Abstract
We investigated the role of ethylene and auxin in regulating the growth and morphology of roots during mechanical impedance by developing a new growing system and using the model plant Arabidopsis (Arabidopsis thaliana). The Arabidopsis seedlings grown horizontally on a dialysis membrane-covered agar plate encountered adequate mechanical impedance as the roots showed characteristic ethylene phenotypes: 2-fold reduction in root growth, increase in root diameter, decrease in cell elongation, and ectopic root hair formation. The root phenotype characterization of various mutants having altered response to ethylene biosynthesis or signaling, the effect of ethylene inhibitors on mechanically impeded roots, and transcription profiling of the ethylene-responsive genes led us to conclude that enhanced ethylene response plays a primary role in changing root morphology and development during mechanical impedance. Further, the differential sensitivity of horizontally and vertically grown roots toward exogenous ethylene suggested that ethylene signaling plays a critical role in enhancing the ethylene response. We subsequently demonstrated that the enhanced ethylene response also affects the auxin response in roots. Taken together, our results provide a new insight into the role of ethylene in changing root morphology during mechanical impedance.
In nature, plant roots navigate through barriers in the soil. Responses of roots to mechanical perturbation are integral features of plant behavior. Indeed, all plants sense and respond to mechanical forces, albeit differentially. The cellular responses of roots during mechanical impedance may be critical for fundamental processes, such as turgor regulation, cellular expansion, morphogenesis, and tropic responses (Okada and Shimura, 1994; Braam, 2005). Signaling molecules and hormones, including intracellular calcium, reactive oxygen species, ethylene, auxin, and abscisic acid, have been implicated in mechanical responses or touch stimulation (Masle, 2002; Braam, 2005). The root cap cells, which are in a dynamic developmental flux and encounter the first obstacles while the roots grow through the soil, are strong candidates for touch sensors (Legué et al., 1997; Fasano et al., 2001). Interestingly, the gravity response of roots has also been suggested to be perceived by the same cap cells (Sack, 1997; Blancaflor et al., 1998). Recently, Massa and Gilroy (2003) further showed that interaction of touch and gravity resides in the cells of the root cap and that the touch sensory system may down-regulate gravity responsiveness in Arabidopsis (Arabidopsis thaliana).
It is generally assumed that mechanical stimulation is perceived via a change in the plasma membrane voltage and transmitted via calcium ion channels (Fasano et al., 2002), which induces fast and transient changes in calcium ions in roots (Knight et al., 1991; Legué et al., 1997) and expressions of some genes, including calmodulin, in shoots (Braam and Davis, 1990; Sistrunk et al., 1994; Xu et al., 1995). Although these studies suggest a few factors playing a regulatory role in this process, the mechanistic bases of touch perception and inter- and intracellular signaling are not well understood. The details of the signaling system involved in the mechanical impedance or touch-induced changes in root morphology also remain elusive. One of the major barriers to this study is to mimic the soil condition in experimental assays. The general approach to study the root physiology is growing the seedlings in vertically oriented agar plates, which provides little resistance to root growth. Growing plants in a horizontal condition can be used as an alternative approach to provide continuous mechanical perturbation. However, in this approach, the roots penetrate the agar and form a coil structure, which makes the study of morphology and growth and development of roots difficult.
In the last decade, Okada and Shimura (1990) developed a unique growth system to provide obstacle-touching stimulus to roots. By using a hard agar (1.5% compared to 1%) surface and inclined plates, they provided touching stimulus to the Arabidopsis roots and found that the roots showed a wavy growth pattern due to a mechanical stimulus avoidance response. Using this system, the authors screened mutants for an abnormal root waving pattern and six mutant lines were reported (Okada and Shimura, 1990). Further studies of these mutants revealed some new candidate proteins involved in the signaling of wavy root growth and transport of auxin (Okada and Shimura, 1994; Mochizuki et al., 2005; Santner and Watson, 2006). However, little is known about the signaling cascade that regulates the root morphology and cellular expansion of roots.
In an effort to understand the effect of continuous mechanical stimulation on Arabidopsis root morphology, we developed a new growing system that provides this stimulation continuously to root tips. In this growing system, Arabidopsis seedlings were grown on a dialysis membrane-covered agar plate and placed vertically (plates on edge) or horizontally (plates laying flat). The horizontally grown root tips continuously perceive mechanical stimulation because the root tips touch the dialysis membrane while bending downward. The presence of the dialysis membrane prevented the root from penetrating inside the agar and gave us an additional advantage to study the root morphology and developmental responses to this stimulation. Our study revealed that continuous mechanical impedance affected both root morphology and elongation. With the aid of available Arabidopsis mutants and gene expression analyses, we also tried to elucidate the role of ethylene and auxin in regulating the growth and development of roots. Our results suggest that ethylene response plays a major role in this process and the auxin effect on the root elongation process during continuous mechanical stimulation is regulated by enhanced ethylene response. In addition, we also provide evidence that ethylene signaling, rather than ethylene synthesis, plays a regulatory role in enhancing the ethylene response.
RESULTS
Root Morphology and Elongation in Horizontally Grown Arabidopsis Seedlings
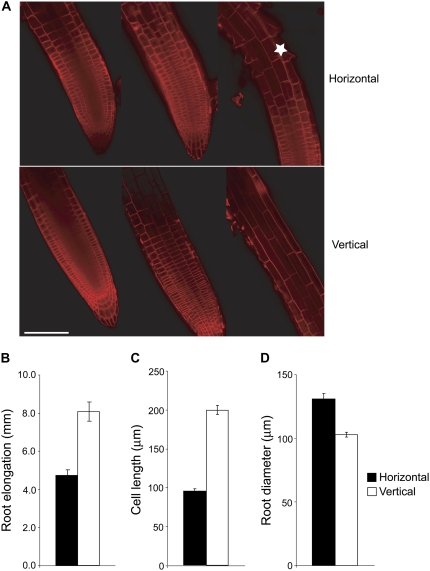
When wild-type Arabidopsis seedlings were placed on dialysis membrane-covered agar plates, the roots grew in the horizontal direction without penetrating into the agar. We compared the root morphology and growth characteristics between horizontally oriented roots affected by continuous mechanical stimulation and vertically oriented roots. Arabidopsis root growth and morphology looked similar while grown vertically in the presence or absence of dialysis membranes (Supplemental Fig. S1; data not shown), confirming that sufficient amounts of nutrients, water, and humidity are constantly available even in the presence of dialysis membranes. Figure 1A represents the morphology of the roots grown in vertical and horizontal conditions. The confocal image of the roots stained with FM1-43 dye, which stains the plasma membrane and endocytic vesicles (Bolte et al., 2004), showed that the elongation zone of the horizontally grown roots was severely shortened compared to that of vertically grown roots. Also, in horizontally grown seedlings, the initiation of root hair was found to be closer to the meristem zone (Fig. 1A). Interestingly, the meristem length and number of cells in the epidermal cell file remained unchanged; the epidermal cell number was 22.33 ± 1.20 (se) for vertically grown roots and 20 ± 1.15 (se) for horizontally grown ones. These results suggest that continuous mechanical stimulation reduced the root length solely by reducing the cell length without affecting the cell division. The physiological analysis of the roots grown in two different conditions revealed clear differences in growth characteristics. The horizontally grown roots showed a 2-fold reduction in root elongation, an increase in root diameter, and a reduction in cell lengths (Fig. 1, B–D). Consistent with the confocal images, we found that mechanical impedance induced a 2-fold reduction in the mature epidermal cell lengths, which accounts for the total reduction of the root growth (Fig. 1B). The morphology and phenotypes of horizontally grown roots show striking similarity to those of ethylene-treated roots (Rahman et al., 2000), indicating that ethylene may play an important role during continuous mechanical stimulation.
Figure 1.
Effect of mechanical impedance on Arabidopsis root morphology and growth. A, Confocal image of primary roots of Arabidopsis grown horizontally (top) or vertically (bottom) on dialysis membrane-covered agar plates. Four-day-old roots were stained in 1 μm FM1-43 dye for 15 min. Images are single confocal sections and representative of three separate imaging runs, with five roots per treatment for each run. The images at the left show the epidermal and cortical cell files of the root meristem, images in the middle are the surface view of images at the left, and images on the right show the root elongation zone. The midpoints of the elongation zone images are approximately 320 and 550 μm away from the root tip, respectively. The asterisk in the top image indicates the root hair initiation zone in mechanically impeded roots. Bar = 100 μm. B to D, Differential response of wild-type seedlings for root length (B), mature epidermal cell length (C), and root diameter (D) grown in the presence or absence of mechanical impedance. Vertical bars = mean ± se. Data are from three to five independent experiments with 10 to 12 seedlings per experiment.
Nullifying the Ethylene Response Restores Normal Root Growth and Morphology in Mechanically Impeded Roots
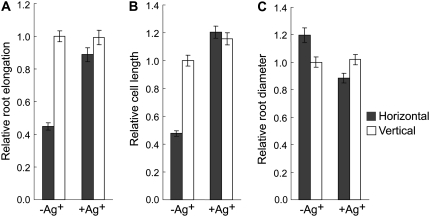
To confirm the role of ethylene in regulating the growth of the roots under continuous mechanical impedance, we took two different approaches. First, we investigated the effects of Ag+, an ethylene action inhibitor (Beyer, 1976), and 1-aminoethoxyvinylglycine (AVG), an ethylene synthesis inhibitor (Yang and Hoffman, 1984), on the growth of the roots. Application of 100 μm Ag+ (Fig. 2) or 3 μm AVG (Supplemental Fig. S2) to horizontal roots increased the root and cell elongation to the levels of vertical control. The root diameter of horizontal roots was also reduced by these treatments to the level of vertical roots, indicating that nullifying the ethylene response can restore the normal root morphology in horizontally grown roots.
Figure 2.
Effect of Ag+ on root length (A), root cell length (B), and root diameter (C) of wild-type seedlings grown in the presence or absence of mechanical impedance. Arabidopsis wild-type seedlings were grown in the presence or absence of 100 μm Ag+ in horizontal or vertical conditions for 3 d under continuous light. Vertical bars = mean ± se. Data are from three independent experiments with 10 to 12 seedlings per experiment.
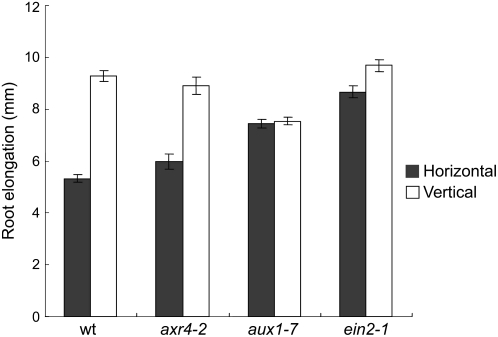
Further confirmation of this idea came from the physiological analyses of the mutants having altered response to ethylene and auxin. To this end, we used three different mutants: axr4-2, aux1-7, and ein2-1 showing differential response to auxin and ethylene (Guzmán and Ecker, 1990; Pickett et al., 1990; Hobbie and Estelle, 1995). The roots of ein2-1, which is a strong ethylene-resistant mutant, showed insensitivity to mechanical perturbations. In contrast to the wild type, the horizontally grown ein2-1 roots showed a similar growth pattern and morphology like the vertical ones (Fig. 3). A similar result was obtained with aux1-7, which shows strong resistance to both ethylene and auxin in root growth. In sharp contrast to aux1-7, axr4-2, which is also an auxin uptake mutant having a defect in localizing the AUX1 in epidermal cells and shows only auxin resistance in root growth but a normal response to ethylene (Hobbie and Estelle, 1995; Bennett et al., 1996; Yamamoto and Yamamoto, 1999; Dharmasiri et al., 2006; wild-type-like ethylene response of axr4-2 roots was also confirmed by us [data not shown]), responded to mechanical stimulations like wild type. The horizontally grown axr4-2 roots showed a 2-fold reduction in root growth and an increase in root diameter compared to vertically grown roots (Fig. 3; data not shown). Taken together, these results strongly suggest that ethylene and auxin play a primary role in regulating the root developmental process during the presence of continuous mechanical perturbations.
Figure 3.
Root growth response of various auxin and ethylene response mutants to mechanical impedance. Mutant seedlings were grown for 3 d in horizontal or vertical conditions under continuous light. Vertical bars = mean ± se. Data are from three independent experiments with 10 to 12 seedlings per experiment.
Ethylene- and Auxin-Responsive Gene Expression under Continuous Mechanical Stimulation
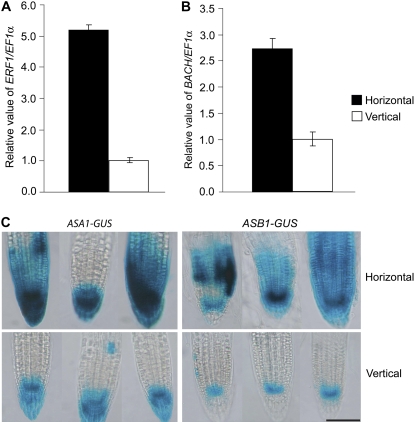
To understand the molecular consequences of mechanical impedance on root growth, we quantified the steady-state level of the transcripts of both the ethylene- and auxin-responsive genes by using quantitative reverse transcription (qRT)-PCR and imaged the GUS staining pattern in respective reporter lines. First, to compare the status of ethylene response in horizontally and vertically grown roots, we investigated the endogenous transcript levels of ETHYLENE RESPONSE FACTOR1 (ERF1) and BASIC CHITINASE (BACH), and next imaged the GUS staining patterns in two reporter lines containing transcriptional fusion of ANTHRANILATE SYNTHASE (AS)-α and AS-β to GUS (ASA1-GUS and ASB1-GUS), which are specifically induced by ethylene (Guo and Ecker, 2004; Stepanova et al., 2005). As shown in Figure 4, A and B, the expression of ERF1 and BACH was 5.2- and 2.7-fold greater in horizontal roots compared to the vertical control, respectively. A similar difference was observed in ASA1 and ASB1 reporter lines. Compared to vertical roots, a significant induction in GUS expression was observed in horizontally grown roots (Fig. 4C). Taken together, these results strongly suggest that the ethylene response is greatly enhanced in roots during continuous mechanical stimulation.
Figure 4.
Mechanical impedance enhances ethylene response in Arabidopsis roots. Real-time PCR analyses of the expression of ERF1 (A) and BACH (B) genes in horizontally or vertically grown roots. The copy number of ERF1 and BACH transcripts was calculated by normalizing against the number of copies of the EF1α transcript. Results are expressed as the mean ± se for the ratio of ERF1 or BACH to EF1α copy number (with the vertically grown root ratio assigned a value of 1) from three independent experiments. C, Expression of ASA1 and ASB1 genes during mechanical impedance. Four-day-old ASA1-GUS and ASB1-GUS transgenic seedlings were stained in a buffer containing 1 mm X-gluc for 18 h at 37°C in the dark. These are representative images of 30 seedlings stained in three separate runs. Bar = 100 μm.
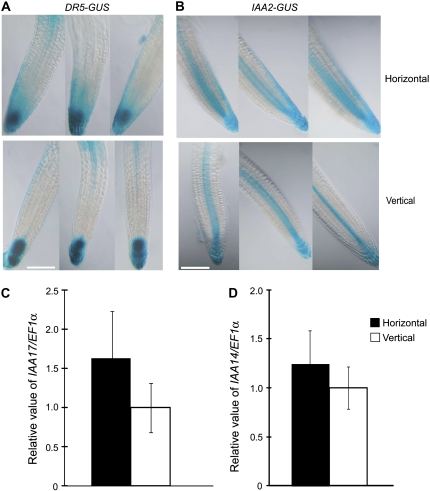
Because auxin has been shown to be a positive regulator of ethylene response for root growth (Rahman et al., 2001) and in horizontally grown roots we observed an induction in GUS staining in ASA1 and ASB1 reporter lines, which catalyze a rate-limiting step of Trp biosynthesis, a precursor of indole-acetic acid (IAA; Stepanova et al., 2005), we next compared the IAA-responsive gene expression in horizontally and vertically grown roots. To this end, we first investigated the auxin-regulated gene expression in horizontally and vertically grown roots using two different auxin-responsive reporter lines, DR5-GUS (Ulmasov et al., 1997) and IAA2-GUS (Luschnig et al., 1998; Swarup et al., 2001), whose activity has been found to correlate well with endogenous auxin (Casimiro et al., 2001; Benkova et al., 2003; Swarup et al., 2005; Grieneisen et al., 2007). A distinct difference in GUS staining pattern was observed between horizontally and vertically grown roots in both reporter lines (Fig. 5, A and B). For instance, in a horizontally grown DR5-GUS reporter line, we observed a spreading of GUS staining from the cells around the root tip toward the cells in the meristem (Fig. 5A), whereas the vertically grown roots showed a characteristic GUS staining pattern, a maximum of GUS activity in the columella initial cells with lower activity in the quiescent cells and mature columella root cap (Fig. 5A; Sabatini et al., 1999). We also observed the formation of a GUS gradient predominantly on the one side of the horizontally grown roots. No such gradient was formed in vertically grown DR5-GUS roots (Fig. 5A). An asymmetric auxin-induced gene expression extending basipetally from the lower side of the root tip to the meristematic region was also observed in horizontally grown IAA2-GUS reporter lines, whereas the vertically grown lines showed a typical staining pattern (Fig. 5B; Swarup et al., 2001). In addition, we observed a slight increase in GUS activity in the central cells of the horizontally grown IAA2-GUS roots (Fig. 5B). Although both reporter lines revealed a similar difference in GUS staining pattern, we found a difference in the sensitivity between these lines. With IAA2-GUS, the auxin gradient in horizontally grown roots could be observed after 1-h staining in 1 mm 5-bromo-4-chloro-3-indolyl β-d-GlcUA (X-gluc), whereas in DR5-GUS 1-h staining did not reveal any difference in the GUS staining pattern between horizontally and vertically grown roots (data not shown). A longer staining time was required to observe any difference and best images were obtained after 15-h staining (data not shown; Fig. 5A).
Figure 5.
Mechanical impedance alters auxin response in Arabidopsis roots. A and B, Expression of DR5-GUS (A) and IAA2-GUS (B) in horizontally and vertically grown roots. C and D, Real-time PCR analyses of the expression of IAA17 (C) and IAA14 (D) genes in horizontally or vertically grown roots. Relative expression was calculated as described in Figure 4. Four-day-old DR5-GUS or IAA2-GUS seedlings were stained in a buffer containing 1 mm X-gluc for 15 and 1 h, respectively, at 37°C in the dark. These are representative images of 30 seedlings stained in two separate runs. Bar = 100 μm.
The effect of mechanical impedance on auxin-responsive genes as observed in DR5 and IAA2-GUS lines could not be confirmed by monitoring the overall transcript levels of other auxin-responsive genes, such as IAA17, IAA14, and IAA11, which belong to the AUX/IAA family (Abel et al., 1995), using qRT-PCR (Fig. 5, C and D; data not shown). Although statistically insignificant (Fig. 5, C and D; 0.50 > P > 0.30 and 0.70 > P > 0.50 for IAA17 and IAA14, respectively), we observed a small but consistent increase in expression of these genes in horizontally grown roots (Fig. 5, C and D). The simplest explanation of this apparent discrepancy is sensitivity of the tissues toward auxin. In GUS reporter lines, the observed difference was confined in the region between approximately 100 to 200 μm from the root tip, whereas the qRT-PCR was performed with mRNA extracted from the whole root. The sensitivity of this qRT-PCR approach was possibly not sufficient to detect the local small change observed by GUS reporter lines. The other explanation of failure to detect a statistically significant difference in these gene expression levels may be due to an asymmetric auxin response, where an increase of expression on one side of the root would be nullified by a reduction in the expression level on the other side. Nevertheless, taken together, these results suggest that mechanical impedance alters the auxin response of roots along with the ethylene response.
Ethylene Signaling Rather than Ethylene Production Is Enhanced by Mechanical Stimulation
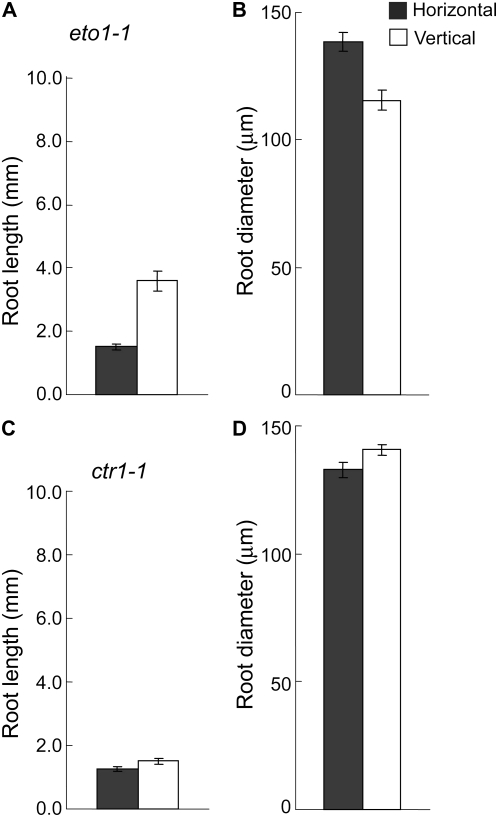
Our physiological and molecular data indicate that ethylene-mediated response is specifically enhanced in Arabidopsis roots encountering continuous mechanical impedance. This enhancement of ethylene response can be achieved by either increasing ethylene biosynthesis or activating the ethylene signaling without altering the biosynthesis. To answer this question, we first characterized the root growth phenotype of two different types of ethylene mutants, ctr1-1, a signaling mutant (Kieber et al., 1993), and eto1-1, an ethylene overproduction mutant (Guzmán and Ecker, 1990), in horizontal and vertical conditions. Compared to vertically grown roots, a 2-fold reduction in root growth, an increase in root diameter, and a decrease in cell length was observed in horizontally grown eto1-1 roots (Fig. 6, A and B; data not shown). These phenotypes are similar to those observed in wild type (compare Figs. 6, A and B, and 1). As opposed to eto1-1, the ethylene signaling mutant ctr1-1 roots showed no differential response to the growth conditions (Fig. 6, C and D). To further clarify the role of ethylene biosynthesis in this process, we next measured the amount of total ethylene accumulated in the seedlings grown vertically and horizontally. We first tried to measure the ethylene content using vertically grown wild-type seedlings, but the data were inconsistent and variable depending on the experiments (data not shown). On the other hand, vertically grown eto1-1 produced at least 6 times more ethylene compared to that of wild type and the data were reproducible. Because eto1-1 showed ethylene phenotypes in our condition, behaved like wild type in horizontal and vertical root phenotype assay, and produced 6 to 10 times more ethylene than Columbia (Col-0) wild type (Figs. 1 and 6; Kieber et al., 1993; Woeste et al., 1999; data not shown), we used this mutant to measure ethylene production with reproducibility. Consistent with the mutant analyses data, we found that the ethylene production from horizontally grown seedlings was the same as that from vertically grown seedlings (Table I). Although these results suggest that possibly the ethylene signaling rather than the ethylene biosynthesis plays a major role in regulating the root morphology during mechanical impedance, two potential problems work against this hypothesis. First, the ethylene biosynthesis and response can be a localized event and ethylene production may only increase in the elongation zone of the mechanically impeded roots. In such a case, the measurement of total ethylene production using the whole seedlings may not reflect the importance of ethylene biosynthesis in regulating root morphology. Second, the observed response of the eto1-1 roots to mechanical impedance may be due to the fact that ethylene concentration is not saturated in this mutant, despite the fact that eto1-1 produces 6 to 10 times more ethylene compared with wild-type seedlings.
Figure 6.
Response of ethylene overproduction mutant eto1-1 and constitutive triple-response mutant ctr1-1 roots toward mechanical impedance. A to D, Root length (A and C) and root diameter (B and D) of eto1-1 and ctr1-1 mutants, respectively. Mutant seedlings were grown for 3 d in horizontal or vertical conditions under continuous light. Vertical bars = mean ± se. Data are from three independent experiments with 10 to 12 seedlings per experiment.
Table I.
Ethylene production from eto1-1 grown in horizontal and vertical conditions
One hundred eto1-1 seedlings were placed on a dialysis membrane-covered agar plate in a sealed plastic container (6 × 4 × 1.8 cm). After 3-d incubation in horizontal or vertical conditions in the light, ethylene accumulation in the plastic container was measured.
| Condition | Ethylene Accumulation/100 Seedlings | Ethylene Accumulation/g Fresh Weight |
|---|---|---|
| nL | nL g−1 | |
| Horizontal | 0.179 ± 0.023 | 7.10 ± 1.30 |
| Vertical | 0.165 ± 0.009 | 7.15 ± 0.59 |
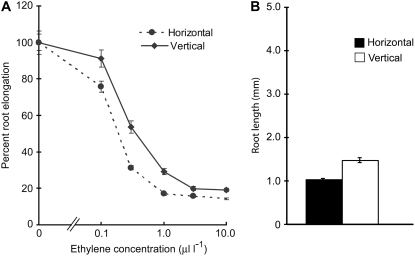
To eliminate these possibilities, we performed an ethylene dose response assay for root elongation and compared the sensitivity of the horizontally and vertically grown roots toward exogenous ethylene (Fig. 7A). The increased sensitivity of the horizontally grown roots toward exogenous ethylene suggests that there is a significant difference in ethylene signal transduction between these two treatments. Further, we compared the root growth phenotype of eto1-1 in the presence of 10 μL L−1 ethylene, which is a saturating concentration for root growth (Fig. 7A). This concentration of ethylene strongly inhibited the vertically grown eto1-1 roots (Fig. 7B; compare with Fig. 6A). However, we could still observe a significant difference in the length of the roots grown in horizontal and vertical conditions (Fig. 7B; P < 0.00005). We also observed a similar difference in the mature root epidermal cell length (data not shown). Collectively, these results suggest that ethylene signaling plays a critical role in sensing the mechanical stimulus in Arabidopsis roots.
Figure 7.
Ethylene dose response assay for root elongation in wild-type seedlings in the presence or absence of mechanical impedance (A) and root growth response of eto1-1 in the presence of 10 μL L−1 ethylene (B). Wild-type or eto-1-1 seedlings were grown for 3 d in horizontal or vertical conditions under continuous light. Vertical bars = mean ± se. Data are from three independent experiments with 10 to 12 seedlings per experiment.
Calcium Ion Channels May Not Be Involved in the Mechanism Behind the Reduced Elongation of Horizontal Roots
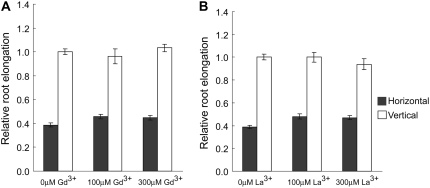
Because the calcium channels have been implicated in the perception of mechanical stimulus of roots, we investigated the role of the calcium ion channels in regulating the elongation of roots grown under continuous mechanical stimulation. The elongation assay of the roots was done in the presence or absence of two widely used calcium ion channel blockers: gadolinium ion (Gd3+) or lanthanum ion (La3+; Haley et al., 1995; Klüsener et al., 1995; Liß et al., 1998; Soga et al., 2005). In contrast to Ag+ ion or AVG, none of the blockers (even at a concentration of 300 μm) could rescue the elongation of the horizontally grown roots to the level of vertical ones (Fig. 8). These results suggest that calcium ion channels on the plasma membrane may not play a major role in regulating the growth and development of roots grown under continuous mechanical impedance.
Figure 8.
Effects of calcium ion channel blockers, gadolinium ion (Gd3+; A) and lanthanum ion (La3+; B), on the growth of Arabidopsis wild-type roots. Wild-type seedlings were grown for 3 d in horizontal or vertical conditions under continuous light in the presence or absence of ion channel blockers. Vertical bars = mean ± se. Data are from three independent experiments with 10 to 12 seedlings per experiment.
DISCUSSION
In a number of species, the morphology and phenotypes of roots during mechanical impedance had been shown to be strikingly similar to those of ethylene-treated seedlings (Goeschl et al., 1966; Kays et al., 1974). It is generally assumed that increased ethylene evolution in mechanically impeded roots is the primary factor in regulating root morphology and development (Goeschl et al., 1966; Kays et al., 1974; Sarquis et al., 1991, 1992; He et al., 1996). However, a controversy exists on this issue because several studies failed to detect any change in ethylene evolution or 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase activities during root mechanical impedance (Lachno et al., 1982; Moss et al., 1988; Hussain et al., 1999, 2000). These results raise a debate as to whether ethylene synthesis or ethylene response is the major factor in regulating the growth and morphology during mechanical impedance. Here, we reexamined this classic issue by developing a new growing system to provide continuous mechanical impedance to the root tip of the model plant Arabidopsis. The availability of the Arabidopsis mutants altered in ethylene signaling and synthesis gave us tools to dissect the role of these two processes in affecting the root development separately.
The observed morphological changes in the horizontally grown roots that include reduction in root elongation, radial root swelling, and induction of ectopic and elongated root hairs suggest that the new growing system can provide an adequate mechanical impedance to root growth (Fig. 1). The characteristic reduction of root elongation in mechanically impeded roots was found to be solely dependent on inhibition of cell length, leaving the cell numbers in meristem intact (Fig. 1). Le et al. (2001) and, more recently, Ruzicka et al. (2007) and Swarup et al. (2007) elegantly showed that ethylene primarily targets the cell elongation machinery to inhibit root growth. The striking similarity of the mechanistic basis of root growth inhibition in our newly developed system and ethylene-treated roots, along with the other observed morphological phenotypes, confirmed that an altered ethylene response regulates the growth and morphology of roots during mechanical impedance.
In principle, the change in the root ethylene responsiveness during mechanical impedance can be explained either by an increase in ethylene production or by an enhancement of ethylene response, possibly mediated via ethylene signaling. The physiological and molecular analyses of various mutants having altered response to ethylene biosynthesis or signaling support the notion that root mechanical impedance response requires an intact ethylene signaling system.
The ethylene overproduction mutant eto1-1 produces 6 to 10 times more ethylene compared to wild type and shows a severe ethylene phenotype (Guzmán and Ecker, 1990; Kieber et al., 1993; Rahman et al., 2000). If the increased ethylene biosynthesis during mechanical impedance turns out to be a major factor in regulating the root morphology as reported by earlier studies (Goeschl et al., 1966; Kays et al., 1974; Sarquis et al., 1991, 1992; He et al., 1996), one would assume that eto1-1 roots will not respond to the mechanical impedance. However, in our experimental condition, eto1-1 roots responded to mechanical stimulus in a wild-type manner not only in the absence of exogenous ethylene, but also in the presence of a saturating concentration of ethylene (Figs. 6 and 7B). In addition, the increased sensitivity of the horizontally grown wild-type roots toward exogenous ethylene (Fig. 7A) and no difference in ethylene production in eto1-1 mutant grown either in horizontal or vertical conditions (Table I) indicate that ethylene biosynthesis plays a minor role in enhancing the ethylene response during mechanical impedance.
The phenotypic characterization of two opposing ethylene signaling mutants, ein2-1 and ctr1-1, suggests that the observed changes in root morphology during mechanical impedance are tightly linked to ethylene signaling. Once ethylene is synthesized, it is perceived by a family of receptors that possess sequence similarity with bacterial two-component His kinases. Ethylene binding results in the inactivation of the receptors and of the receptor-interacting Raf-like protein kinase CONSTITUTIVE TRIPLE RESPONSE1 (CTR1), a negative regulator of the pathway. In the presence of ethylene, CTR1 loses its ability to repress a positive component of the pathway, the membrane protein ETHYLENE INSENSITIVE2 (EIN2; Stepanova et al., 2005). The loss of CTR1 results in constitutive activation of ethylene signaling, whereas the loss of EIN2 results in a loss of ethylene signaling (Kieber et al., 1993; Roman et al., 1995). This opposing effect of the mutations is also apparent in the root phenotypes of ein2-1 and ctr1-1. The roots of ctr1-1 mutant show a typical ethylene phenotype with a severely reduced root length, increased root diameter, and ectopic long root hairs (Kieber et al., 1993; Roman et al., 1995; Masucci and Schiefelbein, 1996; Rahman et al., 2000), whereas the ein2-1 roots exhibit a similar root length and diameter like wild type, but a shorter root hair length (Rahman et al., 2002). In contrast to the ethylene biosynthesis mutant eto1-1, which also shows an ethylene phenotype, none of the signaling mutants, namely, ein2-1 and ctr1-1, responded to mechanical impedance (Figs. 3 and 6), indicating that ethylene signaling is an integral component of this response pathway. Interestingly, although both the ctr1-1 and eto1-1 mutants show a similar ethylene phenotype in roots, only eto1-1 even with a higher ethylene production capability responded to mechanical impedance (Fig. 6). Taken together, these results suggest that ethylene signaling plays a critical role in changing the root ethylene responsiveness during mechanical impedance. However, we do not completely rule out the possibility of involvement of ethylene biosynthesis in this process.
The interaction of auxin and ethylene during root growth has long been known and recent studies further confirmed that they are tightly linked both at response and biosynthesis levels (Rahman et al., 2001, 2002; Stepanova et al., 2005, 2007; Ruzicka et al., 2007; Swarup et al., 2007). Further, in this study we observed an induction in the expression of AS-α and AS-β genes during mechanical impedance (Fig. 4). The AS enzyme catalyzes the first committed step of Trp biosynthesis, the conversion of chorismate to anthralinate (Radwanski and Last, 1995), and has been shown to be ethylene inducible (Stepanova et al., 2005). The induction of AS genes during mechanical impedance, along with the hypothesis that ethylene stimulates the accumulation of IAA in roots (Stepanova et al., 2005, 2007; Ruzicka et al., 2007; Swarup et al., 2007), raises a possibility that IAA may partly contribute to change the morphology of the root.
The GUS expression analysis in DR5-GUS and IAA2-GUS reporter lines revealed that mechanical stimulation, besides altering the ethylene response, also alters the auxin response in Arabidopsis roots. In both marker lines, we observed the formation of asymmetric auxin-induced gene expression in the lower side of the mechanically impeded roots, indicating that the auxin accumulated in the root apex is being redistributed in these roots (Fig. 5). Gravitropic curvature of Arabidopsis roots requires a redistribution of IAA from the root tip (for review, see Muday and Rahman, 2007), and such asymmetric gradient has been shown to be formed in gravity-stimulated or horizontally grown roots (Rashotte et al., 2001; Buer and Muday, 2004). Collectively these results suggest that mechanically impeded roots continuously perceive a gravity response.
Our finding that mechanical impedance increases the auxin-responsive GUS expression, spreading of GUS expression from the root tip to the meristem cells in DR5-GUS, and an increase of GUS activity in the central cylinder cells of IAA2-GUS (Fig. 5) is very important in the context of three recent reports that greatly enhance our understanding about the role of ethylene-auxin in root elongation (Ruzicka et al., 2007; Stepanova et al., 2007; Swarup et al., 2007). The current model suggests that ethylene induces the auxin biosynthesis in different plant organs, including the root apex, which is then redistributed via the auxin transporters AUX1 and PIN2 to the elongation zone where it inhibits the cell expansion. During this process, ethylene enhances both the basipetal and acropetal transport of auxin as judged by the transcriptional up-regulation of auxin transport components, as well as by direct transport assay (Ruzicka et al., 2007; S. Negi and G. Muday, personal communication).
The model of ethylene-induced auxin biosynthesis in the root apex and its redistribution to the elongation zone through auxin transport carriers completely fits with our findings. Mechanical impedance stimulates IAA production in the root apex by enhancing the transcription of AS-α and AS-β genes. This auxin is then transported back to the elongation zone, where it affects cell elongation and hence root growth. The formation of the asymmetric auxin gradient on the lower side of the mechanically impeded roots (Fig. 5, A and B) and the similar GUS expression patterns observed in auxin reporter lines during mechanical impedance and ethylene or ACC treatment (Fig. 5A; Ruzicka et al., 2007; Swarup et al., 2007) support the auxin redistribution hypothesis. This model also explains the observed phenotypic differences between two auxin-resistant mutants, aux1 and axr4. Whereas aux1 shows complete resistance to mechanical impedance, axr4 responded like wild type (Fig. 3). Swarup et al. (2007) suggested that the tissue-specific localization of AUX1 in lateral root cap cells is absolutely required for the redistribution of auxin from the root tip. Because the axr4-2 mutation selectively disrupts the trafficking of AUX1 in epidermal cell files (Dharmasiri et al., 2006), the mutant shows a wild-type sensitivity toward ethylene and hence to mechanical impedance.
In conclusion, the data presented here provide a mechanistic explanation for the role of ethylene in mechanical impedance. Our results strongly suggest that the observed change in root morphology and growth during mechanical impedance is due to enhanced ethylene and auxin responses, regulated largely by ethylene signaling.
MATERIALS AND METHODS
Plant Materials
Wild-type Arabidopsis (Arabidopsis thaliana L. Heynh.), ecotype Col-0, was obtained from Sendai Arabidopsis Seed Stock Center. The ethylene overproduction mutant eto1-1 (Guzmán and Ecker, 1990) and the constitutive triple-response mutant of ethylene, ctr1-1 (Kieber et al., 1993), aux1-7 (Pickett et al., 1990), and axr4-2 (Hobbie and Estelle, 1995) were obtained from the Arabidopsis Biological Resource Center. The ecotype of these mutants is Col-0. Seeds were propagated as described earlier (Rahman et al., 2000).
Growth Test
Test solutions were prepared by dissolving chemicals in 5 mm MOPS buffer (pH 6.6). The buffer was made of 5 mm KNO3, 2 mm Ca(NO3)2, 2 mm MgSO4, 1 mm KH2PO4, and 5 mm MOPS. The pH of the buffer was adjusted with KOH. Arabidopsis seeds were placed in a 2.6-cm petri dish on filter paper (Advantec no. 2; Toyo Roshi Kaisha, Ltd.), wetted with 300 μL of the buffer. Two or 4 d after cold treatment at 4°C under nearly saturating humidity in the dark, seeds were irradiated to germinate for 1 d with white fluorescent lamps (FL 20SS-BRN/18; Toshiba) at an irradiance of about 50 μmol m−2 s−1. The irradiated seeds were placed in a rectangular plastic container (6 × 4 × 1.8 cm) on nutrient agar containing test solutions covered with a dialysis membrane (Spectra/Por 7 regenerated cellulose membrane; molecular weight cutoff 10,000; flat width 24 mm; Spectrum Laboratories). The membrane was stirred in water three times for 10 min, then stirred in a solution containing 2% NaHCO3 and 1 mm EDTA at 60°C for 30 min and followed by washing in autoclaved water three times for 10 min prior to use. Chemicals such as AVG, AgNO3, GdCl3, and LaCl3 were mixed with agar medium, while the temperature of agar was 45°C to 50°C. Seedlings were grown on a dialysis membrane oriented vertically or horizontally at 23°C under continuous irradiation for 3 to 7 d. For ethylene treatment, ethylene was injected with a syringe into each plastic container through a small hole (Tsurumi and Ishizawa, 1997). Ethylene and the air inside the container were refreshed every day during the 3-d incubation period. The length and width of roots were measured under a microscope. The length of mature epidermal cells and the diameter of roots were measured at the longitudinal midpoint of roots. The length of epidermal cells for an individual seedling was the average from 10 cells. Each growth assay was repeated for at least three to five times.
GUS Expression Analysis and Root Imaging
For GUS expression analysis, 4-d-old seedlings were transferred to GUS staining buffer [100 mm sodium phosphate, pH 7.0, 10 mm EDTA, 0.5 mm K4Fe(CN)6, 0.5 mm K3Fe(CN)6, and 0.1% Triton X-100] containing 1 mm X-gluc and incubated at 37°C for specified time. For confocal imaging of roots, 4-d-old seedlings grown in vertical or horizontal conditions were treated with 1 μm FM1-43 dye (Invitrogen) for 15 min and imaged with a spinning disc confocal microscopy (Olympus BX-61 equipped with Bx-DSU; Olympus).
Chemicals
AVG was purchased from Sigma Chemical Co. Other chemicals were from Wako Pure Chemical Industries.
Measurement of Ethylene Production
To measure ethylene production from Arabidopsis seedlings, we used the ethylene overproduction mutant eto1-1. The irradiated 100 seeds of eto1-1 were placed in a rectangular plastic container (described above). The containers were sealed with tape and kept vertically or horizontally in the light at 23°C for 3 d. Ethylene concentration in the container was measured with a GC-14A gas chromatograph (Shimadzu Co.) by injecting a 1-mL gas sample taken from each container as described earlier (Rahman et al., 2000). The experiment was repeated at least three times.
qRT-PCR
Total RNA was extracted from 7-d-old Arabidopsis roots using RNeasy plant mini kit (Qiagen) with on-column DNase digestion to remove residual genomic DNA using RNase-free DNase set according to the provided handbook. Corresponding cDNA was synthesized according to the handbook of Powerscript RT (BD) using 1 μg of total RNA and dT 12-18mer oligonucleotide as a primer. Real-time PCR was performed on the LightCycler (Roche Diagnostics) with the LightCycler FastStart DNA Master Plus SYBR Green I kit (Roche) according to the manufacturer's protocol using the oligonucleotide primer sets (5′-CTTGCTTTCACCCTTGGTGT-3′ and 5′-TCCCTCGAATCCAGAGATTG-3′ for EF1α, 5′-CACCCTTAATCTTCGCTGCTC-3′ and 5′-TTGCATGATCCATGTTTGG-3′ for ERF1, 5′-CGCTTGTCCTGCTAGAGGTT-3′ and 5′-GTCTGGTGCTGTAGCCCATC-3′ for BACH, 5′-GGTCTTACGTTGAGCCTTGG-3′ and 5′-GTGGCTGAAGCCTTAGCTTG-3′ for IAA11, 5′-CATATTCTGATTTAAGACATA-3′ and 5′-AATCAATGCATATTGTCCTCT-3′ for IAA14, and 5′-TTTGTCCAACATGTTCAGCTCT-3′ and 5′-CCAAATCCATCAATTTCCTCTC-3′ for IAA17, respectively). The PCR condition was 95°C for 10 min, followed by a 40 cycles of 10 s at 95°C, 12 s at 58°C, and 10 s at 72°C. The specificity of the PCR amplification was checked with a melting curve analysis program and agarose gel electrophoresis of PCR products. Relative amount of specific mRNA was estimated using a standard cDNA preparation of known size and molecular concentration and normalized to the EF1α mRNA level.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effect of dialysis membrane on root growth.
Supplemental Figure S2. Effect of AVG on growth and development of mechanically impeded roots.
Supplementary Material
Acknowledgments
We thank Gloria Muday (Wake Forest University, Winston-Salem, NC) for critical reading of this manuscript. We are grateful to Tobias I. Baskin (University of Massachusetts, Amherst, MA) and Matsuo Uemura (Iwate University, Morioka, Japan) for invaluable suggestions, Jose Alonso (University of North Carolina, Raleigh, NC) for ASA1-GUS and ASB1-GUS lines, Joe Kieber (University of North Carolina, Chapel Hill, NC) for eto1-1 seeds, Jane Murfett and Tom Gulifoyle (University of Missouri, Columbia, MO) for the DR5-GUS line, Malcolm Bennett (University of Nottingham, Nottingham, UK) for the IAA2-GUS line, Yukio Kawamura (21st COE Program, Iwate University, Morioka, Japan) for assistance with confocal microscopy, the Arabidopsis Biological Resource Center for providing mutant seeds, and the Sendai Arabidopsis Seed Stock Center (Sendai, Japan) for Arabidopsis seeds.
This work was supported in part by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Sports, Culture, Science and Technology of Japan (grant no. 19780246 to A.R.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Abidur Rahman (abidur@iwate-u.ac.jp).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abel S, Nguyen MD, Theologis A (1995) The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol 251 533–549 [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273 948–950 [DOI] [PubMed] [Google Scholar]
- Beyer EM (1976) A potent inhibitor of ethylene action in plants. Plant Physiol 58 268–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S (1998) Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 116 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunmaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plants. J Microsc 214 159–173 [DOI] [PubMed] [Google Scholar]
- Braam J (2005) In touch: plant responses to mechanical stimuli. New Phytol 165 373–389 [DOI] [PubMed] [Google Scholar]
- Braam J, Davis RW (1990) Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell 60 357–364 [DOI] [PubMed] [Google Scholar]
- Buer CS, Muday GK (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16 1191–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inze D, Sandberg G, Casero PJ, et al (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri S, Swarup R, Mockaitis K, Dharmasiri N, Singh SK, Kowalchyk M, Marchant A, Mills A, Sandberg G, Bennett MJ, et al (2006) AXR4 is required for localization of the auxin influx facilitator AUX1. Science 312 1218–1220 [DOI] [PubMed] [Google Scholar]
- Fasano JM, Massa GD, Gilroy S (2002) Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul 21 71–88 [DOI] [PubMed] [Google Scholar]
- Fasano JM, Swanso SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis roots. Plant Cell 13 907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl J, Rappaport L, Pratt HK (1966) Ethylene as a factor regulating the growth of pea epicotyls subjected to physical stress. Plant Physiol 41 877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Maree AFM, Hogeweg P, Scheres B (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449 1008–1013 [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2004) The ethylene signaling pathway: new insights. Curr Opin Plant Biol 7 40–49 [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley A, Russell AJ, Wood N, Allan AC, Knight M, Cambell K, Trewavas AT (1995) Effects of mechanical signaling on plant cytosolic calcium. Proc Natl Acad Sci USA 92 4124–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Finalyson SA, Drew MC, Jordan WR, Morgan PW (1996) Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiol 112 1679–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Estelle M (1995) The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J 7 211–220 [DOI] [PubMed] [Google Scholar]
- Hussain A, Black CR, Taylor IB, Roberts JA (1999) Soil compaction. A role for ethylene in regulating leaf expansion and shoot growth in tomato. Plant Physiol 121 1227–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Black CR, Taylor IB, Roberts JA (2000) Does an antagonistic relationship between ABA and ethylene mediate shoot growth when tomato (Lycopersicon esculentum Mill.) plants encounter compacted soil? Plant Cell Environ 23 1217–1226 [Google Scholar]
- Kays SJ, Nicklow CW, Simons DH (1974) Ethylene in relation to the response of roots to physical impedance. Plant Soil 40 565–571 [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Fledmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72 427–441 [DOI] [PubMed] [Google Scholar]
- Klüsener B, Boheim G, Liß H, Engelberth J, Weiler EW (1995) Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J 14 2708–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352 524–526 [DOI] [PubMed] [Google Scholar]
- Lachno DR, Harrison-Murray RS, Audus LJ (1982) The effects of mechanical impedance on the levels of ABA and IAA in root tips of Zea mays L. J Exp Bot 33 943–951 [Google Scholar]
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP (2001) In the early response of Arabidopsis roots to ethylene, cell elongation is up and down regulated and uncoupled from differentiation. Plant Physiol 125 519–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legué V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S (1997) Cytoplasmic free Ca+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol 114 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liß H, Bockelmann C, Werner N, Fromm H, Weiler EW (1998) Identification and purification of the calcium-regulated Ca2+-ATPase from the endoplasmic reticulum of a higher plant mechanoreceptor organ. Physiol Plant 102 561–572 [Google Scholar]
- Luschnig C, Gaxiola R, Grisafi P, Fink G (1998) EIR1, a root specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masle J (2002) High soil strength: mechanical forces at play on root morphogenesis and in root:shoot branching. In Y Waisel, A Eshel, U Kafkafi, eds, Plant Roots, The Hidden Half, Ed 3. Marcel Dekker, New York, pp 807–819
- Massa GD, Gilroy S (2003) Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana. Plant J 33 435–445 [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW (1996) Hormones act downstream of TTG and GL2 to promote root hair initiation through an auxin and ethylene associated process. Plant Cell 8 1505–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki S, Harada A, Inada S, Sugimoto-Shirasu K, Stacey N, Wada T, Ishiguro S, Okada K, Sakai T (2005) The Arabidopsis WAVY GROWTH 2 protein modulates root bending in response to environmental stimuli. Plant Cell 17 537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss GI, Hall KC, Jackson MB (1988) Ethylene and the responses of roots of maize (Zea mays L.) to physical impedance. New Phytol 109 303–311 [Google Scholar]
- Muday GK, Rahman A (2007) Auxin transport and the integration of gravitropic growth. In S Gilroy, P Masson, eds, Plant Tropisms. Blackwell Publishing, Oxford, pp 47–68
- Okada K, Shimura Y (1990) Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 250 274–276 [DOI] [PubMed] [Google Scholar]
- Okada K, Shimura Y (1994) Modulation of root growth by physical stimuli. In EM Meyerowitz, CR Somerville, eds, Arabidopsis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 665–684
- Pickett FB, Wilson AK, Estelle M (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94 1462–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanski ER, Last RL (1995) Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell 7 921–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Ahamed A, Amakawa T, Goto N, Tsurimi S (2002) Auxin ad ethylene response interactions during root hair development dissected by auxin influx modulators. Plant Physiol 130 1908–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Amakawa T, Goto N, Tsurimi S (2001) Auxin is a positive regulator for ethylene-mediated response in the growth of Arabidopsis roots. Plant Cell Physiol 42 301–307 [DOI] [PubMed] [Google Scholar]
- Rahman A, Tsurumi S, Amakawa T, Soga K, Hoson T, Goto N, Kamisaka S (2000) Involvement of ethylene and gibberellin signalings in chromosaponin I-induced cell division and cell elongation in the roots of Arabidopsis seedlings. Plant Cell Physiol 41 1–9 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubrasky B, Keiber JJ, Rothenberg M, Ecker JR (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel loci integrated into a stress response pathway. Genetics 139 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19 3111–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99 463–472 [DOI] [PubMed] [Google Scholar]
- Sack FD (1997) Plastids and gravitropic sensing. Planta 203 S63–S68 [DOI] [PubMed] [Google Scholar]
- Santner AA, Watson JC (2006) The WAG1 and WAG2 protein kinase negatively regulate root waving in Arabidopsis. Plant J 45 752–764 [DOI] [PubMed] [Google Scholar]
- Sarquis JI, Jordan WR, Morgan PW (1991) Ethylene evolution from maize (Zea mays L.) seedlings roots and shoots in response to mechanical impedance. Plant Physiol 96 1171–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarquis JI, Morgan PW, Jordan WR (1992) Metabolism of 1-aminocyclopropane-1-carboxylic acid in etiolated maize seedlings grown under mechanical impedance. Plant Physiol 98 1342–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistrunk ML, Antosiewicz DM, Purugganan MM, Braam J (1994) Arabidopsis TCH3 encodes a novel Ca2+ binding protein and shown environmentally induced and tissue-specific regulation. Plant Cell 6 1553–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T (2005) Mechanoreceptors rather than sedimentable amyloplasts perceive the gravity signal in hypergravity-induced inhibition of root growth in azuki bean. Funct Plant Biol 32 175–179 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17 2230–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Plame K, Bennett MJ (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in Arabidopsis root apex. Genes Dev 15 2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Kramer EM, Perry P, Knox K, Leyser HMO, Haseloff J, Beemster GTS, Bhalerao R, Bennett MJ (2005) Root gravitropism requires lateral root cap and epidermal cells for transport and response to mobile auxin signal. Nat Cell Biol 7 1057–1065 [DOI] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi S, Ishizawa K (1997) Involvement of ethylene in chromosaponin-induced stimulation of growth in lettuce roots. Plant Cell Physiol 38 668–675 [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste KE, Ye C, Kieber JJ (1999) Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol 119 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Purugganan MM, Polisensky DH, Antosiewicz DM, Fry SC, Braam J (1995) Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell 7 1555–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Yamamoto KT (1999) Effects of natural and synthetic auxins on the gravitropic growth habit of roots in two auxin-resistant mutants of Arabidopsis, axr1 and axr4: evidence for defects in the auxin influx mechanism of axr4. J Plant Res 112 391–396 [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35 155–189 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.