Abstract
Tumor targeting peptides are promising vehicles for site-directed cancer therapy. Pep42, a cyclic 13-mer oligopeptide that specifically binds to glucose-regulated protein 78 (GRP78) and internalized into cancer cells, represents an excellent vehicle for tumor cell-specific chemotherapy. Here, we report the synthesis and evaluation of Pep42-prodrug conjugates that contain a cathepsin B-cleavable linker, resulting in the traceless release of drug inside the cancer cells.
Chemotherapy remains an important treatment option for cancer patients. However, its success is limited by such drawbacks as insufficient intratumor drug concentrations and systemic toxicity due to lack of target specificity. Restricting the action of a cytotoxic drug to the tumor site would greatly alleviate systemic toxicity and improve therapeutic efficacy. Tumor targeting peptides are promising vehicles for site-directed cancer therapy. Recently, we have reported the selection and characterization of Pep42, a cyclic 13-mer oligopeptide, CTVALPGGYVRVC, that specifically binds to glucose-regulated protein 78 (GRP78).1 Furthermore, we have also demonstrated the internalization of Pep42 into a number of cancer cell lines.2 In cancer cells, the overexpression of GRP78, an intracellular chaperone and member of the heat shock protein 70 (HSP70) family, provides a protective cellular response against stress conditions. Indeed, GRP78 overexpression also represents an attractive target as it results in the specific presence of GRP78 molecules on the cancer cell surface. Normal GRP78 expression is maintained at low levels but is upregulated in stress environments and induced in tumor environments. GPR78 overexpression has been demonstrated in a variety of tumors, such as prostate, colon, skin, and breast cancers.3 In human cancers, elevated GRP78 level generally correlates with higher pathologic grade, recurrence, and poor patient survival in breast, liver, prostate, and colon cancers.4, 5
For a drug deliverable release strategy, we focused on a proteolytically cleavable linker as we have shown that Pep42 will be internalized through the GRP78 receptor, endocytosed and trafficked to the lysosomes that contain proteases, such as cathepsin B.6 In terms of proteases, cathepsin B is a ubiquitously expressed cysteine protease located in the lysosomes.7 It is confined strictly to the lysosomes and not found extracellularly, except in pathological conditions such as cancer or rheumatoid arthritis.8 Therefore Pep42-drug conjugates containing cathepsin B-cleavable linkers are likely to be stable in circulation and selectively release their drug specifically in the targeted tissue. For our linker release strategy, we decided to employ the Val-Cit motif that is rapidly cleaved by cathepsin B, but is very stable in plasma.9 The cytotoxic drugs of choice were the two well characterized and widely used anti-tumor agents, paclitaxel (Taxol) and doxorubicin.1, 10
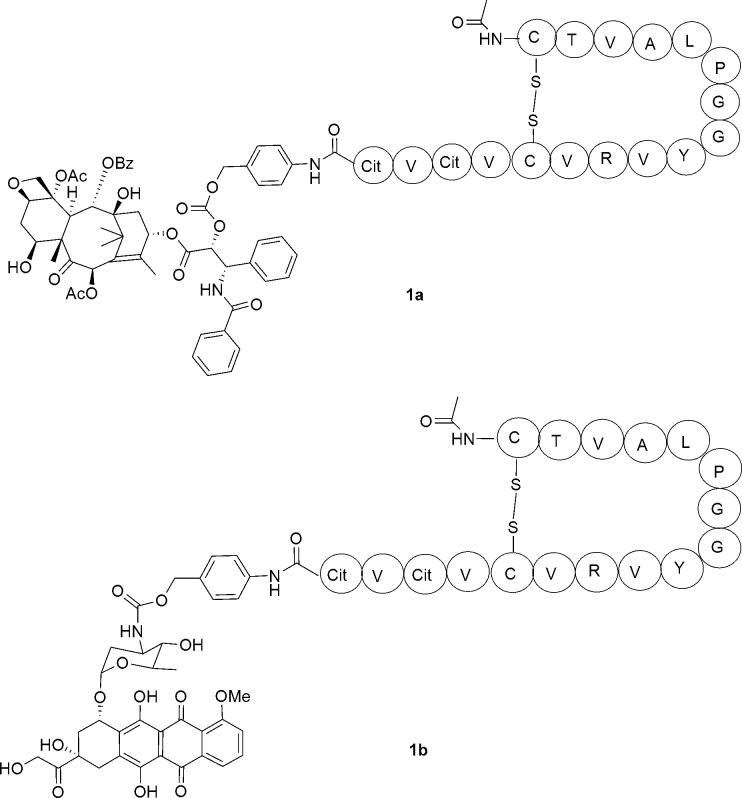
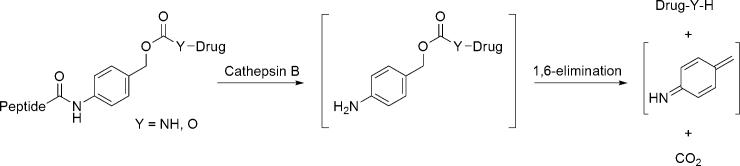
Thus, Pep42 was linked through an amidic bond to p-aminobenzylalcohol, a commonly used self-immolative spacer, attached to paclitaxel through a carbonate and to doxorubicin through a carbamate functionality (Fig. 2).11, 12 The enzymatic cleavage triggers a 1,6-elimination reaction, resulting in the unmodified drug, carbon dioxide and the remnants of the spacer being released (Fig. 3).13
Figure 2.
Conjugates of Pep 42 with taxol (1a) and doxorubicin (1b). Amino acids are described with the appropriate one-letter codes.
Figure 3.
Release cascade of the drug.
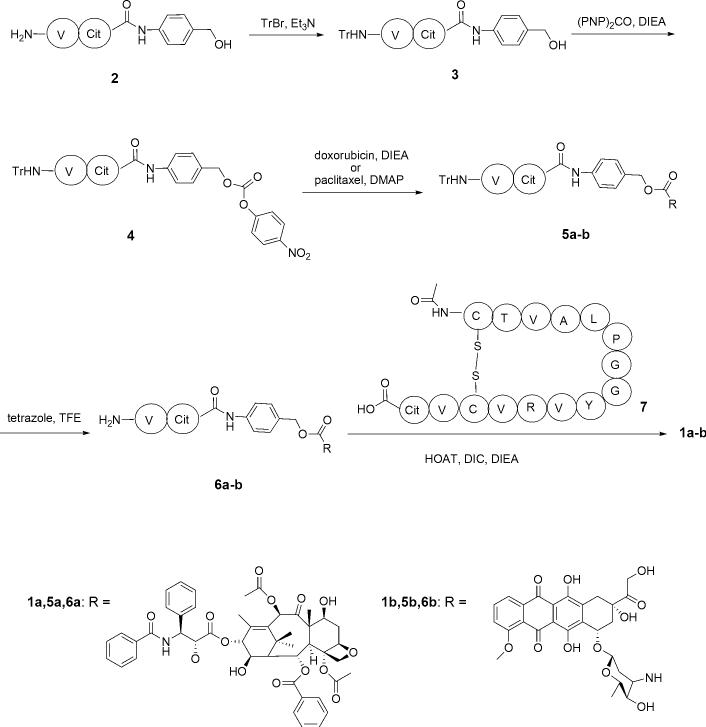
The general synthetic strategy involved coupling of the Pep42-Val-Cit (7) fragment with an appropriately functionalized derivative of paclitaxel or doxorubicin (6a-b) (Scheme 1). The starting Val-Cit-PABOH (2) was readily obtained using a previously described procedure.14 Subsequent protection of the amino group (3) and treatment with bis-PNPC afforded the carbonate (4), which was converted to the corresponding paclitaxel (5a) and doxorubicin (5b) derivatives. Deprotection with tetrazole in TFE (6a-b), followed by coupling with Pep 42-Val-Cit (7), synthesized by standard Fmoc/DIC/HOBt protocols, gave the desired conjugates (1a) and (1b) in good yields (Scheme 1).
Scheme 1.
Synthesis of the Pep 42 conjugates.
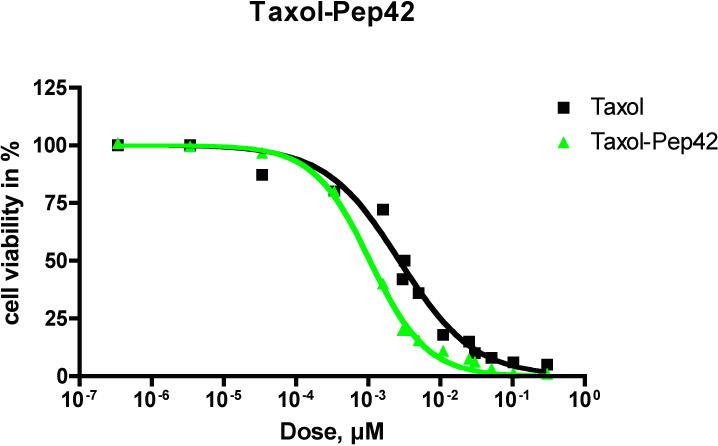
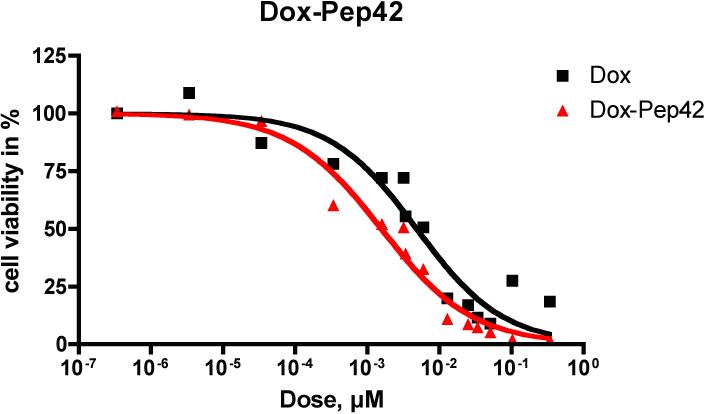
The cytotoxicity of the Pep42-prodrug conjugates on SJSA-1 osteosarcoma cells, a GRP78-expressing cell line, was studied in vitro by using an MTT assay. SJSA-1 cells (5 × 103) were plated in each well of a 96-well tissue culture plate. Medium supplemented with 10% FBS was added, and cells were allowed to adhere for 24 h. Cells were then incubated with serial dilutions of Taxol-Pep42 and doxorubicin-Pep42 for 6 h in triplicate, and a MTT assay was performed (Figures 4 and 5).18 As shown in Figure 4, Taxol-Pep42 showed greater cytotoxicity than free Taxol alone against SJSA-1 cells. At the Taxol equivalent concentration of 10−3 μM, cell viability was about 40% for Taxol-Pep42 treated cells, while the viability of Taxol treated cells decreased to only 80%. The IC50 values for Taxol were 3.2 nM and 1.1 nM for Taxol-Pep42.
Figure 4.
Viability of cells incubated with serial dilutions of Taxol and Taxol-Pep42 by MTT assays.
Figure 5.
Viability of cells incubated with serial dilutions of Doxorubicin and Doxorubicin-Pep42 by MTT assay.
The doxorubicin-Pep42 conjugate also demonstrated enhanced cytotoxicity against SJSA-1 cells (Figure 4). For unconjugated doxorubicin, the IC50 was 3.2 nM, while cells treated with doxorubicin-Pep42 showed a viability of 50% at 1.1 nM (Figure 5). These results are consistent with or superior to previously reported analogous studies.15, 16 When employing the same activation-release cascade, antibody conjugates have demonstrated a higher increase in cytotoxic activity.17 However, here one must also consider that the payload exceed more than one toxin molecule per antibody molecule, while the coupling ratio of prodrug to Pep42 was 1:1.
In summary, we have demonstrated that the peptidic GRP78 ligand Pep42 can be utilized to efficiently deliver two well characterized cytotoxic drugs frequently used in current cancer therapy treatment regimes into cancer cells. Based on our previously reported data we exploited Pep42's translocation into the lysosomal compartment to design prodrug conjugates that specifically release the drugs upon entering the cells. Gratifyingly, this resulted in an increase in cytotoxicity as Pep42 facilitates the uptake of Taxol and doxorubicin into cells and thus, delivers its toxic payload directly into the cancer cell.
Figure 1.
Potent anticancer drugs Taxol and Doxorubicin.
Acknowledgments
This work was supported by The Skaggs Institute for Chemical Biology and the National Institutes of Health (CA 094193).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
References and notes
- 1.Kim Y, Lillo AM, Steiniger SC, Liu Y, Ballatore C, Anichini A, Mortarini R, Kaufmann GF, Zhou B, Felding-Habermann B, Janda KD. Targeting heat shock proteins on cancer cells: selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry. 2006;45(31):9434–44. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Steiniger SC, Kim Y, Kaufmann GF, Felding-Habermann B, Janda KD. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol Pharm. 2007;4(3):435–47. doi: 10.1021/mp060122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin BK, Wang H, Yim AM, Le Naour F, Brichory F, Jang JH, Zhao R, Puravs E, Tra J, Michael CW, Misek DE, Hanash SM. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003;278(9):7607–16. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- 4.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5(7):741–4. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Jiang Y, Jia Z, Li Q, Gong W, Wang L, Wei D, Yao J, Fang S, Xie K. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis. 2006;23(7−8):401–10. doi: 10.1007/s10585-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 6.Schmid B, Chung DE, Warnecke A, Fichtner I, Kratz F. Albumin-binding prodrugs of camptothecin and doxorubicin with an Ala-Leu-Ala-Leu-linker that are cleaved by cathepsin B: synthesis and antitumor efficacy. Bioconjug Chem. 2007;18(3):702–16. doi: 10.1021/bc0602735. [DOI] [PubMed] [Google Scholar]
- 7.Yan S, Sloane BF. Molecular regulation of human cathepsin B: implication in pathologies. Biol Chem. 2003;384(6):845–54. doi: 10.1515/BC.2003.095. [DOI] [PubMed] [Google Scholar]
- 8.Bettio F, Canevari M, Marzano C, Bordin F, Guiotto A, Greco F, Duncan R, Veronese FM. Synthesis and biological in vitro evaluation of novel PEG-psoralen conjugates. Biomacromolecules. 2006;7(12):3534–41. doi: 10.1021/bm060760n. [DOI] [PubMed] [Google Scholar]
- 9.Dubowchik GM, Firestone RA. Cathepsin B-sensitive dipeptide prodrugs. 1. A model study of structural requirements for efficient release of doxorubicin. Bioorg Med Chem Lett. 1998;8(23):3341–6. doi: 10.1016/s0960-894x(98)00609-x. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Sun CZ, Huang HN, Janda K, Edgington T. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Research. 2003;63(11):2957–2964. [PubMed] [Google Scholar]
- 11.Niculescu-Duvaz I, Niculescu-Duvaz D, Friedlos F, Spooner R, Martin J, Marais R, Springer CJ. Self-immolative anthracycline prodrugs for suicide gene therapy. J Med Chem. 1999;42(13):2485–9. doi: 10.1021/jm980696v. [DOI] [PubMed] [Google Scholar]
- 12.Toki BE, Cerveny CG, Wahl AF, Senter PD. Protease-mediated fragmentation of pamidobenzyl ethers: a new strategy for the activation of anticancer prodrugs. J Org Chem. 2002;67(6):1866–72. doi: 10.1021/jo016187+. [DOI] [PubMed] [Google Scholar]
- 13.Dubowchik GM, Mosure K, Knipe JO, Firestone RA. Cathepsin B-sensitive dipeptide prodrugs. 2. Models of anticancer drugs paclitaxel (Taxol), mitomycin C and doxorubicin. Bioorg Med Chem Lett. 1998;8(23):3347–52. doi: 10.1016/s0960-894x(98)00610-6. [DOI] [PubMed] [Google Scholar]
- 14.Dubowchik GM, Firestone RA, Padilla L, Willner D, Hofstead SJ, Mosure K, Knipe JO, Lasch SJ, Trail PA. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug Chem. 2002;13(4):855–69. doi: 10.1021/bc025536j. [DOI] [PubMed] [Google Scholar]
- 15.Safavy A, Raisch KP, Khazaeli MB, Buchsbaum DJ, Bonner JA. Paclitaxel derivatives for targeted therapy of cancer: toward the development of smart taxanes. J Med Chem. 1999;42(23):4919–24. doi: 10.1021/jm990355x. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Plasencia C, Hou Y, Neamati N. Synthesis and biological evaluation of dimeric RGD peptide-paclitaxel conjugate as a model for integrin-targeted drug delivery. J Med Chem. 2005;48(4):1098–106. doi: 10.1021/jm049165z. [DOI] [PubMed] [Google Scholar]
- 17.Jeffrey SC, Nguyen MT, Andreyka JB, Meyer DL, Doronina SO, Senter PD. Dipeptide-based highly potent doxorubicin antibody conjugates. Bioorg Med Chem Lett. 2006;16(2):358–62. doi: 10.1016/j.bmcl.2005.09.081. [DOI] [PubMed] [Google Scholar]
- 18.Cells were plated in 96-well plates (5,000 cells per well) and incubated overnight at 37° C to allow for attachment and spreading of the cells. Serial dilutions of the Pep42, Pep42-prodrug conjugate as well as doxorubicin and paclitaxel were prepared in cell culture medium in a separate plate. The medium of the plate containing the cells were taken out and replaced with medium containing the Pep42-prodrug conjugate dilutions, followed by an incubation period of 6 h at 37° C. The MTT reagent was then added to individual wells and allowed to further incubate for 1 hour at 37° C. Samples were assayed in triplicate and the absorbance was measured at 490 nm. Cells incubated with PBS were used as untreated control cells and cell culture medium was a background control.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.