Abstract
The cabbage aphid, Brevicoryne brassicae, has developed a chemical defence system that exploits and mimics that of its host plants, involving sequestration of the major plant secondary metabolites (glucosinolates). Like its host plants, the aphid produces a myrosinase (β-thioglucoside glucohydrolase) to catalyse the hydrolysis of glucosinolates, yielding biologically active products. Here, we demonstrate that aphid myrosinase expression in head/thoracic muscle starts during embryonic development and protein levels continue to accumulate after the nymphs are born. However, aphids are entirely dependent on the host plant for the glucosinolate substrate, which they store in the haemolymph. Uptake of a glucosinolate (sinigrin) was investigated when aphids fed on plants or an in vitro system and followed a different developmental pattern in winged and wingless aphid morphs. In nymphs of the wingless aphid morph, glucosinolate level continued to increase throughout the development to the adult stage, but the quantity in nymphs of the winged form peaked before eclosion (at day 7) and subsequently declined. Winged aphids excreted significantly higher amounts of glucosinolate in the honeydew when compared with wingless aphids, suggesting regulated transport across the gut. The higher level of sinigrin in wingless aphids had a significant negative impact on survival of a ladybird predator. Larvae of Adalia bipunctata were unable to survive when fed adult wingless aphids from a 1% sinigrin diet, but survived successfully when fed aphids from a glucosinolate-free diet (wingless or winged), or winged aphids from 1% sinigrin. The apparent lack of an effective chemical defence system in adult winged aphids possibly reflects their energetic investment in flight as an alternative predator avoidance mechanism.
Keywords: tritrophic interactions, chemical defences, convergent evolution, glucosinates
1. Introduction
Secondary metabolites form a chemical defence mechanism for a variety of organisms (Berenbaum 1995). In particular, plants produce a vast array of secondary metabolites which function in defence against herbivores and pathogens. Cruciferous plants (Brassicaceae) synthesize glucosinolates, amino acid-derived compounds with a β-thioglucose moiety, a sulphonated oxime and a variable side chain. Myrosinase (β-thioglucoside glucohydrolase, EC number 3.2.1.147) serves a central role in this defence system, catalysing the hydrolysis of glucosinolates. Myrosinase and glucosinolates are spatially separated within plants, but tissue damage brings them together and the reaction has been described as a functional ‘mustard oil bomb’ (Ratzka et al. 2002), yielding a variety of toxic secondary compounds such as isothiocyanates, thiocyanates and nitriles (Bones & Rossiter 1996). The exact nature of the products depends on the nature of the substitution, pH, the concentration of Fe2+, ascorbate and epithiospecifier protein (Lambrix et al. 2001; Zabala et al. 2005; Burow et al. 2006) and the putative thiocyanate-forming factor has recently been characterized (Burow et al. 2007). Specialist crucifer-feeding insects have evolved counter-adaptive biochemical mechanisms to allow feeding on plants containing glucosinolates. Larvae of the cabbage white butterfly, Pieris rapae, possess a nitrile-specifier protein which diverts glucosinolate degradation from isothiocyanates to less-toxic nitriles (Wittstock et al. 2004), while the diamondback moth (Plutella xylostella) desulphates glucosinolates to inactive metabolites (Ratzka et al. 2002). Other crucifer-specialist herbivores, such as the aphid species Brevicoryne brassicae and Lipaphis pseudobrassicae (Lipaphis erysimi), are adapted to exploit glucosinolates. These two aphid species sequester plant glucosinolates and have evolved a specific myrosinase which is distinctly different from plant myrosinase (Jones et al. 2001, 2002; Pontoppidan et al. 2001; Bridges et al. 2002; Husebye et al. 2005). Aphid myrosinase is localized to the sarcoplasm of non-flight muscle, apparently allowing the insects to mimic the host plants' defence mechanism (Bridges et al. 2002), although the location of sequestered glucosinolate within aphids has not been determined. Myrosinase enzyme from the cabbage aphid, B. brassicae, has recently been purified and partially characterized (Jones et al. 2001). The aphid enzyme is capable of hydrolysing a number of common plant glucosinolates including sinigrin (2-propenyl glucosinolate) and glucotropaeolin (benzylglucosinolate; Francis et al. 2002; Jones et al. 2002), and a dual function for aphid myrosinase as an oxidase and thioglucosidase has been proposed (Francis et al. 2002), although this claim requires more rigorous proof.
The aphid glucosinolate–myrosinase system probably plays an important role in protecting aphid colonies from natural enemies. When the aphid body is damaged, volatile isothiocyanates are released as a result of glucosinolate hydrolysis (Francis et al. 2004) and may be directly toxic to natural enemies (Francis et al. 2001). In addition, isothiocyanates have been reported to act as synergists of the aphid alarm pheromone (E)-β-farnesene (Dawson et al. 1986) and the aphid myrosinase may therefore serve a key role in the communication of warning signals between members of a developing colony upon attacks from predators and parasitoids (Bridges et al. 2002). Aphids may also show another, longer-term response to the exposure to natural enemies. By disturbing the aphid colony, aphid antagonists may increase the production of winged forms (Kunert et al. 2005). Although this is a relatively slow predator avoidance mechanism, involving development of the flight apparatus over several days, winged individuals may be less dependent on the glucosinolate–myrosinase system for defence.
In this study, we investigated the ontogeny and functional significance of the glucosinolate–myrosinase system in B. brassicae. Young aphids acquire the defence system very early in their development: embryos express myrosinase in head/thoracic muscle and take up glucosinolate from the maternal haemocoel. Nymphs continue to accumulate dietary glucosinolate very rapidly following birth, but winged and wingless aphid morphs show different uptake patterns. Using an artificial diet system to manipulate aphid glucosinolate content, we demonstrate the effects of the defence system on a natural enemy.
2. Material and methods
(a) Insects and plants
A clone of B. brassicae was maintained on Brassica nigra in a growth room at 18°C and 16L : 8D. Unless stated otherwise, experimental aphids were also reared on B. nigra, as this plant species contains sinigrin as the dominant glucosinolate (Fahey et al. 2001). Throughout this study, experimental aphids developed as a mixture of winged and wingless forms. For convenience, we refer to nymphs with discernable wing buds as ‘winged’ aphids, although true wings do not emerge until the adult stage.
(b) Light microscopy: aldehyde fixation–alcohol dehydration
The conventional fixation and embedding method was the same for light and electron microscopy specimens. Adult aphids were bisected in a droplet of fixative (2.5% paraformaldehyde, 1% glutaraldehyde in 100 mM phosphate buffer, pH 7.2) subjected to light vacuum infiltration for 60 min and dehydrated through an ethanol series (to 70% EtOH v/v). Specimens were embedded in LR white resin and thermally cured. Polymerized blocks were sectioned on an Ultracut E microtome (Reichert Jung) with glass knives to a thickness of 0.5 μm and mounted on poly l-lysine-coated microscope slides.
(c) Immunostaining
All antibody solutions were prepared in 50 mM phosphate-buffered saline pH 7.2, containing 0.005% Tween 20 (v/v) and 0.2% cold water fish gelatin (PBS–GT).
After blocking in 5% normal goat serum (v/v) for 1 h at 37°C, slide-mounted sections were covered in a 20 μl droplet of anti-aphid myrosinase antibody (Jones et al. 2001; diluted to 1 : 1000) and incubated at 37°C for 1 h. After three washes in PBS–GT, sections were incubated in goat anti-rabbit IgG, 10 nm gold diluted to 1 : 200 for 1 h at 37°C. Sections were washed in deionized water before incubation with silver enhancement solution (Amersham, UK). Enhancement was monitored visually under a binocular microscope and halted by repeated rinsing in deionized water. Sections were counterstained in 0.1% toluidine blue, warm-air dried, mounted in Histomount (RA Lamb, Laboratory Supplies, London, UK) and examined using a Nikon Optiphot microscope.
(d) Electron microscopy
Aldehyde-fixed tissue was processed as for light microscopy and sectioned to a thickness of 90 nm on an Ultracut E microtome with a diamond knife. Sections were collected on 400 gauge hexagonal mesh nickel grids and processed through primary antibody solutions as specified above, then with goat anti-rabbit IgG 20 nm gold. Sections were post-stained with uranyl acetate and lead citrate, dried and viewed on a transmission electron microscope (Hitachi-7000, Hitachi, Tokyo, Japan). Controls to ensure antibody specificity were the use of rabbit preimmune serum and the omission of primary antibody.
(e) Western blot analysis
Proteins were separated by SDS–PAGE and transferred onto nitrocellulose membranes (Amersham Biosciences, UK) using a press blot method. Membranes were Ponceau-stained to check transfer efficiency, rinsed out in TBS-T and blocked overnight in 4% (w/v) non-fat dry milk in TBS-T under continuous shaking at 4°C. Primary antibody was diluted to 1 : 5000 in the previous buffer and incubated for 2 h at RT under constant shaking. The filters were then washed twice for 5 min in TBS-T (30 ml each) and a monoclonal anti-rabbit IgG HRP conjugate (Sigma, UK) added at a dilution of 1 : 10 000 and incubated for 1 h at RT. The filters were washed in TBS-T twice as previously described, draining out excess buffer on absorbent paper. Fluorescent development was carried out using ECL Plus kit (Amersham Biosciences) and exposed for 10 min using HyperFilm (Amersham Biosciences).
(f) Sequestration of sinigrin by B. brassicae fed on artificial diets and B. nigra plants
Approximately 10 reproducing, wingless aphids were collected from the stock culture (reared on B. nigra) and put onto an artificial diet (Dadd et al. 1967), presented to the insects across an artificial membrane of stretched Parafilm. Half of the sachets were prepared from diet containing sinigrin (at 1% w/v) and the other half contained no sinigrin. Each diet sachet was placed individually into a small (50 mm diameter) ventilated Petri dish, which was covered with a piece of green semi-transparent plastic to encourage aphid settling. Additionally, approximately 20 reproducing, wingless aphids were transferred to three-week-old B. nigra plants, which were then enclosed within perforated bread bags. All aphids were returned to the same growth room used for the stock culture. After approximately 24 h all adult aphids were removed, leaving the nymphs to develop. Nymphs developing on the artificial diet sachets were transferred to a fresh sachet every 3 days, while those aphids on B. nigra remained on the same plant throughout the experiment. Aphids reared on the artificial diet containing 0% sinigrin were sampled on days 1, 7, 12 and 18, while those reared on the 1% sinigrin diet or on B. nigra were sampled on days 1, 3, 5, 7, 9, 12, 15 and 18. At each time point, aphids were collected from each of three replicates per treatment (plants or artificial diets). On day 1, 20 nymphs per treatment were used for analysis. However, due to limitation in the number of aphids that could be sustained on the diet sachets, this number was gradually reduced during the time course (the minimum total weight analysed throughout the experiment was 1 mg). Aphids sampled on days 1, 3 and 5 were a mixture of winged (alatae) and wingless (aptera) forms (unknown proportions). By day 7, the majority of aphids had reached the fourth instar and it was possible to discriminate between nymphs of winged and wingless forms, based on the presence of wing buds.
(g) Analysis of sinigrin
Aphids were extracted in Eppendorf tubes using boiling 80% methanol and benzylglucosinolate was added as a standard. After 15 min the tubes were centrifuged, the methanol removed and the aphids were extracted once more with 80% methanol. The methanolic extracts were combined and evaporated to dryness at less than 40°C. The residue was redissolved in 1 ml of water, 0.1 ml of a barium/lead acetate solution added as previously described (Rossiter et al. 1990) and the sample was centrifuged. The supernatant was loaded onto DEAE-A25 Sephadex and washed with water. Sulphatase was then added and the desulphoglucosinolates eluted the following day and analysed by HPLC (Rossiter et al. 1990). The sinigrin determinations in the head, thorax and abdomen were carried out on wingless adults. Aphids were first agitated and cornicle exudates collected. Body parts were then separated by slicing the thorax–abdomen interface with a scalpel. The abdomen was gently squeezed to release the haemolymph and embryos and immediately immersed in water. By careful manipulation with a Pasteur pipette, the watery haemolymph was removed and the remaining embryos rinsed with more water. Glucosinolate analysis (as described above) was carried out on the remaining exoskeleton, head and thorax, cornicle exudate, embryos and water/haemolymph extract.
(h) Honeydew analysis
Honeydew collection was carried out using artificial diets and nymphs produced by adult aphids maintained on diet sachets containing 0.1 or 1% sinigrin. On day 9, 15 winged or wingless nymphs were transferred to fresh diet sachets containing 0.1 or 1% sinigrin (three replications for each morph), which were placed on preweighed disks of aluminium foil in separate Petri dishes. After 3 days aphids were removed, weighed and frozen prior to analysis of sinigrin. Foil disks with honeydew were dried overnight in a desiccator and then weighed and transferred into tubes containing water and benzylglucosinolate standard. The contents of the tubes were mixed and the solution was subsequently analysed for sinigrin as above.
(i) GC analysis of volatiles released from aphids either crushed or predated upon by ladybirds
Aphids reared on diet sachets containing 0 or 1% sinigrin were placed into a glass chamber. Aphids were either left undamaged or five adult seven-spotted ladybirds (Coccinella septempunctata) were also placed into the glass chamber. Volatiles were collected onto a Tenax column. Charcoal-filtered air was pumped into the chamber (at 300 ml min−1) and drawn out at 200 ml min−1 through the Tenax column. Volatiles were desorbed using an OPTIC 2 linked to a GC–MS (Hewlett Packard 6890 GC linked to a 5973 MSD). Separation was carried out on a HP-5MS 5% Phenylmethylsiloxane (30 m×250 mm) column in the split mode (25 : 1) injection temperature=260°C, column temperature=50°C for 3 min, 10°C min−1 to 200°C for 2 min. All compounds were identified by using either standards or their mass fragmentation patterns.
(j) Predator survival
Two-spot ladybird beetles (Adalia bipunctata) were maintained on a diet of pea aphids (Acyrthosiphon pisum). Larvae hatching within 24 h of each other, from a number of egg clusters, were placed individually into small glass tubes (18 mm diameter). Winged or wingless adult aphids (approx. 12 days old) were collected from diet sachets containing 0 or 1% sinigrin. For each aphid morph and diet sachet combination, a total of 12 ladybird larvae were provided daily with an excess of aphids. Ladybird larvae were maintained at 19±1°C, recording survival daily throughout the first instar.
3. Results and discussion
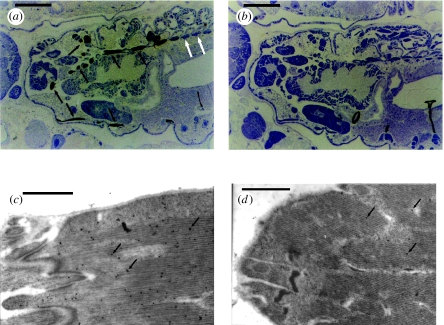
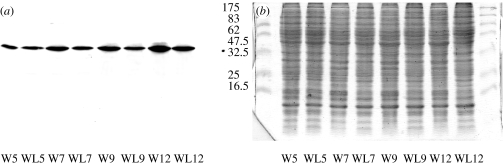
Immunostaining showed that embryos developing within adult B. brassicae already possess myrosinase. The pattern of myrosinase distribution (figure 1a) is similar to that previously described in adult aphids, where labelling is restricted to the head and thorax and abdominal muscle has no myrosinase labelling. Preimmune serum was used as a control and these sections showed no staining in muscle tissue (figure 1b). Using immunocytochemistry at the electron microscopy level, labelling with 20 nm gold particles clearly indicates the presence of myrosinase in non-flight muscle in the embryos (figure 1c), in contrast to control sections (figure 1d) where there is minimal label. Previous work has shown that myrosinase in adult aphids occurs in microcrystalline bodies within the sarcoplasm of non-flight muscle (Bridges et al. 2002). However, these structures were not observed in aphid embryos and it seems that formation of these crystalline bodies occurs at a later developmental stage. We investigated post-natal myrosinase expression and accumulation by western blot analyses (figure 2) of winged and wingless morphs at different growth stages up to the adult stage (day 12). Using a previously described antibody to B. brassicae myrosinase (Jones et al. 2001), this approach revealed an increase in myrosinase expression throughout the developmental stages of both aphid forms (figure 2). Band intensities (relative to day 5 for wingless aphids) increased from 1.0 to 1.9 (D5, 1.0; D7, 1.5; D9, 1.7; D12, 1.9) for the wingless aphids and from 0.9 to 1.5 (D5, 0.9; D7, 1.1; D9, 1.2; D12, 1.5) for winged aphids.
Figure 1.
(a,b) Light and (c,d) electron microscopy sections through wingless adult B. brassicae. Following incubation with sera and antisera, sections were stained with goat anti-rabbit (10 nm gold conjugate) antibody and enhanced with nucleated silver for LM or labelled with goat anti-rabbit (20 nm gold conjugate) for EM. Black arrows indicate the labelled muscles in the head and thorax. White arrows indicate the skeletal muscles in the abdomen. (a) Longitudinal section through a wingless adult showing an embryo stained with anti-myrosinase antibody. (b) Control section with preimmune serum. Scale bars, 100 μm. (c) TEM of immunolocalized myrosinase on the skeletal muscle of a B. brassicae embryo. The arrows indicate the 20 nm colloidal gold secondary antibody on the muscle fibres. (d) Control section with preimmune serum. Scale bars, 1 μm.
Figure 2.
(a) Western blot analysis of protein extracts of winged and wingless aphids for myrosinase expression at 5, 7, 9 and 12 days after birth. (b) Corresponding SDS–PAGE. Gel loading was 30 μg per lane; prestained markers; 175 kDa, MBP-β-galactosidase; 83 kDa, MBP-paramyosin; 62 kDa, glutamic dehydrogenase; 47 kDa, aldolase; 32.5 kDa, trisephosphate; 25 kDa, β-lactoglobulin A; 16.5 kDa, lysozyme. WL, wingless; W, winged, followed by day after birth.
Since myrosinase is highly localized in the aphid, an analysis of sinigrin was carried out on the head, thorax and the abdomen of aphids reared on a host plant (B. nigra). Abdomens were carefully dissected to remove the embryos and the haemolymph and the remaining tissues analysed for glucosinolates. The abdominal tissues, head and thorax and cornicle exudate had no detectable glucosinolate, while the sinigrin content of the haemolymph and embryos was 290 and 19.5 nmol, respectively, for a total of 20 wingless aphids and their embryos. Trophic cords link aphid embryos with the germarium of the ovary and provide nutrients to the youngest embryos (Douglas 2003). Nutrients are allocated to older embryos from the maternal haemocoel, probably involving a selective transport mechanism across the ovariole sheath (Wilkinson & Ishikawa 2000; Douglas 2003). Thus, it would appear that glucosinolates are also probably allocated directly to the developing embryos via one or both of these mechanisms.
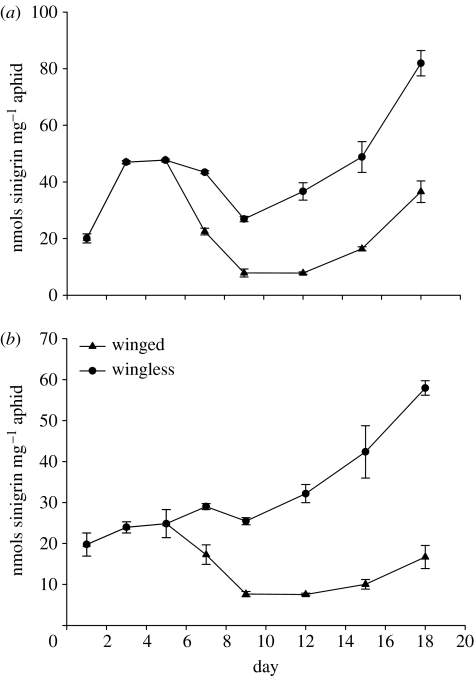
The sequestration of sinigrin by aphids feeding on an artificial diet (Dadd et al. 1967) and B. nigra was monitored over 18 days and expressed as nanomoles sinigrin per milligram aphid. Aphids reared on an artificial diet containing no sinigrin maintain a low but fairly constant residual level of the glucosinolate (approx. 0.1 nmol per aphid) acquired from the abdomen of the mother before birth. The sequestration of sinigrin from an artificial diet containing the compound at 1% is shown in figure 3a and that on B. nigra in figure 3b. The mean weight of aphids on plants was greater than that on the artificial diet by a factor of 2.6 at day 15 (data not shown), although there were no observable differences in mortality rates between aphids fed on the artificial diet and those fed on the host plants (not shown). Despite this difference in body weight, aphids sequestered similar levels of sinigrin (on a nanomoles sinigrin per milligram aphid basis) whether feeding on a 1% sinigrin artificial diet or B. nigra. The sinigrin concentration in B. nigra phloem sap has been estimated at greater than 0.4% (Merritt 1996) and it is probable that the two experimental systems in this study (plants and artificial diets) presented aphids with approximately equal dietary sinigrin concentrations.
Figure 3.
Amount (mean±s.e.) of sinigrin (per milligram of aphid) at different time points for wingless and winged aphids reared on (a) an artificial diet containing 1% sinigrin and (b) B. nigra.
On both plants and the artificial diets, the experimental aphids produced a mixture of winged and wingless offspring. The two morphs were analysed separately for glucosinolate content and showed strikingly different patterns of uptake (figure 3). Wingless aphids sequestered significantly more sinigrin than the winged forms at all time points that the two forms could be discriminated (from day 7). After day 7, the sinigrin content of winged aphids declined while in the wingless form uptake increased substantially. The differences in the amount of sinigrin between the two morphs were significant at the 1% probability level for both the artificial diet and the plants at all time points (p<0.01 at minimum, t-tests). We also examined the amounts of glucosinolate in the body and honeydew of the two morphs reared on artificial diet with 0.1 and 1% sinigrin and results are expressed per milligram aphid (figure 4). At both concentrations of dietary sinigrin, the amount of the glucosinolate in wingless aphids was significantly higher than that in individuals of the winged morph (t-tests; p=0.0032 for 0.1%; p<0.0045 for 1%), in agreement with the previous sinigrin sequestration experiment. The opposite result was obtained for aphid honeydew; the winged morph excreted significantly more sinigrin than the wingless at both 0.1 (p=0.0024) and 1% dietary levels (p=0.013). However, despite these differences, the total sinigrin recovered from aphids (in bodies plus honeydew) was not significantly different between the two morphs (p>0.05). Wingless aphids had sequestered a high proportion (91%) of the total detected sinigrin ingested (in aphid bodies plus honeydew) from the 0.1% diet and maintained a sequestration rate of 69% on the 1% sinigrin diet. Winged aphids also sequestered a high proportion (69%) of sinigrin from the 0.1% diet, but only 22% was detected in the bodies of winged aphids from the 1% diet, and a higher level of sinigrin was then present in the honeydew. Thus it would appear that glucosinolate transport across the guts of the aphids is attenuated in the winged morph by an as yet undetermined mechanism.
Figure 4.
Amount (mean±s.e.) of sinigrin (per milligram of aphid) in aphids, honeydew and their sum for the two morphs of B. brassicae reared on artificial diet containing 0.1 and 1% sinigrin.
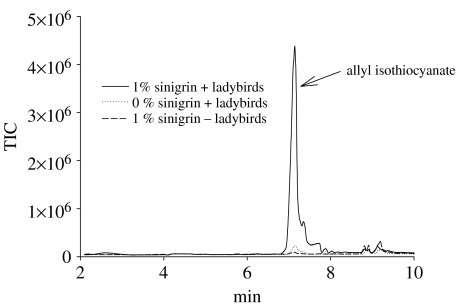
When aphids reared on 1% sinigrin are attacked by ladybirds, the volatile emission (figure 5) comprises mainly allyl isothiocyanate, together with a small amount of (E)-β-farnesene (peak not shown), an aphid alarm pheromone (Pickett et al. 1992). Aphids reared on 0% sinigrin show no detectable emission of allyl isothiocyanate and the artificial diet system therefore provides an opportunity to test the effects of the glucosinolate-based defence system on the aphid's natural enemies. When fed B. brassicae reared on host plants containing high levels of glucosinolates, larvae of the polyphagous ladybird A. bipunctata failed to complete development (Francis et al. 2001). Given that the concentrations of glucosinolates in the two morphs are significantly different, we repeated this approach using both aphid forms. Survival of A. bipunctata larvae provided with a food source of adult winged or wingless aphids reared on diet sachets containing 0 or 1% sinigrin was recorded. Survival of first instar ladybird larvae was high (100%) when fed on both aphid morphs reared on a 0% sinigrin diet. However, when larvae were fed aphids from the 1% sinigrin diet, survival on wingless aphids was zero compared to 83.3% on winged aphids (Χ2=17.1; p<0.001). The high survival rate of ladybird larvae feeding on adult winged aphids reared on a 1% sinigrin diet reflects the low sequestration rate of sinigrin in this aphid morph when compared with the wingless aphid where sinigrin concentrations are high, yielding toxic or repellent quantities of isothiocyanate.
Figure 5.
The volatile profile of adult wingless aphids either intact or undergoing predation. Identification of allyl isothiocyanate was confirmed by mass spectrometry and an authentic standard.
In the same way that the myrosinase–glucosinolate system in cruciferous plants provides an effective defence against many herbivores, through the production of toxic hydrolysis products, it appears that the convergent system in B. brassicae affords a similar defensive function. Aphid colonies risk attack by a wide range of natural enemies from different trophic guilds. It is not yet clear whether the myrosinase–glucosinolate system has an impact on endoparasitoids, as these insects selectively exploit the aphid abdomen for oviposition and larval development (Polaszek 1986) and may therefore avoid aphid myrosinase. However, the compartmentalization of myrosinase and glucosinolate in thoracic muscle and haemolymph ensures that predators causing substantial aphid damage will be exposed to high levels of isothiocyanate. Such damage will be lethal to the attacked individual but aphid colonies are clonal, developing through parthenogenetic reproduction, and traits that are effective in deterring natural enemies will have a clear adaptive advantage via kin selection. The cabbage aphid's exploitation of glucosinolates therefore appears to confer clear defensive advantages to the herbivore. In this particular interaction, the plant's chemical defences appear to be subverted at the expense of plant fitness. However, glucosinolates play important roles in regulating feeding by numerous other herbivore species that are not adapted for dealing with these metabolites.
Thus the cabbage aphid has evolved a highly specialized and unique form of defence which is apparently attenuated during preparation for flight. While the myrosinase–glucosinolate system appears to provide an effective defence mechanism against predators, sequestration of the substrate from plants may also represent a substantial energetic investment. This investment is likely to be particularly worthwhile for wingless aphids, which are committed to a sedentary life and a limited ability to escape predation via dispersal. However, for winged aphids, the development of the flight apparatus also involves substantial energetic costs (Dixon & Kindlmann 1999). During the last few days of nymphal development, winged aphids show a rapid increase in synthesis and storage of lipid reserves, attaining a lipid content of up to 50% dry weight (Liquido & Irwin 1986). This period of maximal investment in the major flight fuel coincides with a decrease in the glucosinolate content of winged B. brassicae (figure 3), possibly representing a trade-off between alternative predator avoidance mechanisms. At this stage, transport of sugars across the gut presumably takes priority over glucosinolate transport in order to increase availability of substrates for lipid biosynthesis (Febvay et al. 1999). While a great deal of work has been carried out on sugar uptake in aphids (Douglas 2003), very little is known about the transport of secondary metabolites and further studies are required to elucidate the mechanism of glucosinolate uptake and its physiological regulation.
Acknowledgments
The authors thank Corrin Pratt for help with volatile analysis. The work was supported by a grant from the Biotechnology and Biological Sciences Research Council.
References
- Berenbaum M.R. The chemistry of defence—theory and practice. Proc. Natl Acad. Sci. USA. 1995;92:2–8. doi: 10.1073/pnas.92.1.2. doi:10.1073/pnas.92.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bones A.M, Rossiter J.T. The myrosinase–glucosinolate system, its organization and biochemistry. Physiol. Plant. 1996;97:194–208. doi:10.1111/j.1399-3054.1996.tb00497.x [Google Scholar]
- Bridges M, et al. Spatial organization of the glucosinolate–myrosinase system in brassica specialist aphids is similar to that of the host plant. Proc. R. Soc. B. 2002;269:187–191. doi: 10.1098/rspb.2001.1861. doi:10.1098/rspb.2001.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow M, Markert J, Gershenzon J, Wittstock U. Comparative biochemical characterization of nitrile-forming proteins from plants and insects that alter myrosinase-catalysed hydrolysis of glucosinolates. FEBS J. 2006;273:2432–2446. doi: 10.1111/j.1742-4658.2006.05252.x. doi:10.1111/j.1742-4658.2006.05252.x [DOI] [PubMed] [Google Scholar]
- Burow M, Bergner A, Gershenzon J, Wittstock U. Glucosinolate hydrolysis in Lepidium sativum—identification of the thiocyanate-forming protein. Plant Mol. Biol. 2007;63:49–61. doi: 10.1007/s11103-006-9071-5. doi:10.1007/s11103-006-9071-5 [DOI] [PubMed] [Google Scholar]
- Dadd R.H, Krieger D.L, Mittler T.E. Artificial feeding of the aphid Myzus persicae. IV. Requirements for water-soluble vitamins and ascorbic acid. J. Insect Physiol. 1967;13:249–272. doi:10.1016/0022-1910(67)90152-7 [Google Scholar]
- Dawson, G. W., Griffiths, D. C., Pickett, J. A., Wadhams, L. J. & Woodcock, C. M. 1986 Plant compounds that synergize activity of the aphid alarm pheromone. In British Crop Protection Conference—Pests and Diseases, Proc. pp. 829–834.
- Dixon A.F.G, Kindlmann P. Cost of flight apparatus and optimum body size of aphid migrants. Ecology. 1999;80:1678–1690. doi:10.2307/176556 [Google Scholar]
- Douglas A.E. The nutritional physiology of aphids. Adv. Insect Physiol. 2003;31:73–140. doi:10.1016/S0065-2806(03)31002-1 [Google Scholar]
- Fahey J.W, Zalcmann A.T, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. doi:10.1016/S0031-9422(00)00316-2 [DOI] [PubMed] [Google Scholar]
- Febvay G, Rahbe Y, Rynkiewicz M, Guillaud J, Bonnot G. Fate of dietary sucrose and neosynthesis of amino acids in the pea aphid, Acyrthosiphon pisum, reared on different diets. J. Exp. Biol. 1999;202:2639–2652. doi: 10.1242/jeb.202.19.2639. [DOI] [PubMed] [Google Scholar]
- Francis F, Lognay G, Wathelet J.P, Haubruge E. Effects of allelochemicals from first (Brassicaceae) and second (Myzus persicae and Brevicoryne brassicae) trophic levels on Adalia bipunctata. J. Chem. Ecol. 2001;27:243–256. doi: 10.1023/a:1005672220342. doi:10.1023/A:1005672220342 [DOI] [PubMed] [Google Scholar]
- Francis F, Lognay G, Wathelet J.P, Haubruge E. Characterisation of aphid myrosinase and degradation studies of glucosinolates. Arch. Insect Biochem. Physiol. 2002;50:173–182. doi: 10.1002/arch.10042. doi:10.1002/arch.10042 [DOI] [PubMed] [Google Scholar]
- Francis F, Lognay G, Haubruge E. Olfactory responses to aphid and host plant volatile releases: (E)-beta-farnesene an effective kairomone for the predator Adalia bipunctata. J. Chem. Ecol. 2004;30:741–755. doi: 10.1023/b:joec.0000028429.13413.a2. doi:10.1023/B:JOEC.0000028429.13413.a2 [DOI] [PubMed] [Google Scholar]
- Husebye H, Arzt S, Burmeister W.P, Haertel F.V, Brandt A, Rossiter J.T, Bones A.M. Crystal structure at 1.1 Å resolution of an insect myrosinase from Brevicoryne brassicae shows its close relationship to beta-glucosidases. Insect Biochem. Mol. Biol. 2005;35:1311–1320. doi: 10.1016/j.ibmb.2005.07.004. doi:10.1016/j.ibmb.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Jones A.M.E, Bridges M, Bones A.M, Cole R, Rossiter J.T. Purification and characterization of a non-plant myrosinase from the cabbage aphid Brevicoryne brassicae (L.) Insect Biochem. Mol. Biol. 2001;31:1–5. doi: 10.1016/s0965-1748(00)00157-0. doi:10.1016/S0965-1748(00)00157-0 [DOI] [PubMed] [Google Scholar]
- Jones A.M.E, Winge P, Bones A.M, Cole R, Rossiter J.T. Characterization and evolution of a myrosinase from the cabbage aphid Brevicoryne brassicae. Insect Biochem. Mol. Biol. 2002;32:275–284. doi: 10.1016/s0965-1748(01)00088-1. doi:10.1016/S0965-1748(01)00088-1 [DOI] [PubMed] [Google Scholar]
- Kunert G, Otto S, Rose U.S.R, Gershenzon J, Weisser W.W. Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecol. Lett. 2005;8:596–603. doi:10.1111/j.1461-0248.2005.00754.x [Google Scholar]
- Lambrix V, Reichelt M, Mitchell-Olds T, Kliebenstein D.J, Gershenzon J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell. 2001;13:2793–2807. doi: 10.1105/tpc.010261. doi:10.1105/tpc.13.12.2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liquido N.J, Irwin M.E. Longevity, fecundity, change in degree of gravidity and lipid-content with adult age, and lipid utilization during tethered flight of alates of the corn leaf aphid, Rhopalosiphum maidis. Ann. Appl. Biol. 1986;108:449–459. [Google Scholar]
- Merritt S.Z. Within-plant variation in concentrations of amino acids, sugar and sinigrin in phloem sap of black mustard, Brassica nigra (L.) Koch (Cruciferae) J. Chem. Ecol. 1996;22:1133–1145. doi: 10.1007/BF02027950. doi:10.1007/BF02027950 [DOI] [PubMed] [Google Scholar]
- Pickett J.A, Wadhams L.J, Woodcock C.M, Hardie J. The chemical ecology of aphids. Annu. Rev. Entomol. 1992;37:67–90. doi:10.1146/annurev.en.37.010192.000435 [Google Scholar]
- Polaszek A. The effects of 2 Species of hymenopterous parasitoid on the reproductive-system of the pea aphid, Acyrthosiphon pisum. Entomol. Exp. Appl. 1986;40:285–292. doi:10.1007/BF00293712 [Google Scholar]
- Pontoppidan P, Ekbom B, Eriksson S, Meijer J. Purification and characterization of myrosinase from the cabbage aphid (Brevicoryne brassicae), a brassica herbivore. Eur. J. Biochem. 2001;268:1041–1048. doi: 10.1046/j.1432-1327.2001.01971.x. doi:10.1046/j.1432-1327.2001.01971.x [DOI] [PubMed] [Google Scholar]
- Ratzka A, Vogel H, Kliebenstein D.J, Mitchell-Olds T, Kroymann J. Disarming the mustard oil bomb. Proc. Natl Acad. Sci. USA. 2002;99:11 223–11 228. doi: 10.1073/pnas.172112899. doi:10.1073/pnas.172112899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter J.T, James D.C, Atkins N. Biosynthesis of 2-hydroxy-3-butenylglucosinolate and 3-butenylglucosinolate in Brassica napus. Phytochemistry. 1990;29:2509–2512. doi:10.1016/0031-9422(90)85177-H [Google Scholar]
- Wilkinson T.L, Ishikawa H. Injection of essential amino acids substitutes for bacterial supply in aposymbiotic pea aphids (Acyrthosiphon pisum) Entomol. Exp. Appl. 2000;94:85–91. doi:10.1023/A:1003909127158 [Google Scholar]
- Wittstock U, Agerbirk N, Stauber E.J, Olsen C.E, Hippler M, Mitchell-Olds T, Gershenzon J, Vogel H. Successful herbivore attack due to metabolic diversion of a plant chemical defence. Proc. Natl Acad. Sci. USA. 2004;101:4859–4864. doi: 10.1073/pnas.0308007101. doi:10.1073/pnas.0308007101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabala M. de T, Grant M, Bones A.M, Bennett R, Lim Y.S, Kissen R, Rossiter J.T. Characterisation of recombinant epithiospecifier protein and its over-expression in Arabidopsis thaliana. Phytochemistry. 2005;66:859–867. doi: 10.1016/j.phytochem.2005.02.026. doi:10.1016/j.phytochem.2005.02.026 [DOI] [PubMed] [Google Scholar]