Abstract
Target-derived factors modulate many aspects of peripheral neuron development including neuronal growth, survival, and maturation. Less is known about how initial target contact regulates changes in gene expression associated with these developmental processes. One early consequence of contact between growing sympathetic neurons and their cardiac myocyte targets is the inhibition of neuronal outgrowth. Analysis of neuronal gene expression following this contact revealed coordinate regulation of a bone morphogenetic protein (BMP)-dependent growth pathway in which basic helix-loop-helix transcription factors and downstream neurofilament expression contribute to the growth dynamics of developing sympathetic neurons. BMP2 had dose-dependent growth promoting effects on sympathetic neurons cultured in the absence, but not the presence, of myocyte targets, suggesting that target contact alters neuronal responses to BMP signaling. Target contact also induced the expression of matrix Gla protein (MGP), a regulator of BMP function in the vascular system. Increased MGP expression inhibited BMP-dependent neuronal growth and MGP expression increased in sympathetic neurons during the period of target contact in vivo. These experiments establish MGP as a novel regulator of BMP function in the nervous system, and define developmental transitions in BMP responses during sympathetic development.
Keywords: Sympathetic neuron, Bone morphogenetic protein, basic helix-loop-helix transcription factor, Matrix Gla protein, Cardiac myocyte, Target contact, Microarray
Introduction
Proper function of the nervous system depends on the appropriate generation and growth of neurons, and on the formation, maturation and modulation of synaptic circuits. Neurons are guided to their targets by the coordinate actions of cell surface attractive and repulsive signals from neuronal and non-neuronal cells and by soluble and cell-surface signals derived from the targets of innervation (O’Leary et al., 1991; Shiga et al., 1997; Xu et al., 2000; Ledda et al., 2002; Haase et al., 2002). Once neurons recognize their appropriate targets, specific neuron-target contacts are established that involve modulation of neurite growth dynamics and the formation of functional synaptic connections (Baird et al., 1996; Mason et al., 1997; Manzini et al., 2006). Developing sympathetic neurons innervate remote targets that include the heart, pineal gland and sweat pad, and factors derived from these targets regulate neuronal growth, neurotransmitter phenotype and synaptic properties (Bharmal et al., 2001; Lockhart et al., 2000; Rowe and Parr, 1980; Cowen, 1996; Stevens and Landis, 1988; Schotzinger et al., 1994). In particular, sympathetic innervation of cardiac tissue has been well-characterized at the morphological and functional levels (Nobin 1997; Lockhart et al., 1997; Lockhart et al., 2000; Carrio 2001), providing an excellent system to examine the initial responses of developing neurons to target contact during development.
Developmentally, neuron-target interactions induce physiological and morphological changes in both neurons and targets in the central and peripheral nervous systems. One of the most distinctive features of neuron-target interactions is the presence of target-derived growth inhibitory signals for growing axons (Lockhart et al., 2000, Porter et al, 1995, Zhang and Mason, 1998). For example, in the CNS, pontine neurite extension is specifically reduced when mossy fibers reach their granule neuron targets (Baird et al., 1992), and ephrins expressed at appropriate thalamic axon targets reduce axonal extension and promote branching density (Mann et al., 2002). At the neuromuscular junction (NMJ) axonal growth of ciliary ganglion motoneurons is inhibited by s-laminin, a component of the synaptic basal lamina in the NMJ (Porter et al., 1995), and by the muscle-specific tyrosine kinase (MuSK) and agrin (Dimitropoulou and Bixby, 2005). In the sympathetic nervous system, the morphology of superior cervical ganglion (SCG) neurons is regulated by targets that include smooth muscle, salivary glands and heart cells (Yawo 1987; Voyvodic 1987; Lockhart 1997). However, the signaling pathways that contribute to target-dependent growth arrest are poorly understood.
Developing rat superior cervical ganglion (SCG) neurons extend postganglionic axons by embryonic day 12 (E12), interacting with sympathetic targets as early as E15 (Rubin 1985a, 1985b). These neuron-target interactions can be recapitulated and examined in vitro as co-cultures of embryonic or neonatal sympathetic neurons and cardiac ventricular myocytes (Conforti et al., 1991; O’Lague et al., 1978). In co-cultures, neonatal sympathetic neurons extend their processes, interact with target cells and form functional synaptic connections onto cardiac myocytes within two days of culture (Lockhart et al., 1997; Lockhart et al., 2000; Conforti et al., 1991). One early effect of neuron-myocyte contact is the decreased growth of sympathetic processes, followed by the development of presynaptic specializations (Lockhart et al., 2000).
A number of different target-derived molecules have been implicated in the development and maturation of sympathetic neurons, although the pathways underlying growth arrest are unknown. Target-derived nerve growth factor (NGF) promotes sympathetic neuron survival (Levi-Montalcini and Angeletti, 1963; Chun and Patterson, 1977), enhances arborization (Snider 1988) and, in conjunction with additional myocyte-derived factors, facilitates the formation of sympathetic presynaptic structures (Lockhart et al., 1997; Lockhart et al., 2000). Bone morphogenic proteins (BMPs) also play critical roles in the development of sympathetic neurons. BMPs in the embryonic environment induce sympathetic neuronal differentiation and noradrenergic properties in neural crest cells (Reissmann et al., 1996; Schneider et al., 1999; Shah et al., 1996). BMPs also increase the expression of neurofilaments in developing sympathetic neurons (Pisano 2000; Yabe et al., 2002) and promote dendritic development, suggesting a role for these factors in neuronal growth as well as early differentiation (Lein et al., 1995; Horbinski et al., 2002; Beck et al., 2001; Lein et al., 2002).
Several transcription factors have been implicated downstream of BMP function during sympathetic development, including Phox2b and Phox2a and the bHLH transcription factors MASH 1 and HAND2 (Howard et al., 2000; Ernsberger et al., 2000; Sarkar and Howard, 2006). HAND2 promotes the expression of NF-M as well as other noradrenergic markers including DBH, TH, and SCG10 (Howard et al., 2000) and has recently been shown to selectively regulate the acquisition of a noradrenergic phenotype in developing mouse and zebrafish sympathetic neurons (Morikawa et al., 2007; Lucas et al., 2006). These signaling molecules are likely to be regulated by target-derived factors that control the sympathetic growth dynamics.
We have reported that sympathetic neurons cultured with cardiac myocytes target cells have reduced neurite extension compared to sympathetic neurons cultured alone (Lockhart et al., 2000). Here we have investigated the signaling pathways regulating neurite dynamics following target contact by examining neuronal gene expression changes in response to myocyte contact. We found that a BMP-signaling pathway is downregulated in cultured neonatal rat sympathetic neurons following myocyte contact, resulting in inhibition of BMP-dependent neuronal growth. Inhibition of this pathway results in decreased expression of neurofilaments and neurite extension. Matrix Gla protein (MGP), a small matrix-associated protein, is induced in the neurons by target contact, and acts as a novel regulator of the neuronal BMP signaling pathway. We demonstrate a shift in sympathetic BMP response following target contact, defining a developmental transition during neuronal maturation.
Materials and Methods
Isolation and culture of primary neonatal sympathetic neurons and ventricular myocytes
The SCG and posterior ventricle were dissected from neonatal (postnatal days 1–3) Simonson Albino rat pups (Simonson Labs, Gilroy, CA). Cell cultures were prepared as previously described (Lockhart et al., 1997). The dissected SCG were incubated for 1hr at 37°C in 1.5 mg/ml collagenase (Worthington, Lakewood, NJ) and 5 mg/ml dispase (Gibco BRL, Invitrogen, Carlsbad, CA), triturated with fire-polished glass pipettes, then preplated on a plastic culture dish and incubated at 37°C for 2 hr. Sympathetic neurons were removed and counted while the more adherent Schwann cells and fibroblasts remained on the dish. The dissected cardiac ventricles were incubated for 13 min at 37 C in 1.5 mg/ml collagenase (Worthington, Lakewood, NJ), followed by a 10 min and 32 min incubation with fresh 1.5 mg/ml collagenase. Myocytes were triturated using fire-polished glass pipettes and strained through Nitex Nylon mesh (Tetko, Lancaster, NY). Myocytes were preplated at 37°C for 1 hr 30 min to remove fibroblasts.
Cells were cultured on 35mm plastic dishes coated with 50 μg/ml rat tail collagen (Type I; Collaborative Biomedical Products/Becton Dickinson Labware, Bedford MA). Sympathetic neurons were plated at 10,000 cells per dish and cardiac myocytes were plated at 50,000 cells per dish. For 60mm dishes the number of cells was scaled up proportionally. Cells were grown in 2X MAH food (Lockhart et al., 1997) with 5 ng/ml of NGF to support neuronal survival. Cytosine arabinofuranoside (1 μM; AraC; Sigma) was added to the dishes 24 hr after plating to stop cell division. For the BMP2 dose experiments, 2.5 – 50 ng/ml of recombinant human BMP2 (gift of Wyeth Pharmaceuticals) was added to the cultures.
In some experiments sympathetic neurons were isolated from neuron-myocyte co-cultures by taking advantage of the differential adhesion of neurons and flat cells. The culture dishes were washed with PBS and incubated with 1 mM EDTA in PBS for 2 min in 37°C. The less adherent sympathetic neurons were then removed from the plate by pipetting 1 mM EDTA in PBS over the dish. Rinsed neurons were collected and centrifuged in a clinical centrifuge at 3,000 rpm for 10 min.
Immuno-isolation of embryonic sympathetic precursor cells
Embryonic sympathetic neuroblasts and developing neurons were immuno-isolated from timed pregnant Simonson Albino rats as previously described (Pisano and Birren, 1999). Briefly, the sympathetic ganglia were dissected from E14, E15, or 19 day embryos, enzymatically dissociated in 250 U/ml type 1 collagenase (Worthington, Lakewood, NJ), triturated with fire-polished glass pipettes, filtered through sterile nylon mesh and labeled with the HNK-1 antibody to label neural crest-derived cells. HNK-1-positive cells were isolated by incubation with a secondary antibody conjugated to paramagnetic beads (Miltenyl Biotech), followed by passage over a column held in a magnetic field. Following this enrichment procedure, the isolated population consisted of 80 – 95% HNK-1-positive cells. RNA was isolated as described below, or cells were cultured as described (Pisano and Birren, 1999) and immuno-labeled with an anti-NCAM antibody (5A5, 1:2 dilution, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) for measurement of neurite outgrowth. At E14 and E15 the isolated HNK-1-positive population contains tyrosine hydroxylase-expressing neuroblasts and differentiating neurons that undergo process outgrowth upon plating (Pisano and Birren, 1999). At E19 the isolated cells show an immature neuronal phenotype when plated (Pisano and Birren, 1999).
Transfection of cultured neurons
A Bio-Rad gene gun was used to transfect plasmid-coated gold particles into cultured cells as described previously (Slonimsky et al., 2006). Two micrograms of DNA was used per milligram of gold particles. The plasmids used were pNF314-CD8-GFP (gift of Gary Banker, Oregon Health Sciences University, Portland, OR), human HAND1 and HAND2-pcDNA3.1/Zeo (gift of Kwang-Soo Kim, McLean Hospital and Harvard Stem Cell Institute, Harvard Medical School, Belmont, Massachusetts), human MGP-pcDNA3.1(+) (gift of Kristina Boström, Division of Cardiology, David Geffen School of Medicine at UCLA, Los Angeles, CA), constitutively active BMPR1a & 1b-pCMV5 (gift of Stephen E. Harris, University of Missouri at Kansas City, School of Dentistry) and E12-pMIW III/myc5 (gift of J. E. Johnson, The University of Texas, Southwestern Medical Center). The GFP construct was loaded alone or co-loaded with other constructs onto the gold particles. Previous studies have shown greater than 90% co-expression under these conditions (Slonimsky and Birren, unpublished).
Measurement of total neurite length
Cultures were transfected with the CD8-GFP construct alone or with other constructs 1 day after plating and maintained in culture for 2 days. GFP-positive sympathetic neurons were observed using an Olympus IX-70 inverted fluorescent microscope with a 10X objective lens and imaged using an Orca-ER CCD digital camera (Hamamatsu, Japan) and OpenLab software (Version 3.0.3; Improvision, Lexington, MA). The multiple images for a single neuron were stitched by Adobe Photoshop 5.0 LE. The total length of neurites was measured by ImageJ 1.34s (Wayne Rasband National Institutes of Health, USA) with a NeuronJ plugin.
Microarray screening
Affymetrix Rat Expression Set 230A oligonucleotide arrays with 15,900 probe sets were screened with probes derived from either neurons grown alone, or neurons isolated from neuron-myocyte co-cultures. Total RNA was extracted using the RNeasy mini kit (Qiagen). Eukaryotic probe preparation, hybridization, array washing, staining and scanning were performed according to Affymetrix manuals (Rev. 5). The synthesis of biotin-labeled cRNA was performed using the BioArray High-Yield Transcript Labeling Kit (Enzo Biochem, New York, NY). Following hybridization, GeneChip CEL files were analyzed by dChip 1.3 (DNA-Chip Analyzer). Probe signal values were normalized across samples using dChip’s invariant set method. Summary values for each probe set were calculated using dChip’s PM-MM difference model. Expression patterns for sympathetic neurons cultured alone were compared with neurons cultured with myocytes. Differentially expressed genes with >1.3 fold change were selected for further analysis. This low cut-off was chosen to allow us to carry out independent verification of candidate genes that might contribute to developmental pathways with relatively small changes in expression levels. In fact, we found that 12 out of 14 these candidates showed a 1.3 or greater fold change in real-time PCR analysis.
Real-Time PCR
RNA was prepared from cultures using the RNeasy mini kit (Qiagen). RNA was reverse transcribed into cDNA using MMLV-reverse transcriptase (Gibco BRL) and random hexamer primers (Lockhart et al., 2000). Real-time PCR was performed using a Rotor-Gene 3000 (Corbett Research). Primer sets were designed using the Whitehead Genome Center Primer3 Program (Table 1). additional primer sets used in this study were: BMP2 5′-AGA CCA CCG GCT GGA GAG-3′, 5′-TGA GAA ACT CAT CAG TAG GGA CA-3′, BMP4 5′-GAG GAG GAA GAA GAG CAG AGC-3′, 5′-GGG ATG CTG CTG AGG TTA AA-3′, BMP7 5′-GTG AGG GAG AGT GTG CCT TC-3′, 5′-AGT AGA GGA CAG ATA TCG CGT TG-3′, BMPR1a 5′-TCG TCG TTG TAT TAC AGG AG-3′, 5′-TTA CAT CCT GGG ATT CAA CC-3′, BMPR1b 5′-TGG AGC AGT GAT GAG TGT CT-3′, TCT GGG TTC CTC TGT GTC TG-3′, BMPR2 5′-CGC AGA ATC AAG AAC GGC TAT G-3′, 5′-TGA ATG AGG TGG ACT GAG TGG T-3′. The 2−ΔΔCT method was used for real-time PCR analysis (Livak and Schmittgen, 2001). For each primer set, PCR reactions were run in triplicate and normalized to the average of triplicate reactions run with GAPDH (glyceraldehyde 3-phosphate dehydrogenase). Data was averaged from a minimum of three independent RNA preparations which did not include the RNA preparation used for microarray analysis.
Table 1.
Verification of microarray screening using real-time PCR. The positive numbers in microarray fold change and real-time PCR fold change indicate the increases in mRNA level in neurons cultured with myocytes. The primers were designed by Primer3 software. The 2−ΔΔCT method was used for real-time PCR analysis and PCR reactions for each gene were normalized to GAPDH expression. Real-time PCR data is shown as the mean of at least 3 experiments ± s.e.m.
| Accession Number | Gene name | Microarray
|
Real-time PCR
|
Primer sequence (5′ to 3′) | Length of amplicon (bp) | ||
|---|---|---|---|---|---|---|---|
| Fold change | Fold Change | S.E.M. | |||||
| NM_031984 | calbindin 1 | −1.53 | −5.33 | 0.01 | Left
Right |
gcacttctgtgctgtttcca
agccagcttgaaccaacagt |
174 |
| NM_022696 | HAND2 protein | −1.59 | −1.43 | 0.11 | Left
Right |
acatcgcctacctcatggat
tggttttcttgtcgttgctg |
162 |
| NM_021592 | HAND1 protein | −1.39 | −2.54 | 0.06 | Left
Right |
caaggatgcacaagcaggt
cagccagtgcgtcctttaat |
162 |
| NM_031050 | lumican | 1.81 | 1.72 | 0.97 | Left
Right |
ttggaagtccaccaggtttc
tggtatgttgcaaatcactgg |
174 |
| NM_012862 | matrix Gla protein | 2.85 | 6.5 | 1.71 | Left
Right |
gccctgatctacgggtacaa
catgtgaggaacaagcaacg |
169 |
| XM_215469 | microtubule-associated protein 1b | −1.45 | 1.54 | 0.71 | Left
Right |
gaccacaccacaggatttga
ggtcggcaaaaattgagaaa |
165 |
| NM_031783 | neurofilament, light polypeptide | −1.37 | −2.24 | 0.12 | Left
Right |
ctcagagctcgcaggtcttt
agctttcgtagcctcaatgg |
152 |
| NM_013191 | S100 calcium-binding protein, beta (neural) | 2.23 | 4.38 | 1.47 | Left
Right |
acaccattgtccccatagga
catctcagtggcccttcatt |
165 |
| AB001382 | secreted phosphoprotein 1 | 1.7 | 2.25 | 0.38 | Left
Right |
tgaggctctcaaggtcatcc
gactgctcagtgctctcgtg |
177 |
| NM_022669 | secretogranin II | −1.72 | −2.86 | 0.05 | Left
Right |
gaagggaacatcttgccaaa
ccccaaaactatctggagtca |
179 |
| NM_019159 | synapsin 2 | −1.65 | −1.46 | 0.19 | Left
Right |
atggcaaagatggcaaagac
gttccactctgtggctgttg |
190 |
| L38247 | synaptotagmin 4 | −1.4 | −1.27 | 0.3 | Left
Right |
cgcagtgttcaacgaactgt
ccaccacttccttctgctgt |
153 |
| U73184 | syndecan 3 | −1.58 | −1.77 | 0.19 | Left
Right |
tcccagctgatgtcagtcac
gacaacccacacagctcctt |
152 |
| NM_012700 | Syntaxin 2 | −1.41 | −2.2 | 0.15 | Left
Right |
tctccctttccttgcctctt
ccgaccatcttgtcctctgt |
151 |
Western blot Analysis
Protein extracts from equal numbers of cells were prepared by diluting in sample buffer (10% glycerol, 50 mM Tris pH 6.8, 2 mM EDTA, 2% SDS, 144 mM 2-mercaptoethanol, 0.01% bromophenol blue), followed by boiling for 5 min. Protein extracts were loaded for SDS/PAGE followed by transfer to Hybond-P membrane (Amersham Pharmacia Biosciences). Monoclonal anti-NF-M (Sigma) 1:500 and monoclonal anti-actin (Sigma) 1:1000 were used as primary antibodies. Horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Amersham Pharmacia Biosciences) 1:2000 was used as a secondary antibody. Proteins were visualized and quantified with chemiluminesence using ECL substrate (Amersham Pharmacia Biosciences) and Bio-Rad Chemi-Doc using Quantity One software.
Immunocytochemistry and analysis
Immunocytochemistry was performed as previously described (Slonimsky et al., 2003). Briefly, monoclonal anti-NF-M (Sigma) 1:500 in 5% donkey serum in PBS was used as a primary antibody and visualized using FITC-conjugated anti-mouse IgG (Jackson Immunoresearch, West Grove, PA) 1:500. For the quantification of NF-M expression, random neurites in each condition were visualized with exposure times that were kept constant across conditions. The image with NF-M staining was cropped to contain same length of single neurite (108X36 pixels). Background subtraction was applied to the cropped images and mean pixel intensity was analyzed by ImageJ 1.34s (Wayne Rasband National Institutes of Health, USA).
Statistics
Significance was analyzed by Student’s t-tests or, for comparisons across multiple conditions, Kruskal-Wallis ANOVA on ranks followed by Dunn’s Method for pairwise comparison using SigmaStat 2.0 (Systat Software, Point Richard, CA).
Results
Contact with cardiac myocytes limits neurite extension of sympathetic neurons
We tested the effect of myocytes on neurite extension of co-cultured sympathetic neurons isolated from neonatal rat superior cervical ganglia (SCG). Sympathetic neurons were cultured alone or co-cultured with myocytes (Fig. 1A) and transfected with EGFP-CD8 to provide fluorescent labeling that extended throughout the neuritic arbor. In some experiments, neurons were co-cultured with CHO cells to provide a cell-based growth substrate that did not include normal targets of sympathetic innervation. Cultures were transfected 24 hours after plating and 24 hours later the entire neuritic arbor was imaged and captured using a cooled CCD camera and OpenLab software (Fig. 1B). The total neurite length of individual transfected cells was measured using Image J software (Fig. 1C). The average total neurite length was significantly shorter for neurons grown in the presence of myocytes compared to neurons grown alone. Neurons co-cultured with CHO cells also had a greater average total neurite length than neurons cultured with myocytes. These data suggest preferential growth of sympathetic neurons on a cell-based substrate and demonstrate that cardiac myocytes provide signals to globally limit the growth of sympathetic fibers. As previously reported, this down-regulation of overall neurite growth is associated with local arborization onto the myocytes and the development of functional synaptic transmission between the neurons and the myocytes (Lockhart et al., 1997; Lockhart et al., 2000), suggesting that contact with myocyte targets triggers a transition from a growth to a maturation developmental program.
Figure 1.
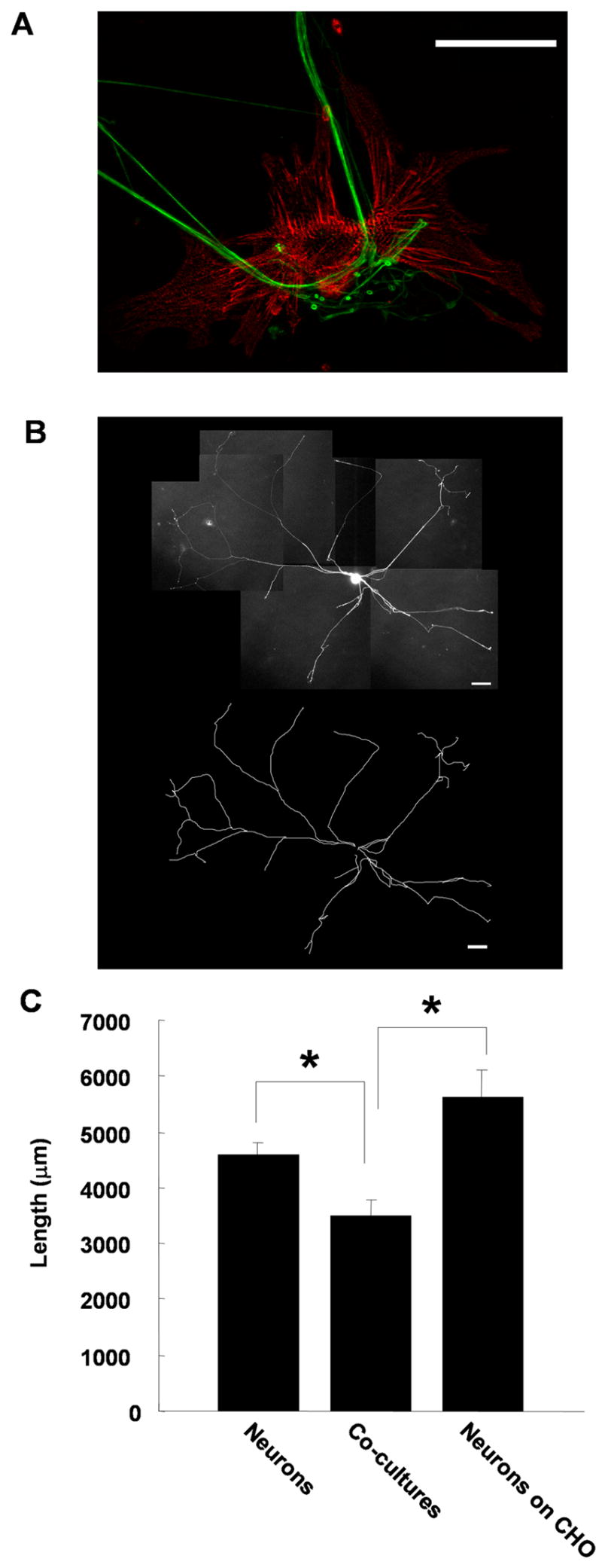
Sympathetic neuron growth is inhibited by contact with co-cultured cardiac myocytes. Neonatal rat sympathetic neurons were cultured alone, with cardiac myocytes or with CHO cells for 2 days. (A) Shows a neuron-myocyte co-culture stained with antibodies against peripherin (green) to stain neuronal fibers and alpha-actinin (red) to stain the cardiac myocyte. Scale bar = 50 μm. Image provided by Jeanine Hinterneder. (B) The neurons were transfected with EGFP-CD8 using a Gene Gun after the first day in culture to label the complete neuritic arbor of a few neurons on the dish and the total process length per neuron was analyzed. An image of EGFP-CD8 transfected neuron is shown with a traced image of the neuronal arbor shown below. Scale bars = 100 μm. (C) A plot of the average neurite length per neuron shows a decrease in total process length for sympathetic neurons cultured with myocytes (Co-cultures) compared to neurons cultured alone or with CHO cells. Data shown is the mean ± s.e.m. of a minimum of 27 transfected cells, p < 0.05.
Regulation of gene expression by target contact
We investigated the molecular basis of the target-regulated growth inhibition program using DNA microarrays to analyze patterns of gene expression following contact of sympathetic neurons with their myocyte targets. Total RNA was isolated from sympathetic neurons cultured alone and from neurons isolated from myocyte co-cultures. These neurons were isolated from co-cultures by taking advantage of the differential adhesion properties neurons and flat cells in culture (see Methods). The two neuron populations showed minimal levels of atrial natriutic peptide (ANF) mRNA, a myocyte marker, compared to myocyte-containing cultures, indicating a low level of myocyte contamination for neurons purified from co-cultures (data not shown).
Isolated total RNA was reverse transcribed, labeled with biotin by in vitro transcription and hybridized to an Affymetrix Rat Expression Set 230A microarray with 15,900 probe sets. The hybridized microarray was washed, stained, scanned and quantified using DNA-Chip Analyzer (dChip) 1.3 (Li and Wong, 2001). We identified 112 known genes and 150 expressed sequence tags (ESTs) showing more than a 1.3 fold change in target-exposed neurons compared to neurons grown in the absence of target. We chose a low cut-off for further validation as relatively small changes in expression of genes involved in developmental pathways could potentially contribute to important developmental transitions. Expression of 36 genes and 49 ESTs was upregulated, while expression of 76 genes and 101 ESTs was downregulated by the presence of target during the culture period (Supplementary Table 1). Overall, these changes represented approximately 1.6% of the probe set. Microarray data can be accessed at http://www.ncbi.nlm.nih.gov/geo/ (Accession numbers: GSE8393, GSM206651, GSM206655).
We verified the expression changes of 14 genes using real-time PCR. The direction of target-induced changes in mRNA levels correlated with microarray analysis with a greater than 1.3 fold change for 12/14 genes tested (Table 1). Several of the genes analyzed, including matrix Gla protein (MGP) and HAND1, showed larger fold changes by real-time PCR compared to the initial microarray result. Interestingly, we found small, but verifiable, decreases in several vesicle-associated proteins, but no increases in expression of genes encoding proteins involved in synaptic function. These data suggest that initial contact between a sympathetic neuron and a myocyte does not act as a trigger for the transcriptional regulation of presynaptic maturation, consistent with our previous study of synaptic maturation of sympathetic neurons (Lockhart et al., 2000).
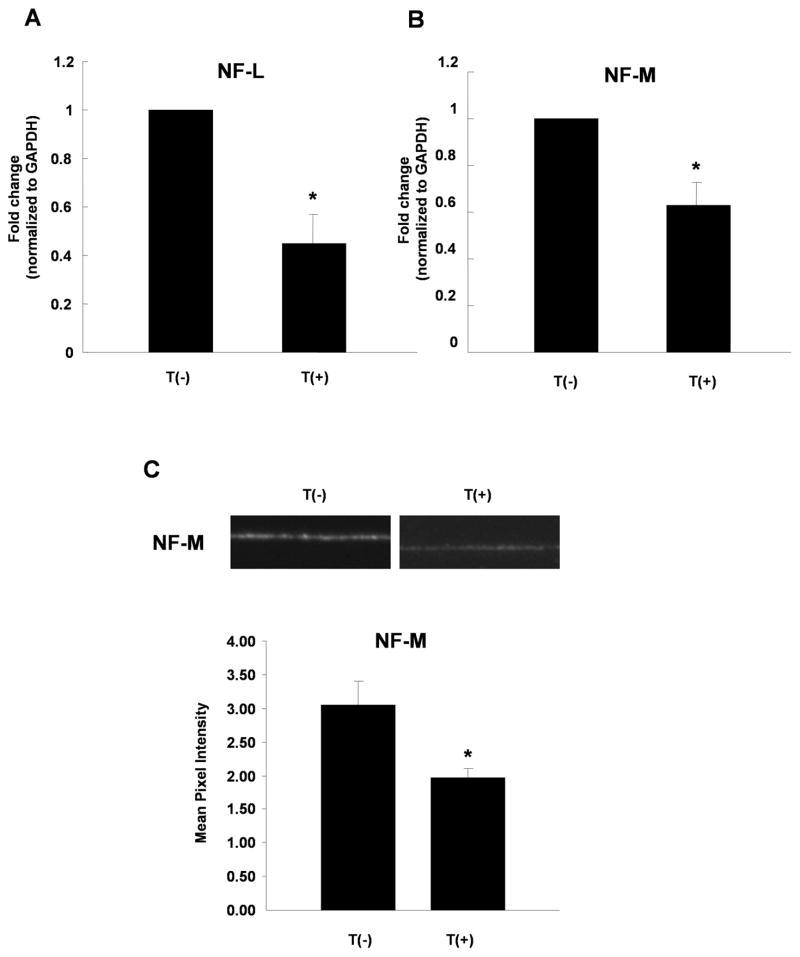
Neurite extension is tightly associated with the expression of neurofilament proteins (Taniwaki and Schwartz, 1995). In addition to decreasing neurite outgrowth, myocyte contact downregulated neurofilament gene expression (Table 1). We further examined the effect of target contact on the expression of mRNAs encoding different neurofilament isoforms using real-time PCR. Both NF-L and NF-M mRNA showed higher expression in neurons grown alone than in neurons grown in the presence of myocytes (Fig. 2A and B). We also asked whether myocyte contact regulated the expression of neurofilament at the protein level by immunostaining neurons grown alone or with myocytes for NF-M. Sympathetic neurons and neuron- myocyte co-cultures were fixed and stained for NF-M following a 2-day culture period. Analysis of matched exposures showed that NF-M was expressed at a lower level in neuronal fibers when myocytes were present (Fig. 2C), suggesting that target-dependent regulation of neurofilaments contributes to changes in the total neurite extension in the presence of myocytes.
Figure 2.
Contact with cardiac myocytes decreases neurofilament expression in sympathetic neurons. Sympathetic neurons were cultured alone, T(−), or were co-cultured with cardiac myocytes, T(+). (A), (B) The mRNA expression levels of NF-L (A) and NF-M (B) were measured by real-time PCR and normalized to GAPDH. Data shown is the mean ± s.e.m. of a minimum of four independent mRNA preparations, p < 0.05. (C) Expression of NF-M protein in sympathetic neurons cultured alone, T(−), or with myocytes, T(+), was analyzed by immunocytochemistry. Cultures were grown for 2 days, fixed and stained with a NF-M antibody. The top panel shows example of images used for quantification, with averaged mean pixel intensity ± s.e.m. of a minimum of 12 images shown below.
Basic helix-loop-helix transcription factors and neurite extension in sympathetic neuron cultures
We identified two bHLH transcription factors, HAND2 (dHAND, Thing2, and Hed) and HAND1 (eHAND, Thing1, and Hxt) as being downregulated by target contact (Table 1). Examination of HAND2 and HAND1 mRNA in neurons cultured alone or co-cultured with myocytes showed that the expression of both genes was significantly decreased by target contact, although HAND1, with a greater than two fold decrease in the presence of target, was down-regulated to a greater extent (Fig. 3A, B). We overexpressed the bHLH proteins in sympathetic neurons to determine whether they participate in the pathways leading to process outgrowth regulation. Neurons were co-transfected with EGFP-CD8 to mark transfected neurons and to label neuronal processes for neurite outgrowth measurements (see Methods). Total neurite length was measured for individual neurons co-expressing EGFP-CD8 and HAND2 or HAND1. Sympathetic neurons overexpressing HAND2 and EGFP-CD8 showed no change in total neurite length compared to neurons expressing EGFP-CD8 alone (Fig. 3C). There was a small, but significant reduction in neurite extension following HAND1 overexpression (Fig. 3D), a surprising result as growth limitation following target contact was correlated with decreased HAND1 expression in our array screen and PCR assays. These data raise the possibility of complex interactions between overexpressed bHLH proteins and endogenous bHLH proteins such as HAND1, HAND2 and the ubiquitously expressed Class A bHLH protein E12 (Firulli, et al., 2003). HAND1 and HAND2 can antagonize the function of other bHLH proteins by competing for E-proteins such as E12 (Bounpheng et al., 2000; Firulli, et al., 2003). Thus, overexpressed HAND proteins might associate with additional bHLH factors involved in process extension, resulting in competition for active complexes. We tested this possibility by overexpressing E12 with EGFP-CD8 and measuring total process outgrowth. E12 overexpression resulted in an increase in total neurite extension compared to control EGFP-expressing neurons (Fig. 3E, F). This suggests that E12 is able to enhance neurite extension by heterodimerizing with Class B bHLH factors, and is consistent with a role for additional bHLH proteins in the target-dependent regulation of neuritic growth.
Figure 3.
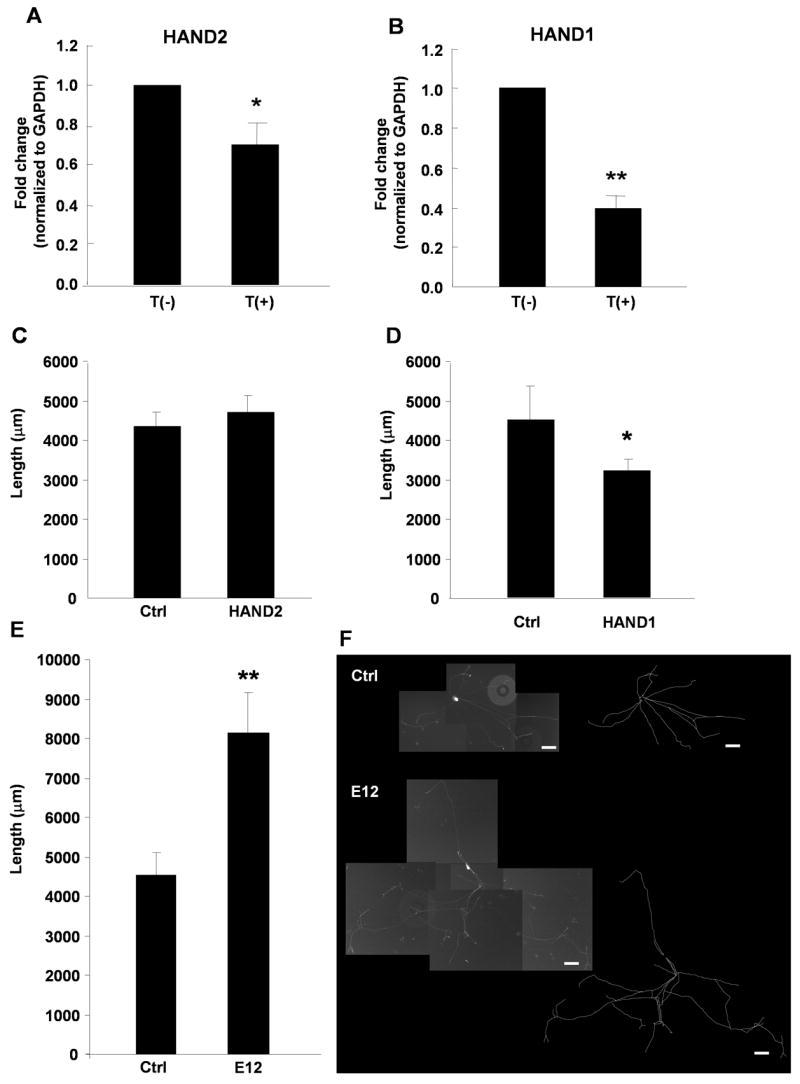
Expression of basic helix-loop-helix (bHLH) transcription factors is regulated by target contact and influence sympathetic neurite extension. (A), (B) Sympathetic neurons were grown in the absence, T(−), or presence, T(+), of myocytes and RNA was isolated after 2 days. The expression of HAND2 (A) and HAND1 (B) mRNA was measured by real-time PCR and normalized to the GAPDH level. Data is shown as the mean ± s.e.m. of a minimum of 8 independent mRNA preparation, * p < 0.05; ** p < 0.01. (C), (D), (E) Sympathetic cultures were co-transfected with EGFP-CD8 and HAND2, HAND1, or E12. In control dishes neurons were transfected with EGRP-CD8 alone (Ctrl). The average neurite length was analyzed for control EGFP-labeled neurons and for neurons co-expressing HAND2, HAND1 or E12. Data is shown as the mean ± s.e.m of a minimum of 13 transfected neurons, *p < 0.05; ** p < 0.01. (F) Images of a neuron expressing EGFP-CD8 (top panel) and a neuron co-transfected with EGFP-CD8 and E12 (bottom panel). Scale bar = 100 μm. Traced arbors are shown to the right of the composite photographs.
BMP2 regulates neurofilament expression in sympathetic neurons cultured without cardiac myocytes
We observed that HAND2, HAND1, and NF, all of which are regulated by BMP signaling in the peripheral nervous system (Schneider et al., 1999; Howard et al., 2000; Wu and Howard, 2002), were coordinately regulated by target contact, raising the possibility of regulation of a BMP-dependent signaling pathway. We investigated the growth effect of BMP2 on cultured sympathetic neurons by measuring the total neurite length per EGFP-labeled neuron. Addition of 10 ng/ml BMP2 to the growth media significantly increased total neurite length in sympathetic neuron cultures (Fig. 4A, B), as did overexpression of constitutively active BMP receptors BMPR1a and BMPR1b (CA-BMPR) (Fig 4C). These data indicate that BMP signaling pathways contribute to the outgrowth of postnatal sympathetic neurons grown in the absence of their cardiac targets. We examined the effect of target contact on BMP2 signaling by analyzing BMP2-dependent changes in neurofilament protein expression. We treated sympathetic neurons grown with or without co-cultured myocytes with BMP2 and extracted total protein following a 2 day culture period. As expected, NF-L protein was significantly higher in neurons grown without myocytes compared to neurons co-cultured with myocytes for neurons grown in the absence of BMP2 (Fig. 4D). BMP2-treatment significantly induced NF-L protein expression in neurons grown alone, however this induction was not seen in neurons cultured with myocytes (Fig. 4D). These data suggest that target contact acts to alter neuronal responses to BMP2, resulting in inhibition of neuritic growth properties.
Figure 4.
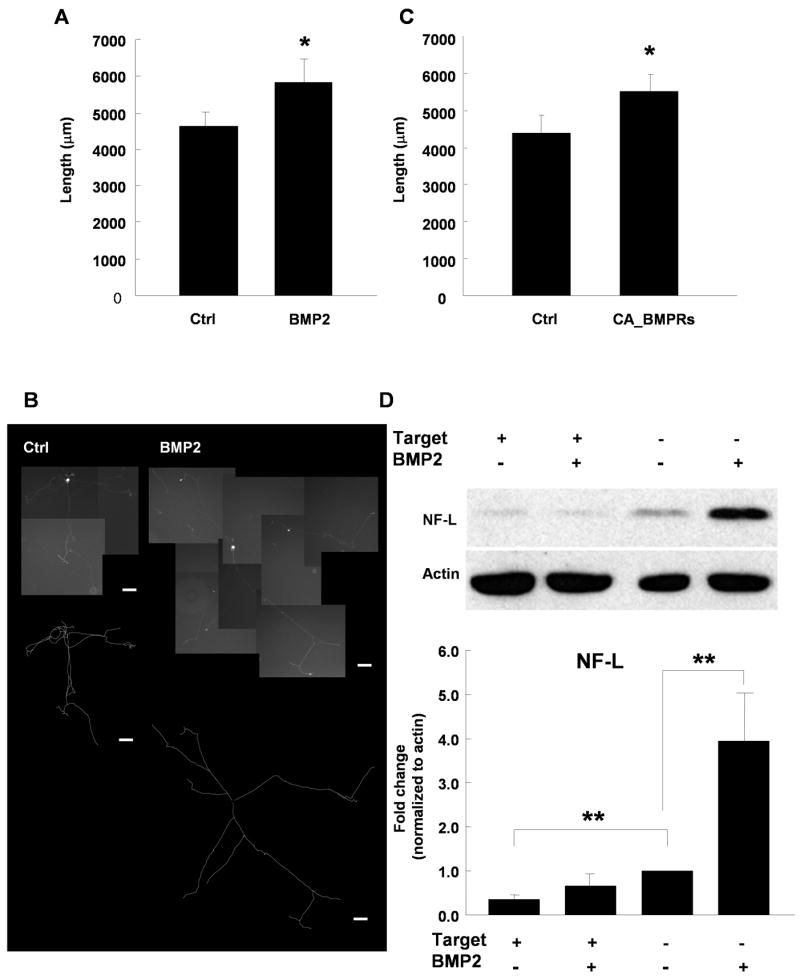
BMP2 regulates the growth of sympathetic neurons in the absence of target. (A) The average neurite length per sympathetic neuron increased for EGFP-labeled neurons grown in absence (Ctrl) or presence of 10 ng/ml BMP2. (B) Images of EGFP-CD8 transfected neurons grown control medium (left panel) and grown with 10 ng/ml BMP2 in medium (right panel). The traced arbors are shown under the composite photographs. Scale bars = 100 μm. (C) Co-expression of constitutively active BMP receptor 1a & 1b with the EGFP label increased total neurite length per neuron. Data is shown as the mean ± s.e.m. of a minimum of 25 transfected neurons, *p < 0.05. (D) Expression of NF-L protein in sympathetic neurons is induced by BMP2 when the neurons were grown in the absence, but not the presence of myocytes. NF-L levels were analyzed by Western blot analysis and normalized to actin. The top panel shows an example of a Western blot, with the averaged data of 4 independent experiments shown below. Data is the mean ± s.e.m., **p < 0.01.
Altered BMP2 response following myocyte contact
The finding that neurons grown with myocytes did not respond to BMP2 with an increase in NF protein suggested that responses to BMP2 changed following target contact. This change could reflect a shift in the dose response, or alternatively, target contact could trigger a developmental change in the neurons resulting in altered BMP responses. We therefore examined the BMP2 dose response for NF-M mRNA expression in the presence and absence of myocytes. In the absence of myocytes, the maximal response for induction of NF-M mRNA was obtained at 2.5 ng/ml BMP2 (Fig. 5A). There was no significant induction of NF-M mRNA at any concentration of BMP2 tested for neurons cultured in the presence of myocytes (Fig. 5B). This suggests that myocyte contact triggers a change in BMP response resulting in the loss of BMP-dependent neurite outgrowth.
Figure 5.
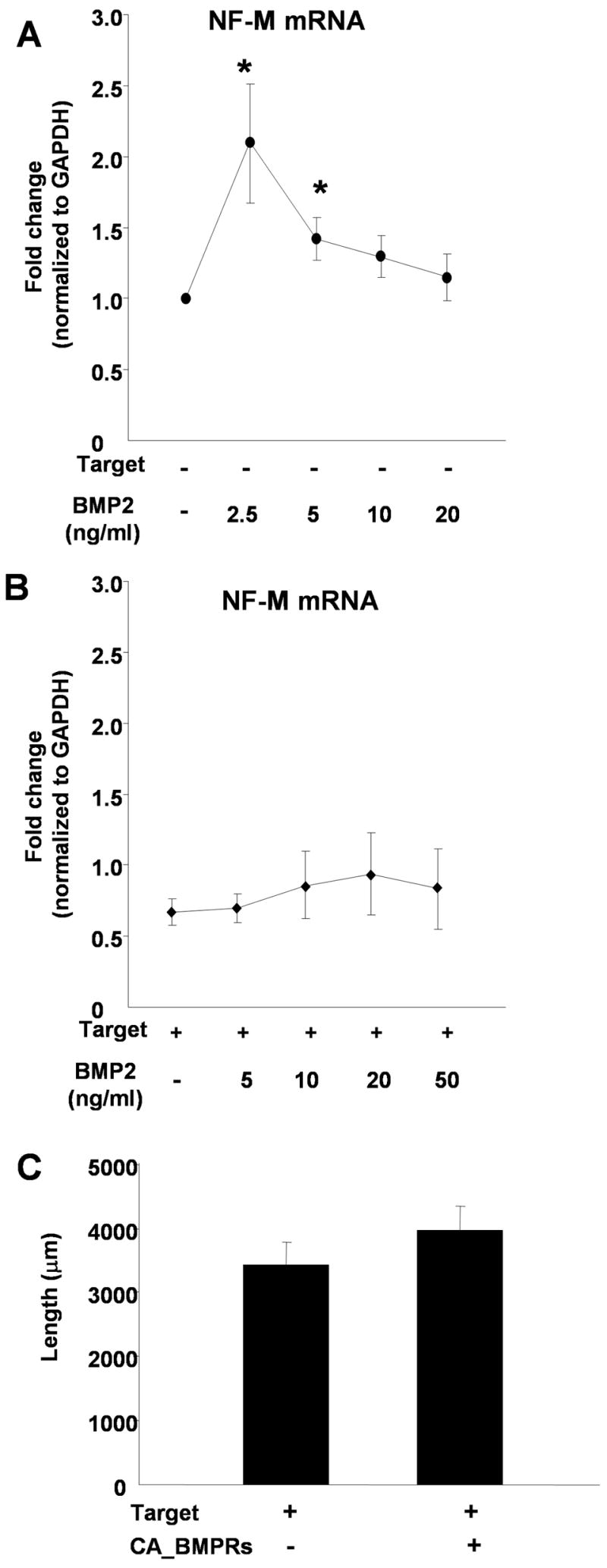
Target contact alters neuronal BMP responses. Sympathetic neurons were cultured alone (A) or with myocytes (B) in different concentrations of BMP2 (0–50 ng/ml). Real-time PCR analysis was used to measure NF-M expression and values were normalized to GAPDH expression levels. (A) BMP2 induced a dose-dependent increase in NF-M mRNA in neurons cultured in the absence of cardiac myocytes. (B) In the presence of co-cultured myocytes, BMP2 does not promote NF-M expression at any concentration tested. Data is shown as the mean of at least 4 experiments ± s.e.m., *p < 0.05. (C) Expression of constitutively active BMP receptors in sympathetic neurons does not increase neurite length for neurons cultured in the presence of myocyte targets. EGFP-CD8 was transfected alone or co-transfected with constitutively active BMP receptor 1a & 1b and the average neurite length of GFP-labeled sympathetic neurons was measured. Data is shown as the mean ± s.e.m. of a minimum of 30 transfected neurons.
We further tested the idea that there was a change in neuron response to BMP2 following myocyte contact by expressing CA-BMPRs in neurons grown in neuron-myocyte co-cultures. There was no significant increase in neurite length in neurons co-expressing CA-BMPRs and EGFP compared to neurons expressing the EGFP construct alone (Fig. 5C), providing further evidence that, following contact with myocytes, BMP signaling no longer promotes the growth of sympathetic neurons.
Regulation of the BMP signal pathway by target contact
One way that target contact might down-regulate a BMP-mediated growth pathway is by reducing the expression of BMPs or BMP receptors in sympathetic neurons. However, we did not observe changes in expression of either BMPs or BMP receptors in our microarray screen. We further examined the expression of BMPs and BMP receptors using real-time PCR on mRNA from neurons isolated from control and myocyte-containing cultures. There was no significant change in the expression of BMP2, BMP4, BMP7, BMPR1a, BMPR1b or BMPR2 mRNA when myocytes were co-cultured with the sympathetic neurons (data not shown).
The lack of BMP or BMP receptor regulation by target contact raised the possibility that myocyte interactions regulated the expression of an inhibitor of BMP signaling. We identified matrix Gla protein (MGP) in our microarray screen as being induced in sympathetic neurons by myocyte contact (Table 1). MGP, a small matrix protein, has direct protein-protein interactions with BMP2 and has been characterized as a matrix-associated modulator of BMP signaling in non-neuronal systems (Wallin et al., 2000; Zebboudj et al., 2002). We hypothesized that MGP expression might regulate neurofilament expression and neurite extension following myocyte contact by modulating the BMP signaling pathway. We used real-time PCR to verify that neuronal MGP mRNA expression was induced by co-culture with myocytes (Fig. 6A and B). Neurons grown without myocytes had low MGP mRNA, as did myocytes grown in the absence of neurons. In contrast, neurons isolated from neuron-myocyte co-cultures had a 6-fold increase in the level of MGP mRNA compared to neurons grown alone.
Figure 6.
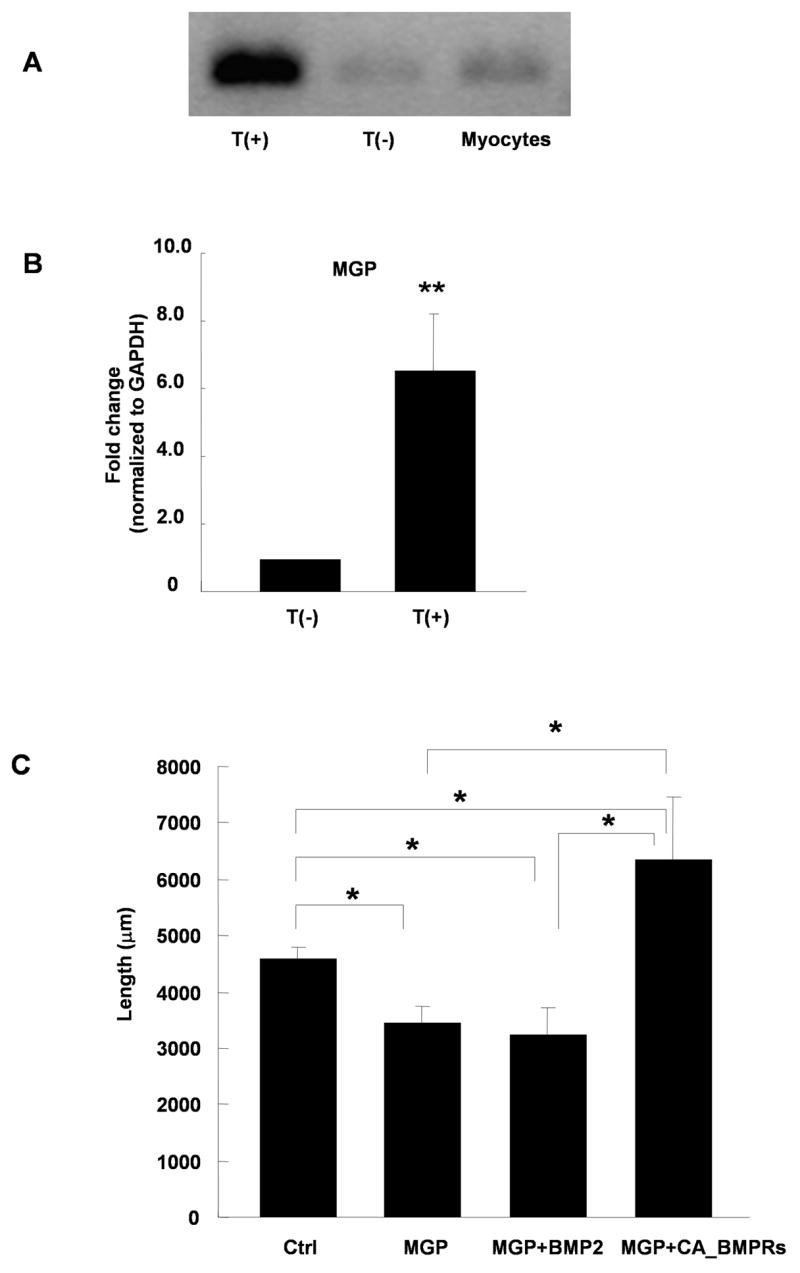
Matrix Gla protein (MGP) is induced by target contact and modulates BMP2 growth responses. (A) Expression of MGP mRNA is increased in sympathetic neurons co-cultured with myocytes T(+) compared to neurons cultured alone T(−) or myocytes cultured alone. (B) Quantification of MGP expression as measured by real-time PCR and normalized to GAPDH levels for neurons grown alone T(−), or in the presence of myocytes T(+). Data shows the mean of 6 experiments ± s.e.m., **p < 0.01. (C) The average neurite length of sympathetic neurons expressing EGFP-CD8 alone (Ctrl) or co-expressing human MGP in the absence (MGP) or presence (MGP+BMP2) of 10 ng/ml BMP2, or co-expressing EGFP, MGP, and constitutively active BMP receptor 1a & 1b (MGP+CA_BMPRs). Neurite growth was inhibited by MGP expression, and this decrease was not rescued by the addition BMP2. Co-expression of CA-BMPRs with MGP resulted in increased neurite growth. Data is shown as the mean ± s.e.m. of a minimum of 17 transfected neurons in each condition, *p < 0.05.
We tested whether increased neuronal MGP expression inhibited neurite growth, and whether such an effect was linked to a BMP-mediated growth pathway. Total neurite outgrowth was decreased in sympathetic neurons transfected with MGP and EGFP-CD8 compared to EGFP-CD8 controls (Fig 6C). We investigated whether MGP affected the neurite promoting effect of BMP2 by culturing MGP-expressing neurons with 10 ng/ml BMP2. BMP2 did not increase neurite growth in MGP-expressing neurons, suggesting decreased BMP2 signaling in the presence of neuronally expressed MGP. Co-expression of CA-BMPRs with MGP resulted in a significant increase in neurite growth compared to both the control and the MGP condition. The finding that MGP blocked signaling by exogenous BMP2 while failing to overcome signaling from constitutively activated receptors shows that MGP interferes with activation of BMP receptors, possibly by sequestering active BMP protein. It is interesting that CA-BMPRs are able to promote process outgrowth in neurons (non-co-cultured) overexpressing MGP (Fig. 6), but not in neurons grown in the presence of co-cultured myocytes (Fig. 5). This suggests that MGP contributes to the inhibition of a BMP-dependent growth pathway through altering BMP availability, but that additional myocyte-derived signals are required for changes in BMP responses in these cells.
MGP expression increases in sympathetic neuron during embryonic development in vivo
Developing neurons of the thoracic sympathetic ganglia begin to make target contacts by embryonic day 15 (Rubin 1985b). Thus, the neonatal neurons used in our experiments have had previous target contact in vivo prior to their isolation. We therefore asked whether neuronal responses to target contact were similar in embryonic and neonatal neurons by investigating whether naïve embryonic neurons showed growth inhibition in response to target contact. We used the cell-surface marker HNK-1 to isolate sympathetic lineage cells at rat embryonic day15 (E15) using a magnetic bead purification system as described in the Methods (Pisano et al., 2000). These E15 HNK-1-expressing neuroblasts undergo neuronal differentiation in culture, extending extensive processes over a three-day culture period. E15 sympathetic cells were cultured for three days in the presence or absence of co-cultured neonatal cardiac myocytes, stained with an anti-NCAM antibody to label all processes, and total process length was measured. Note that the low density of these embryonic cultures made it possible to visualize the entire arbor of individual labeled neurons without transfection with EGFP. We found a significant decrease in total process length in neurons co-cultured with myocytes compared to neurons cultured alone (Fig. 7A), suggesting that the growth responses of neonatal neuron to target contact recapitulate the initial embryonic neuron responses.
Figure 7.
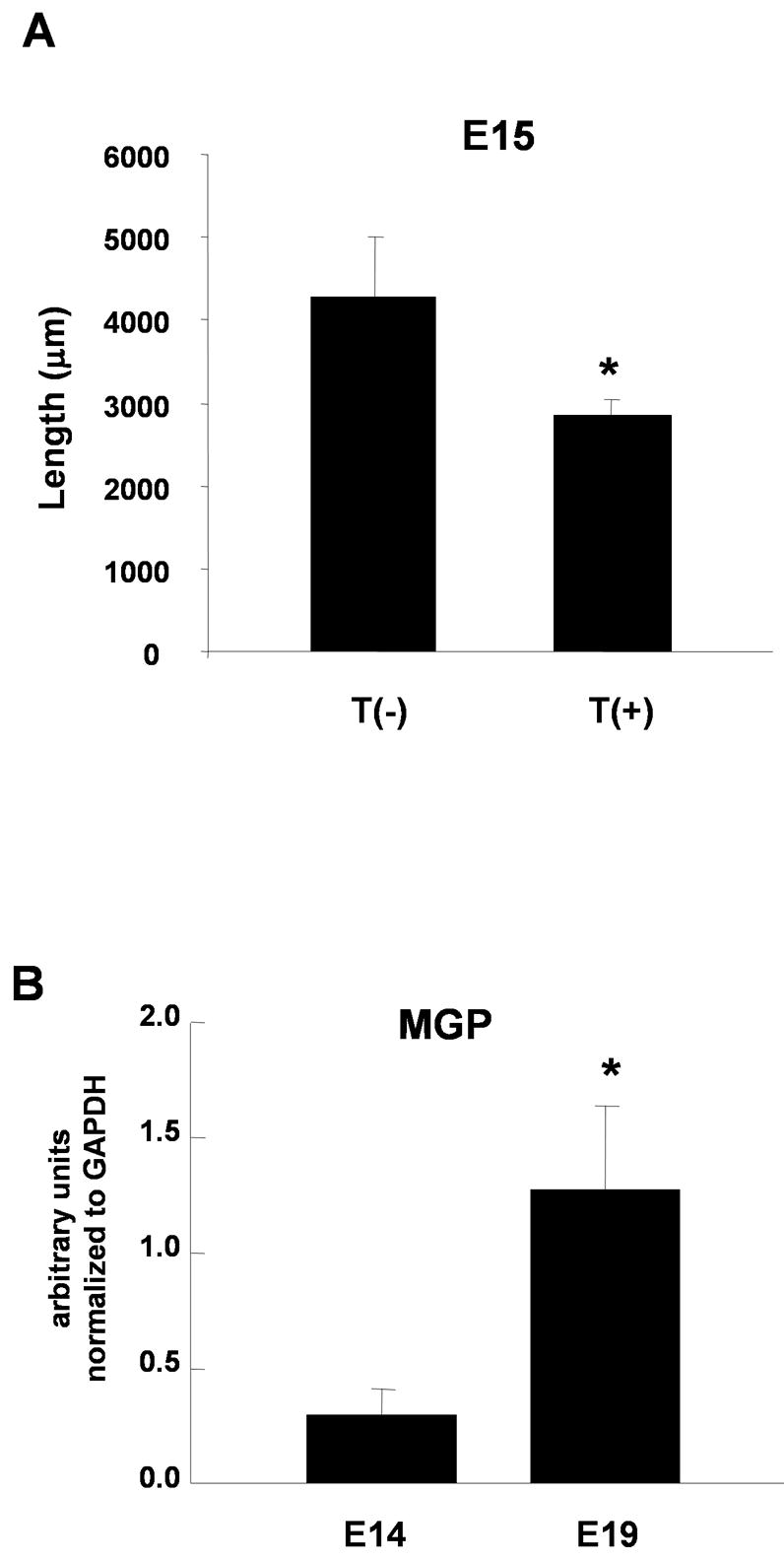
Regulation of growth pathways in embryonic neurons and developmental regulation of MGP expression. A. E15 developing sympathetic neurons were cultured alone T(−) or with neonatal cardiac myocytes T(+) for 3 days. Cultures were stained with a NCAM antibody and the total process length per neuron was analyzed. Data shown is the mean ± s.e.m. of a minimum of 14 labeled neurons, p < 0.05. B. MGP mRNA expression increased in immuno-purified developing embryonic rat sympathetic neurons between E14 and E19, a period in which sympathetic neurons are establishing target contacts. MGP mRNA expression was measured by real-time PCR and was normalized to GAPDH expression. Data is shown as the mean of 4 experiments ± s.e.m., *p < 0.01.
We asked if there was a developmental increase in neuronal MGP expression during the period of target contact, consistent with our observation that MGP expression is induced by target contact in vitro. We examined MGP mRNA expression in sympathetic neurons from embryonic rats at E14 and at E19, a point at which extensive target innervation is already established. HNK-1+ neuroblasts were immuno-isolated from rat sympathetic ganglia at E14 and young HNK-1+ neurons were isolated from E19 embryos. RNA was isolated and subjected to real-time PCR using primers for MGP. MGP mRNA was significantly higher in sympathetic neurons isolated from E19 ganglia than in E14 neuroblasts (Fig. 7B), showing a correlation between the timing of target contact and the expression of a gene implicated in target dependent growth regulation.
Discussion
Bone morphogenetic proteins have been implicated in both early and late stages of peripheral neuron development. BMPs promote the acquisition of a noradrenergic neuronal phenotype and the development of mature neuronal properties of sympathetic neurons (Lein et al., 1995; Schneider et al., 1999; Pisano et al., 2000; Sarkar and Howard, 2006), as well as neurite fasciculation and gangliogenesis in the enteric nervous system (Chalazonitis et al., 2004; Fu et al., 2006; Faure et al., 2007). Here we show that BMP signaling contributes to a sympathetic growth program that is regulated by target interactions, leading to the development of contacts between sympathetic neurons and cardiac myocytes. Target contact induces the expression of MGP, a matrix-associated BMP modulator that is sufficient to suppress BMP-dependent changes in sympathetic neuron growth. Target-dependent growth modulation takes place for embryonic as well as neonatal neurons and the expression of MGP increases in the sympathetic ganglia during the embryonic period of target contact in vivo (Fig. 7). Together, these data suggest a model in which MGP and loss of BMP signaling contribute to a developmental transition between neuronal growth and later maturation events. Changes in BMP-mediated neuronal growth are distinct and separable from the regulation of pre-synaptic proteins and may precede the target-dependent regulation of presynaptic development (Lockhart et al., 2000).
Target contact regulates neuronal growth properties
Several studies have shown that target cells actively halt the growth of their contacting neuronal partners. The growth of pontine mossy fibers is limited by contact with cerebellar granule cells in a process that involves the activation of NMDA receptors (Baird et al., 1996; Mason et al., 1997). Motor neuron growth is halted by muscle contact through an agrin and MuSK-mediated pathway (Dimitropoulou and Bixby, 2005). Initial contact with cardiac myocytes also leads to decreased neurite outgrowth from co-cultured sympathetic neurons (Fig. 1). However, although agrin is expressed in the sympathetic system, the process by which myocytes regulate sympathetic growth properties differs from the motor neuron system. Sympathetic neurons from agrin-deficient mice show normal neuronal process development that is not altered by addition of agrin to the culture medium (Gingras et al., 2002). Together these studies demonstrate that target-dependent regulation of neuronal growth properties is a common feature of nervous system maturation, but show that distinct mechanisms contribute to this regulatory process for different neuron-target interactions.
Initial contact between a developing sympathetic neuron and a myocyte impacts the global growth properties of the neuron, down-regulating growth associated genes and total process outgrowth. In fact, previous work has shown that neurites directly in contact with myocytes may increase their local arborization as measured by the appearance of local, high density neurite networks associated with individual myocytes (Lockhart et al., 2000). Thus, target interactions have multiple, complex effects on the developmental dynamics of contacting neurons. Myocyte contact also results in functional innvervation of the target, leading to the appearance of presynaptic sites in co-cultured sympathetic neurons and functional synaptic transmission (Lockhart et al., 1997; Lockhart et al., 2000; Conforti et al., 1991). Interestingly, while both young neonatal myocytes and mature myocytes regulate neuronal growth properties, the mature myocytes are more effective at promoting presynaptic differentiation (Lockhart et al., 2000). This is similar to the dynamics observed in the cerebellum where immature granule cells arrest mossy fiber outgrowth, while mature granule neurons promote synapse formation (Manzini et al., 2006). Thus, sympathetic neuron maturation following target contact is likely to involve multiple, temporally separate interactions that lead to down-regulation of a growth pathway, local increases in target-associated arborization, and induction of presynaptic specializations.
Target interactions and BMP signaling
BMP signaling has been implicated in multiple developmental events in sympathetic lineage development including neuronal differentiation (Reissmann et al., 1996; Schneider et al., 1999), inhibition of alternative glial differentiation (Dore et al., 2005), dendrite formation (Lein et al., 1995), and neuronal maturation (Pisano et al., 2000). Given the diverse functions of BMPs at consecutive developmental stages, it was surprising that the microarray screen and real-time PCR showed coordinate downregulation of BMP-regulated genes following myoycte contact. Several of these regulated transcripts, including the bHLH transcription factors HAND1 and HAND2 and neurofilament, have been implicated in BMP-regulated events in peripheral nervous system development (Howard et al., 2000; Wu and Howard, 2002). These findings, along with our demonstration of BMP2 as a growth-promoting factor for neonatal sympathetic neurons (Fig. 4), suggests that loss of BMP signaling could contribute to the negative regulation of sympathetic neurite growth following target contact.
BMPs promote the differentiation of noradrenergic sympathetic neurons via a signaling pathway that includes HAND2, a bHLH transcription factor (Reissmann et al., 1996; Howard 2000). HAND2 expression is regulated by BMPs and HAND2 overexpression induces TH and NF-M expression in the chick embryo (Howard et al., 2000; Ernsberger et al., 2000). HAND1 is also capable of modulating noradrenergic properties (Howard et al., 1999) and, like HAND2, was downregulated by target contact. However, we did not observe increased neurite growth following overexpression of either HAND1 or HAND2, and HAND1 expresssion actually down-regulated neurite growth (Fig. 3). HAND1 and HAND2 belong to the Class B tissue-specific bHLH genes, and show broad dimerization characteristics; HAND1 and HAND2 are able to homo- and hetero dimerize with themselves (Firulli et al., 2000) and heterodimerize with other E-proteins (Dai and Cserjesi, 2002; Hill and Riley, 2004). HAND proteins compete for E-protein dimerization, resulting in antagonistic effects on other bHLH transcription factors (Bounpheng et al., 2000). Interestingly, HAND1 heterodimerizes more strongly to E12, a class A bHLH transcription factor, than it homodimerizes to itself (Firulli et al., 2003), suggesting a model in which dimerization between E12 and additional Class B bHLH proteins promote process outgrowth that can be disrupted by competition from overexpressed HAND1 or HAND2. Support for this model comes from our finding that overexpression of E12 strongly promoted neurite outgrowth in sympathetic neurons (Fig. 3). While the identity of the bHLH protein regulating sympathetic growth is unknown and the level of overexpression of the different bHLH proteins could affect the availability of different binding partners, our data suggest that BMPs may utilize different bHLH proteins at different points of sympathetic development, with HAND2 promoting early differentiation and additional factors driving long-range process extension.
Matrix Gla protein and BMP signaling
One way to control BMP signaling is through regulation of a modulator of the BMP pathway. MGP, a known modulator of BMP signaling in non-neuronal systems, showed increased expression in sympathetic neurons following myocyte contact (Fig. 6). MGP is a small, matrix-associated protein containing carboxylated glutamic acid (Gla) residues (Hauschka et al., 1989) that acts as an inhibitor of extracellular matrix mineralization in bone and cartilage (Murshed et al., 2004). MGP binds to BMP2 (Wallin et al., 2000), and inhibits BMP2-induced vascular calcification (Zebboudj et al., 2002). MGP also modulates BMP2-induced differentiation of C3H10T1/2 cells (Bostrom et al., 2001). Interestingly, overexpression of MGP has different effects on BMP-dependent gene expression associated with different cell lineages, with extensive inhibition of osteogenic genes such as osteocalcin and collegen IX and only partial inhibition of PPARγ2, a gene associated with differentiation of the adipocyte lineage. This suggests that MGP may affect BMP signaling in a lineage specific manner, perhaps as a consequence of modulating the availability of local of BMPs (Zebboudj et al., 2002).
The function of MGP in the nervous system has not been established. However, one recent microarray screen showed that MGP expression was modulated by axotomy of SCG neurons (Del Signore et al., 2006). While there was no further investigation of MGP function in this system, it was interesting that an intermediate filament protein gene increased within a day of axotomy, while increased MGP expression was delayed by several days. This finding is consistent with the idea that MGP acts following a period of axonal growth. Here we have shown that overexpression of MGP in sympathetic neurons is sufficient to limit overall neurite outgrowth, an effect that is overcome by expression of constitutively active BMP receptors (Fig. 6C). This implicates MGP in the regulation of BMP signaling in a neuronal system and suggests an action in blocking growth effects of BMP following target contact. The finding that MGP expression significantly increased in developing sympathetic neurons during the period of initial target contact (Rubin, 1985b) and that these embryonic neurons down-regulate their growth in response to myocyte contact (Fig. 7A), suggests that MGP may play a role in the regulation sympathetic-target interactions in vivo.
Changing responses to BMP signaling during development
BMPs can have different effects over the course of development of a single neuronal lineage. In the olfactory epithelium, BMPs inhibit neurogenesis of precursor cells, but support the survival of newly generated olfactory neurons (Shou et al., 2000). BMPs also promote cell cycle withdrawal of ventricular zone cells while later promoting differentiation and maturation (Mehler et al., 2000). In the chick sympathetic nervous system, BMP4 coordinately promotes the expression of TH and dopamine β-hydroxylase (DBH) in the early sympathetic ganglia, while showing differential effects on the two proteins in cells from later embryonic stages (Ernsberger et al., 2000). Differential actions of BMPs may reflect changes in the repertoire of BMP receptors expressed (Panchision et al., 2001), or a range of dose-dependencies for different effects (Mehler et al., 2000; Shou et al., 2000). The finding that BMP2 does not promote NF-M expression at any concentration following target contact and the result that CA-BMPRs do not increase process extension in neurons co-cultured with cardiac myocytes (Fig. 5), provide evidence that myocytes induce an intrinsic change in BMP response. Myocyte-induced expression of neuronal MGP is not sufficient to drive this developmental transition as CA-BMPRs promote process outgrowth in neurons overexpressing MGP. This suggests that additional myocyte-derived signals are required for a developmental transition in BMP responses.
Supplementary Material
Acknowledgments
We would like to thank Jessica Pisano, Frances Colon and Justin Dore for embryonic RNAs and Eva Nokes, Jeanine Hinterneder and Jason Luther for critical reading of the manuscript. We also thank Jeanine Hinterneder for helpful discussions and for providing the co-culture image in Figure 1. This work was supported by NIH HD042716 and NS40168 to S.J.B., and by a grant for Core Facilities for Neuroscience at Brandeis NIH P30 NS45713.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baird DH, Hatten ME, Mason CA. Cerebellar target neurons provide a stop signal for afferent neurite extension in vitro. J Neurosci. 1992;12:619–34. doi: 10.1523/JNEUROSCI.12-02-00619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird DH, Trenkner E, Mason CA. Arrest of afferent axon extension by target neurons in vitro is regulated by the NMDA receptor. J Neurosci. 1996;16:2642–8. doi: 10.1523/JNEUROSCI.16-08-02642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck HN, Drahushuk K, Jacoby DB, Higgins D, Lein PJ. Bone morphogenetic protein-5 (BMP-5) promotes dendritic growth in cultured sympathetic neurons. BMC Neurosci. 2001;2:12. doi: 10.1186/1471-2202-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharmal S, Slonimsky JD, Mead JN, Sampson CP, Tolkovsky AM, Yang B, Bargman R, Birren SJ. Target cells promote the development and functional maturation of neurons derived from a sympathetic precursor cell line. Dev Neurosci. 2001;23:153–64. doi: 10.1159/000048707. [DOI] [PubMed] [Google Scholar]
- Bostrom K, Tsao D, Shen S, Wang Y, Demer LL. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276:14044–52. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- Bounpheng MA, Morrish TA, Dodds SG, Christy BA. Negative regulation of selected bHLH proteins by eHAND. Exp Cell Res. 2000;257:320–31. doi: 10.1006/excr.2000.4898. [DOI] [PubMed] [Google Scholar]
- Carrio I. Cardiac neurotransmission imaging. J Nucl Med. 2001;42:1062–76. [PubMed] [Google Scholar]
- Chalazonitis A, D’Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA, Gershon MD. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24:4266–82. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LL, Patterson PH. Role of nerve growth factor in the development of rat sympathetic neurons in vitro. I. Survival, growth, and differentiation of catecholamine production. J Cell Biol. 1977;75:694–704. doi: 10.1083/jcb.75.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Tohse N, Sperelakis N. Influence of sympathetic innervation on the membrane electrical properties of neonatal rat cardiomyocytes in culture. J Dev Physiol. 1991;15:237–46. [PubMed] [Google Scholar]
- Cowen T, Thrasivoulou C, Shaw SA, Abdel-Rahman TA. Transplanted sweat glands from mature and aged donors determine cholinergic phenotype and altered density of host sympathetic nerves. J Auton Nerv Syst. 1996;58:153–62. doi: 10.1016/0165-1838(95)00127-1. [DOI] [PubMed] [Google Scholar]
- Dai YS, Cserjesi P. The basic helix-loop-helix factor, HAND2, functions as a transcriptional activator by binding to E-boxes as a heterodimer. J Biol Chem. 2002;277:12604–12. doi: 10.1074/jbc.M200283200. [DOI] [PubMed] [Google Scholar]
- Del Signore A, De Sanctis V, Di Mauro E, Negri R, Perrone-Capano C, Paggi P. Gene expression pathways induced by axotomy and decentralization of rat superior cervical ganglion neurons. Eur J Neurosci. 2006;23:65–74. doi: 10.1111/j.1460-9568.2005.04520.x. [DOI] [PubMed] [Google Scholar]
- Dimitropoulou A, Bixby JL. Motor neurite outgrowth is selectively inhibited by cell surface MuSK and agrin. Mol Cell Neurosci. 2005;28:292–302. doi: 10.1016/j.mcn.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Dore JJ, Crotty KL, Birren SJ. Inhibition of glial maturation by bone morphogenetic protein 2 in a neural crest-derived cell line. Dev Neurosci. 2005;27:37–48. doi: 10.1159/000084531. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Reissmann E, Mason I, Rohrer H. The expression of dopamine beta-hydroxylase, tyrosine hydroxylase, and Phox2 transcription factors in sympathetic neurons: evidence for common regulation during noradrenergic induction and diverging regulation later in development. Mech Dev. 2000;92:169–77. doi: 10.1016/s0925-4773(99)00336-6. [DOI] [PubMed] [Google Scholar]
- Faure C, Chalazonitis A, Rheaume C, Bouchard G, Sampathkumar SG, Yarema KJ, Gershon MD. Gangliogenesis in the enteric nervous system: Roles of the polysialylation of the neural cell adhesion molecule and its regulation by bone morphogenetic protein-4. Dev Dyn. 2007;236:spc1. doi: 10.1002/dvdy.20943. [DOI] [PubMed] [Google Scholar]
- Firulli BA, Hadzic DB, McDaid JR, Firulli AB. The basic helix-loop-helix transcription factors dHAND and eHAND exhibit dimerization characteristics that suggest complex regulation of function. J Biol Chem. 2000;275:33567–73. doi: 10.1074/jbc.M005888200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firulli BA, Howard MJ, McDaid JR, McIlreavey L, Dionne KM, Centonze VE, Cserjesi P, Virshup DM, Firulli AB. PKA, PKC, and the protein phosphatase 2A influence HAND factor function: a mechanism for tissue-specific transcriptional regulation. Mol Cell. 2003;12:1225–37. doi: 10.1016/s1097-2765(03)00425-8. [DOI] [PubMed] [Google Scholar]
- Fu M, Vohra BP, Wind D, Heuckeroth RO. BMP signaling regulates murine enteric nervous system precursor migration, neurite fasciculation, and patterning via altered Ncam1 polysialic acid addition. Dev Biol. 2006;299:137–50. doi: 10.1016/j.ydbio.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras J, Rassadi S, Cooper E, Ferns M. Agrin plays an organizing role in the formation of sympathetic synapses. J Cell Biol. 2002;158:1109–18. doi: 10.1083/jcb.200203012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Rueger D, Higgins D. Osteogenic protein-1 and related bone morphogenetic proteins regulate dendritic growth and the expression of microtubule-associated protein-2 in rat sympathetic neurons. Neurosci Lett. 1998;245:131–4. doi: 10.1016/s0304-3940(98)00192-x. [DOI] [PubMed] [Google Scholar]
- Haase G, Dessaud E, Garces A, de Bovis B, Birling M, Filippi P, Schmalbruch H, Arber S, deLapeyriere O. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35:893–905. doi: 10.1016/s0896-6273(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- Hill AA, Riley PR. Differential regulation of Hand1 homodimer and Hand1-E12 heterodimer activity by the cofactor FHL2. Mol Cell Biol. 2004;24:9835–47. doi: 10.1128/MCB.24.22.9835-9847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbinski C, Stachowiak EK, Chandrasekaran V, Miuzukoshi E, Higgins D, Stachowiak MK. Bone morphogenetic protein-7 stimulates initial dendritic growth in sympathetic neurons through an intracellular fibroblast growth factor signaling pathway. J Neurochem. 2002;80:54–63. doi: 10.1046/j.0022-3042.2001.00657.x. [DOI] [PubMed] [Google Scholar]
- Howard M, Foster DN, Cserjesi P. Expression of HAND gene products may be sufficient for the differentiation of avian neural crest-derived cells into catecholaminergic neurons in culture. Dev Biol. 1999;215:62–77. doi: 10.1006/dbio.1999.9450. [DOI] [PubMed] [Google Scholar]
- Howard MJ, Stanke M, Schneider C, Wu X, Rohrer H. The transcription factor dHAND is a downstream effector of BMPs in sympathetic neuron specification. Development. 2000;127:4073–81. doi: 10.1242/dev.127.18.4073. [DOI] [PubMed] [Google Scholar]
- Ledda F, Paratcha G, Ibanez CF. Target-derived GFRalpha1 as an attractive guidance signal for developing sensory and sympathetic axons via activation of Cdk5. Neuron. 2002;36:387–401. doi: 10.1016/s0896-6273(02)01002-4. [DOI] [PubMed] [Google Scholar]
- Lein P, Johnson M, Guo X, Rueger D, Higgins D. Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron. 1995;15:597–605. doi: 10.1016/0896-6273(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Beck HN, Chandrasekaran V, Gallagher PJ, Chen HL, Lin Y, Guo X, Kaplan PL, Tiedge H, Higgins D. Glia induce dendritic growth in cultured sympathetic neurons by modulating the balance between bone morphogenetic proteins (BMPs) and BMP antagonists. J Neurosci. 2002;22:10377–87. doi: 10.1523/JNEUROSCI.22-23-10377.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R, Angeletti PU. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol. 1963;7:653–9. doi: 10.1016/0012-1606(63)90149-0. [DOI] [PubMed] [Google Scholar]
- Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98:31–6. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lockhart ST, Mead JN, Pisano JM, Slonimsky JD, Birren SJ. Nerve growth factor collaborates with myocyte-derived factors to promote development of presynaptic sites in cultured sympathetic neurons. J Neurobiol. 2000;42:460–76. [PubMed] [Google Scholar]
- Lockhart ST, Turrigiano GG, Birren SJ. Nerve growth factor modulates synaptic transmission between sympathetic neurons and cardiac myocytes. J Neurosci. 1997;17:9573–82. doi: 10.1523/JNEUROSCI.17-24-09573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas ME, Muller F, Rudiger R, Henion PD, Rohrer H. The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development. 2006;133:4015–24. doi: 10.1242/dev.02574. [DOI] [PubMed] [Google Scholar]
- Mann F, Peuckert C, Dehner F, Zhou R, Bolz J. Ephrins regulate the formation of terminal axonal arbors during the development of thalamocortical projections. Development. 2002;129:3945–55. doi: 10.1242/dev.129.16.3945. [DOI] [PubMed] [Google Scholar]
- Manzini MC, Ward MS, Zhang Q, Lieberman MD, Mason CA. The stop signal revised: immature cerebellar granule neurons in the external germinal layer arrest pontine mossy fiber growth. J Neurosci. 2006;26:6040–51. doi: 10.1523/JNEUROSCI.4815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CA, Morrison ME, Ward MS, Zhang Q, Baird DH. Axon-target interactions in the developing cerebellum. Perspect Dev Neurobiol. 1997;5:69–82. doi: 10.1080/0907676x.1997.9961300. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhu G, Gokhan S, Kessler JA. Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev Neurosci. 2000;22:74–85. doi: 10.1159/000017429. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, D’Autreaux F, Gershon MD, Cserjesi P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol. 2007;307:114–126. doi: 10.1016/j.ydbio.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–30. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobin A, Falck B, Ingemansson S, Jarhult J, Rosengren E. Organization and function of the sympathetic innervation. Acta Physiol Scand Suppl. 1977;452:103–6. [PubMed] [Google Scholar]
- O’Lague PH, Potter DD, Furshpan EJ. Studies on rat sympathetic neurons developing in cell culture. I. Growth characteristics and electrophysiological properties. Dev Biol. 1978;67:384–403. doi: 10.1016/0012-1606(78)90208-7. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Heffner CD, Kutka L, Lopez-Mascaraque L, Missias A, Reinoso BS. A target-derived chemoattractant controls the development of the corticopontine projection by a novel mechanism of axon targeting. Development. 1991;2(Suppl):123–30. [PubMed] [Google Scholar]
- Panchision DM, Pickel JM, Studer L, Lee SH, Turner PA, Hazel TG, McKay RD. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 2001;15:2094–110. doi: 10.1101/gad.894701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano JM, Birren SJ. Restriction of developmental potential during divergence of the enteric and sympathetic neuronal lineages. Development. 1999;126:2855–68. doi: 10.1242/dev.126.13.2855. [DOI] [PubMed] [Google Scholar]
- Pisano JM, Colon-Hastings F, Birren SJ. Postmigratory enteric and sympathetic neural precursors share common, developmentally regulated, responses to BMP2. Dev Biol. 2000;227:1–11. doi: 10.1006/dbio.2000.9876. [DOI] [PubMed] [Google Scholar]
- Porter BE, Weis J, Sanes JR. A motoneuron-selective stop signal in the synaptic protein S-laminin. Neuron. 1995;14:549–59. doi: 10.1016/0896-6273(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Reissmann E, Ernsberger U, Francis-West PH, Rueger D, Brickell PM, Rohrer H. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development. 1996;122:2079–88. doi: 10.1242/dev.122.7.2079. [DOI] [PubMed] [Google Scholar]
- Rowe V, Parr J. Pineal cells enhance choline acetyltransferase activity in sympathetic neurons. J Neurobiol. 1980;11:547–56. doi: 10.1002/neu.480110605. [DOI] [PubMed] [Google Scholar]
- Rubin E. Development of the rat superior cervical ganglion: ingrowth of preganglionic axons. J Neurosci. 1985;5:685–96. doi: 10.1523/JNEUROSCI.05-03-00685.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E. Development of the rat superior cervical ganglion: initial stages of synapse formation. J Neurosci. 1985;5:697–704. doi: 10.1523/JNEUROSCI.05-03-00697.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AA, Howard MJ. Perspectives on integration of cell extrinsic and cell intrinsic pathways of signaling required for differentiation of noradrenergic sympathetic ganglion neurons. Auton Neurosci. 2006:126–127. 225–31. doi: 10.1016/j.autneu.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Schneider C, Wicht H, Enderich J, Wegner M, Rohrer H. Bone morphogenetic proteins are required in vivo for the generation of sympathetic neurons. Neuron. 1999;24:861–70. doi: 10.1016/s0896-6273(00)81033-8. [DOI] [PubMed] [Google Scholar]
- Schotzinger R, Yin X, Landis S. Target determination of neurotransmitter phenotype in sympathetic neurons. J Neurobiol. 1994;25:620–39. doi: 10.1002/neu.480250605. [DOI] [PubMed] [Google Scholar]
- Shah NM, Groves AK, Anderson DJ. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell. 1996;85:331–43. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- Shiga T, Lustig M, Grumet M, Shirai T. Cell adhesion molecules regulate guidance of dorsal root ganglion axons in the marginal zone and their invasion into the mantle layer of embryonic spinal cord. Dev Biol. 1997;192:136–48. doi: 10.1006/dbio.1997.8742. [DOI] [PubMed] [Google Scholar]
- Shou J, Murray RC, Rim PC, Calof AL. Opposing effects of bone morphogenetic proteins on neuron production and survival in the olfactory receptor neuron lineage. Development. 2000;127:5403–13. doi: 10.1242/dev.127.24.5403. [DOI] [PubMed] [Google Scholar]
- Slonimsky JD, Mattaliano MD, Moon JI, Griffith LC, Birren SJ. Role for calcium/calmodulin-dependent protein kinase II in the p75-mediated regulation of sympathetic cholinergic transmission. Proc Natl Acad Sci U S A. 2006;103:2915–9. doi: 10.1073/pnas.0511276103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Nerve growth factor enhances dendritic arborization of sympathetic ganglion cells in developing mammals. J Neurosci. 1988;8:2628–34. doi: 10.1523/JNEUROSCI.08-07-02628.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LM, Landis SC. Developmental interactions between sweat glands and the sympathetic neurons which innervate them: effects of delayed innervation on neurotransmitter plasticity and gland maturation. Dev Biol. 1988;130:703–20. doi: 10.1016/0012-1606(88)90362-4. [DOI] [PubMed] [Google Scholar]
- Taniwaki T, Schwartz JP. Somatostatin enhances neurofilament expression and neurite outgrowth in cultured rat cerebellar granule cells. Brain Res Dev Brain Res. 1995;88:109–16. doi: 10.1016/0165-3806(95)00090-z. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. Development and regulation of dendrites in the rat superior cervical ganglion. J Neurosci. 1987;7:904–12. doi: 10.1523/JNEUROSCI.07-03-00904.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R, Cain D, Hutson SM, Sane DC, Loeser R. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2) Thromb Haemost. 2000;84:1039–44. [PubMed] [Google Scholar]
- Wu X, Howard MJ. Transcripts encoding HAND genes are differentially expressed and regulated by BMP4 and GDNF in developing avian gut. Gene Expr. 2002;10:279–93. doi: 10.3727/000000002783992361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Fisher DA, Zhou L, White FA, Ng S, Snider WD, Luo Y. The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J Neurosci. 2000;20:2638–48. doi: 10.1523/JNEUROSCI.20-07-02638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T, Samuels I, Schwartz JP. Bone morphogenetic proteins BMP-6 and BMP-7 have differential effects on survival and neurite outgrowth of cerebellar granule cell neurons. J Neurosci Res. 2002;68:161–8. doi: 10.1002/jnr.10210. [DOI] [PubMed] [Google Scholar]
- Yawo H. Changes in the dendritic geometry of mouse superior cervical ganglion cells following postganglionic axotomy. J Neurosci. 1987;7:3703–11. doi: 10.1523/JNEUROSCI.07-11-03703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebboudj AF, Imura M, Bostrom K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem. 2002;277:4388–94. doi: 10.1074/jbc.M109683200. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Mason CA. Developmental regulation of mossy fiber afferent interactions with target granule cells. Dev Biol. 1998;195:75–87. doi: 10.1006/dbio.1997.8837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.