Abstract
To test directly whether fibrin(ogen) is a key binding site for apolipoprotein(a) [apo(a)] in vessel walls, apo(a) transgenic mice and fibrinogen knockout mice were crossed to generate fibrin(ogen)-deficient apo(a) transgenic mice and control mice. In the vessel wall of apo(a) transgenic mice, fibrin(ogen) deposition was found to be essentially colocalized with focal apo(a) deposition and fatty-streak type atherosclerotic lesions. Fibrinogen deficiency in apo(a) transgenic mice decreased the average accumulation of apo(a) in vessel walls by 78% and the average lesion (fatty streak type) development by 81%. Fibrinogen deficiency in wild-type mice did not significantly reduce lesion development. Our results suggest that fibrin(ogen) provides one of the major sites to which apo(a) binds to the vessel wall and participates in the generation of atherosclerosis.
An elevated plasma level of lipoprotein(a) [Lp(a)] is one of the major risk factors for atherosclerosis and its manifestations, myocardial infarction, stroke, and restenosis (see refs. 1 and 2 and references therein). Lp(a) particles contain the lipid and protein components of low-density lipoprotein plus apolipoprotein(a) [apo(a)]. It has been postulated that Lp(a) induces atherosclerosis through its plasminogen-like component apo(a). The human apo(a) gene has been successfully introduced into the mouse, an animal species that normally lacks this gene, and these apo(a) mice develop fatty-streak type lesions in aorta when maintained on a high-fat diet for several months (3). Further experiments in this mouse model have shown a coincidence of apo(a) deposition, decreased level of plasmin, decreased level of active transforming growth factor β, and increased level of smooth muscle cell activation and fatty streak type of lesion development in the vessel walls (4). Because of the extensive sequence homology between apo(a) and plasminogen, a potential mechanism by which apo(a) promotes atherosclerosis is to inhibit plasminogen activation to plasmin through competitively inhibiting binding of plasminogen to sites such as fibrin. This hypothesis has been supported by a number of in vitro studies (5–9). The development of fibrinogen-deficient mice (Fib−/− mice) allows direct testing of the interaction of fibrin and apo(a) in vivo (10). To test directly whether fibrin(ogen) is a key binding site for apo(a), apo(a) vascular accumulation and lipid lesion were measured in Fib−/−/apo(a) mice and control mice.
MATERIALS AND METHODS
Mice.
The human apo(a) mice created in the C57BL/6SJL background (3, 11) were backcrossed to C57BL/6 mice for six generations. The Fib−/− mice originally made in the 129/Ola background (12) were backcrossed to C57BL/6 mice for eight generations. Apo(a) mice were bred with Fib−/− mice to produce offspring both hemizygous for apo(a) and heterozygous for the null allele [apo(a)/Fib+/−]. The apo(a)/Fib−/−, apo(a), Fib−/−, and wild-type control mice used in this study were produced by crossing apo(a)/Fib+/− mice either with Fib+/− or Fib−/− mice. Some of the apo(a) and wild-type control mice were generated by interbreeding apo(a) to wild-type siblings. The fibrinogen null genotype was identified by PCR of DNA derived from ear or tail biopsies using oligonucleotides described by Suh et al. (10). Apo(a) mice were identified by measuring plasma apo(a) concentration using a commercial ELISA detection kit Macra Lp(a) (Strategic Diagnostic, Newark, DE). Mice of all genotypes were housed together and were fed a standard low-fat diet (Purina Mills, Richard, IN) until 6–8 weeks of age when they were switched to an atherogenic high-fat diet containing 1.25% cholesterol, 0.5% cholic acid, and 15% fat (ICN) (3) for 2 months.
Lipid Analysis.
Total plasma cholesterol levels and high density lipoprotein (HDL) cholesterol levels were measured by using an enzymatic method (Sigma). HDL was measured after the precipitation of low density lipoprotein and very low density lipoprotein fractions from the plasma with phosphotungstic acid and magnesium chloride (Sigma).
Lesion Detection.
Aortic sectioning, lipid staining, and lesion scoring in a blinded fashion were performed similar to previously described (13, 14). Briefly, the heart and attached aorta from mice were rinsed with PBS and immediately frozen in OCT embedding medium (Miles). Ten-micrometer frozen sections were taken and fixed with 10% phosphate-buffered formalin. The first and most proximal section of the aorta was taken where the aorta becomes rounded and the aortic valves distinct. Sections were stained with oil-red O and hematoxylin and counterstained with light green. Lesion areas, as determined by oil-red O staining, were measured by using a calibrated eyepiece at ×100 magnification. The mean lesion area per section per animal was determined for each individual animal.
Immunohistochemical Staining for Fibrin(ogen).
Adjacent sections to those used for oil-red O staining were fixed with 10% phosphate-buffered formalin for 10 min. After three washes with Tris-buffered saline (TBS), sections were incubated with 3% BSA for 30 min, then with rabbit anti-mouse fibrinogen antibody (12) diluted 1:1,000 in TBS, 3% BSA (overnight at 4°C) followed by a 4-hr incubation at room temperature with rabbit anti-rabbit alkaline phosphatase-conjugated IgG (Sigma) diluted 1:800 in TBS, 3% BSA. Color was developed by using alkaline phosphatase substrate tablets set (Sigma). Sections were counterstained with hematoxylin.
Quantitative Vessel Wall Fluorescence Assays for Apo(a).
Assays were performed essentially as described (14) with modifications. Briefly, sections adjacent to those used for lesion quantification were fixed in ice-cold acetone for 90 s. The dilution for the primary antibody was 1:1,000, and 1:80 for the secondary antibody (fluorescein isothiocyanate conjugated). The antibody incubation conditions were identical to those described for the fibrinogen staining. Two images of the vessel wall, covering ≈75% of the entire vessel cross section, were randomly selected under phase contrast (×20 objective) and then captured by using a laser confocal microscope system. The gain of the photomultiplier was set so that less than 2% of the intensity signals were saturated. Two sections (80 μm apart) were used for each animal. The average apo(a) staining intensity for each mouse was determined as follows. For each image, a fluorescent intensity histogram representing the distribution of staining in the vessel wall was constructed by using NIH Image software and analyzed by using an igorpro (WaveMetrics, Lake Oswego, OR) program modified by our group. To decrease contributions from nonspecific staining and autofluorescence, only intensity above a threshold was integrated to determine total staining per image. The threshold was determined to exclude 95% of the fluorescence observed in sections from nontransgenic mice by using the same staining procedure. Total staining intensities from different images were combined and normalized to the total vessel areas analyzed for the mouse to obtain the average intensity of the vessel wall for that mouse. The average intensity of background-fluorescence in nontransgenic mice then was subtracted from this value to give the average specific intensity.
Statistical Analysis.
All data were presented as mean ± SEM unless otherwise indicated. The significance levels of the mean of average lesion area per mouse were determined by the Mann–Whitney u test, whereas the significance of the mean of the lipid levels and the mean of the average fluorescent intensity level per mouse were determined by t test.
RESULTS
Generation of Apo(a)/Fib−/− Mice.
Apo(a)/Fib−/− and their control mice were generated by interbreeding fibrinogen knockout mice and apo(a) mice. As previously reported for Fib−/− mice (10, 15), all progeny appeared normal at birth, but within several days, a fraction of the Fib−/− mice developed spontaneous bleeding events. Mice that survived the neonatal period attained normal body weights and developed into healthy-looking adults. The introduction of apo(a) transgene did not affect the survival and general appearance of Fib−/− mice. After 2 months on the high-cholesterol atherogenic diet, about one-third of Fib−/− or apo(a)/Fib−/− mice died of multiple bleeding events that most commonly occurred in liver, intestine, brain, lung, or skin. On neither the chow nor high-cholesterol diet did the addition of the apo(a) transgene have a significant effect on the rate of death because of spontaneous bleeding that occurs in mice lacking a functional fibrinogen gene. The plasma levels of total cholesterol, HDL cholesterol and apo(a) in apo(a)/Fib−/− mice were not decreased as compared with the corresponding Fib+/+ mice that carry or lack the apo(a) transgene (Table 1). These data suggest that, although the health condition of Fib−/− mice was unstable, they are able to maintain a lipid profile and a plasma apo(a) concentration that are similar to that of Fib+/+ (wild type) and apo(a) mice.
Table 1.
Plasma apo(a) and lipid analyses on fibrinogen-deficient mice
| Genotype | Apo(a), mg/dl | Total cholesterol, md/dl | HDL cholesterol, mg/dl |
|---|---|---|---|
| Apo(a)/Fib−/− | 6.9 ± 0.7 (n = 8) | 128 ± 28 (n = 7) | 45 ± 10 (n = 7) |
| Apo(a) | 6.0 ± 0.5 (n = 17) | 124 ± 9 (n = 19) | 51 ± 5 (n = 19) |
| Fib−/− | N/A | 178 ± 31 (n = 13) | 69 ± 17 (n = 12) |
| Wild type | N/A | 149 ± 25 (n = 20) | 58 ± 7 (n = 20) |
N/A, not available.
Colocalization of Fibrin(ogen), Apo(a), and Lipid Lesion in the Vessel Walls.
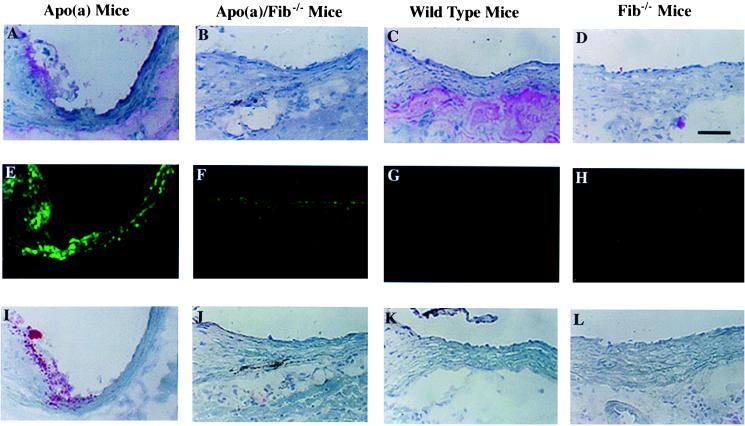
Immunohistochemical staining was performed to examine the locations of fibrin(ogen), apo(a) accumulation, and lipid lesion formation in apo(a), apo(a)/Fib−/−, wild-type, and Fib−/− mice. In the aortic wall of apo(a) mice, focal fibrin(ogen) staining (Fig. 1A) usually is colocalized with focal apo(a) accumulation (Fig. 1E) and fatty streak type lesions (Fig. 1I). This finding is consistent with previous reports that local fibrin(ogen) is present within early and advanced atherosclerotic lesions in mouse and human subjects, and the location overlaps with apo(a) deposition in human atherosclerotic plaques (15–17). This finding is also consistent with the proposal that fibrin(ogen) is an important site for apo(a) binding in vivo.
Figure 1.
Immunofluorescent and histochemical staining of fibrin(ogen) and apo(a) accumulation and lipid deposition in proximal sections of mouse aorta after 2 months of an atherogenic diet. Representative adjacent sections are shown for the three different types of staining. (A–D) Immunohistochemical staining of fibrin(ogen) in apo(a), apo(a)/Fib−/−, wild-type, and Fib−/− mice, respectively. (E–H) Immunofluorescent staining of apo(a) accumulation. (I–L) Oil-red O staining for fatty streak lesions. Note more lipid is in the apo(a) mice, including the edge of the valve and the vessel walls. Fibrin(ogen) staining is essentially colocalized with apo(a) accumulation and fatty streak lesions. (Scale bar equals 50 μm.)
Fibrinogen Deficiency Decreases Apo(a) Accumulation in Aortic Walls.
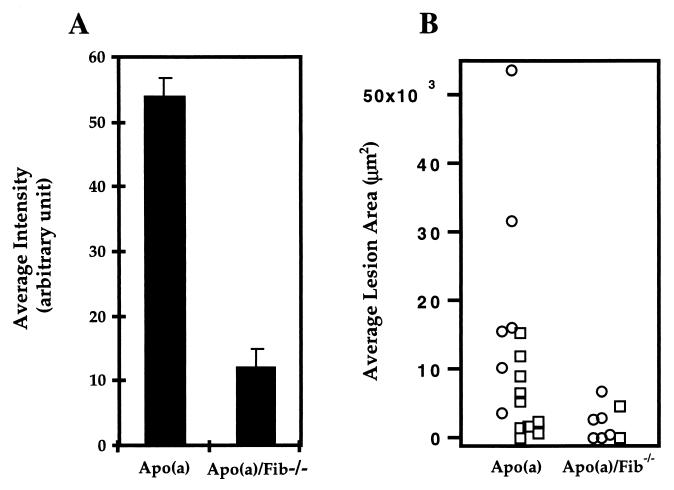
After 2 months on an atherogenic diet, mice were euthanazied, and proximal aortic sections were prepared as described in Materials and Methods. Quantitative immunofluorescent analyses were used to directly measure the vascular accumulation of apo(a) in apo(a) mice and apo(a)/Fib−/− mice. A total of 66 images were quantified for 11 apo(a) mice and eight apo(a)/Fib−/− mice (for details about the measurements, see Materials and Methods). Average intensity of immunofluorescent staining for apo(a) in apo(a)/Fib−/− mice was 78% decreased compared with apo(a) mice expressing fibrinogen (Fig. 2A). This difference was highly significant (P < 0.001). No difference was observed between males and females within each group. As shown in Fig. 1E, the intensity of apo(a) staining in apo(a)/Fib+/+ mice vessel walls is uneven: some regions were much more intense than others. In contrast, the apo(a) staining in apo(a)/Fib−/− mice was distributed in a scattered manner (Fig. 1F) and the intensity is relatively even. This result strongly supports the hypothesis that fibrin(ogen) is one of the major sites to which apo(a) binds in the vessel walls.
Figure 2.
Quantitative analyses of immunofluorescent-stained apo(a) accumulation and oil-red O-stained lipid lesions in the proximal aorta of apo(a) and apo(a)/Fib−/− mice all maintained on high-fat diet. (A) Group mean of average vessel wall intensity [arbitrary units (1–250)/area (number of pixels)] in apo(a) (n = 11) and apo(a)/Fib−/− mice (n = 8). The mean of average intensity for apo(a) mice is 53.7 ± 3.3; for apo(a)/Fib−/− mice it is 11.8 ± 2.2 (P = 1.17 × 10 −8). The staining was performed as one batch. (B) Average lipid lesion area per section per mouse (females, ○; males, □). The mean of the average lesion area per section per mouse is 11,584 ± 3,465 μm2 for the apo(a) mice and 2,161 ± 889 μm2 for the apo(a)/Fib−/− mice. The median is 7,865 μm2 (range: 0–53,560) for the apo(a) mice and 1,560 μm2 (range: 0–6,760) for the apo(a)/Fib−/− mice (P = 0.02).
Fibrinogen Deficiency Reduces Lesion Development in Apo(a) Mice.
To confirm the relationship between vascular apo(a) deposition and lesion development reported previously by our laboratory and others (3, 14, 18), we examined the lesion sizes in sections adjacent to those used for apo(a) quantification, following the protocol originally established by Paigen et al. (19). The area of oil-red O staining per mouse (fatty streak lesion) for the apo(a) and the apo(a)/Fib−/− mice is shown in Fig. 2B. The apo(a)/Fib−/− mice had an average lesion area 81% smaller than that of the apo(a) mice. The mean of the average lipid staining lesion area per section per mouse was 11,584 ± 3,465 μm2 for the apo(a) mice (n = 16 mice), 2,161 ± 889 μm2 for the apo(a)/Fib−/− mice (n = 8 mice), 2,041 ± 853 μm2 for the wild-type mice (n = 20 mice), and 162 ± 112 μm2 for the Fib−/− mice (n = 9 mice). The lipid staining lesion area in the apo(a)/Fib−/− mice was significantly smaller than that in the apo(a) mice (P < 0.05), but not different from that in the wild-type (P > 0.05) or the Fib−/− mice (P > 0.05) by Mann–Whitney u test. When gender was taken into consideration, the difference in lesion area between the female apo(a)/Fib−/− mice and female apo(a) mice was very significant (P < 0.001), whereas the difference between apo(a)/Fib−/− female and wild-type or Fib−/− female remained insignificant (P > 0.05). No comparison was done for male mice because of insufficient numbers of mice. These results suggest that fibrin(ogen) contributes to the atherosclerosis in apo(a) mice. Although Fib−/− mice had even fewer lesions than wild-type mice, this difference did not reach statistical significance (P > 0.05), except when the comparison was made only for females (P < 0.05; n = 7 for Fib−/− mice and n = 8 for wild-type mice). These results are consistent with the report of Xiao et al. (15), which showed that lesion development was delayed only in female Fib−/−/apoE-deficient mice at the earliest time point tested. Taken together, these results suggest that fibrin(ogen) plays an important role in the atherogenicity of apo(a) by serving as one of the major sites for apo(a) to binding to the vessel walls.
DISCUSSION
Lp(a) has been proposed to accelerate atherosclerosis by inhibiting fibrinolysis, inhibiting plasmin and active transforming growth factor β generation, and/or promoting foam cell generation (4, 20–22). Based on in vitro data, apo(a) has been reported to bind to a number of targets including fibrin, fibronectin, tetranectin, glycosaminoglycans, macrophages, and endothelial cells. The results of this study provided direct evidence that fibrin(ogen) promotes the binding of apo(a) to the vessel walls in vivo and is necessary for its resulting pathological effects.
It should be noted that human apo(a) does not form a covalent linkage to mouse apoB, as it does to the human counterpart (11). Although in human blood most apo(a) circulates in association with apoB in the Lp(a) lipoprotein particle, the nature of this linkage in plasma and in the vessel wall is complex. Apo(a) has a strong noncovalent linkage to apoB, and studies in human subjects have found that the covalent bond between the two proteins is exchangeable in circulation (23–25). In the future, transgenic mice expressing human apoB could be bred to apo(a) transgenic and fibrinogen knockout mice to evaluate the effects of fibrinogen deficiency on apo(a) in this context. To date, it has been shown that disruption of the lysine binding in apo(a) kringle IV-10 decreases the vascular accumulation of apo(a) in mice both lacking and including human apoB (14, 26). As another future study, apo(a) transgenic mice could be mated with plasminogen-deficient mice to test whether apo(a) possesses atherogenic properties in addition to its inhibitory effect on plasminogen activation.
In the interpretation of animal model results, the genetic background of the animals always should be considered (27). The genetic background of apo(a)/Fib−/−, apo(a), Fib−/−, and wild-type mice used in this study were essentially identical, because the progenitors of both apo(a) and Fib−/− mice had been backcrossed to C57BL/6 mice for at least six generations such that they are identical to C57BL/6 across more than 98% of the genome (28). In addition, the apo(a)/Fib−/− mice possessed no lower levels of total cholesterol or HDL that might affect the vascular lesion development (29). Therefore, the reduced lesion development in apo(a)/Fib−/− mice as compared with apo(a) mice was most likely directly because of the lack of fibrin(ogen). Fibrin(ogen) conceivably could influence vascular lesion development by mechanisms that require apo(a) or are independent of apo(a) (3, 14, 17, 30–32). Conceivably, mice lacking fibrin could resist atherosclerosis caused by any stimulus. However, a recent study has shown that fibrin(ogen) is not strictly required for lesion development in apoE−/− mice (15). Thus, fibrin deficiency does not protect mice from all atherogenic stimuli. It should be noted that plasma cholesterol levels are exceedingly high in apoE−/− mice (≈900 mg/dl; ref. 15), which may mask the effects of less-potent risk factors. In the context of the apo(a) transgenic model, fibrin appears to be an essential component of the atherogenic accumulation of apo(a) in the vessel wall and resulting fatty streak development.
Animal model systems of human disease frequently have some limitations, yet serve to direct further research. Limitations of the current model include: a diet not typical for humans, a lack of covalent attachment of apo(a) to mouse low density lipoprotein, and the general hemorrhagic risk of mice lacking fibrinogen. Despite this latter consideration, mice lacking fibrinogen still develop atherosclerosis in context of apoE deficiency (15) and achieve levels of plasma lipids on the high-fat diet comparable to fibrinogen containing litter mates (this study). Therefore, the current data imply that fibrin(ogen) is a major requirement for the concentration of apo(a) in vessel walls and can significantly contribute to the development of atherosclerosis.
Acknowledgments
We thank Dr. D. Boffelli and K. Schwartz for critical discussion and technical support. This research was supported by National Institutes of Health Grants HL-50590 to R.M.L. and HL-47826 to J.L.D. and an American Heart Association Postdoctoral Fellowship Award to X.J.L.
ABBREVIATIONS
- apo(a)
apolipoprotein(a)
- Lp(a)
lipolipoprotein(a)
- Fib−/− mice
fibrinogen-deficient mice
- HDL
high density lipoprotein
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Bostom A G, Cupples L A, Jenner J L, Ordovas J M, Seman L L, Wilson P W F, Schaefer E J, Castelli W P. J Am Med Assoc. 1996;276:544–548. doi: 10.1001/jama.1996.03540070040028. [DOI] [PubMed] [Google Scholar]
- 2.Assmann G, Schulte H, Eckardstein A V. Am J Cardiol. 1996;77:1179–1184. doi: 10.1016/s0002-9149(96)00159-2. [DOI] [PubMed] [Google Scholar]
- 3.Lawn R M, Wade D P, Hammer R E, Chiesa G, Verstuyft J G, Rubin E M. Nature (London) 1992;360:670–672. doi: 10.1038/360670a0. [DOI] [PubMed] [Google Scholar]
- 4.Grainger D J, Kemp P R, Liu A C, Lawn R M, Metcalfe J C. Nature (London) 1994;370:460–462. doi: 10.1038/370460a0. [DOI] [PubMed] [Google Scholar]
- 5.Edelberg J M, Gonzalez-Gronow M, Pizzo S V. Thromb Res. 1990;57:155–162. doi: 10.1016/0049-3848(90)90203-o. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Gronow M, Edelberg J M, Pizzo S V. Biochemistry. 1989;28:2374–2377. doi: 10.1021/bi00432a005. [DOI] [PubMed] [Google Scholar]
- 7.Hajjar K A, Gavish D, Breslow J L, Nachman R L. Nature (London) 1989;339:303–305. doi: 10.1038/339303a0. [DOI] [PubMed] [Google Scholar]
- 8.Loscalzo J, Weinfeld M, Fless G M, Scanu A M. Arteriosclerosis. 1990;10:240–245. doi: 10.1161/01.atv.10.2.240. [DOI] [PubMed] [Google Scholar]
- 9.Miles L A, Fless G M, Levin E G, Scanu A M, Plow E F. Nature (London) 1989;339:301–303. doi: 10.1038/339301a0. [DOI] [PubMed] [Google Scholar]
- 10.Suh T T, Holmback K, Jensen N J, Daugherty C C, Small K, Simon D I, Potter S S, Degen J L. Genes Dev. 1995;9:2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- 11.Chiesa G, Hobbs H H, Koschinsky M L, Lawn R M, Maika S D, Hammer R E. J Biol Chem. 1992;267:24369–24374. [PubMed] [Google Scholar]
- 12.Bugge T H, Flick M J, Daugherty C C, Degen J L. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 13.Rubin E, Krauss R, Spangler E, Verstuyft S, Clift S. Nature (London) 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 14.Boonmark N W, Lou X J, Yang Z J, Schwartz K, Zhang J-L, Rubin E M, Lawn R M. J Clin Invest. 1997;100:558–564. doi: 10.1172/JCI119565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao Q, Daton M J S, Witte D P, Kowala M C, Valentine M T, Degen J L. J Clin Invest. 1998;101:1184–1194. doi: 10.1172/JCI1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichikawa K, Nakagawa K, Hirano K, Sueishi K. Pathol Res Pract. 1996;192:224–232. doi: 10.1016/S0344-0338(96)80225-1. [DOI] [PubMed] [Google Scholar]
- 17.Bini A, Kudryk B J. Ann NY Acad Sci. 1995;748:461–471. doi: 10.1111/j.1749-6632.1994.tb17342.x. [DOI] [PubMed] [Google Scholar]
- 18.Lawn R M, Pearle A D, Kunz L L, Rubin E M, Reckless J, Metcalfe J C, Grainger D J. J Biol Chem. 1996;271:31367–31371. doi: 10.1074/jbc.271.49.31367. [DOI] [PubMed] [Google Scholar]
- 19.Paigen B, Morrow A, Holmes P A, Mitchell D, Williams R A. Atherosclerosis. 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 20.Scanu A M, Fless G M. J Clin Invest. 1990;85:1709–1715. doi: 10.1172/JCI114625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Utermann G. Science. 1989;246:904–910. doi: 10.1126/science.2530631. [DOI] [PubMed] [Google Scholar]
- 22.Miles L A, Plow E F. Thromb Haemostasis. 1990;63:331–335. [PubMed] [Google Scholar]
- 23.Trieu V N, Zioncheck T F, Lawn R M, McConathy W J. J Biol Chem. 1991;266:5480–5485. [PubMed] [Google Scholar]
- 24.Knight B L, Perombelon N Y F, Soutar A K, Wade D P, Seed M. Atherosclerosis. 1991;87:227–237. doi: 10.1016/0021-9150(91)90025-x. [DOI] [PubMed] [Google Scholar]
- 25.Bader, G., Edelstein, C., Samburek, R. D., Nishiwaki, M., Nazih, H., Schwartz, C., Scanu, A. M. & Brewer, H. B. (1996) Circulation 94, Suppl., 1–39.
- 26.Hughes S D, Lou X J, Ighani S, Verstuyft J, Grainger D J, Lawn R M, Rubin E M. J Clin Invest. 1997;100:1493–1500. doi: 10.1172/JCI119671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paigen B, Ishida B Y, Verstuyft J, Winters R B, Albee D. Arteriosclerosis. 1990;10:316–323. doi: 10.1161/01.atv.10.2.316. [DOI] [PubMed] [Google Scholar]
- 28.Silver L M. Mouse Genetics. New York: Oxford Univ. Press; 1995. [Google Scholar]
- 29.Grundy S M. Arch Intern Med. 1997;157:1177–1184. [PubMed] [Google Scholar]
- 30.Languino L R, Plescia J, Duperray A, Brian A A, Plow E F, Geltosky J E, Altieri D C. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- 31.Stirk C M, Kochhar A, Smith E B, Thompson W D. Atherosclerosis. 1993;103:159–169. doi: 10.1016/0021-9150(93)90259-w. [DOI] [PubMed] [Google Scholar]
- 32.Naito M, Nomura H, Iguchi A. Thromb Res. 1996;84:129–136. doi: 10.1016/0049-3848(96)00168-5. [DOI] [PubMed] [Google Scholar]