusion hcf136-Ac transcript that is likely unstable and degraded. A ZmHcf136-specific probe was used to map the locus to the short arm of chromosome 1 (Bin 1.01) using the intermated B73 × Mo17 recombinant inbred mapping population (Lee et al., 2002).
Figure 1.
Sequence analysis of the ZmHcf136 homolog. A, The Ac element, shown as an arrowhead, is inserted in the sixth exon of ZmHcf136. Exons are represented by gray boxes and introns by solid lines. B, Protein alignment of HCF136 homologs from maize, sorghum, rice, Arabidopsis, G. theta, and Synechocystis sp. PCC 6803. Residues identical in at least three sequences are shaded black. Predicted mature end of ZmHCF136 is indicated by the black arrowhead.
HCF136 Proteins Are Highly Conserved
As shown in Figure 1B, HCF136 homologs are highly similar across monocots, dicots, algae, and cyanobacterial species. TargetP predicts that ZmHCF136 is chloroplast localized with a 25-amino-acid transit peptide (Emanuelsson et al., 2000). When the predicted N-terminal transit peptide is excluded from sequence comparisons, AtHCF136 and ZmHCF136 share 87% identity or 96% similarity across their entire length. Studies in Arabidopsis have suggested that HCF136 interacts directly with the PSII reaction core proteins D2 and Cyt b559 at the lumenal side of the thylakoid membrane (Plucken et al., 2002). Sorghum (Sorghum bicolor) and maize HCF136 proteins are 96% identical, including the transit peptide. HCF136 from both the alga Guillardia theta and the cyanobacterium Synechocystis sp. PCC 6803 are 43% identical to maize. This high degree of sequence similarity between divergent species suggests a conserved and ancestral function for the HCF136 protein.
Loss of HCF136 Affects PSII Function and Grana Formation
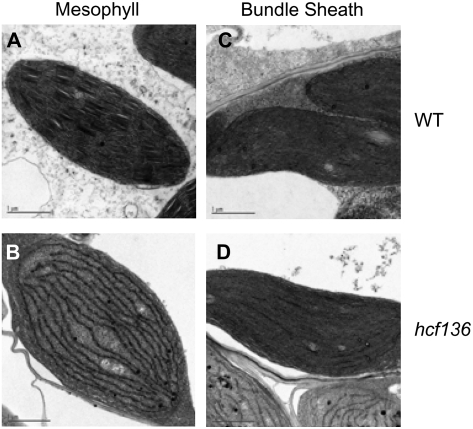
Using in vivo fluorescence induction curves, we examined the functional status of PSII in hcf136 leaves (see “Materials and Methods”). Mutant seedlings displayed hcf, but no variable fluorescence, consistent with the absence of PSII activity (hcf136 Fv/Fm = 0; wild type = 0.8). Light microscopy of cross sections of wild-type and mutant leaf tissue revealed smaller chloroplasts in both M and BS cells of hcf136 (Supplemental Fig. S1B). Plastid ultrastructure was examined in greater detail using transmission electron microscopy (Fig. 2). In the hcf136 mutant, grana are absent or display aberrant ultrastructure in M plastids (Fig. 2B). In contrast, plastid ultrastructure in hcf136 BS cells appears normal (Fig. 2D). These results are consistent with the prediction that the primary defect in Zmhcf136 is a disruption in PSII assembly and accumulation.
Figure 2.
Plastid ultrastructure in second leaf tip of 10-d-old hcf136 mutant and wild-type siblings. A to D, Transmission electron micrographs from seedlings grown under 80 μmol s−1 m−2 light in 16-h days at 50% humidity. A to D, M plastids of wild type (A) and hcf136 mutants (B); bundle sheath plastids of wild type (C) and hcf136 mutants (D).
ZmHcf136 Transcripts Accumulate Preferentially in M Cells
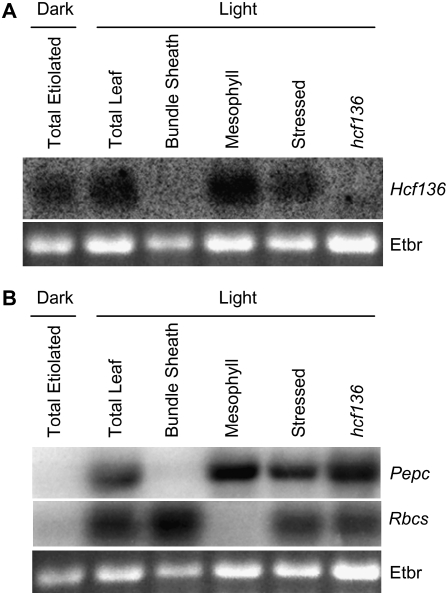
To determine whether ZmHcf136 transcript accumulation is M cell specific, RNA-blot analysis of several cell types and tissues was performed using an Hcf136-specific probe (Fig. 3). RNA was isolated from light-grown wild-type M cell protoplasts, BS strands, total leaf tissue, total hcf136 mutant leaf tissue, and total leaf tissue from wild-type dark-grown plants. To control for changes in gene expression due to M cell protoplast isolation, RNA was also extracted from total wild-type light-grown tissue that was stress-treated by a mock protoplast digestion (see “Materials and Methods”). The RNA samples were hybridized with probes derived from the cell-specific markers Pepc and Rbcs, which accumulate preferentially in M and BS cell types, respectively (Sheen and Bogorad, 1987; Langdale et al., 1988b). As shown in Figure 3B, there is little cross-contamination in our cell preparations, and Hcf136 transcripts clearly accumulate to higher levels in M relative to BS cells (Fig. 3A). Additionally, Hcf136 transcript accumulates in the dark, but is more abundant in light-grown tissues.
Figure 3.
RNA-blot analysis of Hcf136 transcript accumulation. Approximately 5 μg of total RNA was fractionated on 1.5% agarose gels and transferred to nitrocellulose membrane. Separate filters were hybridized to radiolabeled fragments of Hcf136 (A) and Pepc and Rbcs (B). Ethidium bromide-stained 18S rRNA (Etbr) is shown as a loading control.
The psbB-psbH-psbT-petB-petD Polycistron Is Misprocessed in M Cells
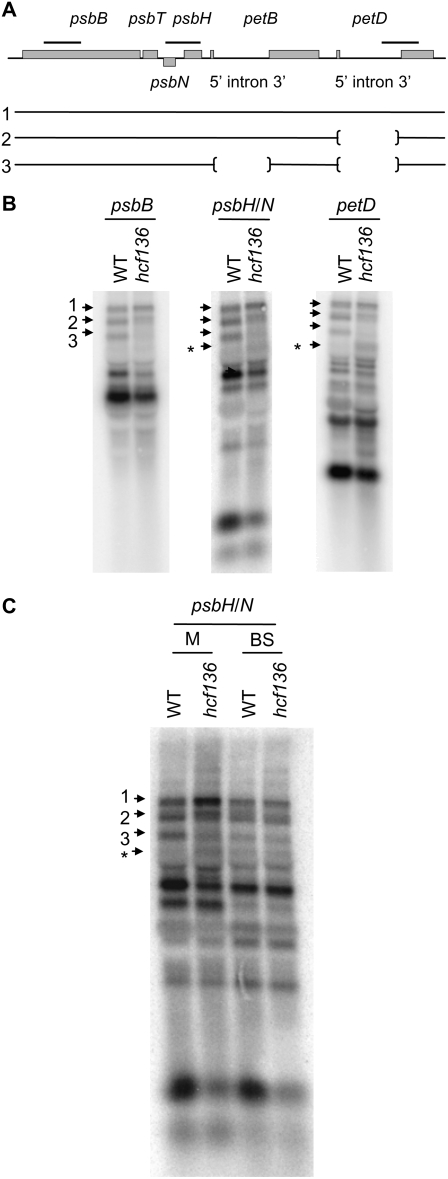
When analyzing the mutant for changes in PSII transcript regulation, we unexpectedly observed a defect in the processing/stability of the psbB-psbH-psbT-petB-petD polycistron (Fig. 4A). Components of PSII (psbB, psbH, psbN, and psbT) and Cyt b6f (petB and petD) are encoded by this polycistron, which is processed into many overlapping RNAs that are capable of directing protein synthesis (Barkan, 1988). As seen in Figure 4B, band 3 accumulates to much lower levels in the mutant leaf RNA, indicating a processing defect of the petB intron in hcf136. In addition, a fourth band (shown by asterisk) aberrantly accumulates in mutant M leaf tissue (Fig. 4C). In contrast, the polycistron is processed similarly in mutant and wild-type BS cells. No processing defects were detected in hcf136 mutants for other polycistronic transcripts examined, including those encoding the core of PSII (psbC and psbD), and other members of the group II intron family (atpF/H and psaAB; Rock et al., 1987; Cushman et al., 1988; Kuck, 1989; Kim and Hollingsworth, 1993). These data are shown in Supplemental Figure S2. Because a processing defect was only detected in the psbB-psbH-psbT-petB-petD polycistron, these findings suggest that a disruption in HCF136 function specifically affects psbB-psbH-psbT-petB-petD processing in M cells.
Figure 4.
psbB-psbT-psbH-petB-petD processing in hcf136. A, Schematic shows polycistronic organization to scale with probe locations marked above the gene by a thick black bar. Genes that are encoded on the plus strand are labeled above their corresponding box, and the minus strand gene is labeled below. Exons and introns of petB and petD are also labeled below their corresponding gene. Numbered lines represent bands in the blots shown in B and C. B, RNA from total leaf tissue of WT and hcf136 was hybridized to fragments of psbB, psbH/N, and petD. C, RNA from separated M and BS cells from wild type and hcf136 was hybridized to psbH/N. Processed fragments shown in A and B are marked by numbered arrows and an unidentified band is marked by an asterisk.
Zmhcf136 Lacks HCF136 and PSII Proteins
To examine the accumulation and localization of ZmHCF136, the profiles of wild-type and hcf136 stroma-enriched and thylakoid peripheral and lumenal proteins were compared by two-dimensional (2D) gel electrophoresis with immobilized pH gradient (IPG) strips in the first dimension and SDS-PAGE in the second dimension. A single spot was identified in Sypro Ruby-stained 2D gels as a spot that is present in plastid protein extracts of wild-type plants, but absent in hcf136 mutants (Supplemental Fig. S3). This spot was excised, trypsin digested, and analyzed by electrospray ionization-tandem mass spectrometry (ESI-MS/MS) and identified as HCF136, confirming the identity of the Ac-tagged gene (TC296744; http://ppdb.tc.cornell.edu; Supplemental Table S1.
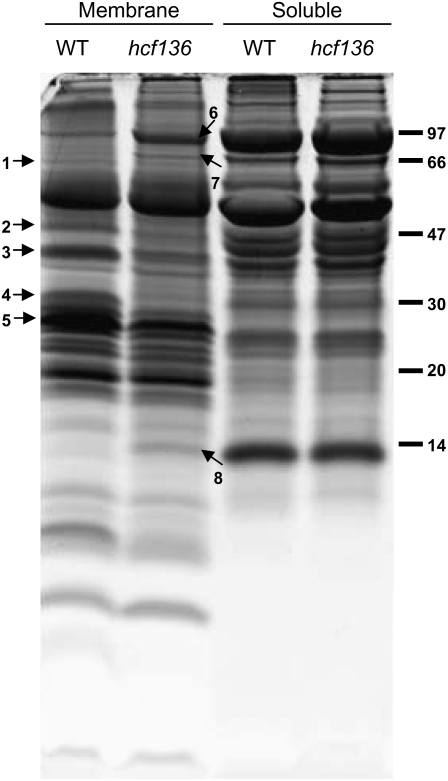
To identify plastid-localized proteins that differentially accumulate in the hcf136 mutant, thylakoid membranes were isolated, subfractionated into membrane and soluble components, and separated by one-dimensional (1D) SDS-PAGE (Fig. 5). Strong differential accumulation was observed for a number of bands in the membrane fractions, but not in the soluble fraction. Eight major bands showing differential accumulation were identified by peptide mass finger printing (PMF) using a matrix-assisted laser-desorption ionization-time-of-flight (MALDI-TOF) mass spectrometer (Supplemental Table S2) as FtsH1 (band 1, TC292243), CP47 (band 2, NP_043049.1), OEC33-like (band 3, TC279249), PSII-D2 (band 4, NP_043009.1), light-harvesting complex (LHCII-1; band 5, TC286614), pyruvate, orthophosphate dikinase (PPDK; band 6, TC286559), cpHSP70 (band 7, TC293193), and small subunit of Rubisco (RBCS; band 8, TC286731). These identifications likely represent the most abundant protein in the band. In hcf136, FtsH1 metalloprotease accumulation is reduced, and the CP47, OEC33-like, and D2 subunits of PSII are absent or dramatically reduced. A slight reduction in the accumulation of the major LHCII-1 band is observed likely due to the absence of accumulation of its interacting PSII complex. PPDK, cpHSP70, and RBCS proteins have increased accumulation in the hcf136 membrane fraction, but no differential accumulation in the soluble fraction, suggesting that these proteins interact more strongly with thylakoid membranes in plastids that lack PSII or grana. It is unlikely that a treatment effect from thylakoid preparation accounts for this result because other abundant chloroplast-soluble components are not found in the membrane fraction.
Figure 5.
Electrophoretic pattern of wild-type (WT in the image) and hcf136 proteins obtained by SDS-PAGE (tricine 12%) and stained with Sypro Ruby fluorescent dye. Total thylakoid membrane vesicles were isolated on Percoll cushions and then treated with a Dounce homogenizer followed by differential ultracentrifugation to collect membrane and soluble fractions. Bands displaying strong differential accumulation were excised and proteins digested and analyzed by MALDI-TOF MS PMF. Identified proteins are: (1) FtsH1 (TC292243); (2) CP47 (NP_043049.1); (3) OEC33-like (TC279249); (4) PSII-D2 (NP_043009.1); (5) LHCII-1 (TC286614); (6) PPDK (TC286559); (7) cpHsp70 (TC293193), and (8) RBCS (TC286731). These proteins are labeled with numbered arrows. Protein markers in kilodaltons are indicated on the right.
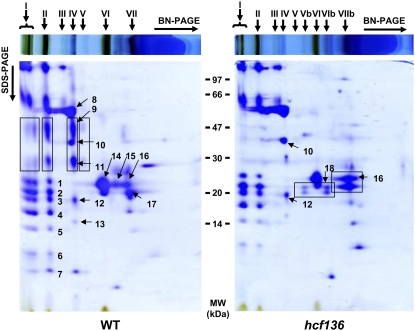
To improve resolution of the thylakoid proteome analysis and to determine the assembly state of the major photosynthetic complexes in wild type and hcf136, thylakoids were solubilized with the nonionic detergent n-dodecyl β-d-maltoside and analyzed by Blue Native (BN) gel electrophoresis followed by SDS-PAGE (2D BN SDS-PAGE; Fig. 6). Major photosynthetic complexes in wild-type and mutant tissues were identified by PMF analysis (Supplemental Table S3). In hcf136, PSII reaction center and core subunits are absent from thylakoid membranes, but there is no dramatic effect on the accumulation of PSI. The hcf136 mutant also has a different oligomeric assembly state of the major LHCII, which is present in a monomeric form rather than the trimeric form typical of wild-type thylakoids (Dekker and Boekema, 2005). This may be a consequence of the loss of its interaction partner, PSII core complex. Alternatively, the absence of membrane stacking may lead to increased accessibility to detergent and destabilization of LHC oligomers during membrane preparation. Other protein complexes, including ATP synthase and Cyt b6f, accumulate to similar levels in both wild-type and mutant plastids.
Figure 6.
BN gel electrophoresis of thylakoid membranes from wild type and hcf136 mutants. Equivalent amounts of wild type and hcf136 thylakoid membranes (700 μg of protein) were solubilized with n-dodecyl β-d-maltoside and separated on native gels in the first dimension. Gel strips were reduced and alkylated in a solubilization buffer and separated by second dimension SDS-PAGE (tricine 12%). Proteins were identified by in-gel digestion, followed by MALDI-TOF MS PMF. Protein complexes were identified as: (I) PSI and PSII “supercomplexes”; (II) PSI and PSII dimers; (III) partially assembled PSI; (IV) PSII, ATP-synthase, and Cyt b6f; (V) partially assembled PSII; and (VI and VII) LHCII. The hcf136 mutant has additional complexes: (Vb–VIb) LHCI-4 and (VIIb) low molecular weight form of the LHCII-1 complex. In the wild-type gel, black boxes indicate the different forms of PSII present in wild type but absent in hcf136. In the hcf136 gel, black boxes indicate changes in LHCI accumulation and in the assembly state of LHCII. Spot identities are as follows (see also Supplemental Table S3); (1) LHCI-3 (TC286618); (2) PsaD-2 PSI subunit II (TC293201, TC293200); (3) PsaD-2 PSI subunit II (TC293201, TC293200); (4) PsaF PSI subunit III (TC299208, TC299217, TC299206); (5) PsaE-2 PSI subunit IV (TC279867); (6) unknown; (7) unknown; (8) CF1β (atpB, TC279356) and CF1α (atpA, TC303520); (9) CP47 (psbB, TC283413); (10) CF1γ (AtpC, TC287102); (11) D1 (psbA, TC290677); (12) Cyt b6f Rieske iron-sulfur (TC286511); (13) unknown; (14) LHCII-1 (TC299123); (15) LHCII-1 (TC286602); (16) LHCII-1 (TC299123); (17) LHCII-3 (TC286603); (18) LHCI-4 (TC279557). Protein marker positions in kilodaltons are indicated between the two gel images. [See online article for color version of this figure.]
Changes in Protein Accumulation Do Not Correlate with RNA Levels
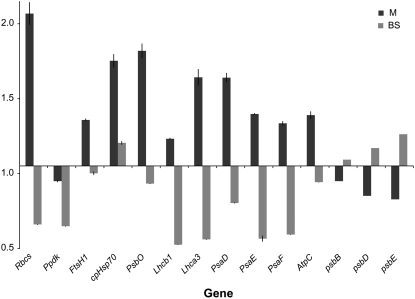
To determine the extent of transcriptional control on the observed changes in protein accumulation, the relative abundance of several transcripts was measured by quantitative real-time PCR (qPCR) in M and BS cell preparations (see “Materials and Methods”). Relative transcript levels were assayed using primer pairs specific to the following nuclear-encoded genes (Supplemental Table S4): Rbcs (TC286731), Ppdk (TC286559), FtsH1 (TC292243), cpHsp70 (TC293193), PsbO OEC33 (TC279249), Lhcb1 (TC286614), Lhca3 (TC286618), PsaD (TC293201, TC293200), PsaE (TC279867), PsaF (TC299208, TC299217, TC299206), and AtpC (TC287102). The plastid-encoded genes psbB (NP_043049), psbD (NP_043009), and psbE (TC279867) were also assayed (see Supplemental Table S2). A comparison of transcript levels in M and BS cells of hcf136 and wild type is shown in Figure 7. A value >1 indicates transcripts were more abundant in the mutant than in the wild type, and a value <1 indicates transcript abundance was greater in the wild type. These data show that the observed disruption in plastid protein accumulation does not correspond to a general reduction in corresponding transcript accumulation. Examination of the qPCR data shown in Figure 7 indicated that, in general, transcripts accumulated to higher levels in mutant M cells and lower levels in BS. This finding suggests that disrupting PSII activity can enhance the differential expression of M-enriched transcripts (e.g. psbB; Kubicki et al., 1994), whereas decreasing the differential expression of BS-enriched transcripts (e.g. rbcS; Sheen and Bogorad, 1986; Langdale et al., 1988a).
Figure 7.
Transcript abundance in M and BS cells of the hcf136 mutant relative to wild type. Fold-change values greater than one correspond to greater transcript abundance in hcf136 tissues relative to wild type. Means of three biological replicates and two technical replicates of qPCR are shown with se estimates. A list of primer sequences is given in Supplemental Table S4.
Loss of PSII Leads to Changes in C4 Spatial Regulation
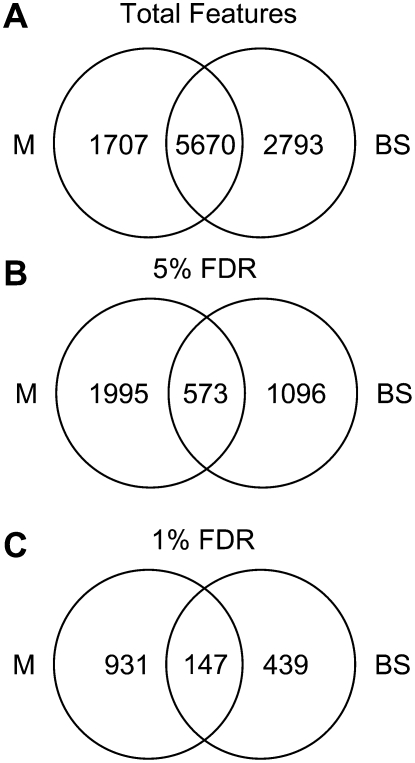
To further explore the disruption of PSII activity on gene expression, transcript profiles from separated M and BS cells were examined using two-label microarray analysis (see “Materials and Methods”). To avoid confounding treatment effects associated with direct comparisons of M and BS transcriptomes (Sawers et al., 2007), comparisons were only made using the same cell type across the hcf136 and wild-type sibling genotypes. After normalization and filtering, 7,377 and 8,463 features were considered for further analysis from the M and BS experiments, respectively (Supplemental Tables S5 and S6). These two data sets share 5,670 common features as summarized in Figure 8A.
Figure 8.
Venn diagrams demonstrating unique expression profiles of hcf136 M and BS cells. A, Total features detectable in M and BS cells. B, Features differentially expressed in hcf136 at a 5% FDR. C, Features differentially expressed in hcf136 at a 1% FDR.
Using a false discovery rate (FDR) of 5%, we identified 2,568 differentially expressed features between hcf136 and wild type in the M cell data set. When a more stringent 1% FDR cutoff is applied, 1,078 features are differentially expressed of which 162 have at least a 2-fold change in expression and 773 are more abundant in the mutant relative to wild type. In the BS experiment, 1,669 features are differentially expressed between hcf136 and wild type at a 5% FDR and 586 at a 1% FDR. In the 1% FDR BS data set, 195 features change by at least 2-fold relative to wild type and 306 are more abundant in the mutant relative to wild type. When the differentially expressed genes are compared at a 5% FDR between M and BS data sets, 573 features are identified that are common to both cell types (Fig. 8B). This overlap is reduced to 147 features when significance is controlled at a 1% FDR (Fig. 8C). Because only 14% or 9% (high and low FDR, respectively) of differentially expressed features are shared, these data suggest there is a cell-specific transcriptional response to the loss of PSII.
A comparison of these data to a previous study (Sawers et al., 2007) shows that BS:M ratios of 129 features that preferentially accumulate in M cells and 167 that preferentially accumulate in BS cells are altered in the hcf136 mutant relative to wild type (Supplemental Table S7). For example, Phosphoenolpyruvate carboxykinase (MZ00013532), which has a wild-type BS:M ratio of 2.82 and a predicted hcf136 ratio of 1.39, is less differentially expressed in the mutant. Conversely, Carbonic anhydrase (MZ00042197), which has a BS:M ratio of 0.24 in wild type and a predicted ratio of 0.18 in hcf136, is more differentially expressed in the hcf136 mutant. Of the 129 M-enriched transcripts, 57% are more differentially expressed in the mutant relative to wild type (e.g. M:BS mutant > M:BS wild type). Conversely, of the 167 BS-enriched transcripts, only 29% are more differentially expressed in the hcf136 mutant. These results suggest that when PSII function is disrupted, the directionality of transcriptional responses in M and BS cells differs.
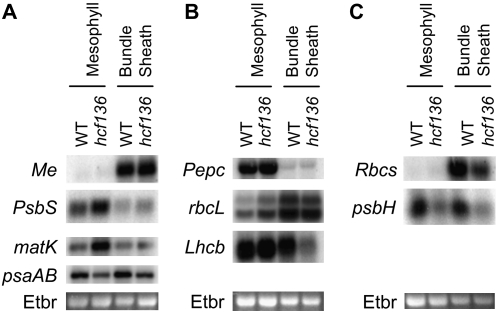
To verify the altered transcriptional profiles determined by microarray analysis, RNA blots were performed (Fig. 9). Probes were designed to a number of plastid- and nuclear-encoded genes with highly abundant transcripts involved in photosynthesis that are differentially expressed between hcf136 and wild type at a 5% FDR in at least one cell type. From the M cell data, chloroplast-encoded psaAB, rbcL, psbH, matK and nuclear-encoded Lhcb were chosen for verification. From the BS data, chloroplast-encoded rbcL and matK and nuclear-encoded PsbS, Lhcb, and Rbcs were chosen for confirmation. As shown in Figure 9, RNA-blot analysis confirmed the differential accumulation of these genes between wild-type and mutant plants. The expression change of PsbS in BS cells was at the limit of detection (Fig. 9), but these data were confirmed using qPCR (Supplemental Fig. S4). Collectively, these data validate a subset of the microarray results indicating differential responses of M and BS cells to a loss of HCF136 function.
Figure 9.
RNA-blot analysis of differentially expressed genes. A to C, RNA blots of separated M and BS cells of hcf136 and wild-type siblings were sequentially hybridized with radiolabeled gene fragments shown. Each blot was first probed with a cell-specific marker to ensure isolation purity (Me, Pepc, Rbcs). The nuclear (N)- and chloroplast (C)-encoded genes include PsbS (N), matK (C), psaAB (C), rbcL (C), Lhcb (N), and psbH (C). Ethidium-bromide stained (Etbr in the image) 18S RNA is shown as a loading control.
DISCUSSION
HCF136 Function in Maize
Using the transposable element Ac as a molecular tag, the ZmHcf136 gene was cloned and characterized. The pale green, seedling-lethal Zmhcf136 mutant displays reduced thylakoid stacking in M plastids, an absence of PSII complexes, and no detectable PSII reaction center functionality (Fv/Fm = 0). These data are consistent with the previously assigned function of HCF136 as a PSII reaction center assembly or stability factor (Meurer et al., 1998; Plucken et al., 2002). In maize, PSII activity is largely restricted to the M cells, resulting in a cell-specific defect in mutant leaf tissues.
Loss of PSII Protein Accumulation in hcf136
Although PSII reaction center and core proteins fail to accumulate to detectable levels in hcf136, the corresponding transcripts of both nuclear and chloroplast genes accumulate to near wild-type levels. This lack of correlation between proteome and transcriptome profiles is likely a consequence of protein degradation of unassembled PSII reaction center and core proteins in the chloroplast. In contrast, nuclear-encoded protein components of PSI (PsaD, E, F) and ATP synthase (CF1γ–AtpC) accumulate to similar levels in mutant and wild-type tissues, but the corresponding transcript profiles are altered in the hcf136 mutant. Transcripts for PSI and ATP synthase-associated proteins tend to accumulate to higher levels in mutant M cells and lower levels in mutant BS cells relative to wild type. Thus, the nuclear-plastid transcriptional networks in these two cell types respond selectively to a loss of PSII function.
Altered Transcript Patterns in hcf136
The microarray data revealed that, whereas many features are detectable in both M and BS cells of hcf136 (5670), only 573 features are differentially expressed between wild type and mutant at a 5% FDR and 147 at a 1% FDR. These data suggest that M and BS cells are responding differently to a perturbation in HCF136 function. Striking examples of these differences in regulation can be observed in transcripts encoded by the plastid genome (5% FDR). For instance, different sets of genes encoding PSII components are misregulated in hcf136 M and BS plastids. In mutant M cells, psbH, J, M, and N are differentially expressed relative to wild type, whereas in BS cells, psbD, E, J, and K show altered accumulation profiles. Also, three components of the ATP synthase (atpA, B, E) and four components of the NADH dehydrogenase (ndhE, F, G, I) are differentially expressed in M cells, but not in the BS. Additionally, significant changes in psaB, petA, petD, rpoA, rpoB, and infA expression are only detected in M cell comparisons. In contrast, transcripts for psaJ, rpoC2, atpI, and ndhJ are differentially expressed solely in the BS.
Another striking trend in the M cell expression data is that nearly twice as many features are differentially expressed in M cells (2,568) relative to the BS (1,669) at a 5% FDR. This trend is most evident for plastid-encoded transcripts, where 57 genes are differentially expressed between wild-type and mutant in M chloroplasts and only 18 genes are differentially expressed in BS plastids. For example, of 21 rpl and rps genes detected in both cell types, all 21 are differentially expressed in the M cells, but only two of those 21 are differentially expressed in BS strands. In general, transcripts encoded by the plastid genome are more abundant in the mutant relative to wild type when differentially expressed. Specifically, only psbH, psaB, ndhJ, atpI, rps14, and rbcL are less abundant in hcf136 than in wild type. Consequently, these data suggest that pools of plastid mRNA, particularly in the M, are responding in concert and are either more stable or more highly expressed in the mutant. It is possible that the smaller global response in the BS may reflect its naturally PSII-depleted state.
A comparison of M and BS cell data sets shows that a greater percentage of differentially expressed features change by more than 2-fold in BS relative to M cells at a 1% FDR (33% versus 15%). This indicates that BS features are capable of a strong transcriptional response to the loss of PSII. For example, putative maize homologs of Phosphatidylcholine acyltransferase (MZ00018920), Peroxidase (MZ00015594), U2 snRNP auxiliary factor (MZ00006052), H2B histone (MZ00013518), and BTH-induced ERF transcriptional factor1 (MZ00017004) are increased in accumulation by more than 2-fold only in the BS. Similarly, Phosphenolpyruvate carboxykinase (MZ00013533) and a putative Inositol 1,3,4-trisphosphate 5/6-kinase (MZ00029181) decrease by more than 2-fold in hcf136 BS cells. In addition, some features are differentially expressed in both cell types, but the magnitude of the response is greater in the BS. For example, Thylakoid formation1 (MZ00043318) increases 2.6-fold in the BS and only 1.9-fold in M cells. Similarly, Cytochrome c (MZ00013468) increases 2.3-fold in BS and 1.5-fold in M hcf136 cells. Thus, M and BS cells are capable of independently regulating gene expression in response to a disruption of PSII.
We identified 296 features that were previously shown to differentially accumulate in BS and M cells (Sawers et al., 2007) and were misregulated in the hcf136 mutant. As described above, the general trend is for less differential expression of BS-enriched features (118/167) and more differential expression of M-enriched features (73/129) in hcf136 relative to wild type. Eighty-five of 118 BS-enriched features are less differentially expressed in hcf136 due to an increase in expression in M cells. For example, cytochrome c oxidase subunit2 (MZ00034818) accumulates to similar levels in mutant BS cells as in wild type, but is more abundant in M cells of hcf136. This bias toward greater differential accumulation in hcf136 M cells coincides with the observation that 72% of M cell features show increased expression in the hcf136 mutant.
The Role of Cellular Environment in C4 Differentiation
Current models propose that the evolution of C4 biology from the basal C3 state requires the recruitment of cis- and trans-acting regulatory elements to alter gene expression (Sage, 2004). However, a recent transcript-profiling experiment indicates that nearly 18% of the leaf transcriptome is differentially expressed between M and BS cells (Sawers et al., 2007). Given the vast numbers of regulatory elements necessary to establish this magnitude of differential expression, we suggest that recruiting thousands of cis- and trans-acting elements to mediate transcriptional change is not a parsimonious explanation for how such a large percentage of the genome is spatially controlled. Rather, key regulatory changes may have resulted in novel M and BS cellular environments and in response extant C3 networks may have been recruited during evolution of C4 photosynthesis (Sheen, 1999; Hibberd and Quick, 2002), accounting for the majority of observed transcriptional changes. Factors that may drive M and BS gene expression include differential protein complex formation (e.g. OEC and PSII in M cell plastids), plastid redox status, and sugar and energy metabolite concentrations.
An example of misexpression due to a change in cellular environment may be the aberrant processing of the psbB-psbH-psbT-petB-petD polycistron detailed in Figure 4. This defect is likely due to a change in the environment of M cell plastids that is associated with the loss of PSII (e.g. pH change, redox poise, thylakoid membrane structure). Although we have not ruled out a direct role for HCF136 in RNA metabolism, the psbB polycistron is aberrantly processed in several nonallelic hcf mutants, including hcf2, hcf38, and hcf43 (Barkan et al., 1986). The less differentially expressed M- and BS-enriched features may also constitute a class of genes that are responding to a loss of C4 cellular environments in the mutant. For instance, genes with BS:M expression ratios that are closer to 1 in hcf136 relative to wild type (e.g. Pyruvate, orthophosphate dikinase [MZ00007665] and Phosphoenolpyruvate carboxykinase [MZ00013532]) may represent a reversion to a more basal C3 state (Langdale et al., 1988b). Together, these findings suggest that a general disruption of photosynthetic electron transport leads to altered processing of the psbB polycistron (Barkan et al., 1986) and deregulation of some spatially restricted transcripts.
Many nuclear genes respond to plastid-derived signals that are integrated through a common pathway in the chloroplast (Koussevitzky et al., 2007). In Arabidopsis, genome uncoupled (gun) mutants have been used to dissect plastid to nucleus retrograde signals. Susek et al. (1993) found that Lhcb and Rbcs expression is unchanged or elevated when gun mutants are treated with the herbicide Norflurazon. Using microarray analysis, Strand et al. (2003) identified 322 genes that are misregulated following Norflurazon treatments, 152 of which do not respond appropriately in at least one gun mutant. Recent studies of plastid-nuclear signaling in Arabidopsis have defined GUN1 as a central integrator of tetrapyrrole metabolism, redox, and plastid gene expression state within the plastid. These multiple inputs are somehow transduced into a signal that is transmitted to transcriptional regulators, including ABI4, to regulate nuclear gene expression (Koussevitzky et al., 2007). It is possible that a disruption of PSII is similarly sensed by a plastid factor and this information is relayed to the nucleus. The slight, but significant, increase in the expression of many nuclear-encoded M-enriched transcripts in hcf136 may be the consequence of the perturbation of PSII activity and loss (or reduction) of a plastid-derived signal that typically negatively regulates gene expression. Furthermore, the reduction in abundance of several transcripts in BS cells may be a secondary response to a loss of reducing equivalents or sugar metabolites rather than a direct response to the absence of PSII function.
In summary, the hcf136 mutant has provided an opportunity to examine the effects of altered M and BS cellular environments on C4 differentiation. The loss of PSII impacts M and BS protein composition, PET, redox poise, energy, and sugar metabolite gradients. As a result, there is a general increase in RNA transcript accumulation in the M cell, and M- and BS-enriched genes become more and less differentially expressed, respectively. Additionally, altering the BS cellular environment results in decreased transcript accumulation for a number of features and this may reflect a shift to a more basal C3 state in this cell type.
MATERIALS AND METHODS
Identification of ZmHcf136
The maize (Zea mays) homolog of Arabidopsis (Arabidopsis thaliana) Hcf136 was identified as part of a regional mutagenesis screen using Ac/Dissociation transposition in the W22 inbred line of maize. The mutant family JK03-77.24 was created by selecting transposition events from bti00228∷Ac and subsequent screening of self-pollinated populations (Kolkman et al., 2005).
DNA-blot analysis was performed using an Ac-specific fragment (Ac900; Kolkman et al., 2005) to identify an EcoRI RFLP that cosegregated with the mutant phenotype. This fragment was cloned using inverse PCR as previously described (Kolkman et al., 2005). BLAST sequence comparisons in available databases revealed similarity to HCF136 protein homologs. A ZmHcf136-specific DNA fragment was mapped using RFLP analysis (Lee et al., 2002) with forward primer, JK03-77.24@FlAc900 (5′-CCGCCAATCTCTACTCCGTCAAGT) and reverse primer, Hcf136 3′-untranslated region (UTR) Common (5′-GGTTTTCAAGTTCCTAAGCAAGCAG).
ZmHcf136 Sequence Assembly
Ac casting was used to obtain genomic DNA sequence for the full ZmHcf136 gene (Singh et al., 2003). Nested PCR was performed using gene-specific primers in combination with Ac internal primers. The gene-specific primers were 5′ Ac Casting 1 (5′-AGTCGATGGGCAGGAAGAT), 5′ Ac Casting 2 (5′-GCCGTCTTTCGTCTCCAGTA), 5′ Ac Casting 3 (5′-TCTGCTCCCCAGTAGCTTTT), 5′ Ac Casting 4 (5′-ACCGCTAATGCCACTTGAAA), and GC-HCF136 Common Exon (5′-AAAGTCCACCGTCCGCTCTC). Downstream primers were 3′ Ac Casting 1 (5′-GATGCATGTGCTGCTTGC), 3′ Ac Casting 2 (5′-GCGTGTTGCTTCGGTATCTT), and GC-HCF136 3′-UTR Common (5′-CTGCTTGCTTAGGAACTTGAAAACC). PCR products were gel purified using QiaEXII (QIAGEN), cloned into pGEM (Promega), or TOPO vectors (Invitrogen). Plasmids were purified using the QIAprep Spin Miniprep kit (QIAGEN) according to manufacturer's recommendations. The DNA was sequenced as previously described (Singh et al., 2003).
Plant Growth Conditions
Plants were grown in 16-h days and constant 28°C under low-light conditions of 80 μmol m−2 s−1 for fluorescence, electron microscopy, and protein analyses and 40 μmol m−2 s−1 for all other experiments. Etiolated seedlings were grown in darkness at 28°C until their light-grown siblings were at the third leaf-emerging stage. Mutants were identified from segregating families, and near-isogenic comparisons made with phenotypically wild-type siblings.
Fluorescence Measurements
In vivo fluorescence induction curves for Fv/Fm were obtained at room temperature from the second leaf tip of seedlings at the third leaf-emerging stage of development using an actinic light source and bright saturating pulse as previously described (Maxwell and Johnson, 2000). The leaf area assayed was dark adapted for at least 15 min prior to illumination. Fv/Fm measurements were obtained with a modulated fluorescence apparatus (model no. FMS2; Hansatech Instruments).
Electron Microscopy
Electron microscopy was performed on wild-type and mutant plants at the third leaf-emerging stage of development. Tips of the second leaves of 10-d-old wild-type and mutant seedlings were harvested in the morning to deplete overnight starch reserves. Samples were fixed for 0.5 h at room temperature and 1.5 h at 4°C in 2.5% glutaraldehyde in 0.1 m sodium cacodylate, pH 6.8. The samples were rinsed at 4°C in 0.1 m sodium cacodylate buffer, pH 6.8, fixed in 1% osmium tetroxide, and rinsed in 0.1 m sodium cacodylate buffer, pH 6.8. Samples were dehydrated in a graded ethanol series, and then infiltrated with Spurr's resin. Sections were cut on a Reichert OmU2 Ultramicrotome and contrasted with uranyl acetate and lead citrate. The sections were viewed on a Tecnai 12 Biotwin transmission electron microscope (FEI Corporation). Digital images were acquired using a Gatan Multiscan Camera (model 791).
Cell Preparation
M protoplasts, BS strands, and the control stressed total tissue were prepared from second leaf blades as previously described (Markelz et al., 2003).
RNA Isolation and Blot Analysis
Total RNA was isolated and analyzed by RNA blot as previously described (Sheehan et al., 2004). Leaf tissue from light-grown plants was harvested when the third leaf was emerging. Dark-grown seedling tissue was harvested above the mesocotyl on the same day. Cell-specific markers were assayed to monitor the integrity of the M and BS preparations (Pepc, Rbcs, Me). Approximately 5 μg of total RNA were loaded for the initial analysis of Hcf136 transcript accumulation (Fig. 3). All other gel blots were prepared using 10 μg of total RNA.
DNA probes used in RNA-blot analysis include Hcf136, Pepc, Rbcs, Me, Lhcb-m7, rbcL, PsbS, matK, psaAB, psbH, petD, psbB, psbD, petB, psbA, psbC, and atpF/H. The Hcf136 probe was a 648-bp fragment amplified from the 3′-end of the gene using the forward primer Hcf136 Common Exon (5′-GAGAGCGGACGGTGGACTTT) and reverse primer Hcf136 3′-UTR Common (5′-GGTTTTCAAGTTCCTAAGCAAGCAG). The rbcL probe was amplified from genomic maize DNA using the primers 5′-GCAGTAGCTGCGGAATCTTCTACT and 5′-GGTGAATGTGAAGAAGTAGGCCGT. PsbS was amplified using 5′-TCTCCATCATCGGCGAGATCATCA and 5′-TACAAGCAGACAACCCAACG. Other fragments were as previously described (Roth et al., 1996) or were generated using gene-specific primers to published maize plastid sequences. DNA probes were generated by PCR using GoTaq Green Master Mix (Promega), gel purified with QiaEXII (QIAGEN), and radiolabeled according to Sheehan et al. (2004).
Protein Characterization of Zmhcf136
Plants were grown as described above and tissue harvested for 2D IPG SDS-PAGE when the third leaf was emerging and for 2D BN SDS-PAGE and 1D SDS-PAGE when the fifth leaf was emerging. Proteins were extracted from whole seedlings for 2D IPG SDS-PAGE and from apical regions about 4 cm from the tips of third and fourth leaves for other PAGE experiments. The total leaf microsomal fraction was isolated in grinding buffer (350 mm sorbitol, 50 mm HEPES-KOH, pH 8, 2 mm EDTA, 5 mm ascorbic acid, 5 mm l-Cys) in a blender at half speed, followed by Miracloth filtration and low-speed centrifugation (1,000g). The thylakoid membrane fraction was purified from the microsomal pellet on discontinuous Percoll gradients as previously described (Friso et al., 2004). Thylakoid membrane vesicles were treated with a Dounce homogenizer followed by differential ultracentrifugation (100,000g) to collect membrane and soluble fractions. Protein concentrations were determined with the bicinchoninic acid assay (Smith et al., 1985).
For 1D SDS-PAGE separation, proteins were equilibrated with SDS (0.2%), Na2CO3 (100 mm), dithiothreitol (100 mm), and Suc (10%) and separated on 12% Tricine gels (Schägger and von Jagow, 1987). Gels were stained with fluorescent Sypro Ruby (Molecular Probes). 2D IPG SDS-PAGE protein separation was performed on the thylakoid soluble fraction using 150 μg of protein per IPG strip as previously described (Majeran et al., 2005). For 2D BN SDS-PAGE, equivalent amounts of wild type and hcf136 thylakoid membranes (700 μg of protein) were solubilized with n-dodecyl β-d-maltoside and separated in the native first dimension according to Schägger et al. (1994). Gel strips issued from the native dimension were reduced and alkylated in a solubilization buffer according to Majeran et al. (2005) and separated by second dimension SDS-Tricine 12% gels (Schägger and von Jagow, 1987). Gels were stained with Coomassie Brilliant Blue R-250 (USB Corporation).
For protein identification, Coomassie Brilliant Blue or Sypro Ruby stained spots were picked manually. Spots were automatically washed and digested with modified trypsin (Promega) as previously described (Shevchenko et al., 1996), and peptides were extracted using a ProGest robot (Genomic Solutions), dried, and resuspended in 5% formic acid. Protein identification was performed by PMF using MALDI-TOF MS in reflectron mode (Perseptive Biosystems Voyager DE-STR Workstation) and online liquid chromatography-ESI-MS/MS (Micromass Q-TOF) according to Majeran et al. (2005). The MS or MS/MS spectra were searched against the maize EST assembly from The Institute for Genomic Research (www.tigr.org; ZmGI, version 1.6) supplemented with maize chloroplast genome sequences obtained from National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov) using an in-house installation of Mascot (www.matrixscience.com).
Microarray
Total RNA was isolated from the second leaf of plants as described above. Six biological replicates were used to compare wild-type and mutant transcript profiles in separate M and BS experiments. To maximize biological replication, different seedling pools were used for each of the 12 hybridizations. Microarray experiments and analyses were performed according to Sawers et al. (2007) using the Maize Array Consortium oligonucleotide platform (www.maizearray.org). Feature intensity values were log-transformed and corrected for local background signal, and a LOWESS procedure (Dudoit et al., 2002) was used to normalize between channels. Features with either low or saturating signal intensity were discarded from further analysis. High expression filtering was less stringent to avoid elimination of previously characterized, high abundance, C4 cell-specific transcripts. After filtering, features that were not assigned an MZ number by the Maize Array Consortium were discarded from further analysis. The moderated t test (Smyth, 2004) using the R package limma was applied to identify differentially expressed features. The P values for each test (feature) were converted to q values for FDR analysis as described by Storey et al. (2004).
SYBR Green qPCR
Three biological replicates were used for qPCR, with two internal technical replicates for each reaction. Total RNA (8 μg) was treated with 3 units of DNase I amplification grade enzyme (Invitrogen) at 37°C for 30 min to remove contaminating DNA in the presence of 80 units of RNaseOUT (Invitrogen). Enzymes and salts were removed from the RNA with TRIzol reagent (Invitrogen). One microgram of purified RNA was incubated at 70°C for 10 min with 50-ng random hexamers and the reaction cooled on ice. Additional reagents were added to a final concentration of 5 mm MgCl2, 0.01 m dithiothreitol, 0.5 mm dNTP, 40 units of RNaseOUT, and 200 units of SuperScript III reverse transcriptase (Invitrogen). Water was substituted for enzyme in the negative control. cDNA synthesis was performed by incubation at 25°C for 10 min, 50°C for 50 min, 80°C for 5 min, and a 4°C soak. Upon completion, the RNA template was destroyed with 2 units Escherichia coli RNase H, and cDNA was diluted with 60 μL of water. For qPCR reactions, the template was further diluted with three parts water, and the SYBR Green JumpStart Taq ReadyMix without MgCl2 kit (Sigma) was used with final concentrations of 2.3 mm MgCl2 and 24 ng/μL forward and reverse primers. Primer sequences are available in Supplemental Table S4. An internal reference dye was used to measure data quality. Samples were run at 95°C for 2 min, cycled 47 times between 95°C for 15 s and 60°C for 1 min, followed by a dissociation stage of 95°C for 15 s, 60°C for 15 s, and 95°C 15 s on an ABI Prism 7900HT sequence detection system (Applied Biosystems). Data were analyzed using ABI Prism SDS 2.1 software. Results were normalized using 18S rRNA reactions as a control.
Sequence data for the maize homolog of Hcf136 can be found in the GenBank library under accession number EF587243. The data discussed in this publication have been deposited in the NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) and are accessible through GEO Series accession number GSE9698. For Figure 1B, HCF136 homologs were aligned using the following accessions: Z. mays (ABQ53629), Oryza sativa (BAD62115.1), Arabidopsis (O82660), G. theta (NP_113453.1), and Synechocystis sp. PCC 6803 (NP_440411). S. bicolor protein information was assembled from CN132236, CN142773, CN142842, CN145337, CN150433, CN150507, and CN148500.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Light micrographs of 1-μm-thick cross sections from the leaf blade tips of 10-d-old wild type (A) and hcf136 mutants (B) at 400× magnification.
Supplemental Figure S2. RNA-blot analysis of polycistronic transcripts.
Supplemental Figure S3. A comparison of 2D electrophoresis (2-DE) gels from wild type and hcf136 mutant tissues.
Supplemental Figure S4. qPCR of relative transcript levels of PsbS between wild type and hcf136 within M and BS cell types.
Supplemental Table S1. Identification of the HCF136 protein in wild-type samples by ESI-MS/MS.
Supplemental Table S2. Identification of differentially accumulating proteins by MALDI-TOF MS PMF in the hcf136 mutant relative to wild type.
Supplemental Table S3. Identification of differentially accumulating proteins by MALDI-TOF MS PMF in the hcf136 mutant relative to wild type.
Supplemental Table S4. Primer sequences for SYBR Green qPCR.
Supplemental Table S5. Comparison of transcript profiles between hcf136 and wild-type M cells.
Supplemental Table S6. Comparison of transcript profiles between hcf136 and wild-type BS cells.
Supplemental Table S7. Comparison of cell-specific expression profiles between hcf136 and wild type.
Supplementary Material
Acknowledgments
We gratefully thank Dr. Thomas Owens, Dr. Robert Turgeon, Dr. Tesfamichael Kebrom, Ms. Phyllis Farmer, and Dr. Qi Sun for technical advice and helpful discussions, Shannon Caldwell and Anita Aluisio at the Cornell Integrated Microscopy Center (Veterinary Medical Center, Cornell University) for the electron micrographs, and Dr. Katia Wostrikoff for helpful discussions.
This work was supported by the National Science Foundation (grant no. DBI–0211935 to T.P.B. and K.J.v.W.) and the Natural Sciences and Engineering Research Council of Canada (to S.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Thomas P. Brutnell (tpb8@cornell.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Andersen KS, Bain JM, Bishop DG, Smillie RM (1972) Photosystem II activity in agranal bundle sheath chloroplasts from Zea mays. Plant Physiol 49 461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A (1988) Proteins encoded by a complex chloroplast transcription unit are each translated from both monocistronic and polycistronic mRNAs. EMBO J 7 2637–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Miles D, Taylor WC (1986) Chloroplast gene expression in nuclear, photosynthetic mutants of maize. EMBO J 5 1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TW, Perro-Rechenmann C, Suzuki A, Hirel B (1993) Subcellular and immunocytochemical localization of the enzymes involved in ammonia assimilation in mesophyll and bundle-sheath cells of maize leaves. Planta 191 129–136 [Google Scholar]
- Brutnell TP, Sawers RJ, Mant A, Langdale JA (1999) BUNDLE SHEATH DEFECTIVE2, a novel protein required for post-translational regulation of the rbcL gene of maize. Plant Cell 11 849–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgener M, Suter M, Jones S, Brunold C (1998) Cyst(e)ine is the transport metabolite of assimilated sulfur from bundle-sheath to mesophyll cells in maize leaves. Plant Physiol 116 1315–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet R (1973) Photosynthetic carbon metabolism in isolated maize bundle sheath strands. Plant Physiol 51 787–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman JC, Hallick RB, Price CA (1988) The two genes for the P700 chlorophyll a apoproteins on the Euglena gracilis chloroplast genome contain multiple introns. Curr Genet 13 159–171 [DOI] [PubMed] [Google Scholar]
- Dekker JP, Boekema EJ (2005) Supramolecular organization of thylakoid membrane proteins in green plants. Biochim Biophys Acta 1706 12–39 [DOI] [PubMed] [Google Scholar]
- Dudoit S, Yang YH, Callow MJ, Speed TP (2002) Statistical methods for identifying differentially expressed genes in replicated cDNA microarray experiments. Statist Sinica 12 111–139 [Google Scholar]
- Edwards GE, Franceschi VR, Ku MS, Voznesenskaya EV, Pyankov VI, Andreo CS (2001. a) Compartmentation of photosynthesis in cells and tissues of C4 plants. J Exp Bot 52 577–590 [PubMed] [Google Scholar]
- Edwards GE, Furbank RT, Hatch MD, Osmond CB (2001. b) What does it take to be C4? Lessons from the evolution of C4 photosynthesis. Plant Physiol 125 46–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GE, Walker D (1983) C3, C4: Mechanisms, Cellular and Environmental Regulation of Photosynthesis. University of California Press, Berkeley, CA
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300 1005–1016 [DOI] [PubMed] [Google Scholar]
- Friso G, Giacomelli L, Ytterberg AJ, Peltier JB, Rudella A, Sun Q, Wijk KJ (2004) In-depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts: new proteins, new functions, and a plastid proteome database. Plant Cell 16 478–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumoto T, Hata S, Izui K (2000) Isolation and characterization of cDNAs for differentially accumulated transcripts between mesophyll cells and bundle sheath strands of maize leaves. Plant Cell Physiol 41 1200–1209 [DOI] [PubMed] [Google Scholar]
- Ghirardi ML, Melis A (1983) Localization of photosynthetic electron transport components in mesophyll and bundle sheath chloroplasts of Zea mays. Arch Biochem Biophys 224 19–28 [DOI] [PubMed] [Google Scholar]
- Gregory RP, Droppa M, Horvath G, Evans EH (1979) A comparison based on delayed light emission and fluorescence induction of intact chloroplasts isolated from mesophyll protoplasts and bundle-sheath cells of maize. Biochem J 180 253–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel E, Lea PJ, Miflin BJ (1977) The localization of enzymes of nitrogen assimilation in maize leaves and their activities during greening. Planta 134 195–200 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Quick WP (2002) Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415 451–454 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Hatch MD (1974) C4-acids as the source of carbon dioxide for Calvin cycle photosynthesis by bundle sheath cells of the C4-pathway species Atriplex spongiosa. Biochem Biophys Res Commun 59 1326–1332 [DOI] [PubMed] [Google Scholar]
- Kim JK, Hollingsworth MJ (1993) Splicing of group II introns in spinach chloroplasts (in vivo): analysis of lariat formation. Curr Genet 23 175–180 [DOI] [PubMed] [Google Scholar]
- Kirchanski SJ (1975) The ultrastructural development of the dimorphic plastids of Zea mays L. Am J Bot 62 695–705 [Google Scholar]
- Kolkman JM, Conrad LJ, Farmer PR, Hardeman K, Ahern KR, Lewis PE, Sawers RJ, Lebejko S, Chomet P, Brutnell TP (2005) Distribution of Activator (Ac) throughout the maize genome for use in regional mutagenesis. Genetics 169 981–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J (2007) Signals from chloroplasts converge to regulate nuclear gene expression. Science 316 715–719 [PubMed] [Google Scholar]
- Ku MS, Kano-Murakami Y, Matsuoka M (1996) Evolution and expression of C4 photosynthesis genes. Plant Physiol 111 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki A, Steinmuller K, Westhoff P (1994) Differential transcription of plastome-encoded genes in the mesophyll and bundle-sheath chloroplasts of the monocotyledonous NADP-malic enzyme-type C4 plants maize and sorghum. Plant Mol Biol 25 669–6798061319 [Google Scholar]
- Kuck U (1989) The intron of a plastid gene from a green alga contains an open reading frame for a reverse transcriptase-like enzyme. Mol Gen Genet 218 257–265 [DOI] [PubMed] [Google Scholar]
- Langdale JA, Rothermel BA, Nelson T (1988. a) Cellular pattern of photosynthetic gene expression in developing maize leaves. Genes Dev 2 106–115 [DOI] [PubMed] [Google Scholar]
- Langdale JA, Taylor WC, Nelson T (1991) Cell-specific accumulation of maize phosphoenolpyruvate carboxylase is correlated with demethylation at a specific site greater than 3 kb upstream of the gene. Mol Gen Genet 225 49–55 [DOI] [PubMed] [Google Scholar]
- Langdale JA, Zelitch I, Miller E, Nelson T (1988. b) Cell position and light influence C4 versus C3 patterns of photosynthetic gene expression in maize. EMBO J 7 3643–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Sharopova N, Beavis WD, Grant D, Katt M, Blair D, Hallauer A (2002) Expanding the genetic map of maize with the intermated B73 x Mo17 (IBM) population. Plant Mol Biol 48 453–461 [DOI] [PubMed] [Google Scholar]
- Leto KJ, Miles D (1980) Characterization of three photosystem II mutants in Zea mays L. lacking a 32,000 Dalton lamellar polypeptide. Plant Physiol 66 18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran W, Cai Y, Sun Q, van Wijk KJ (2005) Functional differentiation of bundle sheath and mesophyll maize chloroplasts determined by comparative proteomics. Plant Cell 17 3111–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markelz NH, Costich DE, Brutnell TP (2003) Photomorphogenic responses in maize seedling development. Plant Physiol 133 1578–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51 659–668 [DOI] [PubMed] [Google Scholar]
- Meurer J, Plucken H, Kowallik KV, Westhoff P (1998) A nuclear-encoded protein of prokaryotic origin is essential for the stability of photosystem II in Arabidopsis thaliana. EMBO J 17 5286–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KR, Miller GJ, McIntyre KR (1977) Organization of the photosynthetic membrane in maize mesophyll and bundle sheath chloroplasts. Biochim Biophys Acta 459 145–156 [DOI] [PubMed] [Google Scholar]
- Plucken H, Muller B, Grohmann D, Westhoff P, Eichacker LA (2002) The HCF136 protein is essential for assembly of the photosystem II reaction center in Arabidopsis thaliana. FEBS Lett 532 85–90 [DOI] [PubMed] [Google Scholar]
- Rathnam CK, Edwards GE (1975) Intracellular localization of certain photosynthetic enzymes in bundle sheath cells of plants possessing the C4 pathway of photosynthesis. Arch Biochem Biophys 171 214–225 [DOI] [PubMed] [Google Scholar]
- Rathnam CK, Edwards GE (1976) Distribution of nitrate-assimilating enzymes between mesophyll protoplasts and bundle sheath cells in leaves of three groups of C4 plants. Plant Physiol 57 881–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CD, Barkan A, Taylor WC (1987) The maize plastid psbB-psbF-petB-petD gene cluster: spliced and unspliced petB and petD RNAs encode alternative products. Curr Genet 12 69–77 [DOI] [PubMed] [Google Scholar]
- Romanowska E, Drozak A, Pokorska B, Shiell BJ, Michalski WP (2006) Organization and activity of photosystems in the mesophyll and bundle sheath chloroplasts of maize. J Plant Physiol 163 607–618 [DOI] [PubMed] [Google Scholar]
- Roth R, Hall LN, Brutnell TP, Langdale JA (1996) bundle sheath defective2, a mutation that disrupts the coordinated development of bundle sheath and mesophyll cells in the maize leaf. Plant Cell 8 915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161 341–370 [DOI] [PubMed] [Google Scholar]
- Sawers RJ, Liu P, Anufrikova K, Hwang JT, Brutnell TP (2007) A multi-treatment experimental system to examine photosynthetic differentiation in the maize leaf. BMC Genomics 8 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner AR, Sheen J (1991) Maize rbcS promoter activity depends on sequence elements not found in dicot rbcS promoters. Plant Cell 3 997–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner AR, Sheen J (1992) Maize C4 photosynthesis involves differential regulation of phosphoenolpyruvate carboxylase genes. Plant J 2 221–232 [DOI] [PubMed] [Google Scholar]
- Schägger H, Cramer WA, von Jagow G (1994) Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem 217 220–230 [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166 368–379 [DOI] [PubMed] [Google Scholar]
- Schuster G, Ohad I, Martineau B, Taylor WC (1985) Differentiation and development of bundle sheath and mesophyll thylakoids in maize. Thylakoid polypeptide composition, phosphorylation, and organization of photosystem II. J Biol Chem 260 11866–11873 [PubMed] [Google Scholar]
- Sheehan MJ, Farmer PR, Brutnell TP (2004) Structure and expression of maize phytochrome family homologs. Genetics 167 1395–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J (1991) Molecular mechanisms underlying the differential expression of maize pyruvate, orthophosphate dikinase genes. Plant Cell 3 225–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J (1999) C4 gene expression. Annu Rev Plant Physiol Plant Mol Biol 50 187–217 [DOI] [PubMed] [Google Scholar]
- Sheen J, Bogorad L (1986) Expression of the ribulose-1,5-bisphosphate carboxylase large subunit gene and three small subunit genes in two cell types of maize leaves. EMBO J 5 3417–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen JY, Bogorad L (1987) Differential expression of C4 pathway genes in mesophyll and bundle sheath cells of greening maize leaves. J Biol Chem 262 11726–11730 [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68 850–858 [DOI] [PubMed] [Google Scholar]
- Singh M, Lewis PE, Hardeman K, Bai L, Rose JK, Mazourek M, Chomet P, Brutnell TP (2003) Activator mutagenesis of the pink scutellum1/viviparous7 locus of maize. Plant Cell 15 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LH, Langdale JA, Chollet R (1998) A functional Calvin cycle is not indispensable for the light activation of C4 phosphoenolpyruvate carboxylase kinase and its target enzyme in the maize mutant bundle sheath defective2-mutable1. Plant Physiol 118 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150 76–85 [DOI] [PubMed] [Google Scholar]
- Smyth GK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3 Article 3 [DOI] [PubMed]
- Stockhaus J, Schlue U, Koczor M, Chitty JA, Taylor WC, Westhoff P (1997) The promoter of the gene encoding the C4 form of phosphoenolpyruvate carboxylase directs mesophyll-specific expression in transgenic C4 Flaveria spp. Plant Cell 9 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD, Taylor JE, Siegmund D (2004) Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. J R Stat Soc Ser B 66 187–205 [Google Scholar]
- Strand A, Asami T, Alonso J, Ecker JR, Chory J (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421 79–83 [DOI] [PubMed] [Google Scholar]
- Susek RE, Ausubel FM, Chory J (1993) Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74 787–799 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.