Abstract
Direct-infusion mass spectrometry (MS) was applied to study the metabolic effects of the symbiosis between the endophytic fungus Neotyphodium lolii and its host perennial ryegrass (Lolium perenne) in three different tissues (immature leaf, blade, and sheath). Unbiased direct-infusion MS using a linear ion trap mass spectrometer allowed metabolic effects to be determined free of any preconceptions and in a high-throughput fashion. Not only the full MS1 mass spectra (range 150–1,000 mass-to-charge ratio) were obtained but also MS2 and MS3 product ion spectra were collected on the most intense MS1 ions as described previously (Koulman et al., 2007b). We developed a novel computational methodology to take advantage of the MS2 product ion spectra collected. Several heterogeneous MS1 bins (different MS2 spectra from the same nominal MS1) were identified with this method. Exploratory data analysis approaches were also developed to investigate how the metabolome differs in perennial ryegrass infected with N. lolii in comparison to uninfected perennial ryegrass. As well as some known fungal metabolites like peramine and mannitol, several novel metabolites involved in the symbiosis, including putative cyclic oligopeptides, were identified. Correlation network analysis revealed a group of structurally related oligosaccharides, which differed significantly in concentration in perennial ryegrass sheaths due to endophyte infection. This study demonstrates the potential of the combination of unbiased metabolite profiling using ion trap MS and advanced data-mining strategies for discovering unexpected perturbations of the metabolome, and generating new scientific questions for more detailed investigations in the future.
With the advent of metabolomics, methods for the simultaneous analysis of a large number of small molecules (metabolites) have been developed and improved, providing more details about the metabolism of complex biological systems (Sumner et al., 2003; Dettmer et al., 2007). Metabolite fingerprinting methods provide relatively unbiased and high-throughput information on complex biological systems and have been used for yeast (Saccharomyces cerevisiae) strain classification (Allen et al., 2003) and annotation of gene functions (Raamsdonk et al., 2001). Comprehensive and unbiased metabolite analysis is also an indispensable tool for systems biology together with transcriptomics and proteomics (see commentary by Sauer et al., 2007). Direct-infusion (without prior chromatographic separation) electrospray ionization (ESI) mass spectrometry (MS) was introduced as a tool for the identification of novel fungal metabolites in culture (Smedsgaard and Frisvad, 1996) and is now widely applied in metabolomics (for a recent review, see Dettmer et al., 2007). High resolution MS instrumentation such as time-of-flight MS (Dunn et al., 2005) or Fourier transform ion cyclotron resonance MS (FT-ICR-MS; Aharoni et al., 2002) is often preferred for the specificity provided by resolution of isobaric ions and highly accurate estimates of mass-to-charge (m/z) ratios. We recently applied direct-infusion ESI MS using an ion trap MS (DIMSn) to determine metabolic differences between endophyte-infected and endophyte-free perennial ryegrass (Lolium perenne) seed samples (Koulman et al., 2007b). This study established that DIMSn is a powerful tool for determining metabolic profiles, even though ion trap MS is a low resolution MS technology. Its advantage lies in the capacity of the ion trap to rapidly collect fragmentation data on large numbers of ions (more than 200) selected from the MS1 spectrum using automated data-dependent scanning, thereby facilitating structural classification and identification of metabolites of interest. Direct-infusion ESI MS/MS with an ion trap has been used to discover unknown drug metabolites (e.g. Tozuka et al., 2003), but its potential for metabolome investigations warrants further exploration and development. Here, we have employed DIMSn technology to detect and investigate a wide range of metabolites involved in an association between perennial ryegrass and its endophytic fungus, Neotyphodium lolii.
Symbiotic associations between fungal endophytes and grasses are widespread and have been estimated to occur in 20% to 30% of all grass species (Leuchtmann, 1992), and are therefore of wide interest to studies on plant-fungal interactions. The two most widely studied associations are the perennial ryegrass-N. lolii symbiosis in Australasia and tall fescue (Lolium arundinaceum)-Neotyphodium coenophialum in North America (Christensen et al., 1993). These two associations are of particular interest to agricultural pastoral systems because the fungal endophytes have been implicated in the toxicity of grazing livestock including ryegrass staggers and fescue foot, but also found to confer a range of agronomic benefits to their grass hosts, mainly through toxicity and feeding deterrent activities toward invertebrate herbivores. These antiherbivore activities have been associated with specific alkaloids produced by the fungi within the plant (Bush et al., 1997; Lane et al., 2000; Malinowski and Belesky, 2000; Schardl et al., 2004). The major known alkaloids produced by N. lolii in perennial ryegrass are peramine, lolitrem B, and ergovaline. Peramine, a pyrrolopyrazine alkaloid, protects the grass host from herbivorous insects such as the Argentine stem weevil (Listronotus bonariensis; Rowan and Gaynor, 1986; Fletcher and Easton, 1997) and has been shown to be exuded in the guttation fluid of endophyte-infected perennial ryegrass (Koulman et al., 2007a). The indole-diterpene lolitrem B acts as a neurotoxin and causes ryegrass staggers in grazing livestock (Gallagher et al., 1984). The peptide alkaloid ergovaline has been associated with heat stress and poor liveweight gains in livestock grazing endophyte-infected perennial ryegrass (Easton et al., 1986; Fletcher and Easton, 1997) and with fescue foot, a severe mammalian disorder that can lead to considerable productivity losses in livestock raised on endophyte-infected tall fescue (Lyons et al., 1986; Strickland et al., 1996). Considerable research efforts have been focused on the biosynthesis, accumulation, and ecological consequences of these fungal alkaloids (for review, see Schardl, 2001; Clay and Schardl, 2002; Schardl et al., 2004). Much less is known about the impacts of fungal endophytes on general host plant performance and metabolism, and recent publications indicate that the effects of fungal endophytes on plant metabolism might be of importance to the understanding of ecosystem-wide impacts of the grass-endophyte symbiosis (Hunt et al., 2005; Cheplick, 2007; Krauss et al., 2007; Rasmussen et al., 2007, 2008).
In this article, we present exploratory data analysis approaches to investigate how metabolites analyzed by DIMSn differ between endophyte-infected and uninfected ryegrass plants in three tissue samples corresponding to three developmental stages (immature leaf, blade, and sheath) of the symbiosis of perennial ryegrass and N. lolii. We have also taken advantage of the MS/MS spectral information to aid metabolite identification and determine the homogeneity of the spectra, and hence uniformity of the metabolite species across the samples. Available software for automating the processing of liquid chromatography (LC)-MS data including commercial software such as MassFrontier (http://www.highchem.com/) and freeware and open source software such as MetAlign (http://www.metalign.nl), XCMS (Smith et al., 2006), and MZmine (Katajamaa and Orešič, 2005) is not applicable to infusion profiles of the type generated in our experiments, as these programs are designed to search for peaks in both the time and mass domain. Thus, computational tools for harnessing the raw data from DIMSn experiments have been developed in this study and will be made available upon request.
RESULTS
The approach to DIMSn data analysis in this article is as follows: (1) statistical analysis of MS1 data, with the range of 150 to1,000 m/z, to select nominal m/z bins that differ significantly in intensity between the groups of four samples from infected and uninfected plants within each of three tissue types (immature leaf, blade, and sheath); (2) analysis of the MS2 spectra deriving from the same parent MS1 m/z bin across all the samples using purpose-built computational tools to determine their similarity and aid identification of components in the fragmentation data; and (3) correlation network analysis of all MS1 bins to identify metabolite relationships not revealed by feature selection based on the statistical ranking.
MS1 Data Analysis and the Selection of Differentiated MS1 m/z Bins
The MS1 spectrum of each sample was obtained from the raw data as described in “Materials and Methods” (“Data Analysis”). To handle the low resolution infusion data we were able to adopt simple processing procedures (compare with Enot et al., 2006) compared to those required for handling high resolution LC-MS data (Hansen and Smedsgaard, 2004), where alignment in the time and mass domains can be challenging. The MS1 spectra were collated in nominal m/z bins in keeping with the 1 m/z resolution of the machine (called MS1 bins thereafter). After background subtraction, the median value of the ion abundance in each bin was determined. Each MS1 bin is thus likely to include ion signals from more than one metabolite. On the other hand, each metabolite is likely to contribute to signals for more than one MS1 bin due to the occurrence of isotopologue ions, hydrogen transfers in the source, and the formation of salt adduct ions. Both aspects must be taken into account for the interpretation of a nominal MS1 bin.
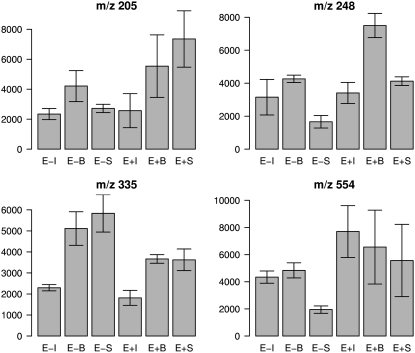
Our experiment was designed to analyze how the metabolome of the symbiosis changes upon endophyte infection in different tissues, i.e. immature leaf, blade, and sheath (see Supplemental Table S1 for sample description). Our first data exploration based on principal component analysis revealed that the main variations (73.45% of the total variation) were explained by metabolic differences between tissues. The infected (E+) and uninfected (E−) samples were not resolved in the PC1-PC2 score plot (Supplemental Fig. S1). We therefore used an empirical Bayes moderated t test (Smyth, 2004) to investigate which metabolites were differentially expressed between E+ and E− in immature leaf, blade, and sheath tissue, respectively. This approach was devised to identify differentially expressed genes across specified conditions in designed microarray experiments, and it provides more stable inference when the sample size is small (Smyth, 2004). Significant differential MS1 bins were selected based on an adjusted P value <0.05 using Benjamin and Hochberg's false discovery rate to control false positives. Ten MS1 bins were identified based on this criterion, of which seven (m/z 230, 248, 205, 231, 335, 189, and 554) were significantly different between E+ and E− in sheath, four in immature (m/z 209, 297, 223, and 230), but none in blade tissue. The distributions of the ion abundance of MS1 bins of m/z 205, 248, 335, and 554 in different treatment groups are shown in Figure 1. See Supplemental Figure S2 for the distribution of the other significant MS1 bins (m/z 230, 231, 189, 209, 223, and 297) and Table I for a summary of all the MS1 bins identified as being significantly different between infected and uninfected perennial ryegrass tissues.
Figure 1.
The distribution of MS1 ion abundance in different treatment groups. MS1 bins m/z 205, 248, 335, and 554 differ significantly (false discovery rate adjusted P value <0.05) between E+ and E− in sheath tissue. The barplot is based on the median and median absolute deviation values of the replicates (n = 4, median ± median absolute deviation). Labels of E+ and E− refer to presence and absence of endophyte, and I, B, and S refer to plant tissue immature leaf, blade, and sheath, respectively. See all sample names in Supplemental Table S1.
Table I.
A summary of MS1 bins discussed in this study
| MS1 Bin | Isotopologue | Ion Adduct | Putative Metabolitea | Ion Fragmentation | Tissueb |
|---|---|---|---|---|---|
| m/z | |||||
| 189 | Unknown (no MS2) | Sheath | |||
| 205 | [182Na]+ | Mannitolp,f | Supplemental Figure S3 | Sheath | |
| 209 | Unknown (heterogeneous bin) | Figure 3 | Immature | ||
| 223 | Unknown (no MS2) | Immature | |||
| 230 | Unknown (no MS2) | Sheath/immature | |||
| 231 | Unknown (no MS2) | Sheath | |||
| 248 | Peraminef | Supplemental Figure S7 | Sheath | ||
| 297 | 296 | 5-Hydroxyferulyl sinapinep | Immature | ||
| 335 | 333, 334 | Perlolinep | Sheath | ||
| 543 | 544, 545, 546 | [504K]+ | Trihexosidep | Sheath | |
| 554 | 555 | [1,107H2]++ | Cyclic peptidef | Supplemental Figure S4, a and b | Sheath |
| 705 | 706 | [666K]+ | Tetrahexosidep | Supplemental Figure S5a | Sheath |
| 867 | 868, 869, 870 | [828K]+ | Pentahexosidep | Supplemental Figure S5a | Sheath |
| 779 | 781 | [666K2Cl]+ | Tetrahexosidep [K2Cl]+ cluster | None | |
| 941 | 943 | [828K2Cl]+ | Pentahexosidep [K2Cl]+ cluster | None |
Superscript labels p and f indicate plant and fungal origin, respectively.
Tissue in which the bins show a statistically significant difference between E+ and E− material. MS1 bins m/z 209, 223, 297, and 335 are significantly higher in E− samples and all other MS1 bins are higher in E+ samples.
Based on the nominal mass of an MS1 bin only, it is impossible to determine the chemical identity of a selected ion in a single experiment. For the MS1 bins of m/z 230, 231, and 189, no MS2 data were generated and no putative identification can be suggested. We have shown previously that DIMSn with our instrumentation allows MS2 product ion spectra for selected MS1 features to be checked manually to identify unique MS1 ion species by their fragmentation pathway (Koulman et al., 2007b). Here, we developed a computational method to utilize the fragmentation data from all the samples where MS2 data are available and circumvent manual comparison of individual spectra from different samples.
MS2 Data Analysis and the Identification of Selected MS1 Ions
As noted above, MS1 bins of different m/z could arise from the same metabolite due to isotopic ions, hydrogen transfers, or salt adducts. To identify a metabolite we need to first identify the relevant monoisotopic (12C, 1H, 14N, 16O) MS1 bin. This can be partially addressed using correlation analysis (Enot et al., 2006) as bins deriving from the same metabolite should be highly correlated, and in the mass range we are investigating, the monoisotopic MS1 bin will be of highest intensity. Thus, to assist in the identification of ions in the significant MS1 bins, we have considered concurrently correlated MS1 bins of adjacent mass (possible isotopologues) as well as MS1 bins corresponding to salt (e.g. K+ and Na+) adducts. We have also compared their MS2 product ion spectra as an aid to identifying isotopologues, adducts, and binning anomalies.
Analysis of the MS2 data can also assist in addressing the alternative scenario, namely, that any one MS1 bin (a nominal unit m/z) is also likely to contain signals for a number of different metabolites not resolved by instrument resolution (and also binning artefacts). If the MS1 bin of a nominal m/z contains ions from different metabolites in different samples, or from the same metabolites but in different concentrations, then the MS2 product ion spectra are likely to differ between samples. Thus we have developed a method for automated comparison of MS2 data across a sample set to investigate ion homogeneity within selected MS1 bins.
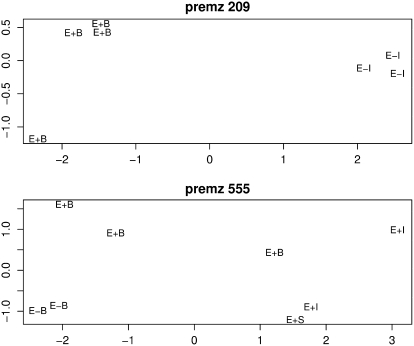
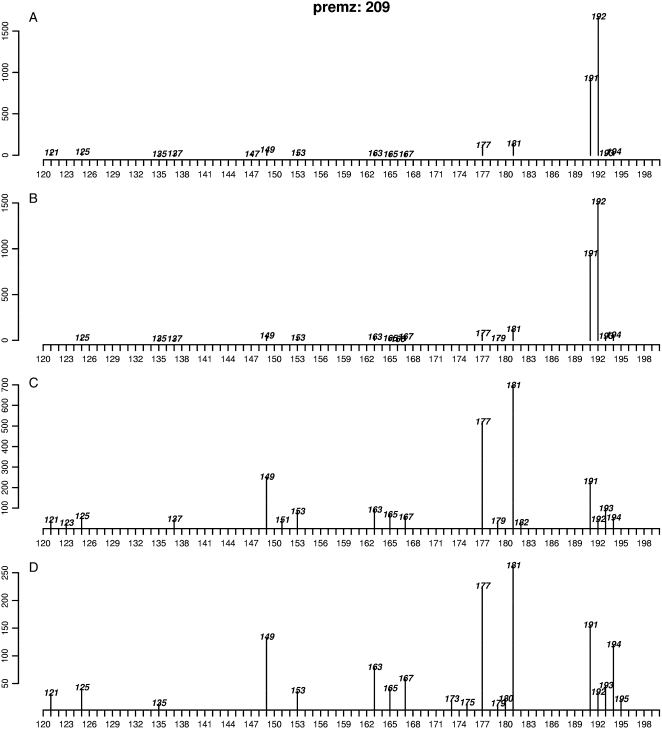
The method is based on the modified Manhattan distance as a measurement of the similarity of MS2 spectra from ions in a given parent MS1 bin. The procedure is described in detail in “Materials and Methods.” In brief, MS2 data for a parent MS1 bin of interest were pairwise compared between samples. Only the 20 most intense fragment ions in each MS2 spectrum were used for the comparison. The sum of the absolute values of the difference in normalized intensities was used as a distance score. From a set of pairwise differences, a distance matrix was obtained for statistical classification (hierarchical clustering or multidimensional scaling [MDS], etc.) to assess the homogeneity of each MS1 bin. For most MS1 bins, no distinct groups were seen, indicating that the MS2 spectra were consistent, and thus these MS1 bins are homogeneous and likely to be dominated by a single ion species. However, there were a number of MS1 bins for which different MS2 spectral patterns were observed for different samples. The differences between MS2 spectra in samples can be visualized with clustering analysis methods such as hierarchical clustering or MDS. MDS preserves the distance metric, and the cluster structures are revealed in different directions in the manner of principal component analysis (Lattin et al., 2003). The m/z 209 MS1 bin, which showed significant differences in ion abundance between E+/E− in the immature tissues (see the plot in Supplemental Fig. S2), also exhibited different MS2 patterns in E+ and E− samples (Fig. 2, premz 209, where premz means precursor m/z in MS2 plots). Inspection of individual MS2 spectra from the MS1 bin m/z 209 (Fig. 3) suggests there are at least two metabolites within the m/z 209 MS1 bin present in different concentrations in the E+ and E− samples (e.g. fragment ions m/z 191 and 192; in comparison with m/z 149, 177, and 181). The MS2 spectra for another significant MS1 bin m/z 554 and the MS1 bin of adjacent mass m/z 555 (see the following discussion) also showed different patterns between E+ and E− samples (Fig. 2, premz 555). See Supplemental Figure S4 for the detailed MS2 spectra of m/z 555.
Figure 2.
The clustering of MS2 spectra derived from the parent MS1 bins m/z 209 and m/z 555 by MDS based on modified Manhattan distances, suggesting there are different MS2 spectral patterns in E+ and E− groups.
Figure 3.
Plots of MS2 spectra derived from the parent MS1 bin m/z 209 in E− immature tissue (spectra A and B) and E+ blade tissue (spectra C and D) showing different relative intensities of product ions indicative of different metabolite compositions within this bin in the two classes of samples. MS2 data were not obtained for other samples.
Many distance metrics have been proposed to measure the similarity of spectra such as the dot product (Stein and Scott, 1994) and correlation-based distance metrics such as 1-correlation coefficient or 1-cosine (Tabb et al., 2003). We observed that fragment masses in MS2 spectra often showed mismatches between samples, and major fragment masses needed to be treated individually. The top 20 ions in each MS2 spectrum were retained and compared in this study, but this number could be extended or decreased to any arbitrary number. However, McLafferty et al. (1999) reported that 18 peaks were 97% as effective as 150 peaks for searching and comparing electron impact mass spectra. In standard practice of chemical analysis, only a few major MS2 product ions are used for confirmation of the identity of the parent MS1 species (Allwood et al., 2006; Koulman et al., 2007b).
The predominant component of the m/z 248 MS1 bin in the endophyte-infected samples is peramine, which is a known fungal alkaloid. Peramine has a guanidinium moiety that undergoes distinctive neutral losses of 17 and 42. Its MS2 product ion spectrum is in accordance with previous findings (Koulman et al., 2007a); see also Supplemental Figure S7 in which m/z 248 is used as an example for MS2 data handling.
The MS2 fragmentation pattern of the m/z 205 MS1 bin showed a clear water loss. Weakly basic metabolites such as alcohols are prone to form sodium adducts rather than [MH]+ ions (Jemal et al., 1997). We propose the actual nominal mass of the metabolite to be 182 (i.e. m/z 205 is [MNa]+). A logical candidate is mannitol or a related sugar alcohol. To test this hypothesis, we infused a water solution of mannitol into the mass spectrometer and observed a sodiated ion of m/z 205 with a highly similar MS2 spectrum to that of the m/z 205 MS1 bin in the endophyte-infected samples (Supplemental Fig. S3). However, as there are several naturally occurring hexitols (10 possible stereoisomers) with similar MS2 product ion spectra (based on data from triple quadrupole MS; see www.hmdb.ca and www.massbank.jp), the MS2 spectrum may be derived from a combination of several unresolved sugar alcohols in the m/z 205 MS1 bin.
No direct fragmentation data are available for the m/z 335 MS1 bin that was detected at higher abundance in endophyte-free tissue (Fig. 1, m/z 335). However, consideration of MS1 bins of adjacent masses suggested the ions of m/z 335 are mainly isotopologues of the ryegrass alkaloid perloline. The m/z 335 MS1 bin is highly correlated with the MS1 bins of m/z 333 (r = 0.86, r is the Pearson's correlation coefficient, thereafter) and 334 (r = 0.93). The major ion detected at m/z 333 in positive ESI MS is assigned as the anhydrocation perloline (C20H17N2O3+), with predicted isotopologue ions at m/z 334 (13C1 and 15N1) and m/z 335 (13C1, 15N1, 13C2, 15N2, and 18O1). The expected relative intensities of m/z 333, 334, and 335 are 1:0.24:0.03. The MS1 bins of m/z 333, 334, and 335 were observed to have a mean relative intensity of 1:0.37:0.07. The measured ratios show higher abundances for the higher mass ions than the theoretical prediction, which suggests ions from additional compounds have also been detected in the m/z 335 MS1 bin. Thus, although modeling isotopic distribution has been attempted for high resolution MS data (Böcker et al., 2006), for low resolution infusion MS data this may be confounded by interfering components isobaric with isotopologue peaks.
The m/z 554 MS1 bin is correlated with the m/z 555 MS1 bin with r = 0.72. MS2 product ion spectra of m/z 554 were available only from four E+ samples and were very similar to the MS2 product ion spectra of m/z 555 in E+ samples (m/z 555 represents a different compound in E− samples as noted above; see Fig. 2; Supplemental Fig. S4). For both MS1 bins, the MS2 spectra in E+ samples show a series of product ions with a higher m/z than the parent ion (Supplemental Fig. S4), suggesting this is a doubly charged ion. Manual examination of these ions showed that the parent ion occurred at m/z 554.5 and its monoisotopologue at m/z 555.1. Due to the limited precision of the mass spectrometer, the measured m/z varied between 554.22 and 554.55 and therefore caused binning problems with bins of unit m/z. The doubly charged state was confirmed by the occurrence of high mass product ions in the MS2 and MS3 data. There was a significant product ion of m/z 904.3 and several other high mass ions in the MS2 spectrum from m/z 554.5 and in the MS3 spectrum from its major MS2 product of m/z 516.7 (Supplemental Fig. S4, a and b). The exact structure of the compound remains to be elucidated, but the complex pattern of product ions suggests that it is a cyclic oligomer of amino acids.
Differentially expressed ions in E+ versus E− immature leaves were observed in MS1 bins of m/z 209, 297, 223, and 230 (230 is also different in E+/E− sheaths). MS2 spectra for the m/z 297 MS1 bin were observed in only two samples. The dominant product ions were of m/z 104,105, 237, and 238. This occurrence of pairs of fragment ions in the MS2 spectrum suggested that the m/z 297 MS1 bin comprised isotopologues of ions in the m/z 296 bin, and the m/z 297 MS1 bin was highly correlated with the m/z 296 MS1 bin with r = 0.82. The m/z 296 MS1 bin abundance is higher in endophyte-free samples and its MS2 spectrum showed a dominant m/z 104 ion as well as a clear m/z 59 loss. The MS3 spectrum of the m/z 104 ion showed a major fragment of m/z 60. These fragmentations are all highly indicative of a choline group (http://metlin.scripps.edu). This appears to be a novel compound, as no plant or fungal compounds with a corresponding mass and a choline group have been reported. One possibility is a 5-hydroxyferulyl analog of sinapine. We also remain uncertain about the chemical identity of the major metabolites detected in the m/z 209 and 223 MS1 bins, although MS2 product ion spectra (data not shown) were obtained in this study.
Correlation Network Analysis of MS1 Bins
Feature selection based on statistical ranking or machine learning algorithms is an important step in high-throughput data analysis. However, no golden rules exist for choosing a cutoff of P values or ranking scores and alternative approaches other than statistical ranking may be of use. Correlations among variables are sometimes considered as redundancy and often one feature (variable) is selected from a correlative group for further analysis (Zou and Hastie, 2005). However, correlations between MS1 bins deriving from different metabolites may provide insights into the functional dependency of these metabolites.
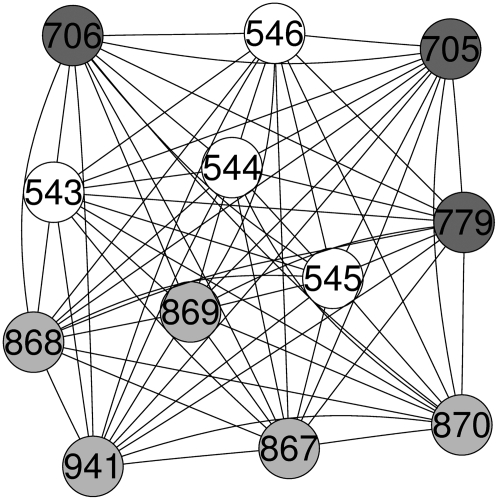
With the aid of network analysis tools (Carey et al., 2005), correlation (or relevance) networks (Butte et al., 2000) among all the measured MS1 bins in all the samples were investigated. The correlation networks constructed in this way should reveal when a group of MS1 bins exhibit the same pattern of relative concentration (ion abundance) across samples. Based on the criterion of r > 0.9, many isolated correlation units were found to be composed of MS1 bins of adjacent mass, which are likely to be due to natural isotopologues. A large highly connected subgraph (clique) in the network was identified with 12 nodes (Fig. 4). In this subgraph component, some correlations between these MS1 bins are due to naturally occurring isotopes. The m/z 543, 544, 545, and 546 MS1 bins belong to one group, the m/z 705 and 706 MS1 bins to another, and m/z 867, 868, 869, and 870 MS1 bins to a third group. That these subgroupings (highlighted by different gray scale in Fig. 4) are due to isotopologues was also supported by MS2 spectral information, as within each group the MS2 spectra for the higher mass MS1 bins were similar to that from the lowest mass (monoisotopic) MS1 bin but with additional isotopologous fragments. The core correlation is between the three most intense MS1 bins of the lowest mass in each group, corresponding to the monoisotopic ions (m/z: 543, 705, and 867; see Table I).
Figure 4.
A group of highly correlated MS1 ions identified by correlation network analysis. The ions with the same gray scale are the same metabolites with different m/z due to the presence of natural isotopologues or salt adducts.
These three MS1 bins differ by a mass of 162, and this corresponds to the mass of a hexose (180) − H2O. The MS2 and MS3 data showed consecutive losses of 162 from the high mass ions (Supplemental Fig. S5a). Ions of these m/z ratios have been reported by Enot et al. (2006) for potassiated fructan oligomers detected in DIMS of genetically modified potatoes (Solanum tuberosum; [504K]+, [666K]+, and [828K]+; potassiated trihexose, tetrahexose, and pentahexose, respectively). Perennial ryegrass is known to produce a range of fructan oligomers from degree of polymerization (DP) 3 to >8 (Pavis et al., 2001), and the identification of these MS1 bins as deriving from fructan oligomers was supported by comparison of MS2 and MS3 spectra with those obtained by infusion and MS/MS analysis of aqueous KCl solutions of 1-kestose, 1,1-tetrakestose, and 1,1,1-pentakestose (Supplemental Fig. S5b).
We considered the possibility that the two other MS1 bins m/z 779 and 941 in the correlation network might derive from glycerol adducts of the oligosaccharides, as they differed in mass from the m/z 705 and 867 species by 74 units. Glycosylglycerides have recently been reported by Yamamoto et al. (2006) as synthetic products of kojibiose phosphorylase from Thermoanaerobacter brocki. However, the MS2 spectrum of the m/z 779 and 941 species in the plant extracts showed in each case only a neutral loss of 74, while synthetic glucosyl-, maltosyl-, and maltotriosylglycerol showed neutral losses of 92 and 162 on MS/MS analysis. Further, m/z 779 and 941 ions were also observed in the DIMSn profiles of 1,1-tetrakestose and 1,1,1-pentakestose, respectively, in aqueous KCl (above), and were accompanied by ions of lower intensity of m/z 781 and 943, respectively, in the DIMSn profiles of both the standard solutions and plant extracts. These higher mass ions fragmented with a sole neutral loss of 76. In each case the product ion underwent subsequent MS fragmentation as for the potassiated oligosaccharide. As neutral losses of 74 and 76 correspond to the two chlorine isotopologues of KCl, we conclude the m/z 779 and 941 species are KCl cluster adducts of the potassiated oligosaccharides of the m/z 705 and 867. Similar adduct ions were present for the potassiated trihexose, although they were not detected as part of the correlation network.
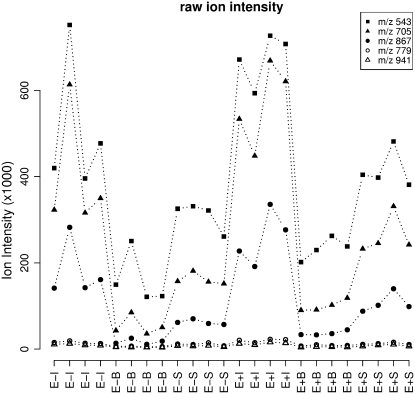
The levels of the oligohexoses were low in the blades and high in immature tissue and present at intermediate levels in sheath tissue (Fig. 5). When considering the endophyte effect, concentrations of the oligosaccharides were significantly higher in the endophyte-infected sheaths by a simple t test, with P values of 0.0082, 0.0048, and 0.0075 for the m/z 543, 705, and 867 MS1 bins, respectively. However, there were no significant (P values >0.05) endophyte infection effects in immature leaf and blade.
Figure 5.
A plot of raw ion abundance of five correlated MS1 bins (represented by m/z 543, 705, 867, 779, and 941) showing that these metabolites accumulate to higher levels in immature leaves, lower levels in blade, and intermediate levels in sheath tissue.
DISCUSSION
Metabolite Identification and Measurement
The identification of metabolites from raw signals detected by mass spectrometers is a challenging task in metabolomics that still demands considerable effort (Schauer and Fernie, 2006). Two types of MS technologies are in general use for high-throughput metabolomics analyses. One type is high-accuracy MS using ICR-MS or time-of-flight MS (Dettmer et al., 2007). Highly accurate m/z measurements narrow down the search space by providing a short list of chemical formulae (elemental compositions), although without additional isotope abundance data this list may remain extensive (Kind and Fiehn, 2006). The other type exploited in metabolomics is tandem MS (MS/MS or MSn) using an ion trap that can provide fragmentation data on the initial MS1 ions, although in practice this capacity is often foregone (Enot et al., 2006). As demonstrated here, these fragmentation data are useful for the elucidation and classification of chemical structures. Technologies combining these two features, e.g. ion trap with FT-ICR-MS, are also available to provide high mass resolution MS1 profiles and information on fragmentation patterns. However, applying this combination to obtain high resolution data on both MS1 and MS2 ions for large numbers of samples is impractical due to the slow scan speeds required by the FT-ICR-MS. All of these technologies generate complex data sets and there are many steps in translating raw signals into chemical entities. With access to the raw data now readily available in standard formats such as mzXML (Pedrioli et al., 2004), novel or improved algorithms can be employed in many steps of data analysis, such as data preprocessing (including baseline detection and removal, peak detection, peak, or retention time alignment, etc.) and inference of the identity of components (e.g. Listgarten and Emili, 2005; for a recent review of LC-MS data processing for metabolomics and currently available software, see Katajamaa and Orešič, 2007).
We recently applied direct-infusion ESI MS using DIMSn to determine metabolic differences between endophyte-infected and endophyte-free ryegrass seed samples (Koulman et al., 2007b). In this study, we have extended this approach by developing tools to analyze the raw MS data from DIMSn and to compare MS2 spectra derived from the same parent MS1 bin from different samples. This has enabled us to handle the collected data appropriately. Rather than seeking alignment in the time domain as addressed by programs such as XCMS and MZmine, our software bins the data into unit m/z bins and finds the median intensity in each m/z bin over the course of the infusion. The challenges of mass alignment and binning of data collected at high mass resolution (e.g. Hansen and Smedsgaard, 2004) are also much less for data collected at unit m/z resolution. Developing methods for automating the handling of MS2 data has enabled us to determine if the assignment of MS1 signal variations to treatment effects on a specific metabolite is justified, as it has allowed us to distinguish whether these MS1 signals were derived from the same metabolite(s) in all the samples or whether there were different metabolites in different samples detected within the same MS1 bin. For chemical identification, an MS2 product ion spectrum derived from an isotopically and chemically homogenous MS1 bin is desirable. An MS2 spectrum derived from an MS1 bin comprising a mixture of isotopologue ions will show an anomalous pattern, as noted above for the m/z 297 MS1 bin. Thus prior to investigating the MS2 data, it is useful to screen candidate MS1 bins for the existence of highly correlated MS1 bins of adjacent mass and higher intensity that are likely candidates for the monoisotopic species. Correlation analysis can also reveal the presence of ESI adducts such as the [K2Cl]+ cluster adducts reported here. The development of software to automate the discovery of isotopologues and adducts is an area of current active research (Tautenhahn et al., 2007). While facilities for comparison of MSn data (ion trees) and construction of libraries are available in commercial software (e.g. MassFrontier), further refinement and extension of the tool developed here to automate the construction of MSn libraries to facilitate metabolite identification would be a valuable tool for the analysis of DIMSn data.
Metabolites have diverse physical and chemical properties and a wide range of concentrations in a biological system, so any single analytical technique cannot detect all the metabolites of biological relevance. Using DIMSn, we have identified or classified a number of metabolites known to be present in endophyte-infected grass samples such as peramine and a sugar alcohol putatively annotated as mannitol (but we cannot exclude other sugar alcohols). Other well-known metabolites, e.g. the alkaloids lolitrem B and ergovaline, although of high biological significance were not detected in this experiment. Indolediterpenes like lolitrem B are lipophilic and not sufficiently extracted by the extraction solvents used in this study. Ergopeptides (like ergovaline) are usually present at very low concentrations in the symbiotum and their MS signals are within the noise range of DIMSn data. Therefore, the analysis of these classes of metabolites requires the deployment of dedicated approaches (Lehner et al., 2005; Spiering et al., 2005). In this study we only used positive ionization and only one type of extraction procedure. We believe that alternative extraction procedures and ionization methods would deliver additional information on other classes of metabolites.
Quantitation in infusion ESI MS is subject to signal suppression or enhancement in the source (see Dettmer et al., 2007), and ionization from an infused mixture is likely to be selective and dependent on the ability of a molecule to capture a charge in the source. Thus the detection of species such as the peramine and perloline cations and the doubly charged putative peptide ion is not unexpected, and other species less prone to forming cations are likely to have been underrepresented in the profile. Developments in nanoscale ESI may provide improved performance in this regard. The experiments described here were carried out with a standard capillary and flow rates of 5 μL/min. Nanospray technology utilizing very low flow rates (<20 nL/min) can reduce and perhaps eliminate analyte suppression (Schmidt et al., 2003) and may provide a less biased profile. An implementation in a microchip-mounted microfluidic device has shown promise for drug metabolite discovery (Trunzer et al., 2007) and may provide advantages for metabolomics.
The other factor confounding quantitation in ion trap DIMSn is the presence of multiple components within each 1 m/z bin. Thus, while the endophyte effect on the m/z 248 MS1 bin can be attributed to peramine, the differences in intensity between tissue types (Fig. 1) appear to derive from the unknown plant components also detected in this bin. Concentrations of peramine in these samples estimated by HPLC with photo diode array detection (L. Johnson, unpublished data) were similar in the three tissue types as reported by Spiering et al. (2005).
Although we have clearly demonstrated the usefulness of fragmentation data for the classification and structural elucidation of metabolites, the method is of limited use for characterizing metabolites that do not show a fragmentation (e.g. m/z 230, 189). For such metabolites the MS1 data provide a lead to further investigation using other methods. Indeed for all putative novel metabolites, additional data such as accurate mass MS, and targeted isolation and structure elucidation by, for example, NMR spectroscopy is necessary for their complete chemical characterization.
Biological Implications of Identified Metabolites
Several metabolites identified in this study have interesting implications for the metabolic regulation of the perennial ryegrass-endophyte symbiotum. As discussed in detail above, the hexitol (m/z 205) present in endophyte-infected plants only is probably mannitol. Mannitol appears to be a very common polyol in fungi (Lewis and Smith, 1967) and has been reported to accumulate in endophyte-infected tall fescue (Richardson et al., 1992) and perennial ryegrass plants (Harwood, 1954; Johnson et al., 2006; Rasmussen et al., 2008). Although mannitol has been implicated as an osmoprotectant in the resurrection plant Myrothamnus flabellifolia (Bianchi et al., 1993) and in transgenic Arabidopsis (Arabidopsis thaliana) expressing a celery (Apium graveolens) Man-6-P reductase (Sickler et al., 2007) as well as in Nicotiana tabacum expressing a mannitol-1-P dehydrogenase (Karakas et al., 1997), a study in tall fescue indicates that mannitol levels in endophyte-infected plants are not increased under drought stress (Richardson et al., 1992). A recent review (Solomon et al., 2007) also questions this role for mannitol as well as other claimed functions like fungal carbohydrate storage (Voegele et al., 2005) or NADPH regeneration (Hult and Gatenbeck, 1978; Hult et al., 1980). Solomon et al. (2007) conclude that the role and requirements for mannitol seem to differ depending on the species of fungus. In Aspergillus niger, mannitol is involved in conidial oxidative and high temperature stress protection (Ruijter et al., 2003), and in the wheat (Triticum aestivum) pathogen Stagonospora nodorum it is required for asexual sporulation (Solomon et al., 2006). Clearly, more studies are needed to understand the function of mannitol in endophyte-infected grasses.
Peramine (m/z 248) has been shown to be the likely agent to confer improved insect resistance to endophyte-infected plants affecting Argentine stem weevil and a range of other insects (Rowan and Gaynor, 1986; Latch, 1993; Rowan, 1993; Rowan and Latch, 1994). Recently, it was shown that peramine is produced by an endophyte-specific two-module nonribosomal peptide synthetase (perA) and that an Epichloë festucae mutant deleted for perA lacks detectable levels of peramine (Tanaka et al., 2005). It was also shown that plant material containing this mutant endophyte was as susceptible to Argentine stem weevil feeding as endophyte-free plants, demonstrating unambiguously that peramine confers resistance to this insect. Peramine is also the most abundant alkaloid produced by this endophyte in infected plants; its concentration is usually an order of magnitude higher than for the other endophyte-specific alkaloids (Spiering et al., 2005) and it is detectable in plant fluids from cut leaf and in guttation fluid (Koulman et al., 2007a).
Perloline (m/z 333), a diazaphenanthrene alkaloid, is produced by the grass plant and has been isolated from both ryegrass and tall fescue plants (Grimmett and Waters, 1943; Jeffreys, 1964; Bush and Jeffreys, 1975). Our results suggest that perloline concentrations are reduced in endophyte-infected mature blades and sheaths; the mechanism for this effect remains to be elucidated. Not much is known about the biosynthesis or function of perloline, although it has been implicated in effects on fall armyworm (Spodoptera frugiperda JE Smith) performance (Salminen et al., 2005) and to stimulate prolactin secretion in rats (Strickland et al., 1992). Earlier reports on the function of perloline, such as causing ryegrass staggers, are questionable due to the possible presence of endophytes in the studied material unknown at the time (Fairbourn, 1962; Aasen et al., 1969).
The putative oligopeptide (m/z 554.5) identified in this study accumulates exclusively in endophyte-infected tissues and is therefore most probably an endophyte-produced metabolite. Recently, a novel cyclic peptide, epichlicin, inhibiting spore germination of Cladosporidium phlei, a pathogenic fungus of timothy grass (Phleum pratense), was isolated from timothy grass infected with Epichloë typhina (Seto et al., 2007), a relative of N. lolii investigated in this study. Oligopeptides have been isolated from fungal endophytes previously, e.g. leucinostatin A, a phytotoxic, anticancer, and antifungal peptide (Arai et al., 1973), and from Acremonium sp., a fungus infecting Taxus baccata (Strobel et al., 1997). This mycotoxin causes necrotic symptoms in nonhost plants, presumably because these plants, unlike T. baccata, are not able to transform it into the less toxic leucinostatin A-β-di-O-glucoside (Strobel and Hess, 1997; Tan and Zou, 2001). The antimicrobial cyclic echinocandin peptides have been isolated from endophytic Cryptosporiopsis sp. and Pezicula sp. in Pinus sylvestris and Fagus sylvatica (Noble et al., 1991) and the antifungal cyclopeptide cryptocandin from the endophytic Cryptosporiopsis compared with quercina of redwood (Strobel et al., 1999). We are currently isolating the oligopeptide identified in this study from endophyte-infected perennial ryegrass to elucidate its structure and to test its potential antimicrobial activity.
Correlation Network Analysis and Its Biological Implications
Correlation analysis of metabolites has been used previously to explore the functional dependency of metabolites, and it was shown that this type of analysis allowed, for example, the reconstruction of the metabolic pathway leading to the biosynthesis of glucosinolates in Arabidopsis (Keurentjes et al., 2006). It has been proposed that the construction of correlation networks based on metabolic fingerprinting might help to uncover underlying enzymatic reaction networks (Steuer et al., 2003), although other origins of correlations between metabolites within a physiological state have also been discussed (Camacho et al., 2005). However, in this study comparing different plant tissues and endophyte infection status, physiological differences are likely to be the dominant factor.
In this study we have identified three MS1 bins representing monoisotopic ions of different metabolites that correlate significantly in our sample set. Mass fragmentation indicates that these metabolites are potassiated tri-, tetra-, and pentahexosides and their identification as fructans of DP 3, 4, and 5 was supported by the comparison with DIMSn of solutions of standards in aqueous KCl. Many cool-season C3 grasses accumulate fructans (Suc derived Fru polymers) as storage carbohydrates in their vegetative tissue, especially in mature sheaths (Pollock and Cairns, 1991). The genera Lolium and Festuca accumulate appreciable amounts of low DP fructans belonging to the inulin series, inulin neoseries, and levan neoseries, which differ in the position of Glc (terminal or internal) and the linkage type of Fru residues (β2,1 or β2,6; Pollock, 1982; Pavis et al., 2001). It was also shown that the proportion of low DP fructans (DP < 6) was more prominent in bases of elongating leaves than in leaf sheaths, and that mature leaf blades accumulate predominantly 6G-kestotriose and 1- and 6G-kestotetraose. The limited MSn data obtained here do not provide direct information on linkage and branching type of these Fru-containing polymers, but differences in the relative intensity of product ions in the MS2 and MS3 spectra between extracts and standards (Supplemental Fig. S5, a and b) may reflect the mixed isomer composition of the plant fructans. Recent developments in the elucidation of carbohydrate structures by MSn without chemical derivatization (e.g. Fang and Bendiak, 2007) suggest more extensive MSn analysis may provide additional structural information. As was shown previously (Rasmussen et al., 2007, 2008), endophyte infection resulted in higher levels of some of the sugars, which might indicate that the increased sink strength in the infected tissue results in a higher turnover of high DP fructans with a concomitant increase in low DP oligosaccharides. Although the exact mechanism for this pattern of accumulation remains to be elucidated, it has been documented (for review, see Chalmers et al., 2005) that the base of youngest leaves and the sheath of the more mature leaves represent the organs where fructosyltransferase activities, fructan accumulation, and remobilization in perennial ryegrass is most active, and where several fructan metabolism genes are expressed.
CONCLUSION
Our results extend our current knowledge on the metabolites involved in the symbiosis of the fungus N. lolii and its host perennial ryegrass. Using unbiased metabolite profiling (DIMSn) and advanced data-mining strategies, we have been able to uncover a number of unexpected perturbations of the metabolome upon endophyte infection. Based on the MS1 spectra we have found several metabolites that were significantly different between endophyte-infected and endophyte-free samples. New methods for automated processing of MS2 data have proved useful in detecting whether ions in a unit m/z MS1 bin represent a single major component across a sample set, or a heterogeneous mixture. With the aid of the MS2 product ion spectra we could readily identify some MS1 ions on the top of the list such as the known metabolites peramine and mannitol. The analysis has also revealed some new metabolites that are present in endophyte-infected plants, such as putative cyclic oligopeptides, and plant compounds present at reduced levels in infected plants such as a novel putative choline derivative. The identification of unknown MS1 bins as being statistically significantly different in uninfected compared to infected tissues also provides justification for their further characterization using more targeted approaches.
Linear correlation network analysis revealed the effect of the endophyte on a range of oligosaccharides, giving us new clues on how the endophyte utilizes plant carbohydrates. The methodology has proved to be a powerful tool for discovering leads to novel chemistry associated with the symbiosis, and these demand further chemical and biological investigation.
MATERIALS AND METHODS
Experimental Design and Sampling
Clonal perennial ryegrass plants (Lolium perenne ‘Nui’), either infected with the fungal endophyte Neotyphodium lolii (strain Lp19) or endophyte free, were used in this study. Endophyte-free perennial ryegrass was obtained as described by Tanaka et al. (2005). A 2 × 3 factorial design was applied with endophyte-infected (E+) and endophyte-free (E−) plants, and three tissue types, namely, immature leaf, blade, and sheath. Four individual tillers, either all E+ or all E− perennial ryegrass, were planted in pots, to give four replicate pots of E+ and E− material. The three tissue types were dissected from each of the plants in each pot and pooled for analysis (see also Supplemental Table S1 for sample description). Thus, 24 ryegrass samples in total were examined in this study. These plants were grown in a controlled environment chamber with 14-h daylength (653 μmol m−2 s−1 of light intensity), a temperature of 20°C day/10°C night, and supplied with a modified Hoagland nutrient solution. The tissue samples were harvested and immediately frozen in liquid nitrogen, and stored at −80°C for subsequent analysis.
Direct-Infusion MS
Plant tissue samples were ground using pestle and mortar in liquid nitrogen and stored at −80°C. Fifty milligrams of ground samples were extracted with 1.5 mL of MeOH. The extract was partitioned between water and dichloromethane. The aqueous phase was lyophilized and redissolved in 1.5 mL of MeOH. The infusion solvent (MeOH) was pumped at 20 μL min−1 flow to a T junction just in front of the ESI source where 5 μL min−1 MeOH with 2% formic acid was added. A 100-μL aliquot of each sample was injected using an autosampler. After 10 min a MeOH blank was injected and run at 200 μL min−1 flow rate for 3 min.
A linear ion trap mass spectrometer (Thermo LTQ) coupled to a Thermo Finnigan Surveyor HPLC system was used. Thermo Finnigan Xcalibur software (version 1.4) was used for data acquisition. The mass spectrometer was set for ESI in positive mode. Samples were infused through a polyimide-coated glass capillary (0.1 mm i.d., 0.19 mm o.d.) at a flow rate of 5 μL/min. The spray voltage was 5.0 kV and the capillary temperature 275°C. The ion optics were tuned using paxilline. The flow rates of sheath gas, auxiliary gas, and sweep gas were set (in arbitrary units/min) to 20, 5, and 12, respectively. For the first 0.9 min after injection only MS1 spectra were recorded; for the period from 0.9 to 10 min the mass spectrometer was set up in data-dependent mode to collect one MS1 spectrum, followed by the isolation (2 m/z) and fragmentation (35% CE; relative collision energy) of the most intense ion from the MS1 spectrum, followed by the isolation (2 m/z) and fragmentation (35% CE) of the most intense ion from the MS2 spectrum. A new MS1 spectrum was then recorded, followed by the repetitive isolation (2 m/z) and fragmentation (35% CE) of the most intense ions from that MS1 spectrum and the most intense MS2 product ion. When an MS1 ion with a specific mass had been isolated and fragmented for the second time, it was placed on an exclusion list for the duration of the run. In total up to approximately 200 MS2 spectra were recorded in an average run.
Samples of glucosyl-, maltosyl-, and maltotriosylglycerol (provided by H. Nakano, Osaka Municipal Technical Research Institute, Osaka) and samples of standard 1-kestose, 1,1-tetrakestose, and 1,1,1-pentakestose (Megazyme International Ireland Ltd.; 4 μg mL−1) in aqueous KCl (50 mm) were infused and analyzed under the similar conditions.
Data Analysis
The raw data (Xcalibur raw file, in centroid mode) were converted into mzXML data format (Pedrioli et al., 2004) using software ReAdw available from http://sashimi.sourceforge.net/software_glossolalia.html. mzXML is a standard data format for tandem mass spectrometric data and relatively easy to manipulate.
MS1 Data Analysis
All the MS1 scans were retrieved from mzXML and the original data could also be checked manually using the Thermo proprietary software Xcalibur. Given the 1 m/z resolution of the machine, we binned the data to unit nominal m/z. Thus in each sample, for a given ion, for example, m/z 248, all the measured m/z values (e.g. 247.98, 248.12, 248.35) were rounded to an integer value of 248 (equivalent to binning with 1 m/z width), and the median (rather than the average) of the abundance of corresponding ions within a sample was taken as a robust statistical estimate to reduce potential rounding (or binning) artefacts. The resulting bins are described here as MS1 bins. For each MS1 bin, the first 300 scans (see Supplemental Fig. S6) were removed because they were background signals from solvent (noise). For each MS1 bin, the median value of these first 300 scans was then used as a baseline value, and subtracted from the ion abundance values for subsequent scans. Any negative values (below the background noise) and weak signals (less than the 10% quantile) were removed. The median value of the ion abundance of all the remaining scans in each sample was used as the representative MS1 bin abundance for that sample. The ion abundance values for MS1 bins over the range m/z 150 to 1,000 were determined for each sample to generate an MS1 data matrix of 24 × 851 for statistical analysis.
The abundance of each MS1 bin was normalized against the median of the observations in all the samples, using log2 [x(i)/median (x)], where vector x comprises the abundance measurements of each MS1 bin, and x(i) is the abundance of each individual treatment with i from 1 to 24.
Empirical Bayes moderated t statistics (Smyth, 2004) were applied to identify MS1 ions that were differentially expressed between E+ and E− across the three developmental stages (immature leaf, blade, and sheath). The algorithms are implemented and available as R package Limma (Smyth, 2004). MS1 data have been provided as Supplemental Data Set S1.
MS2 Data Analysis
All the MS2 data were retrieved by querying the mzXML data. MS2 spectra derived from an MS1 bin in one sample (e.g. 248.35, 248.39; see Supplemental Fig. S7) were merged by rounding to nominal m/z MS2 bins and assigning the median abundance value for each bin. For each sample, only the top 20 most abundant ions (MS2 bins) derived from an MS1 bin were retained for comparisons in this report. For a given parent MS1 bin, all derived MS2 spectra in each sample, if available, were pairwise compared based on a customized distance metric. The measurement of similarity of MS2 spectra is complicated not only by variation in ion abundance, but also by the occurrence of different fragment ions in spectra from the same MS1 bin in different samples and by carry over from adjacent MS1 bins of high intensity (as for isotopologues) as the isolation width of the ion trap was set to a range of 2 m/z for efficient capture and fragmentation of ions. The procedure for the distance metric of two MS2 spectra is as follows: (1) normalize each spectrum to its maximum abundance value and sort each spectrum based on its intensity, up to 20 most intense fragment ions are retained; (2) if the number of MS2 bins is still different, low intensity ions in the longer spectrum are truncated; (3) for the matched nominal ions (MS2 bins of same nominal m/z) occurring in both spectra, the Manhattan distance 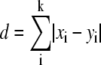 is calculated, where the two spectra are denoted as x = (x, …, xm) and y = (y, …, yn), and k is the number of shared MS2 bins [with m, n ≤ 20; k < min(m, n)]. For any unmatched MS2 bins, the normalized intensity is added to the distance.
is calculated, where the two spectra are denoted as x = (x, …, xm) and y = (y, …, yn), and k is the number of shared MS2 bins [with m, n ≤ 20; k < min(m, n)]. For any unmatched MS2 bins, the normalized intensity is added to the distance.
Step 3 provides a simplified way for m/z alignment. A similar idea was also employed by Zhang et al. (2005). The calculated pairwise distances for all samples were used for clustering analysis.
All the software functions for handling and analysis of MS1and MS2 data, and correlation network analysis were written in R2.5 (R Development Core Team, 2007) based on a number of R packages.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. PCA analysis of normalized MS1 data.
Supplemental Figure S2. Abundance of MS1 bins in treatment groups.
Supplemental Figure S3. MS2 spectra of parent MS1 m/z 205 [182Na]+ and mannitol standard.
Supplemental Figure S4. MS2 and MS3 spectra of parent m/z 555.
Supplemental Figure S5. MS2 and MS3 spectra of potassium adducts of oligosaccharide and oligokestose standards.
Supplemental Figure S6. Intensity plot of the nominal ion m/z 248 across all scans.
Supplemental Figure S7. Spectral processing of MS2 using nominal MS1 ion m/z 248.
Supplemental Table S1. Designation and description of experimental material.
Supplemental Data Set S1. MS1 data matrix.
Supplementary Material
Acknowledgments
We acknowledge Karl Fraser for the operation and maintenance of the mass spectrometer and the DIMSn analysis of fructan standards; Mike Christensen and Catherine Tootil for the maintenance of plant materials; and Hirofumi Nakano at Osaka Municipal Technical Research Institute, Osaka, for providing synthetic glucosyl-, maltosyl-, and maltotriosylglycerol. We appreciate Drs. Brian Tapper and Silas Villas-Boas for reviewing the manuscript and providing useful aspects for discussion.
This work was supported by a grant from the New Zealand Foundation for Research Science and Technology (contracts C10X0203 and AGRX0204) and conducted at AgResearch Grasslands, Palmerston North, New Zealand.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Susanne Rasmussen (susanne.rasmussen@agresearch.co.nz).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aasen AJ, Culvenor CCJ, Finnie EP, Kellock AW, Smith LW (1969) Alkaloids as a possible cause of ryegrass staggers in grazing livestock. Aust J Agric Res 20 71–86 [Google Scholar]
- Aharoni A, de Vos R, Verhoeven H, Maliepaard C, Kruppa G, Bino R, Goodenowe D (2002) Non-targeted metabolic profiling using fourier transform ion cyclotron mass spectrometry (FTMS). OMICS 6 217–234 [DOI] [PubMed] [Google Scholar]
- Allen J, Davey HM, Broadhurst D, Heald JK, Rowland JJ, Oliver SG, Kell DB (2003) High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat Biotechnol 21 692–696 [DOI] [PubMed] [Google Scholar]
- Allwood JW, Ellis DI, Heald JK, Goodacre R, Mur LAJ (2006) Metabolomic approaches reveal that phosphatidic and phosphatidyl glycerol phospholipids are major discriminatory non-polar metabolites in responses by Brachypodium distachyon to challenge by Magnaporthe grisea. Plant J 46 351–368 [DOI] [PubMed] [Google Scholar]
- Arai T, Mikami Y, Fukushima K, Utsumi T, Yazawa K (1973) A new antibiotic, leucinostatin, derived from Penicillium lilacinum. J Antibiot (Tokyo) 26 157–161 [DOI] [PubMed] [Google Scholar]
- Bianchi G, Gamba A, Limiroli R, Pozzi N, Elster R, Salamini F, Bartels D (1993) The unusual sugar composition in leaves of the resurrection plant Myrothamnus flabellifolia. Physiol Plant 87 223–226 [Google Scholar]
- Böcker S, Letzel MC, Lipták Z, Pervukhin A (2006) Decomposing metabolomic isotope patterns. In P Bücher, BME Moret, eds, Proceedings of the Workshop on Algorithms in Bioinformatics, Vol 4175. Springer, Berlin, pp 12–23
- Bush LP, Jeffreys JAD (1975) Isolation and separation of tall fescue and ryegrass alkaloids. J Chromatogr A 111 165–170 [DOI] [PubMed] [Google Scholar]
- Bush LP, Wilkinson HH, Schardl CL (1997) Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol 114 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte AJ, Tamayo P, Slonim D, Golub TR, Kohane IS (2000) Discovering functional relationships between RNA expression and chemotherapeutic susceptibility using relevance networks. Proc Natl Acad Sci USA 97 12182–12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho D, de la Fuente A, Mendes P (2005) The origin of correlations in metabolomics data. Metabolomics 1 53–63 [Google Scholar]
- Carey VJ, Gentry J, Whalen E, Gentleman R (2005) Network structures and algorithms in Bioconductor. Bioinformatics 21 135–136 [DOI] [PubMed] [Google Scholar]
- Chalmers J, Lidgett A, Cummings N, Cao Y, Forster J, Spangenberg G (2005) Molecular genetics of fructan metabolism in perennial ryegrass. Plant Biotechnol J 3 459–474 [DOI] [PubMed] [Google Scholar]
- Cheplick GP (2007) Costs of fungal endophyte infection in Lolium perenne genotypes from Eurasia and North Africa under extreme resource limitation. Environ Exp Bot 60 202–210 [Google Scholar]
- Christensen MJ, Leuchtmann A, Rowan DD, Tapper BA (1993) Taxonomy of Acremonium endophytes of tall fescue (Festuca arundinacea), meadow fescue (Festuca pratensis) and perennial ryegrass (Lolium perenne). Mycol Res 97 1083–1092 [Google Scholar]
- Clay K, Schardl C (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160 S99–S127 [DOI] [PubMed] [Google Scholar]
- Dettmer K, Aronov PA, Hammock BD (2007) Mass spectrometry-based metabolomics. Mass Spectrom Rev 26 51–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WB, Overy S, Quick WP (2005) Evaluation of automated electrospray-TOF mass spectrometry for metabolic fingerprinting of the plant metabolome. Metabolomics 1 137–148 [Google Scholar]
- Easton HS, Lane GA, Tapper BA, Keogh RG, Cooper BM, Blackwell M, Anderson MR, Fletcher L (1986) Ryegrass endophyte-related heat stress in cattle. In Proceedings of the New Zealand Grassland Association, Vol 57. New Zealand Grassland Association, Dunedin, New Zealand, pp 37–41
- Enot DP, Beckmann M, Overy D, Draper J (2006) Predicting interpretability of metabolome models based on behavior, putative identity, and biological relevance of explanatory signals. Proc Natl Acad Sci USA 103 14865–14870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbourn ML (1962) Alkaloid affects in vitro dry matter digestibility of Festuca and Bromus species. J Range Manage 35 503–504 [Google Scholar]
- Fang TT, Bendiak B (2007) The stereochemical dependence of unimolecular dissociation of monosaccharide-glycolaldehyde anions in the gas phase: a basis for assignment of the stereochemistry and anomeric configuration of monosaccharides in oligosaccharides by mass spectrometry via a key discriminatory product ion of disaccharide fragmentation, m/z 221. J Am Chem Soc 129 9721–9736 [DOI] [PubMed] [Google Scholar]
- Fletcher LR, Easton HS (1997) The evaluation of use of endophytes for pasture improvement. In CW Bacon, NS Hill, eds, Neotyphodium/Grass Interactions. Plenum Press, New York, pp 209–228
- Gallagher RT, Hawkes AD, Steyn PS, Vleggaar R (1984) Tremorgenic neurotoxins from perennial ryegrass causing ryegrass staggers disorder of livestock: structure elucidation of Lolitrem B. J Chem Soc Chem Commun 9 614–616 [Google Scholar]
- Grimmett RE, Waters DF (1943) A fluorescent alkaloid in ryegrass (Lolium perenne L.). II. Extraction from fresh ryegrass and separation from other bases. NZ J Sci Tech 24 151B [Google Scholar]
- Hansen ME, Smedsgaard J (2004) A new matching algorithm for high resolution mass spectra. J Am Soc Mass Spectrom 15 1173–1180 [DOI] [PubMed] [Google Scholar]
- Harwood VD (1954) Analytical studies on the carbohydrates of grasses and clovers. VII. The isolation of D-mannitol from perennial ryegrass (Lolium perenne L.). J Sci Food Agric 5 453–455 [Google Scholar]
- Hult K, Gatenbeck S (1978) Production of NADPH in the mannitol cycle and its relation to polyketide formation in Alternaria alternata. Eur J Biochem 88 607–612 [DOI] [PubMed] [Google Scholar]
- Hult K, Veide A, Gatenbeck S (1980) The distribution of the NADPH regenerating mannitol cycle among fungal species. Arch Microbiol 128 253–255 [DOI] [PubMed] [Google Scholar]
- Hunt MG, Rasmussen S, Newton PCD, Parsons AJ, Newman JA (2005) Near-term impacts of elevated CO2, nitrogen and fungal endophyte-infection on Lolium perenne L. growth, chemical composition and alkaloid production. Plant Cell Environ 28 1345–1354 [Google Scholar]
- Jeffreys JAD (1964) 859. The alkaloids of perennial rye-grass (Lolium perenne L.). Part I. Perloline. J Chem Soc 4504–4512 [DOI] [PubMed]
- Jemal M, Almond RB, Teitz DS (1997) Quantitative bioanalysis utilizing high-performance liquid chromatography/electrospray mass spectrometry via selected-ion monitoring of the sodium ion adduct [M + Na]+. Rapid Commun Mass Spectrom 11 1083–1088 [DOI] [PubMed] [Google Scholar]
- Johnson RD, Bassett S, Cao M, Christensen MJ, Gaborit C, Johnson LJ, Koulman A, Rasmussen S, Voisey C, Bryan G (2006) A multidisciplinary approach to dissect the molecular basis of the Neotyphodium lolii/ryegrass symbiosis. In CF Mercer, ed, Advances in Pasture Plant Breeding, Grassland Research and Practice Series No. 12. New Zealand Grassland Association, Dunedin, New Zealand, pp 107–114
- Karakas B, Ozias-Akins P, Stushnoff C, Suefferheld M, Rieger M (1997) Salinity and drought tolerance of mannitol-accumulating transgenic tobacco. Plant Cell Environ 20 609–616 [Google Scholar]
- Katajamaa M, Orešič M (2005) Processing methods for differential analysis of LC/MS profile data. BMC Bioinformatics 6 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katajamaa M, Orešič M (2007) Data processing for mass spectrometry-based metabolomics. J Chromatogr A 1158 318–328 [DOI] [PubMed] [Google Scholar]
- Keurentjes JJB, Fu J, de Vos CHR, Lommen A, Hall RD, Bino RJ, van der Plas LHW, Jansen RC, Vreugdenhil D, Koornneef M (2006) The genetics of plant metabolism. Nat Genet 38 842–849 [DOI] [PubMed] [Google Scholar]
- Kind T, Fiehn O (2006) Metabolomic database annotations via query of elemental compositions: mass accuracy is insufficient even at less than 1 ppm. BMC Bioinformatics 7 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulman A, Lane GA, Christensen MJ, Fraser K, Tapper BA (2007. a) Peramine and other fungal alkaloids are exuded in the guttation fluid of endophyte-infected grasses. Phytochemistry 68 355–360 [DOI] [PubMed] [Google Scholar]
- Koulman A, Tapper BA, Fraser K, Cao M, Lane GA, Rasmussen S (2007. b) High-throughput direct-infusion ion trap mass spectrometry: a new method for metabolomics. Rapid Commun Mass Spectrom 21 421–428 [DOI] [PubMed] [Google Scholar]
- Krauss J, Harri SA, Bush L, Husi R, Bigler L, Power SA, Müller CB (2007) Effects of fertilizer, fungal endophytes and plant cultivar on the performance of insect herbivores and their natural enemies. Funct Ecol 21 107–116 [Google Scholar]
- Lane GA, Christensen MJ, Miles CO (2000) Coevolution of fungal endophytes with grasses: the significance of secondary metabolites. In CW Bacon, JF White, eds, Microbial Endophytes. Marcel Dekker, New York, pp 341–388
- Latch GCM (1993) Physiological interactions of endophytic fungi and their hosts: biotic stress tolerance imparted to grasses by endophytes. Agric Ecosyst Environ 44 143–156 [Google Scholar]
- Lattin J, Carroll JD, Green PE (2003) Analyzing Multivariate Data. Duxbury Applied Series, Thomson Learning, Pacific Grove, CA
- Lehner AF, Craig M, Fannin N, Bush L, Tobin T (2005) Electrospray[+] tandem quadrupole mass spectrometry in the elucidation of ergot alkaloids chromatographed by HPLC: screening of grass or forage samples for novel toxic compounds. J Mass Spectrom 40 1484–1502 [DOI] [PubMed] [Google Scholar]
- Leuchtmann A (1992) Systematics, distribution, and host specificity of grass endophytes. Nat Toxins 1 150–162 [DOI] [PubMed] [Google Scholar]
- Lewis DH, Smith DC (1967) Sugar alcohols (polyols) in fungi and green plants. I. Distribution, physiology and metabolism. New Phytol 66 143–184 [Google Scholar]
- Listgarten J, Emili A (2005) Statistical and computational methods for comparative proteomics profiling using liquid chromatography-tandem mass spectrometry. Mol Cell Proteomics 4 419–434 [DOI] [PubMed] [Google Scholar]
- Lyons PC, Plattner RD, Bacon CW (1986) Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 232 487–489 [DOI] [PubMed] [Google Scholar]
- Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40 923–940 [Google Scholar]
- McLafferty FW, Staufferb DA, Lohb SY, Wesdemiotis C (1999) Unknown identification using reference mass spectra: quality evaluation of databases. J Am Soc Mass Spectrom 10 1229–1240 [DOI] [PubMed] [Google Scholar]
- Noble HM, Langley D, Sidebottom PJ, Lane SJ, Fisher PJ (1991) An echinocandin from an endophytic Cryptosporiopsis sp. and Pezicula sp. in Pinus sylvestris and Fagus sylvatica. Mycol Res 95 1439–1440 [Google Scholar]
- Pavis N, Chatterton NJ, Harrison PA, Baumgartner S, Praznik W, Boucaud J, Prud'homme MP (2001) Structure of fructans in roots and leaf tissues of Lolium perenne. New Phytol 150 83–95 [Google Scholar]
- Pedrioli PGA, Eng JK, Hubley R, Vogelzang M, Deutsch EW, Raught B, Pratt B, Nilsson E, Angeletti R, Apweiler R, et al (2004) A common open representation of mass spectrometry data and its application in a proteomics research environment. Nat Biotechnol 22 1459–1466 [DOI] [PubMed] [Google Scholar]
- Pollock CJ (1982) Oligosaccharide intermediates of fructan synthesis in Lolium temulentum. Phytochemistry 21 2461–2465 [Google Scholar]
- Pollock CJ, Cairns AJ (1991) Fructan metabolism in grasses and cereals. Annu Rev Plant Physiol Plant Mol Biol 42 77–101 [Google Scholar]
- Raamsdonk LM, Teusink B, Broadhurst D, Zhang N, Hayes A, Walsh MC, Berden JA, Brindle KM, Kell DB, Rowland JJ, et al (2001) A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat Biotechnol 19 45–50 [DOI] [PubMed] [Google Scholar]
- Rasmussen S, Parsons AJ, Bassett S, Christensen MJ, Hume DE, Johnson LJ, Johnson RD, Simpson WR, Stacke C, Voisey CR, et al (2007) High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytol 173 787–797 [DOI] [PubMed] [Google Scholar]
- Rasmussen S, Parsons AJ, Fraser K, Xue H, Newman JA (2008) Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiol 146 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MD, Chapman GW, Hoveland CS, Bacon CW (1992) Sugar alcohols in endophyte-infected tall fescue under drought. Crop Sci 32 1060–1061 [Google Scholar]
- Rowan DD (1993) Lolitrems, peramine and paxilline: mycotoxins of the ryegrass/endophyte interaction. Agric Ecosyst Environ 44 103–122 [Google Scholar]
- Rowan DD, Gaynor DL (1986) Isolation of feeding deterrents against Argentine stem weevil from ryegrass infected with the endophyte Acremonium loliae. J Chem Ecol 12 647–658 [DOI] [PubMed] [Google Scholar]
- Rowan DD, Latch GCM (1994) Utilization of endophyte-infected perennial ryegrasses for increased insect resistance. In CW Bacon, JF White, eds, Biotechnology of Endophytic Fungi in Grasses. CRC Press, Boca Raton, FL, pp 169–183
- Ruijter GJG, Bax M, Patel H, Flitter SJ, van de Vondervoort PJI, de Vries RP, van Kuyk PA, Visser J (2003) Mannitol is required for stress tolerance in Aspergillus niger conidiospores. Eukaryot Cell 2 690–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen SO, Richmond DS, Grewal SK, Grewal PS (2005) Influence of temperature on alkaloid levels and fall armyworm performance in endophytic tall fescue and perennial ryegrass. Entomol Exp Appl 115 417–426 [Google Scholar]
- Sauer U, Heinemann M, Zamboni N (2007) Getting closer to the whole picture. Science 316 550–551 [DOI] [PubMed] [Google Scholar]
- Schardl CL (2001) Epichloë festucae and related mutualistic symbionts of grasses. Fungal Genet Biol 33 69–82 [DOI] [PubMed] [Google Scholar]
- Schardl CL, Leuchtmann A, Spiering MJ (2004) Symbioses of grasses with seedborne fungal endophytes. Annu Rev Plant Biol 55 315–340 [DOI] [PubMed] [Google Scholar]
- Schauer N, Fernie AR (2006) Plant metabolomics: towards biological function and mechanism. Trends Plant Sci 11 508–516 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Karas M, Dülcks T (2003) Effect of different solution flow rates on analyte ion signals in nano-ESI MS, or: when does ESI turn into nano-ESI? J Am Soc Mass Spectrom 14 492–500 [DOI] [PubMed] [Google Scholar]
- Seto Y, Takahashi K, Matsuurai H, Kogami Y, Yada H, Yoshihara T, Nabeta K (2007) Novel cyclic peptide, epichlicin, from the endophytic fungus, Epichloë typhina. Biosci Biotechnol Biochem 71 1470–1475 [DOI] [PubMed] [Google Scholar]
- Sickler CM, Edwards GE, Kiirats O, Gao Z, Löscher W (2007) Response of mannitol-producing Arabidopsis thaliana to abiotic stress. Funct Plant Biol 34 382–391 [DOI] [PubMed] [Google Scholar]
- Smedsgaard J, Frisvad JC (1996) Using direct electrospray mass spectrometry in taxonomy and secondary metabolite profiling of crude fungal extracts. J Microbiol Methods 25 5–17 [Google Scholar]
- Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G (2006) XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching and identification. Anal Chem 78 779–787 [DOI] [PubMed] [Google Scholar]
- Smyth GK (2004) Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 1: Article 3. [DOI] [PubMed]
- Solomon PS, Waters ODC, Jörgens CI, Lowe RGT, Rechberger J, Trengove RD, Oliver RP (2006) Mannitol is required for asexual sporulation in the wheat pathogen Stagonospora nodorum (glume blotch). Biochem J 399 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PS, Waters ODC, Oliver RP (2007) Decoding the mannitol enigma in filamentous fungi. Trends Microbiol 15 257–262 [DOI] [PubMed] [Google Scholar]
- Spiering MJ, Lane GA, Christensen MJ, Schmid J (2005) Distribution of the fungal endophyte Neotyphodium lolii is not a major determinant of the distribution of fungal alkaloids in Lolium perenne plants. Phytochemistry 66 195–202 [DOI] [PubMed] [Google Scholar]
- Stein SE, Scott DR (1994) Optimization and testing of mass spectral library search algorithms for compound identification. J Am Soc Mass Spectrom 5 859–866 [DOI] [PubMed] [Google Scholar]
- Steuer R, Kurths J, Fiehn O, Weckwerth W (2003) Observing and interpreting correlations in metabolomic networks. Bioinformatics 19 1019–1026 [DOI] [PubMed] [Google Scholar]
- Strickland JR, Bailey EM, Abney LK, Oliver JW (1996) Assessment of the mitogenic potential of the alkaloids produced by endophyte (Acremonium coenophialum)-infected tall fescue (Festuca arundinacea) on bovine vascular smooth muscle in vitro. J Anim Sci 74 1664–1671 [DOI] [PubMed] [Google Scholar]
- Strickland JR, Cross DL, Jenkins TC, Petroski RJ, Powell RG (1992) The effect of alkaloids and seed extracts of endophyte-infected tall fescue on prolactin secretion in an in vitro rat pituitary perfusion system. J Anim Sci 70 2779–2786 [DOI] [PubMed] [Google Scholar]
- Strobel GA, Hess WM (1997) Glucosylation of the peptide leucinostatin A, produced by an endophytic fungus of European yew, may protect the host from leucinostatin toxicity. Chem Biol 4 529–536 [DOI] [PubMed] [Google Scholar]
- Strobel GA, Miller RV, Martinez-Miller C, Condron MM, Teplow DB, Hess WM (1999) Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Microbiology 145 1919–1926 [DOI] [PubMed] [Google Scholar]
- Strobel GA, Torczynski R, Bollon A (1997) Acremonium sp.—a leucinostatin A producing endophyte of European yew (Taxus baccata). Plant Sci 128 97–108 [Google Scholar]
- Sumner L, Mendes P, Dixon R (2003) Plant metabolomics: large-scale phytochemistry in the functional genomics era. Phytochemistry 62 807–836 [DOI] [PubMed] [Google Scholar]
- Tabb DL, MacCoss MJ, Wu CC, Anderson SD, Yates III Jr (2003) Similarity among tandem mass spectra from proteomic experiments: detection, significance, and utility. Anal Chem 75 2470–2477 [DOI] [PubMed] [Google Scholar]
- Tan RX, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18 448–459 [DOI] [PubMed] [Google Scholar]
- Tanaka A, Tapper BA, Popay A, Parker EJ, Scott B (2005) A symbiosis expressed non-ribosomal peptide synthetase from a mutualistic fungal endophyte of perennial ryegrass confers protection to the symbiotum from insect herbivory. Mol Microbiol 57 1036–1050 [DOI] [PubMed] [Google Scholar]
- Tautenhahn R, Böttcher C, Neumann S (2007) Annotation of LC/ESI-MS mass signals. In S Hochreiter, R Wagner, eds, Lecture Notes in Computer Science, Vol 4414. Springer, Berlin, pp 371–380
- Tozuka Z, Kaneko H, Shiraga T, Mitani Y, Beppu M, Terashita S, Kawamura A, Kagayama A (2003) Strategy for structural elucidation of drugs and drug metabolites using (MS)n fragmentation in an electrospray ion trap. J Mass Spectrom 38 793–808 [DOI] [PubMed] [Google Scholar]
- Trunzer M, Graf D, Kiffe M (2007) Comparison of a two-dimensional liquid chromatography/mass spectrometry approach with a chip-based nanoelectrospray device for structural elucidation of metabolites in a human ADME study using a quadrupole time-of-flight mass spectrometer. Rapid Commun Mass Spectrom 21 937–944 [DOI] [PubMed] [Google Scholar]
- Voegele RT, Hahn M, Lohaus G, Link T, Heiser I, Mendgen K (2005) Possible roles for mannitol and mannitol dehydrogenase in the biotrophic plant pathogen Uromyces fabae. Plant Physiol 137 190–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Watanabe H, Nishimoto T, Aga H, Kubota M, Chaen H, Fukuda S (2006) Acceptor recognition of kojibiose phosphorylase from Thermoaerobacter brockii: syntheses of glycosyl glycerol and myo-inositol. J Biosci Bioeng 101 427–433 [DOI] [PubMed] [Google Scholar]
- Zhang X, Asara JM, Adamec J, Ouzzani M, Elmagarmid AK (2005) Data pre-processing in liquid chromatography-mass spectrometry based proteomics. Bioinformatics 21 4054–4059 [DOI] [PubMed] [Google Scholar]
- Zou H, Hastie T (2005) Regularization and variable selection via the elastic net. J R Statist Soc B 67 301–320 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.