Abstract
Ethylene is a plant hormone that plays a major role in the elongation of both exploratory and root hair systems. Here, we demonstrate in Brassica napus seedlings that treatments with the ethylene precursor, aminocyclopropane carboxylic acid (ACC) and the ethylene biosynthesis inhibitor, aminoethoxyvinylglycine (AVG), cause modification of the dynamic processes of primary root and root hair elongation in a dose-dependent way. Moreover, restoration of root elongation in AVG-treated seedlings by 1 mm l-glutamate suggested that high concentrations of AVG affect root elongation through nonoverlapping ethylene metabolic pathway involving pyridoxal 5′-P-dependent enzymes of nitrate (N) metabolism. In this respect, treatments with high concentrations of ACC and AVG (10 μm) over 5 d revealed significant differences in relationships between root growth architecture and N uptake capacities. Indeed, if these treatments decreased severely the elongation of the exploratory root system (primary root and lateral roots) they had opposing effects on the root hair system. Although ACC increased the length and number of root hairs, the rate of N uptake and the transcript level of the N transporter BnNrt2.1 were markedly reduced. In contrast, the decrease in root hair length and number in AVG-treated seedlings was overcompensated by an increase of N uptake and BnNrt2.1 gene expression. These root architectural changes demonstrated that BnNrt2.1 expression levels were more correlated to the changes of the exploratory root system than the changes of the root hair system. The difference between treatments in N transporters BnNrt1.1 and BnNrt2.1 gene expression is discussed with regard to presumed transport functions of BnNrt1.1 in relation to root elongation.
In higher plants, root nitrate (N) absorption has to be considered as a function of the number of transporters and their activity per root surface. Until now, the first term in the equation has only been intensively investigated and many positive or negative effectors implicated in regulation of N transporter transcription and/or activity and have been recognized (Touraine et al., 2001; Forde, 2002; Beuve et al., 2004). Recent studies have brought some new insights into the modulation of the root surface equation for terms related to different macronutrients such as N, sulfate (S), and phosphate (P; Lopez-Bucio et al., 2003). The root surface depends on root architecture development and can be divided into two structural components; the exploratory root system component, which is composed of primary roots and lateral roots (LRs) of different orders, and the root hair system component, which is composed of root hair cells. These two components are built progressively over time and composed of the whole root absorbing surface. The exploratory root system requires differentiation of new LR primordia followed by their activation and elongation (Malamy and Benfey, 1997; Casimiro et al., 2003). The root hair system is initiated from files of elongated epidermal root hair cells issued from the functioning of primary and LR meristems (Schiefelbein, 2000; Carol and Dolan, 2002, Ringli et al., 2005).
The LR primodium is initiated after the activation of pericycle cells that are in a specific position with respect to the protoxylem pole within primary root (Casimiro et al., 2003). Genetic approaches have shown that LR initiation was under the control of the auxin transport and signaling pathway but also cell-cycle events (Malamy and Benfey, 1997; Hobbie, 1998; Casimiro et al., 2003). Despite the fact that macronutrients such as N, S, and P also have an important impact on LR development (Lopez-Bucio et al., 2003), it is surprising that no mutants of macronutrients transport have been found to be directly involved in this differentiation processes. However, recent studies have reported that the building of the exploratory root system might also depend on a N signaling pathway (Zhang et al., 1999; Little et al., 2005; Remans et al., 2006a, 2006b). Indeed, mutants of the AtNrt1.1 and AtNrt2.1 N transporters seemed to demonstrate that these transporters were involved in LR initiation independently of any nutritional effect. This assumption, in the case of the AtNrt1.1 N transporter, was supported by several lines of evidence. First, the atnrt1.1 mutant showed a reduced LR growth within the N-rich patch. Secondly, the LR growth of the atnrt1.1 mutant was not due to a decrease in root N uptake activity. Thirdly, the phenotype of the atnrt1.1 mutant was associated with an altered number of LRs. However, these reports failed to distinguish between: (1) the impacts of AtNrt2.1 and AtNrt1.1 mutations on differentiation of LR and root hair; (2) the impacts of AtNrt2.1 and AtNrt1.1 mutations on elongation events during root development; (3) the relationships of hormones and nutrients metabolisms during root development.
In this study, we are mainly interested in the growth/elongation component of root development (ultimately moving toward a root hair system building) in relation to N uptake. Indeed, the modulation of root elongation by pharmacological treatments on ethylene biosynthesis with aminocyclopropane carboxylic acid (ACC; ethylene precursor) and aminoethoxyvinyl-Gly (AVG; ethylene biosynthesis inhibitor) have been recognized for a long time (Smalle and Van Der Straeten, 1997; Schiefelbein, 2000; Casson and Lindsey, 2003). Thus in Arabidopsis (Arabidopsis thaliana), ACC treatment has been shown to induce a reduction of primary and LR elongation (Jackson, 1991; Rodriguez-Pousada et al., 1993; Clark et al., 1999) and the increase of root hair length (Tanimoto et al., 1995; Masucci and Schiefelbein, 1994, 1996; Pitts et al., 1998; Schiefelbein, 2000). On the other hand, the use of AVG, an inhibitor of ACC synthase (ACS; a pyridoxal 5′-P [PLP] amino transaminase that catalyzes the rate-limiting step of ethylene biosynthesis) has been shown to induce a significant increase in primary root elongation and the reduction of root hair length (Abeles and Wydoski, 1987; Satoh and Yang, 1989; Locke et al., 2000; Le et al., 2001).
However, until now these physiological ethylene-induced cell root responses have not been questioned either in relation to shoot versus root N allocation for growth nor in terms of compensatory mechanisms to maintain minimal absorbing surfaces and/or transporter activity for the uptake of nutrients such as N. Our first working hypothesis assumes that metabolic regulation of ethylene biosynthesis by AVG and ACC pharmacological treatments in short-term experiments must contribute to the alteration of root architecture mainly in its elongation component. Our second working hypothesis supposes that in long-term experiments the contrasting modifications to absorbing surfaces (exploratory and root hair systems) via reduction of their elongation must affect N uptake.
In this article, we have first characterized the dose effects of AVG and ACC pharmacological treatments on oilseed rape (Brassica napus) root elongation during 72 h and showed that high concentrations of ACC and AVG affect root system growth through nonoverlapping metabolic pathways.
Secondly, we have determined, during 5 d of treatment, the impact of elongation architectural changes induced by an optimal dose of each treatment on the variations of the net N uptake rate, N translocation from root to shoot, and expression of the N transporter genes BnNrt2.1 and BnNrt1.1.
RESULTS
Dose-Response Curves of Increasing ACC and AVG Concentrations on Primary Root Tip Elongation in Oilseed Rape
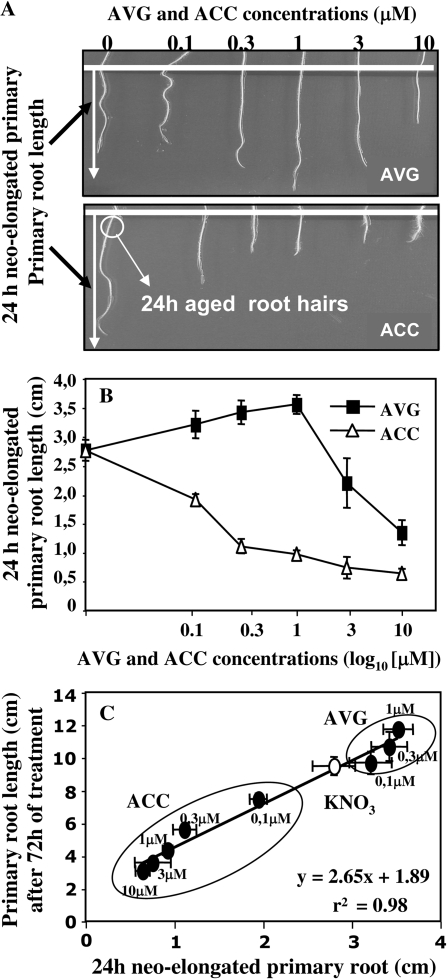
Changes in root architecture in oilseed rape seedlings were examined on vertical agar plates under a homogeneous supply of 1 mm N with different concentrations of the precursor (ACC) or inhibitor (AVG) of ethylene biosynthesis. Optimal pharmacological concentrations for ACC and AVG treatments were first tested for their contrasting effects on primary and root hair systems when compared to KNO3-treated seedlings (control) during 72 h of treatment (Fig. 1, A and B). Neo-elongated primary root length was measured after 24 h of treatments in 2-d-old treated seedlings as shown by the white arrow in Figure 1A. AVG- and ACC-treated seedlings exhibited opposing effects on primary root development up to the 1 μm dose when compared to the control (Fig. 1, B and C). These results of long-term treatment effects (days) in oilseed rape were in agreement with the results of short-term treatment effects (hours) obtained by Le et al. (2001) in Arabidopsis. In our study, under the 1 μm AVG treatment, seedlings showed an increase of 28.5% in neo-elongated root length whereas seedlings treated with 1 μm ACC showed a decrease of 65% in root length compared to the control.
Figure 1.
Impacts of ACC and AVG treatments on dynamic elongation process in primary roots of oilseed rape under homogeneous feeding of N (1 mm) on agarose gel. A, The experimental procedure to measure 24-h-old neo-elongated primary root and root hair lengths after treatments. Root tips of seedlings growing in agarose gel for 48 h were marked (white line) to determine the extent of root elongation between 48 and 72 h of treatment. B, Dose-response curves of ACC or AVG treatments on 24-h neo-elongated primary roots. C, Effects of AVG and ACC treatments on the correlation established between primary root length and 24-h neo-elongated primary roots. Each bar represents the average length per treatment ± se for n = 20 seedlings.
These opposing effects were clearly illustrated by the linear correlation found between the 24-h primary neo-elongated root length and full primary root length (Fig. 1C). This correlation was drawing in opposite direction by AVG doses (0.1–1 μm) and ACC doses (0.1–10 μm) demonstrating that endogenous ethylene levels play a major role in the dynamic process of root elongation (Fig. 1C). Beyond the 1 μm level, AVG-treated seedlings showed a drastic decrease in primary root length by 51.4% for 10 μm compared to the control (Fig. 1B). This unexpected reduction of root elongation suggested that for increasing concentrations, AVG had an inhibitory effect on other metabolic targets that have been implicated in molecular root elongation mechanisms.
Effects of Increasing ACC and AVG Concentrations on the Root Hair System in Oilseed Rape
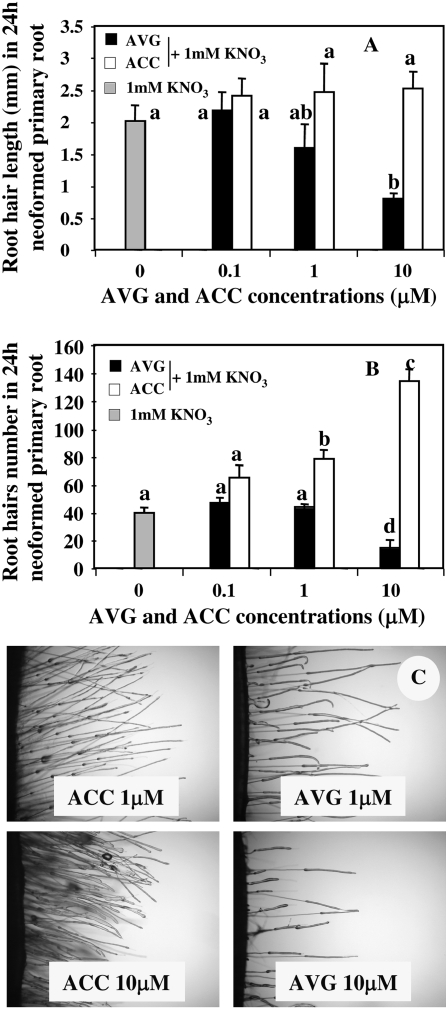
It is well known in Arabidopsis that modifications of ethylene biosynthesis by high ACC treatments promote root hair elongation (Tanimoto et al., 1995; Pitts et al., 1998) and increase in root hair frequency by the induction of ectopic root hair formation in normally atrichoblastic cells (Tanimoto et al., 1995). The role of ethylene biosynthesis in regulation of root hair development was illustrated by AVG treatments, an ACC synthase inhibitor, that blocked root hair formation and elongation at high treatment concentrations (>1 μm; Masucci and Schiefelbein, 1996; Berger et al.,1998). Our results in oilseed rape confirm these previous results obtained in Arabidopsis (Fig. 2, A and B). Up to the level of 1 μm AVG and ACC, root hairs in the upper part of a 24-h neo-elongated primary root (Fig. 1A) were not significantly different in terms of length and frequency between treatments (Fig. 2, A and B). By contrast, seedlings treated with ACC and AVG at doses >1 μm showed a significant increase or decrease in root hair length and number. As illustrated in Figure 2C, treatments with AVG ranging from 1 to 10 μm doses showed a drastic reduction in root hair length and number whereas ACC-treated plants exhibited a significant increase in root hair length and number (Fig. 2, A and B).
Figure 2.
A and B, Dose effects of AVG and ACC root treatments on root hair length (A) and number (B) of oilseed rape seedlings under homogeneous feeding of N (1 mm). C, Morphological changes in root hairs induced by 1 to 10 μm ACC or AVG doses of treatments. Data points represent the mean of the 12 longest and oldest root hairs from six different seedlings (n = 6 × 12 = 72) in the uppermost 3-mm section of 24-h-old neo-elongated primary roots (see white circle in Fig. 1A). Data were analyzed by the nonparametric test of Kruskall Wallis. Then, Mood median tests were used to compare means or medians; bars sharing different letters are significantly different at P = 0.05 (Sokal and Rohlf, 2003).
Ability of l-Glu Treatment to Recover AVG Root Elongation Inhibition
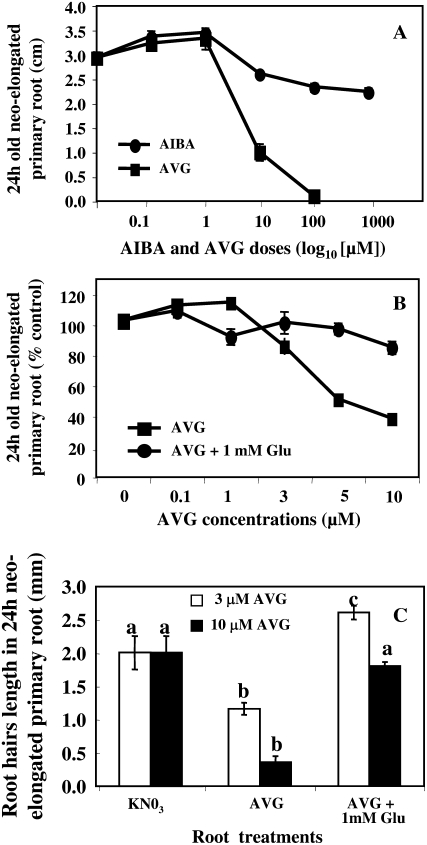
To test if the unexpected reduction of root elongation beyond 1 μm dose of AVG was due to an inhibitory effect on other metabolic targets than ACS protein, we have blocked the second step of the ethylene biosynthesis pathway at the level of ACC oxidase with α-aminoisobutyric acid (AIBA): a structural analog of ACC (Satoh and Esashi, 1982). Comparison of root elongation inhibition induced by AIBA and AVG treatments showed that up to the level of 1 μm, AVG induced a more drastic inhibition than AIBA bringing convincing evidence that AVG inhibited more targets than ACS protein (Fig. 3A). Due to AVG being considered as a potential suicide inhibitor of many PLP (or vitamin B6)-dependent enzymes (enzymes closely related to the subgroup of I aminotransferases such as ACC synthetase; Werck-Reichhart et al., 1988; Bagh et al., 2004), we have examined whether the root supply of l-Glu (the main product of N assimilation) was able to recover AVG primary and root hair growth inhibition during 3 d of treatment. Indeed, key enzymes of N metabolism such as Gln synthase and transaminases involve PLP as a cofactor and use l-Glu as a substrate or as a product. Moreover, it has been recently demonstrated that l-Glu is implicated in both mitotic activity in the apical meristem (Walch-Liu et al., 2006) and dynamic elongation of root cell files via the microtubule cytoskeleton (Sivaguru et al., 2003). Our results showed that the cotreatment of seedlings with different doses of AVG and 1 mm of l-Glu restores primary root elongation beyond 1 μm AVG treatment (Fig. 3B). This restoration of primary root length was also accompanied by a restoration of root hair length (Fig. 3C).
Figure 3.
A and B, Twenty-four-hour elongation responses of primary roots of oilseed rape seedlings treated with different doses of AIBA or AVG (n = 12–16 seedlings; A) or cotreated with different doses of AVG or AVG + 1 mm Glu under homogeneous feeding of 1 mm N (n = 1–4 experiments of n = 12–16 seedlings; B). C, Restoration effects of 72-h cotreatments with 3 μm AVG + 1 mm Glu or 10 μm AVG + 1 mm Glu on root hair lengths in 24-h neo-elongated primary root between 48 and 72 h of treatment (n = 72; see experimental procedure in Fig. 1A). Each bar indicates ± se when larger than the symbol. Mood median tests were used to compare means or medians; bars sharing different letters are significantly different at P = 0.05 (Sokal and Rohlf, 2003).
l-Glu Recovery Effect Is Metabolic and Not Due to an N Nutritional Effect
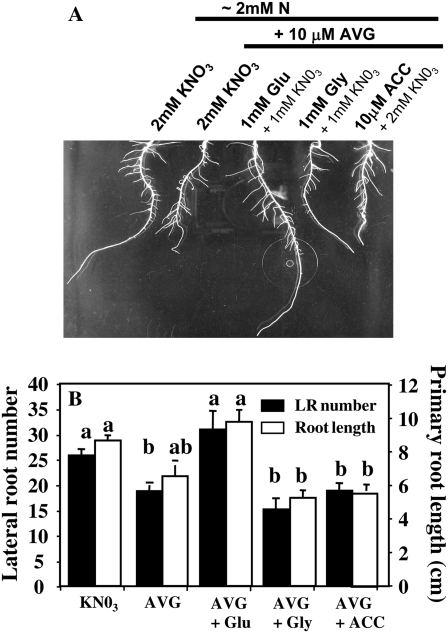
To test if l-Glu recovery effect was metabolic and was not due to an N nutritional effect on root growth induced by l-Glu α amino group, we have compared the effects of treatments on root architecture in the same millimolar range of N supply than the 10 μm AVG + 1 mm Glu + 1 mm KNO3 treatment corresponding to 2 mm supply of N (Fig. 4, A and B). In addition, the time course of experiment was increased from 3 to 5 d to measure the l-Glu effect at the whole root system (primary root and LRs). Comparison of the treatments showed that the l-Glu restoration effect on primary root elongation was not nutritional because AVG control treatment (2 mm KNO3 + 10 μm AVG) was not able to rescue root elongation. Likewise, lack of restoration after 10 μm AVG + 1 mm Gly + 1 mm KNO3 treatment argued in favor of a specific metabolic effect of l-Glu (Fig. 4B). Contrary to l-Glu, ACC treatment was unable to restore root growth of AVG-treated plants.
Figure 4.
Restoration effects of amino acid treatment on exploratory root system in 10 μm AVG-treated seedlings. A, Root architectural changes induced in seedlings cotreated over 120 h with 10 μm AVG plus 1 mm l-Glu, 1 mm Gly, or 10 μm ACC under homogeneous feeding of N (approximately 2 mm). Control- and ACC-treated plants were fed with 2 mm of N to bring similar amounts of N (approximately 2 mm) between treatments. B, Restoration effects of pharmacological cotreatments on LR number and primary root length. Each bar indicates ± se for n = 20 when larger than the symbol.
Effects of High Concentrations of ACC and AVG on Readjustment of Seedling Growth Parameters and Root System Components
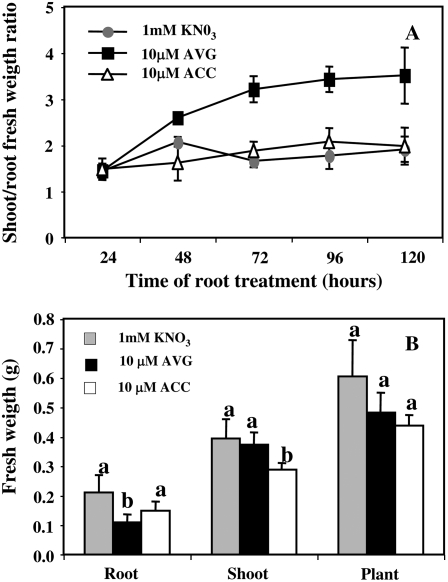
We have investigated how 5 d of treatment with 10 μm ACC and 10 μm AVG treatments might readjust seedling growth parameters and exploratory root architecture. AVG treatment significantly increased the shoot-root fresh weight (FW) ratio (Fig. 5A) compared to ACC- and control (KNO3)-treated plants. This result was explained by a significant decrease in the root FW of AVG-treated plants (Fig. 5B) suggesting that, contrary to ACC, AVG exercised probably its action via modifications of nutrient allocation within the plants.
Figure 5.
A and B, Changes in shoot-root fresh biomass ratio (A) and FW (B) in oilseed rape seedlings treated for 120 h with 10 μm ACC and 10 μm AVG under homogeneous feeding of N (1 mm). Vertical bars indicate average values ± se of n = 3 to 15 repeats of the sum of four seedlings each when larger than the symbol. Data were analyzed by the nonparametric test of Kruskall Wallis. Then, Mood median tests were to compare means or medians; bars sharing different letters are significantly different at P = 0.05 (Sokal and Rohlf, 2003).
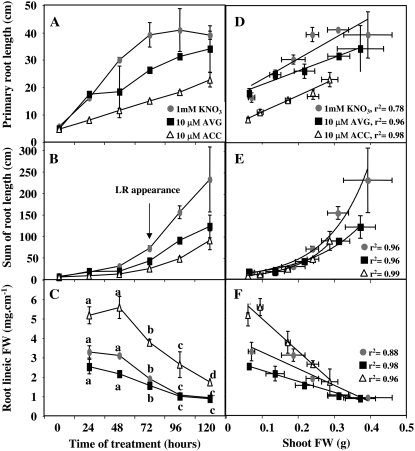
The evolution patterns of primary root length, exploratory root length, and lineic root biomass (defined as the ratio between root FW and the sum of primary root and LR lengths) were measured for the sum of four seedlings (Fig. 6, A–C). The sum of the primary root lengths of four seedlings was more impaired by 120 h of ACC treatment than AVG treatment (Fig. 6A). Likewise, the exploratory root system was severely affected by ACC and AVG treatments (Fig. 6B) as demonstrated by the significant decrease in mean LR length and number between treatments (see below; Fig. 7, A and B). Relative to root FW, the length of the exploratory root system expressed as root lineic FW showed a similar overall decrease for all the treatments (Fig. 6C). This demonstrated that to maintain or increase their shoot-root ratio during growth (Fig. 5A) without impediment of their capacity to explore soil for N resources, seedlings decrease their root lineic biomass and create lighter primary and LRs (Fig. 6C). These biomass readjustments were particularly significant between treatments from 48 to 96 h suggesting that these treatments affect at early stages of development nutrient allocation between root and shoot.
Figure 6.
A to C, Changes in primary root (A), sum of root length (B), and root lineic fresh biomass (C) in oilseed rape seedlings treated with 10 μm ACC and 10 μm AVG under homogeneous feeding of N (1 mm). D to F, Linear and exponential correlations established between primary root (D), sum of root length (E), and root lineic fresh biomass (F) and shoot FW. Vertical bars indicate average values ± se for n = 3 to 15 repeats of the sum of four seedlings each when larger than the symbol.
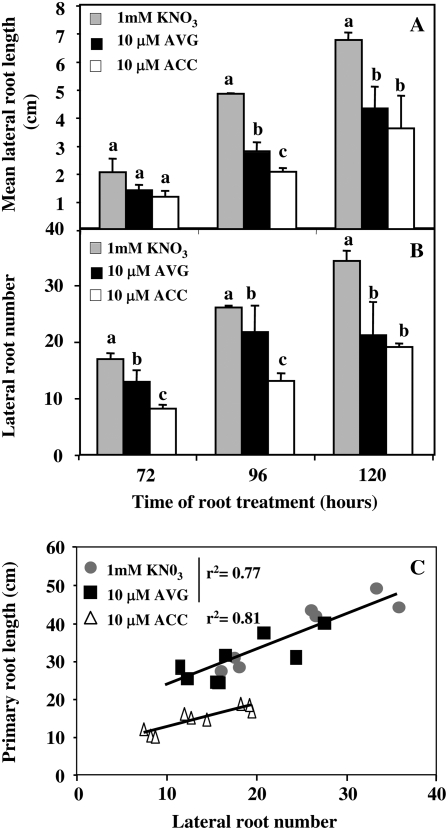
Figure 7.
A and B, Effects of 120 h of treatment with 10 μm ACC and 10 μm AVG on mean LR length (A) and LR number (B) under homogeneous feeding of N (1 mm) in oilseed rape seedlings. C, Changes induced by 10 μm ACC and 10 μm AVG root treatments on the correlation established between primary root length and LR number. Vertical bars indicate ± se for n = 3 repeats of the sum of four seedlings each when larger than the symbol. Data were analyzed by the nonparametric test of Kruskall Wallis. Then, Mood median tests were to compare means or medians; bars sharing different letters are significantly different at P = 0.05 (Sokal and Rohlf, 2003).
Consequently, correlations between shoot FW and exploratory root components were systematically sought. Three significant correlations were found between shoot FW and exploratory root components (Fig. 6, D–F). In comparison to the control plants, ACC and AVG treatments did not impair these correlations but impaired the early amplitude of the architectural root components and affected the overall trends of these correlations. Another linear correlation, found between primary root elongation and LR number, was also modified by root treatments (Fig. 7C). This correlation was similar for KNO3- and AVG-treated seedlings even if in AVG-treated seedlings the reduction of primary root reduced significantly the LR number compared to KNO3 treatment (Fig. 7C). The differences observed after ACC and AVG treatments in terms of LR number (Fig. 7C) and in terms of root hair length and number (Fig. 2, A and B) suggested that the metabolic changes induced by high AVG concentrations on root elongation were clearly different from those caused by high ACC concentrations.
Impacts of Root Architectural Changes Induced by High Concentrations of ACC and AVG on N Uptake and the Expression Level of N Transporter Genes
We have measured the impacts of root architectural adjustments induced by 10 μm ACC and 10 μm AVG treatments on N accumulation, N uptake (by using K15NO3 labeling) and BnNrt2.1 and BnNrt1.1 N transporter gene expression (by quantitative reverse transcription [RT]-PCR) during time-course experiment.
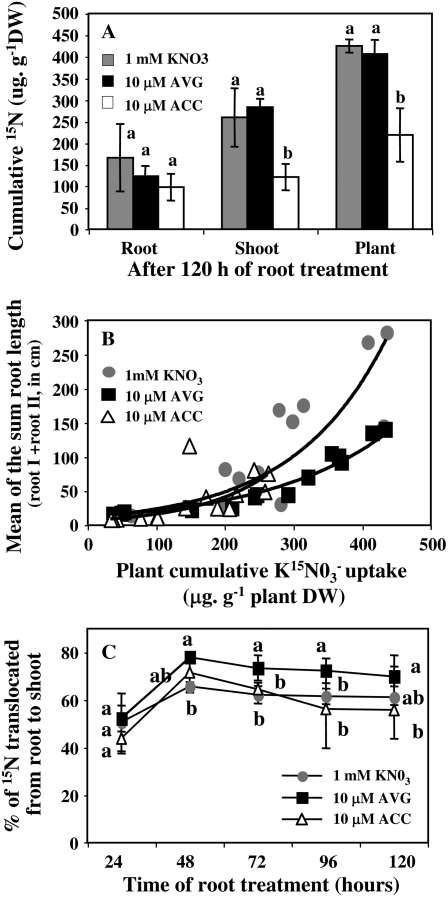
After 120 h of treatment, 10 μm ACC-treated seedlings showed significantly less 15N accumulation in shoot than AVG and control plants (Fig. 8A). Moreover, the exponential relationships established between cumulative 15N g−1 in seedlings dry weight and total root length for the different pharmacological root treatments (Fig. 8B) showed that AVG-treated plants accumulated as much 15N as KNO3 seedlings but with only half of their exploratory (Fig. 6B) and root hair systems (Fig. 2, A and B). Despite a high number of root hairs (Fig. 2, A and B) and a more reduced exploratory root system (Fig. 6B), ACC-treated plants did not up-regulate the capacity of their N uptake systems.
Figure 8.
A, Cumulative amount of N in root, shoot, and whole plant after 120 h of treatment with 10 μm ACC and 10 μm AVG under homogeneous feeding of 1 mm N. B, Relationship between cumulative 15N uptake in seedlings and total roots length (points are the mean value of the sum of four seedlings). C, Curves of the percentage of 15N translocated from root to shoot. Vertical bars indicate ± se for n = 3 repeats of the sum of four seedlings when larger than the symbol.
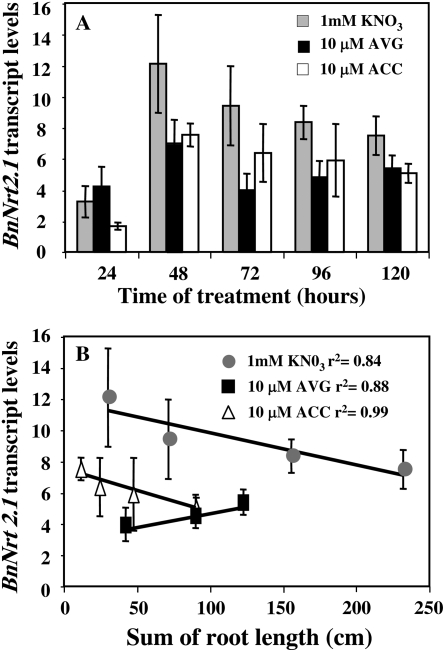
To investigate whether AVG compensatory effect on N uptake can be explained by either posttranscriptional or transcriptional regulation of N transporters, we have examined 15N translocation from root to shoot during the time-course experiment and quantified the transcript expression of the BnNrt2.1 and BnNrt1.1 N transporter genes by quantitative RT-PCR. AVG treatment induced a significant increase in 15N translocation from root to shoot when compared to ACC and control plants (Fig. 8C). In addition, increase of the BnNrt2.1 transcripts from 72 to 120 h (Fig. 9A) in AVG-treated seedlings was positively correlated with the appearance and the growth of LR (Figs. 6B and 9B). In contrast, a negative correlation was found between exploratory root length and BnNrt2.1 expression levels for ACC and KNO3 treatments from 48 to 120 h (Fig. 9B).
Figure 9.
A and B, Expression patterns of BnNrt2.1 transcripts in root (A) and correlation between BnRT2.1 transcript levels and total roots length in 10 μm ACC or 10 μm AVG-treated seedlings under homogeneous feeding of 1 mm N in oilseed rape seedlings (B). Vertical bars indicate ± se for n = 4 experiments with a bulk of four seedlings when larger than the symbol.
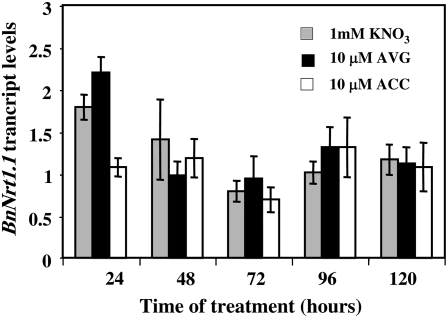
The expression patterns of the N transporter gene BnNrt1.1 were very different from the BnNrt2.1 gene (Fig. 10). Indeed, BnNrt1.1 transcript levels showed an opposite expression compared to BnNrt2.1 during primary root elongation, in the first 48 h of treatments in control- and AVG-treated seedlings (Figs. 9A and 10). The strong inhibition of primary root elongation by ACC treatment (Fig. 1C) during the first 48 h of treatment was also accompanied by a significant decrease of BnNrt1.1 expression (Fig. 10). Thereafter, BnNrt1.1 expression was not significantly modified between the three treatments and a slight increase in BnNrt1.1 expression was observed between 72 and 96 h of treatment during the LR emergence and elongation (Fig. 10).
Figure 10.
Root expression patterns of BnNrt1.1 transcripts in response to 10 μm ACC or 10 μm AVG treatments. Vertical bars indicate ± se for n = 4 experiments with a bulk of four seedlings when larger than the symbol.
DISCUSSION
The aim of this study was to establish a relationship between changes in the root absorbing surface (composed of primary root, LRs, and root hairs) and the activity and gene expression in N transport systems. This objective was first approached via characterization of the pharmacological dose effects of ACC and AVG treatments on root elongation of exploratory and root hair systems in oilseed rape; secondly we used K15NO3 isotope measurement of N uptake in relation to long-time (5 d) pharmacological modifications of root architecture with 10 μm ACC and 10 μm AVG root treatments. Indeed, until now, the physiological ethylene-induced cell root responses of these treatments on root absorbing surfaces (primary root, LR, and root hairs) have not been questioned in terms of transporter activity for the uptake of nutrients such as N.
Modulation of Ethylene Biosynthesis by AVG and ACC Up to 1 μM Level Modifies Primary Root and Root Hair Elongation in Oilseed Rape
We have found that the primary root elongation rate showed opposite dose-response curves when long-time (72 h) AVG and ACC treatments were applied exogenously up to the 1 μm concentration level (Fig. 1A). AVG treatments increased root elongation whereas ACC decreases root length. These data were in agreement with recent results of Le et al. (2001, 2004) who reported after short-time experiments in the Arabidopsis that the elongation rate of primary root exposed from 1 to 5 μm ACC (saturating dose) was quickly and severely reduced in a dose-dependent way whereas AVG-treated plant showed an increase in primary root length for the same treatment dose. These results allow us to establish that in oilseed rape a 1 μm dose corresponds to the saturating condition for ACC and that above a 1 μm dose, AVG treatments inhibit metabolic targets other than the ACS enzyme (Fig. 1B). In addition, we have found the same linear correlation between 24-h primary neo-elongated root length and primary root length for ACC-treated seedlings from 0 to 10 μm dose and AVG-treated seedlings from 0 to 1 μm dose (Fig. 1C). From a dynamic point of view, this correlation brought convincing evidence that endogenous ACC and/or ethylene levels play a major role on root cell elongation processes. This result is in agreement with recent studies demonstrating the regulatory role of ethylene in root development through auxin biosynthesis, transport, and root distribution (Ruzicka et al., 2007; Swarup et al., 2007) but also through microtubule reorientation in epidermal root cells during growth (Roberts et al., 1985; Takahashi et al., 2003; Le et al., 2004, Chilley et al., 2006).
Beyond 1 μm AVG, Changes in Root Architecture Are Not Uniquely Due to ACC Synthase Inhibition But Certainly through a Nonoverlapping Ethylene Pathway
Until now, AVG inhibitory action has been only considered to be specific to ethylene biosynthesis via inhibition of the ACS enzyme (Rando 1974, Satoh and Yang, 1989) but some studies have demonstrated a broader spectrum of action for this zenobiotic molecule (Werck-Reichhart et al., 1988). In fact, AVG is an olifinic Gly analog that inhibits irreversibly PLP (or vitamin B6)-dependent enzymes closely related to the subgroup of I aminotransferases such as Asp aminotransferase (Soper and Manning, 1982; Werck-Reichhart et al., 1988). This group of aminotransferases can also be inhibited by other PLP inhibitors such as allyl-Gly, MSO, and AOA (Bagh et al., 2004). As a consequence there exists a high degree of uncertainty about the targets and specificity of these inhibitors on PLP-dependent enzymes especially on N metabolic aminotransferases using PLP as cofactor. Other intriguing and convincing evidences have been provided from salt overly sensitive4, and pyridoxal phosphate synthase1.1 (pdx1.1) and pdx1.2 mutants that have been implicated in PLP production in higher plants. These genes encode for a PL kinase and pyridoxine (PLP precursor) biosynthesis proteins, respectively (Chen and Xiong, 2005; Titiz et al., 2006; Wagner et al., 2006), and these two mutants showed the same root hair phenotypes as those induced by AVG treatments (Shi and Zhu, 2002).
In consequence, our results were consistent with the view that AVG effects on root development above 1 μm impaired probably not only the ethylene metabolism via ACS inhibition but also other PLP enzyme activities implicated in N metabolism through an independent and nonoverlapping ethylene pathway. First, inhibition of ethylene pathway at ACC oxidase level by 1 mm AIBA treatment (a structural analog of ACC; Satoh and Esashi, 1982) impaired less root elongation than 10 μm AVG treatment (Fig. 3A). Second, l-Glu was able to restore primary root elongation and root hair length only above 1 μm AVG dose (Fig. 3, B and C). Third, Gly and ACC root supplies were unable to restore primary root length and LR number in AVG-treated seedlings (Fig. 4, A and B). Fourth, AVG inhibition induced biomass and N partitioning in favor of the shoot rather than the root (Figs. 5, A and B, and 7A). Taken together, these data demonstrated convincingly that the endogenous l-Glu pool used or produced by the N metabolism PLP enzyme(s) is a key element for normal root elongation. We can also assume that this specific pathway acts upstream from Met synthesis (ethylene precursor) through a nonoverlapping ethylene pathway.
Does Glu Issued from N Metabolism Act on Root Elongation Components of Exploratory and Root Hair Systems?
Restoration of primary root and root hair elongation in 3 and 10 μm AVG-treated seedlings by l-Glu (Fig. 4, A and B) suggests that PLP enzyme(s) of N metabolism that use or produce l-Glu such as transaminases play a central role in elongation of primary root cell files and consequently determination of LR number (Fig. 7C). This assumption agrees for example with aat2 (Asp aminotransferase 2) mutants that showed a significant reduction in root growth (Miesak and Coruzzi, 2002). However, there exist in Arabidopsis at least 44 genes encoding aminotransferases (Liepman and Olsen, 2004) that could be potential targets for AVG or other PLP inhibitors. Likewise, recent studies in Arabidopsis reported that l-Glu modulates both mitotic activity in the apical meristem (Li et al., 2006; Walch-Liu et al., 2006) and dynamic elongation of root cell files via the microtubule cytoskeleton (Sivaguru et al., 2003). The very surprising point of our results is that, in our hands, l-Glu root treatment in the presence of AVG displayed an increase in root elongation and presented exactly the opposite root phenotype that has been reported in plants treated with l-Glu alone (Sivaguru et al., 2003; Walch-Liu et al., 2006). Moreover, l-Glu root supply from 100 to 1 mm in oilseed rape did not induce significant reduction of root elongation as reported in Arabidopsis by Walch-Liu et al. (2006; Supplemental Fig. S1). On the other hand, mutants deficient in high-affinity LYSINE HISTIDINE TRANSPORTER1 (LHT1) that cannot uptake l-Glu from the medium as sole nitrogen source show high-growth defects (Hirner et al., 2007) suggesting that LHT1 instead of GluRs (Glu receptor-like proteins) are probably involved in Glu-root elongation responses. These combined results also suggest that Glu might mediate root-specific growth molecular responses, raising the question of whether Glu cellular concentrations and compartmentation act on root division and/or elongation mechanisms (Qi et al., 2006; Forde and Lea, 2007).
The Increase of Root Hair System Induced by High Concentration of ACC Dramatically Impaired N Uptake via BnNrt2.1 Expression and N Translocation
From our results, it is clear that in ACC-treated seedlings the decrease in their exploratory root system and the increase in root hair number are accompanied by a dramatic decrease instead of an increase in N uptake when compared to AVG and control seedlings (Fig. 8, A and B). Despite that the root lineic biomass was increased by ACC treatment (Fig. 6C), these root morphogenetic changes did not impair shoot-root ratio (Fig. 5A) but impaired N uptake from root (Fig. 8B) and consequently N accumulation in the shoot (Fig. 8A). This reduction in N uptake was only accompanied by a decrease in BnNrt2.1 expression levels (Fig. 9, A and B) without changes in BnNrt1.1 (Fig. 10) suggesting distinct roles and regulation for these two genes in root architectural development. In other respects, the same overall trend observed in root growth parameters when compared to the control seedlings (Figs. 5A, 6, D and E, 7C, 8B, and 9, A and B) pose questions about the effects of high ACC concentrations on plant growth and regulation of the NRT2 protein. Indeed, it is known that high ACC or ethylene concentrations induce severe reduction of growth via inhibition of foliar gas exchange by direct reduction on stomatal conductance and rate of photosynthesis (Pierik et al., 2006). It is also well recognized that ACC treatment dampers the plant growth (Thain et al., 2004; Pierik et al., 2006). Taken together these results suggest clearly that N uptake is coregulated with carbon metabolism. Moreover, the synthesis of this stress hormone is regulated like N uptake in a circadian manner (Malagoli et al., 2004; Thain et al., 2004) and recent work has reported that posttranslational mechanisms are crucial for the fast modulation of NO3− uptake by NRT2 plasma membrane protein in response to environmental changes (Wirth et al., 2007).
Reduction in Root Architecture by High AVG Concentrations Increased N Uptake and BnNrt2.1 Regulation at Transcriptional and Posttranscriptional Levels
Despite a reduction in exploratory and root hair systems, treatments with a high AVG concentration increased N uptake and 15N accumulation in comparison to the control plants (Fig. 8B). This remarkable increase in N uptake after AVG treatment is mainly explained by a posttranscriptional regulation of N transporters probably by a significant translocation of 15N compounds from root to shoot (Fig. 8C) that could alleviate the repression exercised by some N products, such as amino acids, on N transporters (Lejay et al., 1999; Forde, 2002). Indeed, despite an increase in transcription from 72 to 120 h of treatment, the transcription level remains too low when compared to control seedlings to explain the high increase in N uptake (Fig. 9A). Moreover, as observed during ACC treatments these results bring convincing evidence that root hair cells are not the only location of N absorption in the root. Nonhair cells could also be highly involved in N uptake. In this respect, Chopin et al. (2007) reported that in Arabidopsis the N transporter AtNrt2.1 is not specifically targeted to the root plasma membrane of epidermal root cells. Moreover, our results demonstrated that BnNrt2.1 expression levels for all the treatments were more correlated to the changes of exploratory root system (Fig. 9B) than the changes of root hair system (Fig. 2, A and B).
Role of BnNrt2.1 N Transporter in Root Elongation in Relation to N Metabolism
Despite the fact that macronutrients such as N are known to have an important impact on LR elongation (Lopez-Bucio et al., 2003) it is surprising that no mutations of macronutrients (S, P, and N) transport have been directly implicated in the differentiation processes of the LR primordium (Casimiro et al., 2003; Montiel et al., 2004). Our results on root elongation restoration by l-Glu in AVG-treated plants (Fig. 4, A and B) pose questions about recent conclusions about the role of N transporters AtNrt2.1 as a sensor or facilitator in the N signaling specific pathway implicated in LR development (Little et al., 2005; Remans et al., 2006a, 2006b). Thus, it is reported that there is an inhibition of LR primordia initiation in atnrt2.1 mutants that is independent of N uptake at whole root system (Remans et al., 2006a) suggesting a signaling role of N in root development. Surprisingly, Little et al. (2005) reported exactly the reverse phenotype (LR initiation activation) in the lin1 mutant that carries a missense mutation in the AtNrt2.1 gene. However, in these two contradictory studies the number of LR is not presented as a function of primary root elongation. Now there exists in Arabidopsis, as in oilseed rape (Fig. 7C), a similar relationship between primary root length and LR number (Loudet et al., 2005); therefore this correlation could explain the LR number decrease in the atnrt2.1 mutants. Indeed, a reduction in primary root elongation induced by a decrease in N uptake and subsequently production of l-Glu could be the main reason. Moreover, in our results this correlation was established from pharmacological treatments with only one genotype and not from 150 inbred lines (Loudet et al., 2005) demonstrating that AVG inhibition can modify one or several key steps of N metabolism implicated in root architecture formation. In relation to this, our transcriptional data showed that BnNrt2.1 expression levels were markedly different between ACC (ethylene metabolism activation)- and AVG (N metabolism inhibition)-treated seedlings (Fig. 9A).
Role of BnNrt1.1 N Transporter in Root Elongation in Relation to Ethylene Metabolism
In comparison to BnNrt2.1, BnNrt1.1 expression pattern was quite different during the time course of experiment (Figs. 9A and 10) and no correlation can be draw between BnNrt1.1 expression level and exploratory root length. In control- and AVG-treated seedlings, BnNrt1.1 transcript levels showed an opposite expression compared to BnNrt2.1 during the first 48 h of treatments suggesting that transport function of BnNrt1.1 during primary root elongation could be independent of N uptake or N metabolism (Figs. 8A and 9). Furthermore, BnNrt1.1 expression was significantly decreased by ACC treatment during the first 48 h of primary root elongation and showed a slight increase during LR appearance and elongation from 72 to 96 h for all the treatments (Fig. 10). Taken together, these data revealed that BnNrt1.1 is clearly involved in the root elongation process through a function that could be precociously modified by ethylene (Zhang et al., 2007). These results were consistent with the results of Guo et al. (2001) in Arabidopsis who reported by using Nrt1.1-GFP/GUS constructs that the strongest expression of AtNrt1.1 was found in the growing tips of primary root and LRs in the first few days after germination. Likewise, atnrt1.1 mutants were impaired in primary root and LR development during elongation and activation of the root meristems independently of the N uptake capacities and nitrogen source (Guo et al., 2001; Remans et al., 2006b). The activation and function of the Nrt1.1 gene as N transporter during growth of nascent organs such as root tips is not clear (Guo et al., 2001). In fact, the BnNrt1.1 gene belongs not only to the NRT (N transporter) family but also to the PTR (di/tripeptide transporter) family whose members can transport di- or tripeptide, such as γ-glutamyl-Cys and glutathione (GSH), but also indole-3-acetic acid/amino acid conjugates (Tsay et al., 2007). Moreover, GSH and auxin are the main components involved in the G1-S transition in dividing root cells (Den Boer and Murray, 2000), and GSH levels are essential for initiation and maintenance of cell division in root elongation (Sanchez-Fernandez et al., 1997; Vernoux et al., 2000). Because ethylene up-regulates auxin biosynthesis in Arabidopsis seedling conducting to the inhibition of root cell elongation (Ruzicka et al., 2007; Swarup et al., 2007), it is tempting to speculate that Nrt1.1 expression changes induced by ACC treatments during root elongation could be linked directly or indirectly to GSH or auxin conjugates transport.
In conclusion, our investigation of the elongation component of root by activation or inhibition of the ethylene metabolic pathway in relation to N uptake has revealed that some PLP enzyme(s) of N metabolism that use or produce l-Glu could play a central role in root elongation and N uptake. Indeed, the restoration of AVG-treated seedlings by l-Glu supply to the medium suggests that l-Glu acts upstream from hormonal pathways but probably downstream from high-affinity N transporters of the NRT2 family and brings convincing evidence that l-Glu acts as a major endogenous signal in root elongation and N allocation between root and shoot. In addition, BnNrt2.1 expression levels for all the treatments were more correlated to the elongation changes of the exploratory root system than the changes of the root hair system. The N transport function of BnNrt1.1 is less evident and seems to depend on hormonal regulation of root elongation and LR differentiation. In this context, a key challenge for future research will be first to elucidate the role of PLP enzymes of N metabolism that use or produce l-Glu (such as root transaminases) by using pharmacological and genetic approaches on specific targets.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Oilseed rape seeds (Brassica napus ‘Capitol’) were used. The seeds were sterilized with 20% hypochlorite solution (v/v) for 3 min, followed by three rinses in sterile water. The disinfected seeds were placed on imbibed filter paper saturated with sterile water within petri dishes (24 × 24 cm) for 48 h in the dark at room temperature. The germinated seeds were then selected from their radicle length (5–6 mm) and four or five seedlings were transferred on new petri dishes (12 × 12 cm), filled up with 50 mL of solidified culture medium supplemented with or without activator or inhibitor of ethylene biosynthesis, and half-sealed with adhesive tape. Culture solution (50 mL per petri dish) was used for seedlings culture consisting of 0.4 mm KH2PO4, 0.15 mm K2HPO4, 1 mm K2S04, 0.5 mm MgSO4, 3 mm CaCl2, 0.2 mm Fe-Na EDTA, 14 μm H3B03, 5 μm MnSO4, 3 μm ZnSO4, 0.7 μm CuSO4, 0.7 μm (NH4)6 Mo7O24, and 0.1 μm CoCl2 and solidified with 0.8% (w/v) agar (A–7002; Sigma), pH 6.75. The dishes were placed vertically in a growth chamber at 22°C under 16-h/8-h light/dark regime with a light intensity of 145 μmol m−2 s−1 and 70% relative humidity.
Application of the Ethylene Biosynthesis Activator or Inhibitor
ACC (A–3903; Sigma), AIBA (850993; Sigma), and AVG (A–1284; Sigma) were dissolved in sterile distillated water to a final stock concentration of 10 and 100 mm and stored at 4°C, respectively. Chemicals were added to 50 mL autoclaved culture medium with 1 mm N in a falcon tube at 45 to 50°C, mixed, and then poured in petri dishes under laminar sterile air flux. Two-day-old germinated seedlings were grown in the vertical agar plates for 3 or 5 d before root morphometric measurements, 15N labeling, and molecular analyses were undertaken.
Exploratory and Root Hair System Morphometric Analyses
The primary root elongation of 3-d-old treated seedlings with different concentrations of AVG and ACC was only measured for the last 24 h of treatment with a ruler (approximately 24-h neo-elongated primary root). For each dose of treatment, 20 seedlings were treated. Among the 20 seedlings, six showing the same value of primary root elongation than the elongation mean value were chosen for measurement of root hairs length and number. From these six seedlings, the 12 longest root hairs and the total number of root hairs were measured within the primary hemiroot region of 3-mm length at the top of the last 24-h neo-elongated primary root (see experimental procedure in Fig. 1A). Root hairs length and number were measured under a light microscope (CME; Leica) at 40× magnification equipped with a camera (TK–C1481BEG; JVC) and video imaging system (PVM–20L1; Sony Trinitron).
Treatment effects of 10 μm ACC and 10 μm AVG were examined during 5 d to quantify modifications of the exploratory root system (primary and LRs). The three replicated agar plates of four seedlings used for each treatment were photocopied (KM-2030; Kyocera Mita) every day. Daily differences in primary root and LR lengths were obtained by superimposing two successively dated photocopies of the growth on a light bench and determined by a map measurer with a revolution counter (Wedo).
Net K15NO3− Uptake and Isotope Analysis
Three sets of germinated seeds for each treatment (10 μm AVG, 10 μm ACC, and 1 mm KNO3) composed of three replicates of four seedlings were transferred into agar plates for different durations (0, 24, 48, 72, 96, and 120 h). For measurement of cumulative uptake during each point of this time series, the medium was supplemented with K15NO3 (atom % 15N, 1%). At each sampling time, photocopies of each agar plate from the three replicates were made before washing the roots in 1 mm CaSO4 solution for 1 min at room temperature and then harvesting the roots and shoots separately into 2-mL Eppendorf tubes. The FW of organs was measured before drying for 48 h at 60°C. After drying, organ dry weight was measured and then the shoots and roots were ground separately for 2 min to fine powder with inox beads of 0.4-mm diameter in an oscillating grinder (mixer mill MM301; Retsch) before isotope analysis.
The 15N content (μg 15N per plant) was determined for roots, shoots, and seedlings. The 15N analyses were performed using an analyzer (EA 300; Eurovector) coupled with a mass spectrometer (isoprime mass spectrometer; GV Instrument).
RNA Isolation and Quantitative RT-PCR Analysis
For a given date of treatment, total RNA was extracted from 200 to 400 mg of root fresh matter corresponding to three sets of seedlings for each treatment (10 μm AVG, 10 μm ACC, and 1 mm KNO3) composed of four replicates of four seedlings. Fresh root samples frozen in 2-mL Eppendorf tubes in liquid nitrogen were ground for 1 min and 30 s with stainless steel beads of 0.4-mm diameter in an oscillating grinder (mixer mill MM301; Retsch). The resulting powder was suspended in 750 μL of extraction buffer (0.1 m Tris, 0.1 LiCl, 0.01 m EDTA, 1% SDS [w/v], pH 8) and 750 μL of hot phenol (80°C, pH 4). This mixture was vortexed for 30 s. After addition of 750 μL of chloroform/isoamylalcohol (24:1), the homogenate was centrifuged (15,000g, 5 min, 4°C). The supernatant was transferred into 4 m LiCl solution (w/v) and incubated overnight at 4°C. After centrifugation (15,000g, 30 min, 4°C), the pellet was suspended in 250 μL of sterile water. Fifty microliters of 3 m sodium acetate (pH 5.6) and 1 mL of 96% ethanol were added to precipitate the total RNA for 1 h at −80°C. After centrifugation (15,000g, 20 min, 4°C), the pellet was washed with 1 mL of 70% ethanol, then centrifuged at 15,000g for 5 min at 4°C. The resulting pellet was dried for 5 min at room temperature and resuspended in sterile water containing 0.1% SDS and 20 mm EDTA. Quantification of total RNA was performed by spectrophometer at 260 nm (BioPhotometer, Eppendorf) before RT-PCR analysis.
For RT, 1 μg of total RNA was converted to cDNA with an iScript cDNA synthesis kit using the manufacturer's protocol (Bio-Rad). The genes and specific primers selected for the analysis were the following: EF1-a (housekeeping gene; F, 5′-tttcgagggtgacaacatga; R, 5′-ccgttccaataccaccaatc); BnNrt2.1 (F, 5′-tggtggaataggcggctcgagttg; R, 5′-gtatacgttttgggtcattgccat); and BnNrt1.1. (F, 5′-atggtaaccgaagtgccttg; R, 5′-tgattccagctgttgaagc). The subsequent PCR reactions were performed with 4 μL of 200× diluted cDNA, 500 nm of the primers, 1× SYBR Green PCR Master Mix (Bio-Rad) in a total volume of 15 μL. The specificity of PCR amplification was examined by monitoring the melting curves after quantitative PCR reactions using the Chromo4 system (Bio-Rad) and by sequencing the quantitative PCR product to confirm that the correct amplicons were produced from each pair of primers (Biofidal). Comparative relative expression of the various genes was determined using the delta-delta Ct method employing the formula: relative expression = 2−[ΔCt sample-ΔCt control] where Ct refers to the threshold cycle, sample indicates the gene of interest, and control indicates the endogenous housekeeping gene (Livak and Schmittgen, 2001).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers DQ312264, AJ293028, and AJ278966.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effects of l-Glu or l-Gly treatment doses on 24-h neo-elongated primary roots of oilseed rape seedlings under homogeneous feeding of N (1 mm) on agarose gel (n = 12–16 seedlings).
Supplementary Material
Acknowledgments
We thank Professors C. Huault and J.P. Billard of Caen University for critical reading of this manuscript. The authors are grateful to J. Cliquet for permitting us to use the Leica microscope analysis system and to S. Rezé and M.-P. Henry for their technical assistance in quantitative RT-PCR and 15N analyses.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Erwan Le Deunff (erwan.ledeunff@unicaen.fr).
The online version of this article contains Web-only data.
References
- Abeles FB, Wydoski SG (1987) Inhibitors of ethylene synthesis and action: a comparison of their activities in a lettuce root growth model system. J Am Soc Hortic Sci 112 122–125 [Google Scholar]
- Bagh K, Hiraoki T, Thorpe TA, Vogel HJ (2004) Nitrogen-15 NMR studies of nitrogen metabolism in Picea glauca buds. Plant Physiol Biochem 42 803–809 [DOI] [PubMed] [Google Scholar]
- Berger F, Hung CY, Dolan L, Schiefelbein J (1998) Control of cell division in the epidermis of Arabidopsis thaliana. Dev Biol 194 235–245 [DOI] [PubMed] [Google Scholar]
- Beuve N, Rispail N, Lainé P, Ourry A, Le Deunff E (2004) Putative role of γ-aminobutyric acid (GABA) as long distance signal in up-regulation of nitrate uptake in Brassica napus L. Plant Cell Environ 27 1035–1046 [Google Scholar]
- Carol RJ, Dolan L (2002) Building a hair: tip growth in Arabidopsis thaliana root hairs. Philos Trans R Soc Lond B Biol Sci 357 815–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8 165–171 [DOI] [PubMed] [Google Scholar]
- Casson SA, Lindsey K (2003) Gene and signalling in root development. New Phytol 158 11–38 [Google Scholar]
- Chen H, Xiong L (2005) Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stresses. Plant J 44 396–408 [DOI] [PubMed] [Google Scholar]
- Chilley PM, Casson SA, Tarkowski P, Hawkins N, Wang KL-C, Hussey PJ, Beale M, Ecker JR, Sandberg GK, Lindsey K (2006) The POLARIS peptide of Arabidopsis regulates auxin transport and root growth via effects on ethylene signaling. Plant Cell 18 3058–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin F, Wirth J, Dorbe MF, Lejay L, Krapp A, Gojon A, Daniel-Vedele F (2007) The Arabidopsis nitrate transporter AtNrt2.1 is targeted to the root plasma membrane. Plant Physiol Biochem 45 630–635 [DOI] [PubMed] [Google Scholar]
- Clark DG, Gubrium EK, Barett JE, Nell TA, Klee HJ (1999) Root formation in ethylene-insensitive plants. Plant Physiol 121 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Boer BGW, Murray JAH (2000) Triggering the plant cell cycle in plants. Trends Cell Biol 10 245–250 [DOI] [PubMed] [Google Scholar]
- Forde BJ (2002) Local and long-range signalling pathways regulating plant response to nitrate. Annu Rev Plant Biol 53 203–224 [DOI] [PubMed] [Google Scholar]
- Forde BJ, Lea PJ (2007) Glutamate in plants: metabolism, regulation, and signalling. J Exp Bot 58 2339–2358 [DOI] [PubMed] [Google Scholar]
- Guo F-Q, Wang R, Chen M, Crawford NM (2001) The Arabidopsis dual-affinity transporter gene AtNrt1.1 (CHL1) is activated and functions in nascent organ development during vegetative and reproductive growth. Plant Cell 13 1761–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirner A, Ladwig F, Stransky H, Okumoto S, Keinath M, Harms A, Frommer WB, Koch W (2007) Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. Plant Cell 18 1931–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie LJ (1998) Auxin: molecular genetic approaches in Arabidopsis. Plant Physiol Biochem 36 91–102 [Google Scholar]
- Jackson MB (1991) Ethylene in root growth and development. In AK Mattoo, JC Suttle, eds, The Plant Hormone Ethylene. CRC Press, Boca Raton, FL, pp 159-181.
- Le J, Vandenbusche F, Van Der Streaten D, Verbelen JP (2001) In early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiol 125 519–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Vandenbusche F, Van Der Streaten D, Verbelen JP (2004) Position and cell type-dependent microtubule reorientation characterizes the early response of Arabidopsis root epidermis to ethylene. Physiol Plant 121 513–519 [Google Scholar]
- Lejay L, Tillard P, Lepetit M, Francesc Dominingo O, Filleur S, Daniel-Vedele F, Gojon A (1999) Molecular and functional regulation of two NO3− uptake systems by N- and C-status of Arabidopsis plants. Plant J 18 509–519 [DOI] [PubMed] [Google Scholar]
- Li J, Zhu S, Song X, Shen Y, Chen H, Yu J, Yi K, Liu Y, Karplus VJ, Wu P, et al (2006) A rice glutamate receptor-like gene is critical for the division and survival of individual cells in the root apical meristem. Plant Cell 18 340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman AH, Olsen LJ (2004) Genomic analysis of aminotransferases in Arabidopsis thaliana. Crit Rev Plant Sci 23 73–89 [Google Scholar]
- Little D, Rao H, Oliva S, Daniel-Vedele F, Krapp A, Malamy JE (2005) The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutrient cues. Proc Natl Acad Sci USA 102 13693–13698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Locke JM, Bryce JH, Morris PC (2000) Contrasting effects of ethylene perception and biosynthesis inhibitors on germination and seedling growth of barley (Hordeum vulgare L.). J Exp Bot 51 1843–1849 [DOI] [PubMed] [Google Scholar]
- Lopez-Bucio J, Cruz-Ramirez A, Herrera-Estrella L (2003) The role of nutrient in regulating root architecture. Curr Opin Plant Biol 6 280–287 [DOI] [PubMed] [Google Scholar]
- Loudet O, Gaudon V, Trubuil A, Daniel-Vedele F (2005) Quantitative trait loci controlling root growth and architecture in Arabidopsis thaliana confirmed by heterogeneous inbred family. Theor Appl Genet 110 742–753 [DOI] [PubMed] [Google Scholar]
- Malagoli P, Lainé P, Le Deunff E, Rossato L, Ney B, Ourry A (2004) Modeling nitrogen uptake in oilseed rape cv Capitol during a growth cycle using influx kinetics of root nitrate transport systems and field experimental data. Plant Physiol 134 388–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Down and out in Arabidopsis: the formation of lateral roots. Trends Plant Sci 2 390–395 [Google Scholar]
- Masucci JD, Schiefelbein JW (1994) The rdh6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol 106 1335–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW (1996) Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell 8 1505–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesak BH, Coruzzi GM (2002) Molecular and physiological analysis of Arabidopsis mutants defective in cytosolic or chloroplastic aspartate aminotransferase. Plant Physiol 129 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel G, Gantet P, Jay-Allemand C, Breton C (2004) Transcription factor networks pathways to the knowledge of root development. Plant Physiol 136 3478–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Pooter H, Visser EJW, Voesenek ACJ (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11 176–183 [DOI] [PubMed] [Google Scholar]
- Pitts RJ, Cernac A, Estelle M (1998) Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J 16 553–560 [DOI] [PubMed] [Google Scholar]
- Qi Z, Stephens NR, Spalding EP (2006) Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol 142 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando RR (1974) Irreversible inhibition of aspartate aminotransferase by 2-amino-3-butenoic acid. Biochemistry 13 3859–3863 [DOI] [PubMed] [Google Scholar]
- Remans T, Nacry P, Pervent M, Girin T, Filleur S, Diataloff E, Mouinier E, Tillard P, Forde B, Gojon A (2006. b) The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci USA 103 19206–19211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans T, Nacry P, Pervent M, Girin T, Tillard P, Lepetit M, Gojon A (2006. a) A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of root system to nitrogen limitation in Arabidopsis. Plant Physiol 140 909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli C, Baumberger N, Keller B (2005) The Arabidopsis root hair mutants der2-9 are affected at different stages of root hair development. Plant Cell Physiol 46 1046–1053 [DOI] [PubMed] [Google Scholar]
- Roberts IN, Lloyd CW, Roberts K (1985) Ethylene-induced microtubule reorientations: mediation by helical arrays. Planta 164 439–447 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pousada RA, Rycke RD, Dedonder A, Van Caeneghem W, Engler G, Van Montagu M, Van Der Straeten D (1993) The Arabidopsis 1-aminocyclopropane-1-carboxylate synthase gene 1 is expressed during early development. Plant Cell 5 897–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka K, Ljung K, Vanneste S, Podhorskà R, Beeckman T, Frimi J, Benkova E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Fernandez R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inzé D, May MJ (1997) Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc Natl Acad Sci USA 94 2745–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Esashi Y (1982) Effect of α-aminoisobutyric acid and D- and L-amino acids on ethylene production and content of 1-aminocyclopropane-1-carboxylic acid in cotyledonary segments of cocklebur seeds. Physiol Plant 54 147–152 [Google Scholar]
- Satoh S, Yang SF (1989) Inactivation of 1-aminocyclopropane-1-carboxylate synthase by l-vinylglycine as related to the mechanism-based inactivation of the enzyme by S-adenosyl-l-methionine. Plant Physiol 91 1036–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW (2000) Constructing a plant cell. The genetic control of root hair development. Plant Physiol 124 1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Zhu J-K (2002) SOS4, a pyridoxal kinase gene, is required for root hair development in Arabidopsis. Plant Physiol 129 585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Pike S, Gassmann W, Baskin TI (2003) Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol 44 667–675 [DOI] [PubMed] [Google Scholar]
- Smalle J, Van Der Straeten D (1997) Ethylene and vegetative development. Physiol Plant 100 595–605 [Google Scholar]
- Sokal RR, Rohlf FJ (2003) Biometry, Ed 3. Freeman, New York
- Soper TS, Manning JM (1982) Inactivation of pyridoxal phosphate enzymes by gabaculine. J Biol Chem 257 13930–13936 [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedling conducting to the inhibition of root cell elongation. Plant Cell 19 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Kawahara A, Yasunori I (2003) Ethylene promotes the induction by auxin of the cortical microtubule randomisation required for low-pH-induced root hair initiation in lettuce (Lactuca sativa L.) seedlings. Plant Cell Physiol 44 932–940 [DOI] [PubMed] [Google Scholar]
- Tanimoto M, Roberts K, Dolan L (1995) Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J 8 943–948 [DOI] [PubMed] [Google Scholar]
- Thain SC, Vandenbussche F, Laarhoven LJJ, Dowson-Day MJ, Wang ZY, Tobin EM, Harren FJM, Millar AJ, Van Der Straeten D (2004) Circadian rhythms of ethylene emission in Arabidopsis. Plant Physiol 136 3751–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titiz O, Tambasco-Studart M, Warzych E, Apel K, Amrhein N, Laloi C, Fitzpatrick TB (2006) PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J 48 933–946 [DOI] [PubMed] [Google Scholar]
- Touraine B, Daniel-Vedele F, Forde B (2001) Nitrate uptake and its regulation. In PJ Lea, JF Morot-Gaudry, eds, Plant Nitrogen. Springer-Verlag, Berlin, pp 1–37
- Tsay Y-F, Chiu C-C, Tsai CB, Ho C-H, Hsu P-K (2007) Nitrate transporters and peptide transporters. FEBS Lett 581 2290–2300 [DOI] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld J-P, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, et al (2000) The root meristemless1/cadmium sensitive2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Bernhardt A, Leuendorf JE, Drewke C, Lytovchenko A, Mujahed N, Gurgui C, Frommer WB, Leistner E, Fernie AR, et al (2006) Analysis of the Arabidopsis rsr4-1/pdx1-3 mutant reveals the critical function of PDX1 protein family in metabolism, development and vitamin B6 biosynthesis. Plant Cell 18 1722–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Liu P, Liu LH, Remans T, Tester M, Forde BJ (2006) Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant Cell Physiol 47 1045–1057 [DOI] [PubMed] [Google Scholar]
- Werck-Reichhart D, Jones OTG, Durst F (1988) Haem synthesis during cytochrome P-450 induction in higher plants. Biochem J 249 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J, Chopin F, Santoni V, Viennois G, Tillard P, Krapp A, Lejay L, Daniele-Vedele F, Gojon A (2007) Regulation of root nitrate uptake at the NRT2.1 protein level in Arabidopsis thaliana. J Biol Chem 282 23541–23552 [DOI] [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96 6529–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Rong H, Pilbeam D (2007) Signalling mechanisms underlying the morphological responses of root system to nitrogen in Arabidopsis thaliana. J Exp Bot 58 2329–2338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.