Abstract
Abundant, many-flowered plants represent reliable and rich food sources for animal pollinators, and may even sustain guilds of specialized pollinators. Contrastingly, rare plants need alternative strategies to ensure pollinators' visitation and faithfulness. Flower mimicry, i.e. the sharing of a similar flower colour and display pattern by different plant species, is a means by which a rare species can exploit a successful model and increase its pollination services. The relationship between two or more rewarding flower mimic species, or Müllerian mimicry, has been proposed as mutualistic, in contrast to the unilaterally beneficial Batesian floral mimicry. In this work, we show that two different geographical colour phenotypes of Turnera sidoides ssp. pinnatifida resemble co-flowering Malvaceae in colour as seen by bees' eyes, and that these pollinators do not distinguish between them when approaching flowers in choice tests. Main pollinators of T. sidoides are bees specialized for collecting pollen in Malvaceae. We demonstrate that the similarity between at least one of the geographical colour phenotypes of T. sidoides and co-flowering Malvaceae is adaptive, since the former obtains more pollination services when growing together with its model than when growing alone. Instead of the convergent evolution pattern attributed to Müllerian mimicry, our data rather suggest an advergent evolution pattern, because only T. sidoides seems to have evolved to be more similar to its malvaceous models.
Keywords: flower mimicry, Müllerian, mutualism, Malvaceae, pollination, Turneraceae
1. Introduction
To attract animal pollinators, plant species may either exhibit unique flower displays or imitate models that are present in the local environment. The imitation of the flowers of one species by another is known as floral mimicry (Little 1983; Dafni 1984, 1986; Roy & Widmer 1999). Cases of floral mimicry have been classified as Batesian or Müllerian, depending on reciprocity pattern, density balance between mimics in a community and evolutionary origin (Dafni 1984, 1986; Roy & Widmer 1999). In Batesian mimicry, there is a rewarding model and a rewardless mimic. Thus, the latter parasitizes the successful advertisement of the former and enjoys a reproductive benefit as long as it remains at lower densities than the model. Müllerian mimicry is mutualistic since both species reward pollinators and benefit each other by sharing a common advertising display, reaching a higher combined flower density. As pollinator visitation is commonly density dependent, this similarity between flowers implies higher pollination success of both species (Little 1983; Roy & Widmer 1999).
Distinct patterns of reciprocity are expected to have rather different consequences for the fitness of each mimicry partner. In Batesian flower mimicry, directional evolution towards an increasing similarity of the mimic to the model, i.e. advergent evolution, is predicted and often observed. Müllerian floral mimicry, on the other hand, might have originated by evolution to mutual similarity, i.e. convergent evolution (Dafni 1984; Johnson et al. 2003a). However, convergent evolution can also be the result of selection exerted by a guild of shared pollinators to meet their sensory preferences rather than the result of selection by joint conditioning of the signal receiver. The similarity between species resulting from this former mechanism is referred to as flower syndromes, and is only subtly distinct from a Müllerian mimicry system (Johnson et al. 2003a; Jersáková et al. 2006). The dichotomous distinction between Batesian and Müllerian floral mimicries is perhaps more a theoretical perspective than an empirical reality, though being useful by marking the extremes of a reward continuum in floral mimicry as proposed by Johnson et al. (2003a).
In this study, we attempt to demonstrate that the flower resemblance of Turnera sidoides spp. pinnatifida (Poiret) Arbo to different mallow species represents a special case of floral mimicry. In part, it can be regarded as an example of Müllerian mimicry because both mimics and models are rewarding. On the other hand, it does not conform to the strict definition of Müllerian mimicry because the similarity between mimics and models appears to be due to advergent evolution. Thus, the present case of mimicry also shares features of Batesian mimicry.
Several conditions must be met if a pair of species, whose flowers are apparently similar to each other, are to be considered as mimics. First, although flowers of mimics may appear similar to the human eye, they must resemble each other in flower display from the pollinators' perceptual point of view. Second, mimics have to depend on the same pollinating individuals as pollen vectors, which must be able to move freely between mimic species. Third, mimics must have evolved to have high similarity with their model (Batesian) or they must have coevolved to mutual similarity (Müllerian). We therefore addressed the following questions. First, are T. sidoides flower colours similar to those of co-occurring Malvaceae as they are perceived by bees? Second, is colour in different geographical phenotypes of T. sidoides different from other community members and phylogenetic relatives, and can this deviation be attributed to differences in the colour of locally prevailing model species? Third, which pollinators are shared with the model and are pollinators able to differentiate between the mimic and the model? Fourth, are the nectar rewards of both species similar?
Finally, mimicry must have a positive effect on pollination and reproductive success in at least one of the mimics (Johnson 1994; Roy & Widmer 1999). This aspect, though a crucial one, has seldom been explored in flower mimicry systems (Johnson 1994; Roy & Widmer 1999). It has been argued instead that sharing of pollinators by two or more species could mean competition for pollinator services and improper pollen transfer between flowers (Rathcke 1983; Roy & Widmer 1999). Competition would thus represent a factor constraining the evolutionary emergence of mimicry. However, selection towards convergence in flower colour, size and shape is not incompatible with evolutionary strategies to decrease improper transfer of pollen, such as the use of different parts of pollinators' body for pollen transport (Brown & Kodric-Brown 1979; Roy & Widmer 1999). In addition, competition among plants that share pollinators does not seem to be a serious problem in many floral guilds. On the contrary, some studies have provided evidence of facilitation, instead of competition, among plants that share pollinators (Feinsinger 1987; Moeller 2004). Sometimes this is due to the effect of an abundant and highly rewarding magnet species (Thomson 1978; Johnson et al. 2003b).
Thus, we also investigated whether T. sidoides plants co-flowering with malvaceous models have a higher reproductive success or whether reproduction is negatively impacted by improper pollen transfer.
2. Material and methods
Turnera sidoides ssp. pinnatifida is a self-incompatible, heterostylous and stress-tolerant perennial herb, widespread from southern Bolivia to central Argentina. It includes several geographical colour phenotypes (Solís Neffa 2000; Solís Neffa et al. 2004), from yellow in the montane valleys of southern Bolivia and the northern Argentine provinces of Salta and Jujuy, to light orange in central Argentina. To examine flower colours and geographical colour variation, we obtained data from plants of the Sierras de Córdoba mountain range, where light orange-flowered populations of T. sidoides ssp. pinnatifida grow together with Sphaeralcea cordobensis Krapov. (Malvaceae), and from the Salta province, where yellow-flowered populations co-occur with three additional malvaceous species: Modiolastrum malvifolium (Gris.) K. Schum., Sida rhombifolia L., and Malvastrum coromandelianum (L.) Garcke. Data on flowering phenologies and reproductive success were obtained only from the light orange-flowered phenotype in several populations of the Córdoba province.
(a) Floral colours, size and display patterns
We studied the resemblance in visual display from the pollinators' perceptual point of view. Petal reflectances were measured with a S 2000 spectrometer (Ocean Optics, USA). We recorded spectra of flowers from Argentinean populations in Córdoba (31°15′ S; 64°18′ W) and Salta (24°39′ S; 65°22′ W). To investigate whether the colour of mimicry partners is widespread in each community, we recorded the reflectance spectra of co-flowering species in both communities. In addition, to explore the extent of variation of flower colour within Turneraceae, we obtained from the living collection of IBONE (Instituto de Botánica del Nordeste, Corrientes, Argentina) reflectance spectra of eight subspecies and species related to T. sidoides ssp. pinnatifida. The perceptual similarity of the floral colours was estimated using the receptor noise-limited (RNL) model of honeybee colour vision (Vorobyev et al. 2001). The RNL model predicts the discrimination between colours for the honeybee based on parameters obtained from electrophysiological recordings in the bee photoreceptors. It assumes that colour is coded by two colour-opponent mechanisms and only receptor noise limits their accuracy in colour discrimination. The model has successfully predicted a number of experimental results for human, birds and bees (Vorobyev & Osorio 1998; Hempel de Ibarra et al. 2001; Goldsmith & Butler 2003; Niggebrügge & Hempel de Ibarra 2003). We extend our predictions about the similarity of measured colours for honeybees to other bee species because spectral sensitivities of photoreceptors do not vary strongly in Apidae (Menzel et al. 1988; Peitsch et al. 1992; Chittka 1996; Vorobyev & Menzel 1999). Using the model, each spectral reflectance can be characterized by a colour locus in the bee's perceptual colour space. Colour distances between colour loci allow us to predict whether colours are distinguished. In general, the larger the distance, the better the colours are discriminated. However, any loci separated by distances below the threshold value of 2.3 RNL units are not distinguishable for bees (Vorobyev et al. 2001). One unit corresponds to the standard deviation of bee photoreceptor noise. To estimate the spread of colour loci between and within flower species, the mean chromatic distances between all individual loci pairings were calculated. In addition, single floral displays were visualized as they are seen by the bees (Vorobyev et al. 1997, 2001). For this purpose, flowers were imaged through a set of chromatic filters. The calculated bee photoreceptor excitations in the short (S), medium (M) and long (L) wavelength ranges in each pixel of the image were coded with the primary monitor colours blue, red and green, respectively. The optical resolution of a bee compound eye was simulated for a floral display that subtended an angle of 16° (equivalent to viewing from a distance of 6–9 cm). This size lies within the perceptual range of chromatic pattern cues (Hempel de Ibarra et al. 2001). As flower size can also affect flower discrimination by pollinators, we compared petal length in plants of T. sidoides and plants of the presumed models in Córdoba and Salta.
(b) Pollinators and behaviour
To determine whether pollen vectors were shared among mimics and models, we analysed pollinator assemblage, pollen loads on pollinators and flowering phenologies. In 10 mixed populations of the light orange-flowered T. sidoides and S. cordobensis, we captured 63 bees while they were visiting the flowers of either species, and we examined their pollen loads under the microscope. We analysed separately pollen loads from the scopae and the ventral part of the body, because specialist bees collect pollen from few plant species, but may use a variety of plants as nectar sources (Wcislo & Cane 1996). Pollen was identified by comparison with reference pollen samples from the same plant communities. Since pollen can be present on the bees' bodies even if plants are not in flower at the same time, we determined flowering phenologies and examined the hours of opening and closing of flowers of both species. Phenology was determined by checking the number of flowers produced in nine patches at six fortnight periods along the whole flowering season (September–December 2002). The match between phenologies of both species was examined by means of Pearson's correlation measure.
To determine whether pollinators discriminate between flowers of T. sidoides and S. cordobensis, we used a modification of the ‘bee interview’ technique (Thomson 1988; Johnson et al. 2003b). We placed single flowered branches of T. sidoides and S. cordobensis, at 10 cm from each other, in a natural plot with both species flowering. We recorded during a total of 240 min pollinator approaches to flowers (to a distance less than 30 mm without landing) and landings on flower (visitors touching fertile organs) of each species. On data of approaches and landings separately, we analysed with G-tests whether frequencies significantly deviated from the expected 0.5 proportion for each species.
In the yellow-flowered phenotype of T. sidoides, pollinator observations were restricted to the recording of visitation frequencies in two mixed patches of T. sidoides and M. malvifolium during 70 min, and to the analysis of pollen loads on the stigma in an additional mixed population to confirm the pollen transfer between species.
(c) Nectar
To compare reward properties, we covered newly opened flowers for 5 h and quantified with 5 μl microcapillaries nectar amounts of 50 flowers of S. cordobensis and 28 flowers of T. sidoides. Nectar concentrations were measured in 15 flowers of T. sidoides and 15 flowers of S. cordobensis using a hand refractometer (Atago) in Brix % scale. Mann–Whitney U-tests were used to compare nectar volumes and concentrations.
(d) Pollination services and reproductive success
We studied the possible benefit of both species flowering together in terms of pollination services by comparing female reproductive success in plants from mixed and single species patches. For T. sidoides ssp. pinnatifida, two measures, i.e. the number of conspecific pollen grains on the stigma and fruit set, were used. This is because pollen loads on the stigmas are straightforward related to pollinator behaviour, while fruit set can be affected by environmental conditions. Pollen loads were analysed with epifluorescent microscopy (Leica DMLB). For S. cordobensis, the number of conspecific pollen grains on the stigmas was not used because the stigmas are intermingled with numerous anthers making spontaneous pollen transfer within a flower likely. For this reason, we used only fruit set (fruits/flowers) as a measure of female fitness in this species. The effect of flower density of T. sidoides and S. cordobensis on fruit set was analysed for both species in nine mixed patches by means of multiple regression. Density dependence was also tested in 11 single species patches of T. sidoides. In all cases, flower density was log-transformed.
The consequence of improper pollen transfer on reproductive success of T. sidoides was determined by comparing the arcsine-transformed percentage of malvaceous pollen (the percentage of other heterospecific pollen types was negligible) on fruiting and non-fruiting stigmas by means of a t-test. This was possible because stigmas remain undamaged on initiated fruits and wilted flowers of T. sidoides. All S. cordobensis stigmas inspected had only conspecific pollen.
3. Results
(a) Floral colour, size and display pattern
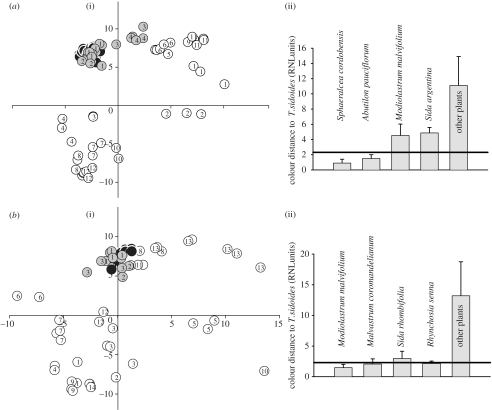
To assess differences between T. sidoides ssp. pinnatifida populations (light orange and yellow), we recorded flower colours at two locations (Córdoba and Salta). The subjective colour difference for bees was determined by calculating the distance between colour loci in the bee's perceptual colour space (RNL model of bee colour vision). The mean colour distance was 3.4±0.9 s.d. in RNL units, which is well above the bee's colour discrimination threshold of 2.3 RNL units (Vorobyev et al. 2001). Both phenotypes of T. sidoides were thus different in colour to bees.
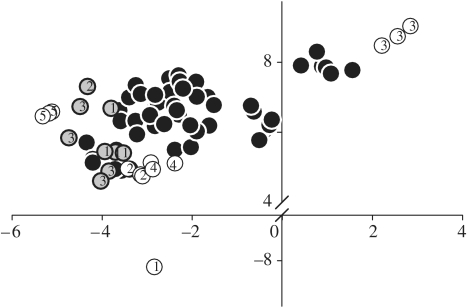
We further recorded flower colours of co-flowering mallows as putative models. Within each phenotype population, the colour loci of the local T. sidoides overlapped strongly with that of co-occurring Malvaceae in the bee's perceptual colour space. Resulting colour distances were well below the discrimination threshold. Thus, for bees, the light orange phenotype of T. sidoides found in the Córdoba population was indistinguishable in colour from the abundant mallow S. cordobensis and the rare mallow Abutilon pauciflorum (figure 1a). Similarly, in the Salta population, the yellow phenotype of T. sidoides was similar in colour to two other Malvaceae: the dominant M. malvifolium and the less abundant M. coromandelianum (figure 1b). One additional mallow, S. rhombifolia, found in the Salta population, was marginally above the discrimination threshold in its mean colour distance, but some individuals of it were indistinguishable from the local T. sidoides. Colour variability in T. sidoides was also very similar to that of the malvaceous models (Córdoba: T. sidoides 0.9±0.5 s.d. and S. cordobensis 0.8±0.5. s.d. Salta: T. sidoides 1.5±1 s.d. and M. malvifolium 0.9±0.4 s.d.). Other co-flowering species in both populations were very dissimilar in colour to the mimic T. sidoides (figure 1 a,b), with the exceptions of the legume Rhynchosia senna and one of the three measured plants of the loosestrife Heimia salicifolia in the Salta population. Not only was petal colour indistinguishable between T. sidoides and its malvaceous models, but bee-views of the concentric floral patterns and floral shape of the mimicry partners were also similar (figure 2). We further discovered that the variation in petal colour among colour phenotypes of T. sidoides ssp. pinnatifida growing in separated geographical locations was wide. In contrast, such a degree of colour polymorphism was not found in any of the other subspecies of T. sidoides (figure 3).
Figure 1.
Two colour phenotypes of T. sidoides ssp. pinnatifida resemble their local models in two distant communities: in (a) the Córdoba and (b) the Salta provinces. (a(i),b(i)) The loci of petal colours in the perceptual colour space of bees (RNL model). A distance of 2.3 RNL units (horizontal line) corresponds to the threshold distance between loci, below which colours are indistinguishable to bees. (a(ii),b(ii)) Mean colour distances (+s.d.) between T. sidoides, the proposed model species, other Malvaceae and other plants in the communities. (a) Plant species of the Córdoba community. T. sidoides spp. pinnatifida (black circles) and co-flowering Malvaceae species (grey circles): (1) S. cordobensis, the proposed model, (2) A. pauciflorum, and (3) the yellow coloured mallows M. malvifolium and (4) Sida argentina. Other co-occurring plants in the community (white circles): (1) Acacia aroma, (2) Amblyopetalum coccineum, (3) Ammi majus, (4) Cirsium vulgare, (5) Gaillardia sp., (6) Hirschfeldia incana, (7) Melilotus albus, (8) Melochia anomala, (9) Oxalis sp., (10) Pfaffia sp., (11) Senecio pampeanus, and (12) Solanum sysimbriifoluim. (b) Plant species of the Salta community. T. sidoides spp. pinnatifida (black circles) and co-flowering Malvaceae species (grey circles): (1) M. malvifolium, (2) M. coromandelianum, and (3) S. rhombifolia. Other co-occurring plants in the community (white circles): (1) Ageratum conyzoides, (2) Argemone subfusiformis, (3) Borreria densiflora, (4) Centaurium pulchellum, (5) Cestrum parqui, (6) Cuphea sp., (7) Cynoglossum amabile, (8) H. salicifolia, (9) Heliotropium amplexicaule, (10) Ludwigia peploides, (11) R. senna, (12) Rorippa nasturtium-aquaticum, (13) Senecio rudbeckiifolius, and (14) Solanum sisymbriifolium.
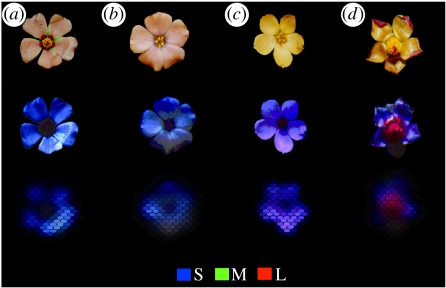
Figure 2.
Floral displays as seen by the human and the bee eye. Floral displays of T. sidoides spp. pinnatifida (b,c) and their respective models, S. cordobensis (a) and M. malvifolium (d), in Córdoba (a,b) and Salta (c,d). Appearance to the human eye (first row), and bee-views of floral displays (second and third rows; Vorobyev et al. 1997), where primary colours (blue, green and red) label the bee photoreceptor excitations (short (S), medium (M) and long (L) wavelength sensitive). The third row simulates the low spatial resolution of a bee eye corresponding to a distance to the flower (16° angular subtense or 6–9 cm distance) where bees start exploiting chromaticity of floral pattern as a visual cue.
Figure 3.
Colour variation among the whole geographical range of T. sidoides ssp. pinnatifida, including pink, light orange and yellow phenotypes (black circles). Other T. sidoides subspecies (grey circles): (1) T. s. integrifolia, (2) T. s. holosericea and (3) T. s. carnea. Other Turneraceae (white circles): (1) T. grandiflora, (2) T. orientalis, (3) T. krapovickasii, (4) T. grandidentata and (5) Piriqueta morongii. The colour loci of their petal reflections are represented in the perceptual colour space of bees (RNL model; Vorobyev et al. 2001).
Petals of T. sidoides were longer than those of its presumed models in both studied populations. In Córdoba, T. sidoides petal length (±s.d.) was 12.3±1.6 mm (n=33) and S. cordobensis petal length was 10.2±1.1 mm (n=25; t-test: d.f.=56, t=−5.78, p<0.0001). In Salta, T. sidoides petal length was 13.8±1.9 mm (n=50) and M. malvifolium petal length was 11.3±1.4 (n=45; t-test: d.f.=93, t=−7.48, p<0.0001; differences with the much smaller flowers of M. coromandelianum and S. rhombifolia not shown). Thus, T. sidoides does not resemble its malvaceous models in petal size, which could potentially enable the bees to discriminate it.
(b) Pollinators and behaviour
The major pollinators captured in Córdoba from mixed populations of T. sidoides and S. cordobensis were solitary Emphorini bees of the genera Diadasia (two species) and Leptometriella (one species), and Colletidae bees of the genus Leioproctus (three species). These six species represented more than 85% of all the captured bees. We found pollen of either T. sidoides or S. cordobensis on all the captured bees, and pollen of both species was found on the majority of them (45 of 63). Pollen of T. sidoides was present in the scopae of all bee species, showing that bees otherwise specialized on malvaceous pollen actively collected its pollen (table 1).
Table 1.
Pollen carried by pollinator species: mean percentage of pollen types in the scopae and on the ventral part of the body of 63 bees captured on T. sidoides or S. cordobensis.
| species | pollen load | n | S. cordobensis | other Malvaceae | T. sidoides | other plants |
|---|---|---|---|---|---|---|
| Diadasia spp. | scopae | 3 | 87.30 | 9.23 | 0.07 | 3.40 |
| body | 12 | 67.75 | 2.71 | 9.33 | 20.21 | |
| Leptometriella separata | scopae | 17 | 92.73 | 0.15 | 7.00 | 0.12 |
| body | 24 | 85.75 | 1.16 | 12.99 | 0.09 | |
| Leioproctus sp. 1 | scopae | 1 | 25.00 | 0.00 | 75.00 | 0.00 |
| body | 8 | 65.48 | 0.00 | 27.76 | 6.76 | |
| Leioproctus sp. 2 | scopae | 7 | 88.18 | 3.85 | 7.98 | 0.00 |
| body | 7 | 87.71 | 4.00 | 8.29 | 0.00 | |
| Leioproctus sp. 3 | scopae | — | — | — | — | — |
| body | 2 | 29.72 | 0.00 | 70.28 | 0.00 | |
| other hymenoptera | scopae | 5 | 47.02 | 1.25 | 37.27 | 14.46 |
| body | 10 | 54.16 | 0.63 | 35.28 | 9.93 | |
| all species | scopae | 33 | 82.29 | 13.23 | 1.92 | 2.56 |
| body | 63 | 73.17 | 19.01 | 1.50 | 6.32 |
Flowering phenologies were significantly correlated between both plant species (r=0.83; d.f.=4; p=0.04). Lifespan of T. sidoides flowers, which extended from 9.00 to 13.00, was completely included within the period of time between flower opening (approx. 9.00) and closure (20.00) of S. cordobensis. Bees could, thus, visit flowers of both species simultaneously.
The bees observed during choice tests in the Córdoba population belonged mainly to the genera Diadasia, Leptometriella and Leioproctus (191 of 200 bees). Frequencies of approaches did not differ significantly from the expected 0.5 proportion for each plant species (53 approaches to T. sidoides; 45 approaches to S. cordobensis; G=0.654; p=0.4, n.s.). Frequencies of landings did differ significantly from this expected proportion, with bees more frequently visiting flowers of T. sidoides (landings on T. sidoides=70; landings on S. cordobensis=33; G=14.504; p=0.0001).
In mixed populations of the yellow-flowered phenotype of T. sidoides and M. malvifolium from the Salta province, we recorded a total of 111 visits. Both species were predominantly visited by Microthurge sp. (Megachilidae, Lithurgini), which accounted for 88% of the visits to T. sidoides and 96% of visits to M. malvifolium. Visitation rates for both species were similar (4.86 visits h−1 per flower for T. sidoides and 4.80 visits h−1 per flower for M. malvifolium). We found that pollen loads on the stigmas of T. sidoides contained malvaceous pollen (6.40±16.48%, n=56) and this represented the main heterospecific pollen type.
(c) Nectar
Nectar concentration differed significantly in the Córdoba community between mimics and models (Mann–Whitney U-test: U=32; p<0.001), with concentration in S. cordobensis (36.79±8.25 Brix %±s.d., n=15) being nearly twice as high as that of T. sidoides (20.97±10.62 Brix %±s.d., n=15). However, this was compensated for by T. sidoides having a much higher nectar volume provided per flower (2.82±1.43 μl±s.d., n=28), approximately twice as much as in S. cordobensis flowers (1.47±0.99 μl±s.d.; Mann–Whitney U-test: U=312.5; p<0.001). Thus, the net reward in terms of sugar amount was similar between flowers of both species.
(d) Pollination service and reproductive success
Patch composition (mixed versus single species) significantly accounted for differences in the mean number of conspecific pollen grains on T. sidoides stigmas. The mean number (±s.d.) of conspecific pollen grains was 32.72±2.6 (n=4) in mixed patches and 25.07±6.0 (n=8) in single species patches (t-test: d.f.=10, t=2.39, p=0.038). This indicates that pollinator visitation of T. sidoides was more efficient in mixed patches. In contrast, patch composition did not account for variation in fruit set, as a measure of reproductive success, neither in T. sidoides (mixed patches=0.21±0.13 fruits/flowers, n=9; single species patches=0.22±0.15 fruits/flowers, n=15; t-test: d.f.=22, t=−0.177, p=0.861) nor in S. cordobensis (mixed patches=0.44±0.14 fruits/flowers, n=11; single species patches=0.54±0.06 fruits/flowers, n=6; t-test: d.f.=15, t=−1.68, p=0.114). Thus, fruit set is not significantly affected when both species simply grow together. However, such comparison did not include the effect of different flower densities among single and mixed patches on fruit set. Regression models showed that fruit set of T. sidoides in mixed patches is not explained by its own flower density but that it is related instead to S. cordobensis flower density (table 2). In single species patches of T. sidoides fruit set is not related to flower density (R2=0.01, P=0.696, n=15), showing a lack of density dependence. S. cordobensis fruit set in mixed patches is better explained by its own flower density; and it is not affected by T. sidoides flower density (table 2). This can be explained by differences in relative abundances: in mixed patches, S. cordobensis is clearly the more common plant, presenting in average 41.24±34.46 s.d. times more flowers than T. sidoides. These results indicate that T. sidoides obtained better pollination services and reproductive success in mixed patches.
Table 2.
Relationship between density and reproductive success. (Multiple regression models showing the effect of flower densities on fruit set in nine mixed patches. *p<0.05, **p<0.01.)
| dependent variable | partial regression coefficients | model | |||
|---|---|---|---|---|---|
| ln (T. sidoides density) | ln (S. cordobensis density) | F2,6 | p | R2 | |
| T. sidoides fruit set | 0.0709 | 0.213* | 4.401 | 0.067 | 0.595 |
| S. cordobensis fruit set | 0.2101 | 0.384** | 18.945 | 0.003 | 0.863 |
As S. cordobensis was much more common, its pollen appeared on most T. sidoides stigmas (121 of 142 flowers). No T. sidoides pollen was found on S. cordobensis stigmas. However, the percentage of improper pollen deposited on T. sidoides stigmas was low (21.36±17.29%, n=142), considering the much higher proportion of S. cordobensis flowers in mixed patches. Also, heterospecific pollen did not affect fruit production since no significant difference was evident in the percentage of heterospecific pollen found on the stigmas of fruiting and non-fruiting flowers of T. sidoides (t-test; d.f.=140; t=−0.41; p=0.68; fruiting flowers: 17.08±16.14%, n=88; non-fruiting flowers: 16.37±15.84%, n=54).
4. Discussion
The following evidence supports the view that T. sidoides ssp. pinnatifida and co-occurring malvaceous species are associated with floral mimicry relationships. First, flower colour and colour patterns of these plants are indistinguishable to bees, according to the predictions of the bee colour vision model and the frequency of approaches to each species in choice tests. Bees landed more often on T. sidoides, evincing partial discrimination between mimics, possibly due to petal size differences, but finally benefiting the mimic. Second, geographical colour variants of T. sidoides matched the different local malvaceous species in colour. Third, pollinators are able to freely move between mimics because flower phenologies are synchronized and the lifespan of the flowers of one species is completely included in that of the other. Fourth, individual pollinators were shared between plant species. Fifth, female reproductive success of at least one colour phenotype of T. sidoides increased with the mallow flower density, and pollinator services are higher when this mallow is present than when it is absent. This shows that at least one of the species obtained a reproductive advantage, such as enhanced pollen loads, when growing in mixed patches. Both T. sidoides and co-occurring mallows offer nectar to pollinators. In one population where nectar reward was measured, the profitability as food source in terms of net sugar amount per flower was similar among mimicry partners.
(a) Effects of patch composition on reproductive success
Flower mimicry should lead to a higher fitness for the plant species involved. Here we found that only the T. sidoides mimic benefits while S. cordobensis is neither favoured nor harmed. The observations that T. sidoides plants have larger pollen loads on their stigmas when growing together with S. cordobensis constitutes evidence of facilitation of pollination. However, this effect is not translated into an increase of T. sidoides fruit set. Patch composition per se was not a significant factor for T. sidoides success in terms of fruit set, but success was associated with S. cordobensis flower density in mixed patches. Since we did not find any evidence of density dependence from its own flower density, reliability of pollination for T. sidoides seems to be secured by establishing a mimicry relationship with S. cordobensis. Thus, when the mallows are present, pollination success is ensured. Whereas when mallows are absent, pollination services are in general poor as shown by pollen loads on the stigmas originating from single patches, and reproductive success in terms of fruit set is subject to chance. Nevertheless, in our study, T. sidoides still achieved the same mean proportion of fruits with and without mallows. The number of pollen grains deposited on the stigmas of a flower might not necessarily correlate with the number of fruits produced, but with the number of seeds per fruit (Quesada et al. 2001). However, this could not be measured in this study. It could thus well be that the number of seeds per fruit differed and hence also the reproductive success differed for T. sidoides in the presence or absence of mallows. Pollen limitation may well affect seed set in this plant, because it is self-incompatible and heterostylous, but experiments to establish the degree of pollen limitation on seed and fruit set remain to be done.
We have shown that pollination services strongly depend on the presence of resident bees specialized in Malvaceae as pollen hosts. These bees also collected pollen from T. sidoides. Thus, isolated T. sidoides patches might not have enough flowers to maintain a resident population of these bees, and would be therefore visited by them only occasionally during flights between mallow patches and less frequently each day when compared with T. sidoides plants growing in mixed patches. This is a further fact supporting our conclusion that pollination is more reliable for T. sidoides in the presence of malvaceous models.
(b) Covariation in flower colour between mimicry partners
We attribute differences in flower colour among geographical races of T. sidoides ssp. pinnatifida to the use of different mallow species as models. We suggest that floral colour variation among populations of T. sidoides is adaptive and that, through colour matching with locally prevalent Malvaceae, these plants engage in different geographical mimicry rings, whose members share a distinctive colour within each community. Such covariations of mimic and model floral traits have also been reported for Batesian mimicry systems: Disa ferruginea (Johnson 1994), in which colour races mimic different models, and Disa nivea (Anderson et al. 2005), in which flower morphology covaries with its model.
Our results suggest that the similarity pattern can best be attributed to evolution of mimic–model system rather than to flower syndromes (the convergent evolution of flowers to meet the sensory preferences of pollinators). First, the convergent evolution hypothesis cannot account for the geographical variation observed in flower colour within T. sidoides ssp. pinnatifida. Second, the light orange and yellow colours of T. sidoides are not common among co-flowering plants of their native communities, except for malvaceous species, suggesting adaptivity of its colour polymorphism rather than just chance. Third, these colours are unique to pinnatifida among subspecies of T. sidoides (Solís Neffa et al. 2004) and probably represent apomorphies of this subspecies. The colour polymorphism found in T. s. pinnatifida contrasts with the rather conserved distribution of petal colours along phylogenetic lines in Turneraceae (Truyens 2005), suggesting that it is maintained through selection towards mimicry with malvaceous models in different geographical regions.
(c) Partial discrimination between model and mimic benefits the mimic
Flower colour of the mimics was indistinguishable from the mallows according to the bee vision model and bees did not prefer any of the mimics when approaching. However, bees preferred the rare mimic T. sidoides when landing, suggesting that there are some short-distance cues that mediated the landing response and allowed the bees to partly distinguish the flowers. Such short-distance cues could be either visual or olfactory (Dobson & Bergström 2000). Flowers of T. sidoides and the malvaceous models are odourless to the human nose, but could produce volatile chemicals detectable by their insect pollinators. This aspect could not be analysed in the present work. However, olfactory mimicry was not found in the only food-deceptive mimicry system studied so far in this respect (Galizia et al. 2005). Considering that in several cases multiple biochemical pathways are involved in fragrance production, accurate strategies for plants to mimic each other are to be scentless or to display high odour variability. Mimics do not need to produce odours if they grow near scented models, as odour acts as a diffuse and long-distance signal. Mimics with weak odour cues cannot be the subject of inhibitory learning by flower visitors and consequently are not easily avoided (Kunze & Gumbert 2001; Galizia et al. 2005).
Flower size could be a visual cue for bees to distinguish between mimics, since T. sidoides flowers are larger on average than those of the proposed models. If large flower size is a facilitating cue for landing decisions, more frequent landings would be on the larger T. sidoides, although the model and the mimic are not distinguishable by colour. Indeed, some authors report a higher visitation rate to large than to small flowers of the same species (see Blarer et al. 2002 and references therein). In our present case, an interesting question for further investigation is whether selection towards larger flowers is acting in either of the two plant species. For T. sidoides, this selective pressure should be stronger since an increased attractiveness might be needed to divert pollinators from the more abundant mallows.
(d) Involved pollinators are pollen specialist among solitary bees
Mimicry between T. sidoides and S. cordobensis is remarkable for the pollinator species involved. In the light orange phenotype populations, the main pollinators found, i.e. Diadasia and Leptometriella, are known as specialists on malvaceous plants as pollen sources (Sipes & Tepedino 2005). Specialist bees' preferences for pollen hosts are genetically constrained, independently of the relative abundance of alternative floral resources (Wcislo & Cane 1996). However, these bees are not specialized in their use of nectar sources (Wcislo & Cane 1996), although both types of food are available on the same plants. Among the bees captured on T. sidoides and S. cordobensis, most carried pollen of both species in the scopae and on the body. While Diadasia carried a quite small percentage of T. sidoides in the scopae, Leptometriella separata appeared with a considerable proportion of T. sidoides pollen in the scopae. Furthermore, the involvement of Leioproctus bees is intriguing. To our knowledge, there are no studies on behaviour of Leioproctus or their specialization to pollen hosts. Thus, it is still unclear whether the similar and high proportion of S. cordobensis and T. sidoides pollen carried by these bees reflects an innate specialism or a circumstantial preference due to local and temporal abundance of these plants. The main pollinator found in the Salta population of yellow T. sidoides, Microthurge sp., is shared by both plant species, but records on its pollen preferences are rare. We only have a report of a Microthurge species from Brazil visiting Malvaceae flowers (Gaglianone 2000). The two remaining genera of Lithurgini bees also include specialist of Malvaceae as pollen hosts (Michener 2000). Importantly, all these solitary bees and pollen specialists are basic elements of the mimicry relationship, since we found that pollen loads on the stigmas of T. sidoides in all populations investigated contained malvaceous pollen. They reliably fulfilled the condition that to maintain mimicry, the same individuals must regularly visit flowers of both species and move freely between them.
(e) Ecology and evolution of floral Müllerian mimicry
In the populations of the light orange phenotype, the model and the mimic are equally profitable to pollinators, suggesting that the mimicry system should be classified as Müllerian. However, two important aspects are more similar to what is expected for a Batesian mimicry system. First, only one partner obtained a reproductive benefit from flower similarity. Second, advergent evolution (Johnson 1994; Johnson et al. 2003a) seems more likely in the present case, i.e. species would have evolved not to mutual similarity, but to greater similarity of one to the other.
Sphaeralcea cordobensis represented the major food source for the involved pollinators owing to its abundance. So it is not surprising that a unilateral adaptation in flower colour of the co-flowering T. sidoides would result in a higher recruitment of bees. The investigated mimicry system is probably maintained because recruited bees consistently forage on a reliable and rich food source that is clearly distinguishable in colour from other co-flowering species in the community. The fact that rare plants may have an advantage in pollination by their flowers being similar to more common ones has been pointed by several authors (Thomson 1983; Waser 1983). For T. sidoides ssp. pinnatifida, which forms low-density populations, a selective pressure would favour colour similarity to the more common mallows, if it could not enhance its reward to ensure a reliable visitation while keeping a different colour (Feinsinger 1983; Gumbert et al. 1999).
The term quasi-Batesian has been proposed for flower mimicry systems where a rewarding species is not common enough to induce foraging constancy by pollinators and hence imitate the signals of more common plants (Johnson et al. 2003a). Mimicry cases of Müllerian type, in which both the model and the mimic are equally rewarding, are less studied than those of Batesian ones in flowers (Little 1983; Roy & Widmer 1999). In most studies, similarity in flower colour, flower shape, pollinator assemblages, nectar rewards, phenology and geographical distribution of plants has been reported (Brown & Kodric-Brown 1979; Bierzychudek 1981; Schemske 1981; Powell & Jones 1983; Dafni et al. 1990). However, as far as we are aware, this is the first report of an advantage in pollination services for a rewarding floral mimic. It has been argued that pollinator sharing by a mimetic species pair carries the problem of improper pollen transfer onto the stigmas, which would impair fruit set and entail a selective factor impeding mimicry (Roy & Widmer 1999). The low amount of improper pollen found on T. sidoides stigmas suggests that a strong mechanism for avoiding stigma clogging with heterospecific pollen must be operating. Future work should determine whether the comb-like structure and the stickiness of T. sidoides stigma are responsible for this mechanism. In addition, heterospecific pollen at the levels found in this study did not seem to impair fruit production of T. sidoides: at least in this model system, there does not appear to be any inhibitory effect of improper pollen.
In the present study system, the key objection against its classification as Müllerian mimicry comes from the advergent pattern of evolution. We argue that pure convergent evolution as proposed for Müllerian mimicry can only happen when both mimicry partners are found in similar densities, which is probably not a common situation. Communities with an abundant model and one or several rare mimics are probably more common. In such circumstances, rare plants could have evolved towards higher similarity with the abundant model, as the present study case exemplifies. Proposed cases of Müllerian flower mimicry should be revised in the light of present results to revaluate the conceptual gap between true Müllerian and Batesian systems to fully understand the evolutionary origin of similarity between flowers.
Acknowledgments
We thank Arturo Roig-Alsina for bee identification, Viviana Solís Neffa for help in locating some T. sidoides populations and providing access to the living collection of IBONE, Randolf Menzel for support and Misha Vorobyev for help with flower imaging. We are grateful to Amots Dafni for helpful comments on earlier versions of the manuscript and Robert A. Harris for help with English. A.A.C. and S.B.V. are a CONICET researcher and fellowship holder, respectively. N.H.I., A.M.W. and A.A.C. were supported by the DAAD.
References
- Anderson B, Johnson S.D, Carbut C. Exploitation of a specialized mutualism by a deceptive orchid. Am. J. Bot. 2005;92:1342–1349. doi: 10.3732/ajb.92.8.1342. [DOI] [PubMed] [Google Scholar]
- Bierzychudek P. Asclepias, Lantana and Epidendrum: a floral mimicry complex? Biotropica. 1981;13:54–58. doi:10.2307/2388070 [Google Scholar]
- Blarer A, Keasar T, Shmida A. Possible mechanisms for the formation of flower size preferences by foraging bumblebees. Ethology. 2002;108:341–351. doi:10.1046/j.1439-0310.2002.00778.x [Google Scholar]
- Brown J.H, Kodric-Brown A. Convergence, competition, and mimicry in a temperate community of hummingbird-pollinated flowers. Ecology. 1979;60:1022–1035. doi:10.2307/1936870 [Google Scholar]
- Chittka L. Does bee colour vision predate the evolution of flower colours? Naturwissenschaften. 1996;83:136–138. doi:10.1007/s001140050263 [Google Scholar]
- Dafni A. Mimicry and deception in pollination. Annu. Rev. Ecol. 1984;15:259–278. doi:10.1146/annurev.es.15.110184.001355 [Google Scholar]
- Dafni A. Floral mimicry–mutualism and unidirectional exploitation of insects by plants. In: Southwood T.R.E, Juniper B.E, editors. The plant surface and insects. Edward Arnold; London, UK: 1986. pp. 81–90. [Google Scholar]
- Dafni A, Bernhard P, Shmida A, Ivri Y, Greenbaum S, O'Toole Ch, Losito L. Red bowl-shaped flowers: convergence for beetle pollination in the Mediterranean region. Isr. J. Bot. 1990;39:81–92. [Google Scholar]
- Dobson H.E.M, Bergström G. The ecology and evolution of pollen odors. Plant Syst. Evol. 2000;222:63–87. doi:10.1007/BF00984096 [Google Scholar]
- Feinsinger P. Co-evolution and pollination. In: Futuyma D.J, Slatkin M, editors. Co-evolution. Sinauer; Sunderland, MA: 1983. pp. 283–310. [Google Scholar]
- Feinsinger P. Effects of plant species on each other's pollination: Is community structure influenced? Trends Ecol. Evol. 1987;2:123–126. doi: 10.1016/0169-5347(87)90052-8. doi:10.1016/0169-5347(87)90052-8 [DOI] [PubMed] [Google Scholar]
- Gaglianone M.C. Biologia floral de espécies simpátricas de Malvaceae e suas abelhas visitantes. Biociências. 2000;8:13–31. [Google Scholar]
- Galizia C.G, Kunze J, Gumbert A, Borg-Karlson A.K, Sachse S, Markl C, Menzel R. Relationship of visual and olfactory signal parameters in a food-deceptive flower mimicry system. Behav. Ecol. 2005;16:159–168. doi:10.1093/beheco/arh147 [Google Scholar]
- Goldsmith T.H, Butler B.K. The roles of receptor noise and cone oil droplets in the photopic spectral sensitivity of the budgerigar, Melopsittacus undulates. J. Comp. Physiol. A. 2003;189:135–142. doi: 10.1007/s00359-002-0385-8. doi:10.1007/s00359-002-0385-8 [DOI] [PubMed] [Google Scholar]
- Gumbert A, Kunze J, Chittka L. Floral colour diversity in plant communities, bee colour space and a null model. Proc. R. Soc. B. 1999;266:1711–1716. doi:10.1098/rspb.1999.0836 [Google Scholar]
- Hempel de Ibarra N, Giurfa M, Vorobyev M. Detection of coloured patterns by honeybees using chromatic and achromatic cues. J. Comp. Physiol. A. 2001;187:215–224. doi: 10.1007/s003590100192. doi:10.1007/s003590100192 [DOI] [PubMed] [Google Scholar]
- Jersáková J, Johnson S.D, Kindlmann P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 2006;81:219–235. doi: 10.1017/S1464793105006986. doi:10.1017/S1464793105006986 [DOI] [PubMed] [Google Scholar]
- Johnson S.D. Evidence of Batesian mimicry in a butterfly pollinated orchid. Biol. J. Linn. Soc. 1994;53:91–104. doi:10.1006/bijl.1994.1062 [Google Scholar]
- Johnson S.D, Alexandersson R, Peter Linder H. Experimental and phylogenetic evidence for floral mimicry in a guild of fly-pollinated plants. Biol. J. Linn. Soc. 2003a;80:289–304. doi:10.1046/j.1095-8312.2003.00236.x [Google Scholar]
- Johnson S.D, Peter C.I, Nilsson L.A, Agren J. Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology. 2003b;84:2919–2927. doi:10.1890/02-0471 [Google Scholar]
- Kunze J, Gumbert A. The combined effect of color and odor on flower choice behaviour of bumble bees in flower mimicry systems. Behav. Ecol. 2001;12:447–456. doi:10.1093/beheco/12.4.447 [Google Scholar]
- Little R.J. A review of floral food deception mimicries with comments on floral mutualism. In: Jones C.E, Little R.J, editors. Handbook of experimental pollination biology. Van Nostrand Reinhold; New York, NY: 1983. pp. 294–309. [Google Scholar]
- Menzel R, Steinmann E, De Souza J, Backhaus W. Spectral sensitivity of photoreceptors and colour vision in the solitary bee, Osmia Rufa. J. Exp. Biol. 1988;136:35–52. [Google Scholar]
- Michener C.D. The bees of the world. The John Hopkins University Press; Baltimore, MD: 2000. [Google Scholar]
- Moeller D.A. Facilitative interactions among plants via shared pollinators. Ecology. 2004;85:3289–3301. doi:10.1890/03-0810 [Google Scholar]
- Niggebrügge C, Hempel de Ibarra N. Colour-dependent target detection by bees. J. Comp. Physiol. A. 2003;189:915–918. doi: 10.1007/s00359-003-0466-3. doi:10.1007/s00359-003-0466-3 [DOI] [PubMed] [Google Scholar]
- Peitsch D, Fietz A, Hertel S, Ventura D.F, Menzel R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A. 1992;170:23–40. doi: 10.1007/BF00190398. doi:10.1007/BF00190398 [DOI] [PubMed] [Google Scholar]
- Powell E.A, Jones C.E. Floral mutualism in Lupinus benthamii (Fabaceae) and Delphinium paryi (Ranunculaceae) In: Jones C.E, Little R.J, editors. Handbook of experimental pollination biology. Van Nostrand Reinhold; New York, NY: 1983. pp. 310–329. [Google Scholar]
- Quesada M, Fuchs E.J, Lobo J.A. Pollen load size, reproductive success, and progeny kinship of naturally pollinated flowers of the tropical dry forest tree Pachira quinata (Bombacaceae) Am. J. Bot. 2001;88:2113–2118. doi:10.2307/3558436 [PubMed] [Google Scholar]
- Rathcke B. Competition and facilitation among plants for pollination. In: Real L, editor. Pollination biology. Academic Press; New York, NY: 1983. pp. 305–338. [Google Scholar]
- Roy B, Widmer A. Floral mimicry. A fascinating yet poorly understood phenomenon. Trends Plant Sci. 1999;418:325–330. doi: 10.1016/s1360-1385(99)01445-4. doi:10.1016/S1360-1385(99)01445-4 [DOI] [PubMed] [Google Scholar]
- Schemske D.W. Floral convergence and pollinator sharing in two bee-pollinated tropical herbs. Ecology. 1981;62:946–954. doi:10.2307/1936993 [Google Scholar]
- Sipes S.D, Tepedino V.J. Pollen–host specificity and evolutionary patterns of host switching in a clade of specialist bees (Apoidea: Diadasia) Biol. J. Linn. Soc. 2005;86:487–505. doi:10.1111/j.1095-8312.2005.00544.x [Google Scholar]
- Solís Neffa V. G. 2000 Estudios Biosistemáticos en el Complejo Turnera sidoides L (Turneraceae, Leiocarpae). PhD thesis, Córdoba: Universidad Nacional de Córdoba.
- Solís Neffa V.G, Panseri A.F, Reynoso W, Seijo J.G. Variación en el color de flores y números cromosómicos en en Noroeste del área de distribución de Turnera sidoides (Turneraceae) Bonplandia. 2004;13:117–128. [Google Scholar]
- Thomson J.D. Effect of stand composition on insect visitation in two-species mixtures of Hieracium. Am. Midl. Nat. 1978;100:431–440. doi:10.2307/2424843 [Google Scholar]
- Thomson J.D. Component analysis of community-level interactions in pollination systems. In: Jones C.E, Little R.J, editors. Handbook of experimental pollination ecology. Van Nostrand Reinhold; New York, NY: 1983. pp. 451–460. [Google Scholar]
- Thomson J.D. Effects of variation in inflorescence size and floral rewards on the visitation rates of traplining pollinators of Aralia hispida. Evol. Ecol. 1988;2:65–76. doi:10.1007/BF02071589 [Google Scholar]
- Truyens S, Arbo M.M, Shore J.S. Phylogenetic relationships, chromosome and breeding system evolution in Turnera (Turneraceae): inferences from its sequence data. Am. J. Bot. 2005;92:1749–1758. doi: 10.3732/ajb.92.10.1749. [DOI] [PubMed] [Google Scholar]
- Vorobyev M, Menzel R. Flower advertisement for insect: bees, a case study. In: Archer S.N, et al., editors. Adaptive mechanisms in the ecology of vision. Kluwer Academic Publishers; London, UK: 1999. pp. 537–553. [Google Scholar]
- Vorobyev M, Osorio D. Receptor noise as determinant of colour thresholds. Proc. R. Soc. B. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. doi:10.1098/rspb.1998.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyev M, Kunze J, Gumbert A, Giurfa M, Menzel R. Flowers through the insect eyes. Isr. J. Plant Sci. 1997;45:93–102. [Google Scholar]
- Vorobyev M, Brandt R, Peitsch D, Laughlin S.B, Menzel R. Colour thresholds and receptor noise: behavior and physiology compared. Vision Res. 2001;41:639–653. doi: 10.1016/s0042-6989(00)00288-1. doi:10.1016/S0042-6989(00)00288-1 [DOI] [PubMed] [Google Scholar]
- Waser N. Competition for pollination and floral character differences among sympatric plant species: a review of evidence. In: Jones C.E, Little R.J, editors. Handbook of experimental pollination ecology. Van Nostrand Reinhold; New York, NY: 1983. pp. 277–293. [Google Scholar]
- Wcislo W.T, Cane J.H. Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stores foods by natural enemies. Annu. Rev. Entomol. 1996;41:257–286. doi: 10.1146/annurev.en.41.010196.001353. [DOI] [PubMed] [Google Scholar]