Abstract
Independent evolutionary lineages often display similar characteristics in comparable environments. Three kinds of historical hypotheses could explain this convergence. The first is adaptive and evolutionary: nonrandom patterns may result from analogous evolutionary responses to shared conditions. The second explanation is exaptive and ecological: species may be filtered by their suitability for a particular type of environment. The third potential explanation is a null hypothesis of random colonization from a historically nonrandom source pool. Here we demonstrate that both exaptation and adaptation have produced convergent similarity in different size-related characters of solitary island lizards. Large sexual size dimorphism results from adaptive response to solitary existence; uniform, intermediate size results from ecological filtering of potential colonizers. These results demonstrate the existence of deterministic exaptive convergence and suggest that convergent phenomena may require historical explanations that are ecological as well as evolutionary.
Keywords: adaptation, Anolis, convergence, ecological filter, exaptation, island
1. Introduction
Adaptation and exaptation are alternative historical explanations for traits (Gould & Vrba 1982). Adaptation, or evolutionary response to selective pressure, is commonly invoked to explain convergence between species (Futuyma 1986). Conversely exaptation, or current utility of a previously evolved character, is infrequently, if ever, associated with convergence. However, convergent exaptation may occur through ecological filtering, whereby only those lineages that possess particular selectively favourable characteristics are able to colonize similar environments (Almada & Santos 1995). Convergence is a fundamentally historical concept, and a phylogenetic approach is necessary to distinguish adaptation from exaptation and both of these phenomena from alternative explanations (Baum & Larson 1991).
Solitary species—those on islands lacking similar species (i.e. congeners)—that evolved from multispecies communities present an excellent system for elucidating instances of exaptation and adaptation. Such species experience competitive release, whereby populations display atypical characteristics in the absence of competitors (Brown & Wilson 1956; Grant 1972). For example, greater sexual size dimorphism (SSD; allowing intraspecific partitioning of resources) and uniform (i.e. energetically efficient) body sizes have been documented in many solitary species (e.g. birds: Selander 1966, Clegg & Owens 2002; mammals: Dayan & Simberloff 1998; lizards: Schoener 1967, 1969a,b). Although these patterns are widely accepted and ecological and energetic explanations have been offered (Schoener 1967, 1969b; Case 1978; Naganuma & Roughgarden 1990), the evolutionary bases for these patterns are poorly understood.
At least three historical hypotheses are consistent with these patterns. First, several authors have postulated that the unusual traits seen in solitary forms are due to competitive release, manifested in evolutionary responses to solitary existence (Schoener 1969a; Grant 1972); this is a hypothesis of adaptation. Second, particular body sizes and greater SSD might predispose species to successfully colonize an environment (Schoener 1969a; Case & Siddell 1983); this is a hypothesis of exaptation or ecological filtering. Third, the seemingly unusual characteristics of contemporary solitary species may simply reflect the ancestral characteristics of a historical species pool (i.e. the pool of potential colonizers has changed over evolutionary time); this is a hypothesis of random colonization.
Anolis lizards are ideal models for examining questions of the evolution of convergence and ecology (Williams 1983). Communities of Anolis are structured by intrageneric competition (Collette 1961; Losos 1994), clades display convergence in morphologies and habitat use across Caribbean islands (Williams 1972; Losos et al. 1998), and species may occur in isolation or coexist with up to 11 sympatric congeners (Williams 1983; Losos et al. 2003). Schoener (1969a) demonstrated that solitary Anolis species in the Caribbean display nonrandom patterns of size and SSD, and Losos & de Queiroz (1997) found that many solitary species were not generalists but rather fit the specialized ‘trunk-crown’ ecomorph.
Here we use modern phylogenetic approaches to show that nonrandom patterns of body size and SSD in solitary island Anolis lizards arise from distinct historical mechanisms. Increased SSD is a consequence of evolutionary change following colonization, whereas uniform size results from ecological filtering of exapted colonizers.
2. Material and methods
(a) Data
We investigated patterns of body size and SSD using a dataset of maximum male and female size for all 25 solitary and 208 nonsolitary Anolis species, including Caribbean and mainland-associated forms. Size data were compiled from species descriptions, Williams et al. (1995) and personal observation. Overall body size was measured as the median value of maximum snout to vent lengths (SVL) for males and females (the same results were obtained using maximum male size). The SSD was measured as the ratio of maximum female size to maximum male size. Distribution data were taken from Schwartz & Henderson (1991) and Losos & de Queiroz (1997).
(b) Tests for nonrandom distribution of SSD and size
In order to test for the patterns of nonrandom size and SSD shown by Schoener (1969a) for Caribbean Anolis using a broader sample, we compared mean SSD of the 25 solitary species to 10 000 random samples of 25 species from the entire pool of 233 species. We tested for the presence of especially uniform size among solitary species by comparing the coefficients of variation (CVs) of size of the solitary species to the CVs of size of 10 000 random samples of 25 species from the entire pool of 233 species. Species within a random sample were drawn without replacement.
(c) Tests of adaptation
We reconstructed a phylogeny for 233 species of Anolis and eight outgroups using parsimony analyses of data from Poe (2004; excluding characters of size and SSD) and Nicholson et al. (2005), and morphological data collected for this paper. This dataset includes 1664 parsimony-informative characters from mitochondrial and nuclear DNA sequences, allozymes, chromosomes and morphology. We performed 100 random additions of taxa and tree-bisection–reconnection branch swapping on PAUP* (Swofford 2002). Mixed model Bayesian analyses were attempted but failed to converge on believable results and/or crashed, apparently due to the size and complexity of the dataset.
We used phylogenetic comparative methods because both SVL and SSD have been shown to display strong phylogenetic signal in Anolis (Poe 2005, unpublished data). Branch lengths were assumed to be equal because our phylogenetic dataset includes varying character coverage across species (some species scored for just morphology, others for DNA and partial morphology, etc.), thus rendering any branch length estimates based on data incomparable across taxa. We tested for a correlation between solitary existence and SSD using generalized least squares (GLS). GLS analyses were performed using Continuous (Pagel 2006) with kappa and delta fixed at 1.0 and lambda estimated using maximum likelihood. We performed a likelihood ratio test comparing a null hypothesis of no correlation between solitary existence (coded as 0, nonsolitary; 1, solitary) and SSD to a model where characters were allowed to covary (see Pagel 1997).
As an alternative test of the hypothesis of adaptive change in SSD, we also reconstructed ancestral SSDs at nodes where solitary colonization occurred and compared these values to solitary SSDs using a Wilcoxon signed-rank test. Transitions to solitary existence were estimated on the optimal tree using likelihood and geographical approaches in Mesquite (Maddison & Maddison 2004) as follows. Transitions were identified at branches where a maximum likelihood estimate of nonsolitary at an ancestral node was followed by a maximum likelihood estimate of solitary at a descendant node, with two exceptions. In the cristatellus-and sagrei-group clades, solitary species are paraphyletic with respect to a nonsolitary species. The geographical distributions of these species suggest that multiple independent solitary invasions are much more likely than the ‘stepping stone’ solitary invasions followed by back-invasion to the mainland that would be interpreted by comparative approaches such as GLS (figure 1). Thus we hypothesized that A. nelsoni and A. luteosignifer each colonized independently from Cuba, and A. ernestwilliamsi, A. desechensis, A. scriptus and A. monensis each colonized independently from Puerto Rico. In these analyses, ancestral states were calculated excluding the solitary species (including solitary species obtained nearly identical results). Ancestral sizes and SSDs were reconstructed using squared-change parsimony in Mesquite (Maddison & Maddison 2004).
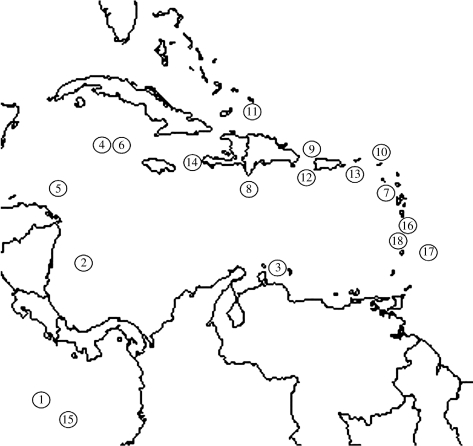
Figure 1.
Map of Caribbean region, showing localities of clades of solitary Anolis.
To test whether colonization by solitary species was accompanied by evolutionary change to a more uniform size distribution, we reconstructed ancestral sizes at nodes where change to solitary existence occurred and compared the distribution of ancestral sizes to the distribution of solitary sizes using Lewontin's (1966) test for equality of variances. As above, we used squared-change parsimony and omitted solitary species from these reconstructions. The expectation under the adaptation/evolutionary change hypothesis is that variance in sizes of solitary species is significantly lower than variance in sizes of ancestors. Size and SSD values were natural log transformed before analyses.
(d) Tests for exaptation
The hypotheses of ecological filtering and random colonization require identification of species pools available for colonization at the time(s) of solitary colonization. Candidate lineages are difficult to identify for mainland sources and data suitable for dating are not available for most mainland species. Therefore, we focused solely on Anolis of the Greater Antilles. This dataset includes eight solitary colonization events and 121 species. We performed a Markov-chain Monte Carlo Bayesian analysis (Huelsenbeck & Ronquist 2001) of mitochondrial sequence data from Nicholson et al. (2005) omitting mainland-associated species and species lacking size data. We used the GTR+G+I model as suggested by MrModeltest (Nylander 2004) and performed 1 000 000 generations, saving one tree every 1000. We used PAUP* (Swofford 2002) to force a molecular clock onto the optimal tree from this analysis using likelihood and the GTR+G+I model with all parameters estimated and rooted with A. occultus. Unresolved nodes were resolved randomly with branch lengths of 0.00001; these were deep in the tree and so did not affect ancestral reconstructions for solitary lineages. Species were coded as Cuban (0), Hispaniolan (1), Puerto Rican (2), Jamaican (3), Lesser Antillean (4) or solitary (5) and ancestral areas and transitions to solitary existence were estimated as above. Thus, A. brunneus and A. longiceps were treated as independent invasions from Cuba in this analysis. Ancestral sizes and SSDs were estimated using weighted squared-change parsimony in Mesquite (Maddison & Maddison 2004).
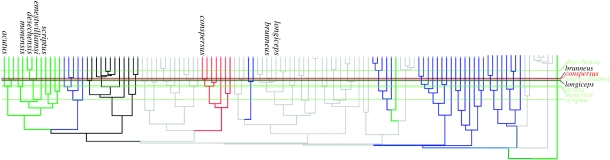
We reconstructed the temporal sequence of solitary invasions on the molecular clock tree by noting ancestral sizes and SSDs for each lineage that was present at the time of each solitary colonization event, that is, when a solitary lineage split from its sister lineage. We assume that the lineages that were extant at the time of each colonization event comprise the pool of available colonists from a particular Greater Antillean island (figure 2). For example, when A. scriptus invaded the Caicos islands from Puerto Rico, seven lineages were present on Puerto Rico: the acutus/evermanni/stratulus lineage, scriptus/desechensis/cristatellus/ernestwilliamsi, cooki/monensis, gundlachi/poncensis, krugi/pulchellus, cuvieri and occultus. For each of 10 000 replicates of Monte Carlo sampling, we chose one lineage at random from each of the eight pools, corresponding to the eight solitary colonization events, and compiled reconstructed SSDs and sizes for those lineages. This resulted in null distributions of body size and SSD; that is, the distributions of body size and SSD expected in the absence of ecological filtering. We compared the CV of sizes and the mean of SSDs from ancestors of solitary lineages to these values measured for their respective null distributions. The exaptation/ecological filter hypothesis predicts that: (i) SSDs reconstructed at nodes that lead directly to solitary lineages are larger than random samples of ancestral SSDs and (ii) sizes reconstructed at nodes that lead directly to solitary lineages show less variance than random samples of ancestral sizes. In contrast, the random colonization hypothesis predicts that ancestral SSDs and ancestral sizes of solitary lineages are representative of the pool of potential colonizers. In this case, the null distributions should accurately predict SSD and variance in size for ancestors of solitary Anolis.
Figure 2.
Phylogeny of Greater Antillean Anolis comparing lineages that colonized solitary islands to potential colonizing lineages, assuming molecular clock; names of nonsolitary species excluded. Horizontal lines mark divergence times of solitary species and cross other lineages that were extant at time of solitary invasion. Colours refer to Puerto Rico (green), Cuba (brown), Jamaica (red), Hispaniola (blue), northern Lesser Antilles (black). All lineages crossed by horizontal lines on a given island represent the pool of potential colonizers from that island when a solitary species invaded from that island.
3. Results
(a) Tests for nonrandom patterns of size and SSD
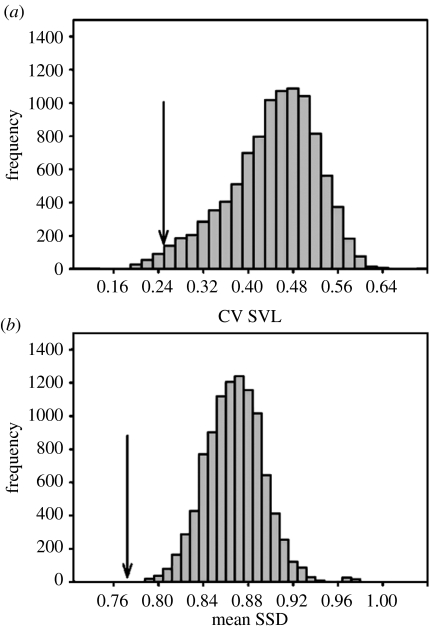
Solitary species display more uniform size (figure 3a; p<0.001) and greater SSD (figure 3b; p<0.001) than nonsolitary species. Mean male SVL of solitary species is 77 mm (72 mm in nonsolitaries), standard deviation is 19.2 (31.2 in nonsolitaries). Mean female SVL of solitary species is 58 (63 in nonsolitaries), standard deviation is 12.4 (26.8 in nonsolitaries).
Figure 3.
Distribution of (a) coefficient of variation of size (observed CV SVL=0.25, p=0.018), (b) and mean SSD (observed mean SSD=0.77, p<0.001) for random selections of 25 Anolis species. The 25 solitary island species display especially large SSD and uniform size.
(b) Tests for adaptation
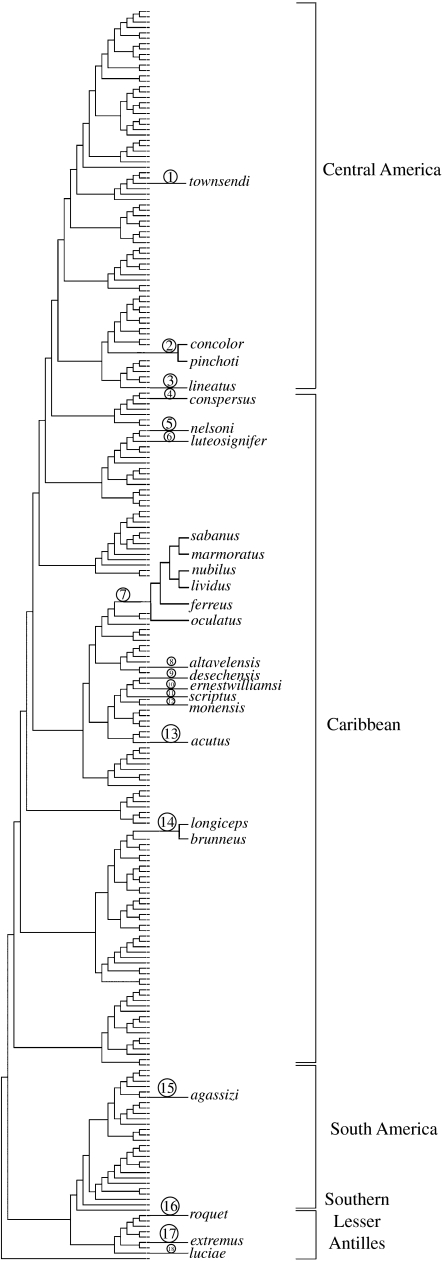
Parsimony analysis resulted in 6240 optimal trees of length 30 139. We chose one tree from this sample at random for analysis because optimal trees did not differ in recent relationships of solitary Anolis and the comparative methods we use require a single tree (figure 4). This tree includes 18 independent solitary colonization events (figure 1).
Figure 4.
Phylogenetic relationships of 233 species of Anolis, excluding outgroups; names of nonsolitary species excluded. Numbered clades are hypothesized transitions to solitary existence. Numbers refer to localities in figure 1.
In the GLS test of adaptation in SSD, the log-likelihood was significantly greater for a correlated model (−138.75) relative to a noncorrelated model (−141.17), indicating significant evolutionary association between solitary existence and increased SSD (p=0.028). In the sign test of association between SSD and solitary existence, solitary SSDs were significantly greater than ancestral SSDs (p=0.043).
Size distributions of ancestors and solitary species were similar (p=0.58), indicating a lack of association between solitary existence and change to a more uniform size distribution. Also, there was no significant trend towards increase or decrease in size with solitary colonization (p=0.31, GLS; p=0.18, sign test).
(c) Tests for exaptation
The CV in size was significantly lower for solitary ancestors relative to the pool of available colonists (p<0.001), supporting the role of ecological filtering in size. The uniform size of solitary colonists is intermediate for Anolis and similar to that recorded by Schoener (1969a; mean male size =77 mm). SSD was not significantly greater in solitary ancestors (p=0.15), and thus we could not reject the null hypothesis of random colonization with respect to SSD.
4. Discussion
Adaptation towards greater SSD appears to accompany the transition to solitary existence, supporting the idea that competitive release can drive evolutionary change (Brown & Wilson 1956; Schoener 1969a; Grant 1972). In the case of Anolis lizards, it seems that the shift to solitary existence does not initiate an evolutionary change in SSD, but rather enhances the SSD that is already present in the colonizing lineages. Solitary species derive from ancestral clades in which males are larger than females. It seems probable that males become larger than females according to some other selective pressure (e.g. increased fitness of larger males in defence of territories (Darwin 1874)) and that solitary existence accentuates this condition to still greater SSD. Following solitary colonization, greater SSD should minimize competition between sexes (Schoener 1967), and both sexual selection and intrasexual competition for resources may shape SSD in the absence of competitive congeners. Instances of increase in male size are no more common than decreases in female size with solitary colonization (data not shown), which is perhaps more consistent with a hypothesis of decreased intrasexual competition for resources than with increased sexual competition among males.
Support for the ecological filter hypothesis in the evolution of uniform size indicates exaptation for size in solitary Anolis. Intermediate size evolves in response to local competition in multispecies communities (Losos 1994), but later is advantageous for solitary existence (Schoener 1967). This result suggests a fundamental shift in understanding the unusual characteristics of solitary species; namely, away from adaptive response to solitary existence towards exaptive conditions that facilitate colonization. For example, the intermediate size of solitary Anolis has been interpreted to represent an energetic optimum towards which species evolve in order to more efficiently exploit resources (Schoener 1969b; Naganuma & Roughgarden 1990). Our results do not contradict the energetic interpretation, but they do suggest that this optimum, if it exists, should be interpreted in terms of dispersal and solitary establishment (if invasion of a barren island occurred) or competitive ability (if vicariance followed by extinction of congeners occurred, or dispersal to an already-inhabited island is followed by extinction of the inhabitant) rather than selective response to reduced competition.
Why would intermediate size be advantageous for dispersal and establishment? Possible explanations include optimal propagule size (i.e. large enough to avoid desiccation, small enough to travel), greater abundance in source populations, optimal habitat use for dispersal (i.e. coastal) and ability to use a broad range of environments. These hypotheses cannot be distinguished on current evidence, but we note that intermediate-sized Anolis may be extremely abundant (e.g. Rodda et al. 2001) as well as present in coastal areas in apparently greater numbers than larger or smaller congeners (e.g. Genet et al. 2001). And, some intermediate-sized species are able to inhabit a great diversity of habitats. For example, A. cristatellus is found in mesic forest, dry forest, open areas and human habitations (Schwartz & Henderson 1991).
Why would intermediate size be competitively advantageous? Perhaps, intermediate-sized Anolis are more catholic in their habitat and/or prey needs and thus able to marginalize more specialized species. This possibility is somewhat consistent with Roughgarden & Pacala's (1988) ‘taxon cycle’. In their scenario, a large species invades an island inhabited by a medium species and outcompetes the medium species while evolving to intermediate size. Under current evidence (i.e. lack of size evolution), a similar scenario can be imagined where an intermediate sized generalist invader outcompetes a specialized inhabitant, then evolves greater specialization. This would be a version of the taxon cycle where ecological specialization, rather than size evolution, drives the cycle.
Finally, the apparent success of intermediate-sized Anolis in solitary colonization could be multifaceted and is probably due to some combination of the above and/or other factors. The most successful recent anoline colonizer, A. sagrei, may possess both dispersal and competitive advantages over some congeners (Williams 1969; Tokarz & Beck 1987).
Co-option of a trait (intermediate size) for the same apparent function (solitary existence) in several independent lineages suggests that solitary Anolis represent a case of widespread exaptive convergence. Thus, repeated similarity need not be adaptive or neutral, but can be exaptive as well. Similarly, exaptation usually is considered to be a stochastic process where some feature is fortuitously advantageous in a new selective regime (Arnold 1994). Our results demonstrate that exaptation, like adaptation, can be deterministic; similar environments favour similar exaptations, in this case via ecological filtering.
5. Conclusions
Nonrandom patterns of body size and SSD in solitary island Anolis lizards arise from distinct historical mechanisms. The phylogenetic pattern of increased SSD is consistent with the predictions of adaptation to a solitary environment, whereas uniform size appears to result from ecological filtering of colonizers exapted for solitary existence. Both these processes are convergent, thus demonstrating that exaptation, like adaptation, may have a deterministic component.
Adaptive convergence appears to be widespread (Futuyma 1986), but further study is necessary to determine the pervasiveness of convergent exaptation via ecological filtering or through other mechanisms. Other candidate systems for convergent exaptation by ecological filtering include mammalian dispersal across landbridges (Webb 1976), establishment of exotic species (Jeschke & Strayer 2006), and size assortment in multispecies communities (Losos 1994). Phylogenetic studies can determine whether these systems require adaptive, exaptive, or, as in this case of solitary Anolis, both kinds of historical explanations.
Acknowledgments
We thank Jim Brown, Jonathan Losos, Eric Schaad, and Ian Latella for their comments on this work.
References
- Almada V.C, Santos R.S. Parental care in the rocky intertidal: a case study of adaptation and exaptation in Mediterranean and Atlantic blennies. Rev. Fish Biol. Fisher. 1995;5:23–37. doi:10.1007/BF01103364 [Google Scholar]
- Arnold E.N. Investigating the origins of performance advantage: adaptation, exaptation and lineage effects. In: Eggleston P, Vane-Wright R, editors. Phylogenetics and ecology. Linnean society of London and Academic Press; London, UK: 1994. pp. 123–168. [Google Scholar]
- Baum D.A, Larson A. Adaptation reviewed: a phylogenetic methodology for studying character macroevolution. Syst. Zool. 1991;40:1–18. doi:10.2307/2992218 [Google Scholar]
- Brown W.L, Wilson E.O. Character displacement. Syst. Zool. 1956;5:49–65. doi:10.2307/2411924 [Google Scholar]
- Case T.J. A general explanation for insular body size trends in terrestrial vertebrates. Ecology. 1978;59:1–18. doi:10.2307/1936628 [Google Scholar]
- Case T.J, Siddell R. Pattern and chance in the structure of model and natural communities. Evolution. 1983;37:832–849. doi: 10.1111/j.1558-5646.1983.tb05604.x. doi:10.2307/2407923 [DOI] [PubMed] [Google Scholar]
- Clegg S.M, Owens I.P.F. The “island rule” in birds: medium body size and its ecological explanation. Proc. R. Soc. B. 2002;269:1359–1365. doi: 10.1098/rspb.2002.2024. doi:10.1098/rspb.2002.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette B.B. Correlations between ecology and morphology in anoline lizards from Havana, Cuba and southern Florida. Bull. Mus. Comp. Zool. 1961;125:137–162. [Google Scholar]
- Darwin C. A.L.Burt; New York, NY: 1874. The descent of man, and selection in relation to sex. [Google Scholar]
- Dayan T, Simberloff D. Size patterns among competitors: ecological character displacement and character release in mammals. Mammal. Rev. 1998;3:99–124. doi:10.1046/j.1365-2907.1998.00029.x [Google Scholar]
- Futuyma D. Sinauer Associates; Sunderland, MA: 1986. Evolutionary biology. [Google Scholar]
- Genet K.S, Genet J.A, Burton T.M, Murphy P.G. The lizard community of a subtropical dry forest: Guanica forest, Puerto Rico. Trop. Ecol. 2001;42:97–109. [Google Scholar]
- Gould S.J, Vrba E.S. Exaptation: a missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- Grant P.R. Convergent and divergent character displacement. Biol. J. Linn. Soc. 1972;4:39–68. [Google Scholar]
- Huelsenbeck J.P, Ronquist F. MrBayes: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. doi:10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- Jeschke J.M, Strayer D.L. Determinants of vertebrate invasion success in Europe and North America. Glob. Change Biol. 2006;12:1608–1619. doi:10.1111/j.1365-2486.2006.01213.x [Google Scholar]
- Lewontin R.C. On the measurement of relative variability. Syst. Zool. 1966;15:141–143. doi:10.2307/2411632 [Google Scholar]
- Losos J.B. Integrative approaches to evolutionary ecology: Anolis lizards as model systems. Annu. Rev. Ecol. Syst. 1994;25:467–493. doi:10.1146/annurev.es.25.110194.002343 [Google Scholar]
- Losos J.B, de Queiroz K. Evolutionary consequences of ecological release in Caribbean Anolis lizards. Biol. J. Linn. Soc. 1997;61:459–483. doi:10.1006/bijl.1997.0137 [Google Scholar]
- Losos J.B, Jackman T.R, Larson A, de Queiroz K, Rodriguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. doi:10.1126/science.279.5359.2115 [DOI] [PubMed] [Google Scholar]
- Losos J.B, Leal M, Glor R.E, de Queiroz K, Hertz P.E, Rodr'guez Schettino L, Chamizo Lara A, Jackman T.R, Larson A. Niche lability in the evolution of a Caribbean lizard community. Nature. 2003;424:542–545. doi: 10.1038/nature01814. doi:10.1038/nature01814 [DOI] [PubMed] [Google Scholar]
- Maddison, W. R. & Maddison, D. R. 2004 Mesquite: a modular system for evolutionary analysis, version 1.04. See http://mesquiteproject.org
- Naganuma K.H, Roughgarden J.D. Optimal body size in lesser Antillean Anolis lizards: a mechanistic approach. Ecol. Monogr. 1990;60:239–256. doi:10.2307/1943046 [Google Scholar]
- Nicholson K.E, Glor R.E, Kolbe J.J, Larson A, Hedges S.B, Losos J.B. Mainland colonization by island lizards. J. Biogeogr. 2005;32:1–10. doi:10.1111/j.1365-2699.2004.01222.x [Google Scholar]
- Nylander, J. A. 2004 MrModelTest. 2.0th edn. Distributed by the author. Evolutionary Biology Centre, Uppsala University, Sweden.
- Pagel M. Inferring evolutionary processes from phylogenies. Zool. Script. 1997;26:331–348. doi:10.1111/j.1463-6409.1997.tb00423.x [Google Scholar]
- Pagel, M. 2006 Continuous See http://www.ams.reading.ac.uk/zoology/pagel
- Poe S. Phylogeny of anoles. Herp. Monos. 2004;18:37–89. doi:10.1655/0733-1347(2004)018[0037:POA]2.0.CO;2 [Google Scholar]
- Poe S. A study of the utility of convergent characters for phylogeny reconstruction: do ecomorphological characters track evolutionary history in Anolis lizards? Zoology. 2005;108:337–344. doi: 10.1016/j.zool.2005.08.002. doi:10.1016/j.zool.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Rodda G, Perry G, Rondeau R.J, Lazell J. The densest terrestrial vertebrate. J. Trop. Ecol. 2001;17:331–338. doi:10.1017/S0266467401001225 [Google Scholar]
- Roughgarden J, Pacala S. Taxon cycle among Anolis lizard populations: review of evidence. In: Otte D, Endler J, editors. Speciation and its consequences. Sinauer; Sunderland, MA: 1988. pp. 403–432. [Google Scholar]
- Schoener T.W. The ecological significance of sexual dimorphism in size in the lizard Anolis conspersus. Science. 1967;155:474–477. doi: 10.1126/science.155.3761.474. doi:10.1126/science.155.3761.474 [DOI] [PubMed] [Google Scholar]
- Schoener T.W. Size patterns in West Indian Anolis lizards I: size and species diversity. Syst. Zool. 1969a;18:386–391. doi:10.2307/2412183 [Google Scholar]
- Schoener T.W. Models of optimal size for solitary predators. Am. Nat. 1969b;103:277–313. doi:10.1086/282602 [Google Scholar]
- Schwartz A, Henderson R.W. University of Florida Press; Gainesville, FL: 1991. Amphibians and reptiles of the West Indies. [Google Scholar]
- Selander R.K. Sexual dimorphism and differential niche utilization in birds. Condor. 1966;68:113–151. doi:10.2307/1365712 [Google Scholar]
- Swofford D.L. Sinauer; Sunderland, MA: 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods) [Google Scholar]
- Tokarz R.R, Beck J.W., Jr Behaviour of the suspected lizard competitors Anolis sagrei and Anolis carolinensis: an experimental test for behavioural interference. Anim. Behav. 1987;35:722–734. doi:10.1016/S0003-3472(87)80108-2 [Google Scholar]
- Webb S.D. Mammalian faunal dynamics of the great American interchange. Paleobiology. 1976;2:216–234. [Google Scholar]
- Williams E.E. The ecology of colonization as seen in the zoogeography of anoline lizards on small islands. Quart. Rev. Biol. 1969;44:345–389. doi:10.1086/406245 [Google Scholar]
- Williams E.E. The origin of faunas: evolution of lizard congeners in a complex island fauna: a trial analysis. Evol. Biol. 1972;6:47–90. [Google Scholar]
- Williams E.E. Ecomorphs, faunas, island size, and diverse end points in island radiations of Anolis. In: Huey R.B, Schoener T.W, editors. Lizard ecology: studies of a model organism. Harvard University Press; Cambridge, MA: 1983. pp. 326–370. [Google Scholar]
- Williams E.E, Rand H, Rand A.S, O'hara R.J. A computer approach to the comparison and identification of species in difficult taxonomic groups. Breviora. 1995;502:1–47. [Google Scholar]