Abstract
DNA polymerase β (pol β) is the most error prone of all known eukaryotic DNA polymerases tested in vitro. Here, we show that cells overexpressing pol β cDNA have acquired a spontaneous mutator phenotype. By measuring the appearance of mutational events using three independent assays, we found that genetic instability increased in the cell lines that overexpressed pol β. In addition, these cells displayed a decreased sensitivity to cancer chemotherapeutic, bifunctional, DNA-damaging agents such as cisplatin, melphalan, and mechlorethamine, resulting in enhanced mutagenesis compared with control cells. By using cell-free extracts and modified DNA substrates, we present data in support of error-prone translesion replication as one of the key determinants of tolerance phenotype. These results have implications for the potential role of pol β overexpression in cancer predisposition and tumor progression during chemotherapy.
Cancer cells differ from normal cells in many important characteristics, including loss of differentiation, increased ability to invade, and decreased drug sensitivity in a chemotherapy pressure context. It was suggested several years ago that these differences do not arise simply from uncontrolled cellular growth, but rather from a mutator phenotype providing a continuing pool of mutants upon which selection could act to promote a tumor (1). The current hypothesis of the mutator phenotype considers the large number of genes required to maintain the integrity of the human genome. For example, mutations in mismatch repair genes have been shown to predispose carriers to cancer, presumably by increasing genomic instability (2, 3). The genes encoding components of cell cycle checkpoints ensuring the order of events in the cell cycle correspond to another category of genes that play a role in genetic stability and cellular evolution (4, 5). Here, we hypothesized that a new category of genetic events, overexpression of DNA polymerase β (pol β), could increase genetic instability and decrease sensitivity to chemotherapeutic agents. Pol β is the base excision repair polymerase (6) that is expressed at a constant low level throughout the cell cycle (7) and is inducible by some genotoxic treatments (8). Features that distinguish pol β from other cellular polymerases are its small size, the lack of associated proofreading activity, its high infidelity in replicating DNA in vitro (9), and its poor ability to discriminate nucleotides at the level of binding (10). In accordance with its low accuracy, pol β exhibits the lowest discrimination against mutagenic analogs of dGTP modified by endogenous processes (11). In addition, we have shown previously that purified pol β has the potential to efficiently catalyze error-prone translesion synthesis in vitro across intrastrand crosslinks (12, 13). Therefore, we investigated whether overexpression of pol β in cells could result in an increased rate of spontaneous mutation and could modulate cellular sensitivity to the anticancer cross-linking agents cisplatin, mechlorethamine, and melphalan, whose therapeutic effects result from their covalent binding to DNA to form mostly intrastrand crosslinks.
MATERIALS AND METHODS
Cell Culture and Drug Survival Analysis.
pol β-overexpressing plasmid pUTpolβ was constructed following a previously described procedure (14) to be stably inserted into the genomic DNA of transfected Chinese hamster ovary (CHO) cells. Pol β cDNA was fused in-frame with the bacterial Sh∷ble gene conferring resistance to the broad-spectral zeocin xenobiotic of the phleomycin family. The fusion did not alter pol β expression (14). For growth rate analysis, cells were seeded (150,000 cells/well) in 6-well dishes and grown at 37°C in growth medium (MEM supplemented with glutamine/8% fetal calf serum/penicillin/streptomycin). Cells were counted for up to 3 days at 24-h intervals. Drug sensitivity of the cell lines was determined by clonogenic assay. Cells (400 per well) were plated in 6-well plates and allowed to attach overnight. Next, they were treated for 1 h with the drugs diluted in complete medium at varying doses. Colonies were fixed and stained after 6 days of posttreatment incubation, and those with >50 cells were scored. For each experiment, three independently transfected clones were used.
Mutagenesis Analysis.
For the ouabain and 6-thioguanine (6-TG)-resistant tests, cell cultures were exposed to 2 mM ouabain and 20 μM 6-TG-containing media to determine the number of Na-K-ATPase and hypoxanthine phosphoribosyltransferase (HPRT) mutants, respectively. Replica cultures were plated at a density of 3 × 106 and 5 × 105 cells for evaluation of ouabain and 6-TG-resistant colonies, respectively. After 1 wk, plates were stained, and colonies of >50 cells were counted. Mutant frequencies were corrected for plating efficiency. Mutation rates were calculated by the method of the mean (15). For the LacZ mutagenesis test, a CHO–AA8 clone was stably transfected with an expression vector harboring the Escherichia coli lacZ gene fused in-frame with the bacterial aminoglycoside phosphotransferase gene conferring resistance to G418; the fusion was under the control of the strong and constitutive promoter of herpes simplex virus-1 thymidine kinase. Clones were picked 10 days after transfection and transferred to 24-well plates for 3 days. Cells were then trypsinized, and 10,000 cells were spread into Petri dishes that were 60 mm in diameter. After 48 h of incubation, 5-bromo-4-chloro-3-indolyl β-d-galactoside staining (X-Gal), and counting, we selected one clone, CJS3, for which only a few white cells were detected as a result of epigenetic inactivation (16). This clone was then transfected with pUT526Δ or pUTpolβ plasmid. Independent, stably cloned cotransfectants were analyzed as described above to determine the proportion of white cells. In all experiments related to this assay, cells were grown in the absence of G418 to avoid selection of missense mutants.
Preparation of Replicative Cell Extracts.
CHO extract preparation was performed according to the previously described protocol (17). Briefly, the cells were grown as monolayer cultures, washed with ice-cold PBS, scraped off the plates, and harvested by centrifugation. The cell pellet was suspended in hypotonic buffer (10 mM Tris⋅HCl, pH 7.5/10 mM KCl/10 mM MgCl2/1 mM DTT) containing protease inhibitors and disrupted in a Dounce homogenizer. Nuclei were harvested by centrifugation, and the nuclear proteins were extracted in hypotonic buffer containing 350 mM NaCl. Cytosolic and nuclear extract proteins were precipitated by addition of ammonium sulfate, and the precipitates were resuspended in dialysis buffer (50 mM Tris⋅HCl, pH 7.5/1 mM EDTA/100 mM mono-K glutamic acid/10% glycerol) and dialyzed for 2 h at 0°C. The extracts were frozen in liquid nitrogen and stored at −70°C.
Translesion DNA Synthesis Assay.
The cisplatin-modified 60 mer was prepared as reported (12) and hybridized to a 5′ 32P-labeled 17-mer primer. Standard 15-μl reaction mixtures contained 5 ng of labeled intact or damaged substrate and 3 μg of cell extract protein in reaction buffer (45 mM Hepes-KOH, pH 7.8/7 mM MgCl2/1 mM DTT/0.4 mM EDTA/2 mM ATP/50–500 μM each of dATP/dCTP/dGTP/dTTP/3.4% glycerol/65 mM mono-K glutamic acid/18 μg of BSA) in the absence or presence of 5 units of calf thymus DNA pol β (a generous gift of V. Hübscher, Zurich, Switzerland). At the end of the reaction, 5 μl of stopping buffer (90% formamide/0.1% xylene cyanol/0.1% bromophenol blue/0.1 mM EDTA) was added. Samples were denatured for 10 min at 70°C and loaded to a 15% polyacrylamide/7 M urea/30% formamide gel.
Mutational Analysis of the Bypass Products.
The bypass products were excised from the gel, and the DNA was extracted. The purified bypass product was PCR-amplified, and the PCR products (74-bp) were then 5′ 32P-labeled by T4 polynucleotide kinase and digested by Aci I that cleaves at the 5′-CCGC-3′ restriction site corresponding to the location of the cisplatin adduct. The products resistant to the Aci I cleavage were gel-purified, PCR-amplified, inserted into the polylinker region of M13 mp18, and sequenced as described (13).
RESULTS AND DISCUSSION
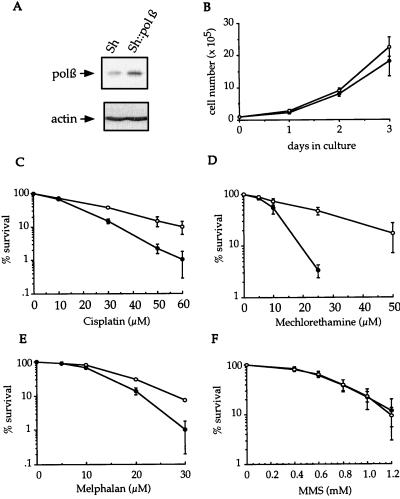
To test whether overproduction of pol β in cells could result in an increased rate of spontaneous mutation, a DNA expression vector was constructed; this vector harbored the cDNA encoding the rat DNA pol β fused in-frame with the ble Sh gene conferring resistance to zeocin. This fusion, driven by the strong constitutive viral herpes simplex virus thymidine kinase enhancer promoter, led to a ≈7-fold overexpression of pol β (Fig. 1A). Growth rate analysis of control Sh and pol β∷Sh-transfected CHO cell lines indicated no difference in doubling time (Fig. 1B), suggesting that the activity of the replicative DNA polymerases (pol ∂ and pol ɛ) is not affected. To determine whether genetic instability increased in the cell lines that overexpressed pol β, we measured the frequency of spontaneous mutations by two conventional methodologies that test the appearance of a mutational event leading to a resistance phenotype. First, we measured the ouabain resistance (OuaR) at the Na-K-ATPase locus and found that the frequency of OuaR mutants was 12.8-fold higher for the overexpressing pol β cell line (Table 1). Second, the mutant frequency was analyzed at the locus encoding the purine salvage enzyme HPRT; a 4.2-fold increase for the pol β∷Sh cells relative to the Sh cells was measured (Table 1). However, the mutation frequency was strongly underevaluted in this case, because we found a hypersensitivity of pol β∷Sh cells to the selecting agent 6-TG (data not shown); this hypersensitivity was probably caused by the capacity of the overexpressed pol β to incorporate toxic levels of the nucleotide analogs into the neosynthetized DNA (14) after HPRT-independent metabolization. Mutation data shown in Table 1 were measured from one clone, but we found comparable OuaR frequencies by using other independent Sh and pol β∷Sh stably transfected clones (data not shown). In addition, we verified the pol β-mediated mutator phenotype by developing a third test that did not require resistance phenotype expression; this test used the E. coli β-galactosidase gene as the mutagenic target. Stable Sh/lacZ∷neo and pol β∷Sh/lacZ∷neo CHO cell lines were constructed by transfecting the Sh and pol β∷Sh CHO cell lines, respectively, with an expression vector that harbors the E. coli β-galactosidase gene fused in-frame with the neo gene conferring resistance to G418. Mutagenesis was analyzed after 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) staining by determining the proportion of white cells (resulting from the inactivation of the β-galactosidase gene) among the blue Lac+ cells. All of the six independent Sh/lacZ∷neo clones we tested contained a relatively high proportion of white cells (17.5 ± 5.3%), presumably because LacZ expression in mammalian cells is subject to epigenetic inactivation (16). Nevertheless, a significant increase in the population of LacZ-defective mutant cells was observed for the seven independent pol β∷Sh/lacZ∷neo clones tested (46.5 ± 13.3%).
Figure 1.
Phenotypic comparison of Sh and pol β∷Sh CHO cell lines. (A), Western blot analysis of pol β expression in Sh and pol β∷Sh stable CHO cell lines. Whole cell extracts (100 μg) were separated on a 12% SDS/PAGE and transferred to a polyvinylidene fluorure) membrane. Expression of pol β was revealed by using a polyclonal Ab (kindly provided by S. Wilson, Research Triangle Park, NC) with the enhanced chemiluminescence system (Amersham). (B), Growth rates of Sh (•) and pol β∷Sh (○) cell lines. Sensitivity of Sh and pol β∷Sh cell lines to cisplatin (C), mechlorethamine (D), melphalan (E), and methyl methanesulfonate (MMS) (F) is shown. Survival is expressed as the relative plating efficiency of treated cells to the control. Results are the mean ± SD of at least three separate experiments performed in duplicate.
Table 1.
Increased spontaneous mutation frequencies and rates in pol β∷Sh cells
|
Sh
|
pol β∷Sh
|
|||
|---|---|---|---|---|
| OuaR | HPRT | OuaR | HPTR | |
| Replica cultures | 35 | 10 | 23 | 10 |
| Cultures with mutants | 8 | 8 | 20 | 10 |
| Mutant colonies | 8 | 21 | 49 | 58 |
| Mutation frequency | 7.6 × 10−8 | 4.2 × 10−6 | 9.75 × 10−7 | 1.8 × 10−5 |
| Mutation rate | 4.74 × 10−8 | 9.69 × 10−7 | 3.8 × 10−7 | 2.92 × 10−6 |
To compare accurately the occurrence of mutations, we calculated the rates of mutation per locus and per generation producing ouabain as well as 6-TG resistances by the method of the mean (15). The mutation rate at the Na-K-ATPase locus in pol β∷Sh was 8-fold greater than the rate measured for the Sh cells (Table 1). For the HPRT locus, a 3-fold increase in the mutation rate was found in pol β∷Sh cells (Table 1). Taken together, these data indicate that an elevated level of pol β in cells results in a spontaneous mutator phenotype. It should be pointed out that the increased level of mutation rate that we observed here by using the OuaR test with pol β is similar to those rates observed in mismatch repair-deficient cell lines, such as the embryonic fibroblast cell line established from mice deficient for Msh2 (18) and the human ovarian adenocarcinoma cell line 2008/A that is deficient for hMlh1 (19).
We have shown previously that purified pol β has the potential to efficiently catalyze error-prone translesion synthesis in vitro across the cisplatin-d(GpG) intrastrand crosslinks (12, 13). Therefore, we investigated whether overexpression of pol β could modulate cellular sensitivity to the anticancer cross-linking agents cisplatin, mechlorethamine, and melphalan, whose therapeutic effects result from their covalent binding to DNA to form mostly intrastrand crosslinks. For all three agents, a reduced drug sensitivity was observed in the pol β∷Sh cells as compared with the control cells (Fig. 1 C–E). In contrast, no differential sensitivity to the monofunctional alkylating agents methyl methanesulfonate (Fig. 1F) and ethyl methanesulfonate (data not shown) were observed. Although the participation of pol β to the base excision repair system of monoadducts has been demonstrated (6), this observation is not surprising if we assume that the rate-limiting step in the base excision repair process is the incision step that precedes the pol β-mediated DNA synthesis.
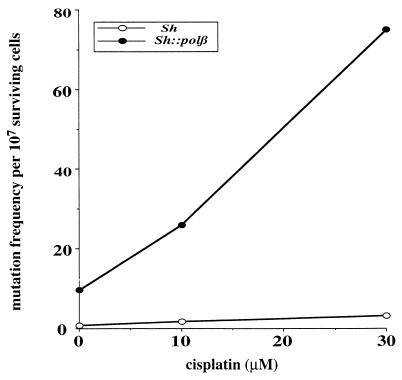
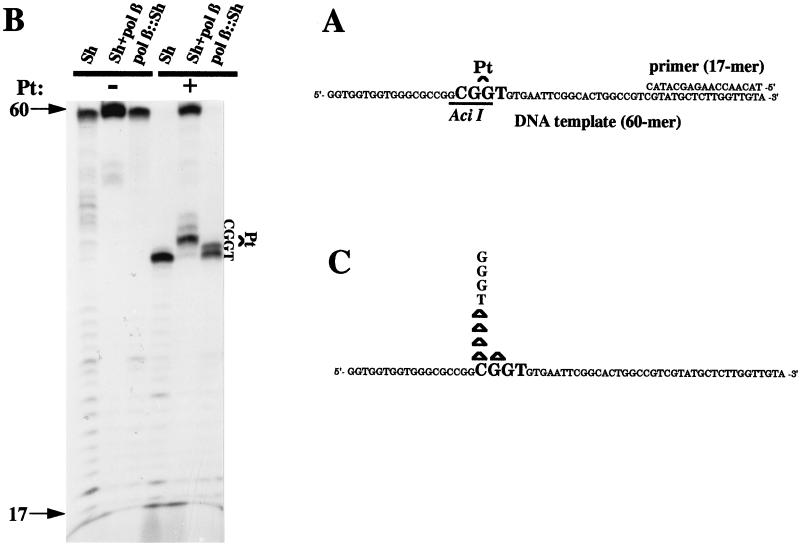
We subsequently determined whether Sh and pol β∷Sh cell lines responded differently to the mutagenic action of cisplatin by examining OuaR frequencies in both cell lines exposed to various doses of the drug (Fig. 2). A clear difference was observed between the dose-dependent increases, suggesting that the mutator phenotype induced by an elevated level of pol β can be potentiated when the cells are exposed to the anticancer drug cisplatin. Therefore, mutation accumulation in cells overexpressing pol β may be enhanced during chemotherapy treatment. Because we showed the capacity of pol β to efficiently catalyze bypass replication in vitro through cisplatin bifunctional lesions (12, 13), we hypothesize that the observed pol β-mediated mutagenic tolerance to cisplatin in cells may result from an elevated translesion DNA synthesis, although a role for pol β in nucleotide excision repair cannot be ruled out. Primer extension experiments were conducted with extracts from control cells and from cells overexpressing pol β by using untreated and site-specific Pt-d(GpG)-damaged, single-stranded DNA templates (Fig. 3 A and B). The control cell extracts stopped opposite to the base immediately preceding the unique Pt-d(GpG) adduct, whereas DNA synthesis by pol β∷Sh CHO cell extracts added one more nucleotide opposite the first platinated guanine (Fig. 3B), which is the first step for a bypass process. Because of the extremely high concentration of lesions per nucleotide (one lesion per 60 nucleotides) in this in vitro assay compared with the in vivo situation, further extension and complete bypass cannot be achieved. Full-size products were generated only when the reaction was performed with the control cell extracts in the presence of excess purified pol β (Fig. 3B); products were not generated in the presence of pol α, pol δ, or pol ɛ (data not shown). To investigate the fidelity of the in vitro bypass replication, the full-size reaction products generated by Sh extract supplemented by purified pol β were purified, PCR-amplified, screened for mutations at the site of the lesion by resistance to Aci I digestion, and analyzed by sequencing. The most frequent modifications were −1 deletions and base substitutions at the cytosine 5′ of the damaged guanines (Fig. 3C). Thus, an excess of pol β in normal cell extracts can perform mutagenic bypass replication in vitro, which suggests that translesion synthesis could be one of the key mechanisms to explain the tolerance phenotype in vivo. Cisplatin, which is mainly used for the treatment of testicular and ovarian cancers, may cause several undesirable side effects, including tumorigenesis and mutagenesis (20). Mutagenic translesion synthesis by overexpressed pol β may be one of multiple reasons why such complications develop. Enhanced bypass synthesis was demonstrated previously to occur in two cisplatin-resistant human ovarian cancer cell lines (21).
Figure 2.
Cisplatin-induced mutagenesis in Sh and pol β∷Sh cell lines. For determination of cisplatin-induced mutagenesis, cells were treated for 1 h with various doses of cisplatin, allowed to grow for 1 wk before plating at 3 × 106 cells in ouabain-supplemented medium, and grown for an additional week. Next, plates were stained, and OuaR mutant colonies were counted.
Figure 3.
Mutagenic in vitro translesion synthesis by pol β∷Sh CHO cell extracts. (A), The 60-mer substrate annealed to a 17-base oligonucleotide primer used in the replication assay. (B), primer extensions of intact and damaged substrates were replicated for 1 hr at 37°C by the indicated cell extracts. The reaction conditions are described. Arrows indicate the position of the primer (17) and the full-size reaction products (60-mer). The position of the platinated guanines is also indicated. (C), Spectrum of mutations induced by the pol β-mediated translesion synthesis. The full-size (60-mer) reaction bypass product was PCR-amplified, and those products resistant to Aci I were subcloned and sequenced. −1 base deletions are shown as Δ, and single base substitutions are indicated by the nucleotide concerned. No mutations were detected when undamaged templates were processed as controls.
We and others found a significant higher expression of DNA pol β in many tumor cell lines (22), such as chronic myeloid leukemia and ovarian cancer cells (Y.C., C.C., and J.S.H., unpublished data), but because of the ploidy phenotype exhibited by most of these cell lines, the implication of this overexpression in tumorigenesis is difficult to evaluate. However, increases in pol β mRNA and protein were observed in human ovarian, colon, and leukemia cancer cells resistant to cisplatin over the sensitive parent cell lines (22), supporting a role for pol β in tolerance and tumor progression.
In conclusion, our experimental data support the concept that cancer may involve a mutator phenotype by overexpression of the error-prone DNA pol β. This genetic event, which can lead to spontaneous mutations and anticancer drug-induced mutations arising during the course of translesion replication, is likely to be an important contributory cause in the maintenance of a tumor cell during chemotherapy pressure.
Acknowledgments
We thank Florence Larminat, Guy Poirier, and Leon Müllenders for helpful comments and discussions. This work was supported in part by La Ligue Contre le Cancer-Comité de la Haute Garonne (fellowship to Y.C.).
ABBREVIATIONS
- pol β
DNA polymerase β
- CHO
Chinese hamster ovary
- 6-TG
6-thioguanine
- OuaR
ouabain resistance
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.L. Loeb L. Cancer Res. 1991;51:3075–3079. [PubMed] [Google Scholar]
- 2.de Wind N, Dekker M, Berns A, Radman M, te Riele H. Cell. 1995;82:321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]
- 3.Edelmann W, Yang K, Umar A, Heyer J, Lau K, Fan K, Liedtke W, Cohen P E, Kane M F, Lipford J R, et al. Cell. 1997;91:467–477. doi: 10.1016/s0092-8674(00)80433-x. [DOI] [PubMed] [Google Scholar]
- 4.Hartwell L H, Kastan M B. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 5.Cahill D P, Lengauer C, Yu J, Riggins G J, Willson J K V, Markowitz S D, Kinzler K W, Vogelstein B. Nature (London) 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 6.Sobol R W, Horton J K, Kühn R, Gu H, Singhal R K, Prasad R, Rajewsky K, Wilson S H. Nature (London) 1996;379:183–186. doi: 10.1038/379183a0. [DOI] [PubMed] [Google Scholar]
- 7.Zmudzka B Z, Fornace A J, Collins J, Wilson S H. Nucleic Acids Res. 1988;16:9589–9596. doi: 10.1093/nar/16.20.9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fornace A J, Zmudzka B, Hollander M C, Wilson S H. Mol Cell Biol. 1989;9:851–853. doi: 10.1128/mcb.9.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunkel T A. J Biol Chem. 1985;260:5787–5796. [PubMed] [Google Scholar]
- 10.Kornberg A, Baker T A. DNA Replication. 2nd Ed. New York: Freeman; 1991. [Google Scholar]
- 11.Kamath-Loeb A S, Hizi A, Kasai H, Loeb L A. J Biol Chem. 1997;272:5892–5898. doi: 10.1074/jbc.272.9.5892. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann J S, Pillaire M J, Maga G, Podust V, Hübscher U, Villani G. Proc Natl Acad Sci USA. 1995;92:5356–5360. doi: 10.1073/pnas.92.12.5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann J S, Pillaire M, Garcia-Estefania D, Lapalu S, Villani G. J Biol Chem. 1996;271:15386–15392. doi: 10.1074/jbc.271.26.15386. [DOI] [PubMed] [Google Scholar]
- 14.Bouayadi K, Hoffmann J S, Fons P, Tiraby G, Reynes J P, Cazaux C. Cancer Res. 1997;57:110–116. [PubMed] [Google Scholar]
- 15.Capizzi R L, Jameson J W. Mutat Res. 1973;17:147–148. doi: 10.1016/0027-5107(73)90265-0. [DOI] [PubMed] [Google Scholar]
- 16.van Sloon P P, Lohman P H, Vrieling H. Mutat Res. 1997;382:21–33. doi: 10.1016/s1383-5726(97)00006-x. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann J S, Pillaire M, Lesca C, Burnouf D, Fuchs R, Defais M, Villani G. Proc Natl Acad Sci USA. 1996;93:13766–13769. doi: 10.1073/pnas.93.24.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reitmair A H, Risley R, Bristow R G, Wilson T, Ganesh A, Jang A, Peacock J, Benchimol S, Hill R P, Mak T W, et al. Cancer Res. 1997;57:3765–3771. [PubMed] [Google Scholar]
- 19.Aebi S, Kurdi-Haidar B, Gordon R, Cenni B, Zheng H, Fink D, Christen R D, Boland C R, Koi M, Fishel R, et al. Cancer Res. 1996;56:3087–3090. [PubMed] [Google Scholar]
- 20.Greene M. J Natl Cancer Inst. 1992;84:306–312. doi: 10.1093/jnci/84.5.306. [DOI] [PubMed] [Google Scholar]
- 21.Mamenta E L, Poma E E, Kaufmann W K, Delmastro D A, Grady H L, Chaney S G. Cancer Res. 1994;54:3500–3505. [PubMed] [Google Scholar]
- 22.Scanlon K J, Kashani-Sabet M, Miyachi H. Cancer Invest. 1989;7:581–587. doi: 10.3109/07357908909017533. [DOI] [PubMed] [Google Scholar]