Abstract
Echolocating big brown bats (Eptesicus fuscus) broadcast ultrasonic frequency-modulated (FM) biosonar sounds (20–100 kHz frequencies; 10–50 μs periods) and perceive target range from echo delay. Knowing the acuity for delay resolution is essential to understand how bats process echoes because they perceive target shape and texture from the delay separation of multiple reflections. Bats can separately perceive the delays of two concurrent electronically generated echoes arriving as little as 2 μs apart, thus resolving reflecting points as close together as 0.3 mm in range (two-point threshold). This two-point resolution is roughly five times smaller than the shortest periods in the bat’s sounds. Because the bat’s broadcasts are 2,000–4,500 μs long, the echoes themselves overlap and interfere with each other, to merge together into a single sound whose spectrum is shaped by their mutual interference depending on the size of the time separation. To separately perceive the delays of overlapping echoes, the bat has to recover information about their very small delay separation that was transferred into the spectrum when the two echoes interfered with each other, thus explicitly reconstructing the range profile of targets from the echo spectrum. However, the bat’s 2-μs resolution limit is so short that the available spectral cues are extremely limited. Resolution of delay seems overly sharp just for interception of flying insects, which suggests that the bat’s biosonar images are of higher quality to suit a wider variety of orientation tasks, and that biosonar echo processing is correspondingly more sophisticated than has been suspected.
Keywords: echo location/target ranging/image resolution
The resolving power of an imaging system is the minimal spacing of two objects for which each object is still registered separately. For perceptual images, this limit is the classical psychophysical “two-point threshold.” We carried out experiments with big brown bats (Eptesicus fuscus) to determine the threshold for resolving two overlapping biosonar echoes received at slightly different delays. Eptesicus broadcasts wide band ultrasonic (20–100 kHz) frequency-modulated sounds and uses echoes of these sounds to orient in the immediate environment and to locate and track flying insect prey (1, 2). Echolocating bats perceive the distance to objects from the delay of echoes (2–4), so resolution of delay is equivalent to resolution of closely spaced reflecting points along the dimension of target range. Thus, two-point resolution is a measure for the quality or sharpness of the bat’s images.
Range resolution is important for perception of target shape or surface texture (2) and for segregating multiple objects located in different directions but at similar distances so that their echoes overlap (3). Echolocating bats indeed can discriminate differences of less than 1 mm in the depth of holes in flat targets (5, 6) or the texture of granular surfaces (7) from the structure of the multiple echoes they reflect (5–8). Moreover, in two-choice discrimination tests, Eptesicus can distinguish one-point echoes from two-point echoes with two-point delay separations as small as 2–5 μs (9–11). However, two-point versus one-point discrimination experiments show only that bats perceive two-point echoes to be different than one-point echoes; they do not show whether bats actually perceive each of the reflecting points making up the two-point target, as is necessary for effective resolution of objects. Bats could perceive the actual distances to individual reflecting surfaces making up the target—in effect, a range image of the object, or they could perceive only the acoustic spectrum of the overlapping, interfering echoes reflected by the object’s complex shape, without explicitly recreating a range image (2, 3). One experiment has examined these possibilities using an echo-delay discrimination procedure modified to incorporate two-point test echoes (12) instead of simple two-point versus one-point discrimination. The results reveal that Eptesicus perceives the delay of each component of two-point echoes separately, at least for the relatively large two-point spacing of 100 μs (equivalent to a range difference of 17 mm). More sensitive experiments using a modified version of a jittered-echo procedure (13–15) show that Eptesicus perceives both components of two-point echoes at even smaller two-point spacings of 10, 20, or 30 μs (range differences as small as 1.7 mm). Our concern here is to determine the limit for the bat’s two-point echo-delay resolution, which must be somewhere between 10 μs and 0, using a method that also determines whether they perceive each point separately.
MATERIALS AND METHODS
The animals used in our experiments were big brown bats, Eptesicus fuscus (Chiroptera: Vespertilionidae; ref. 16), obtained from houses in southeastern New England. Eptesicus is a relatively docile insectivorous bat, and many psychophysical studies of echolocation use this species (4). Care of animals used in this research was in accordance with the guidelines of the Brown University Institutional Animal Care and Use Committee.
Electronic Target Simulator for Jittering Echoes.
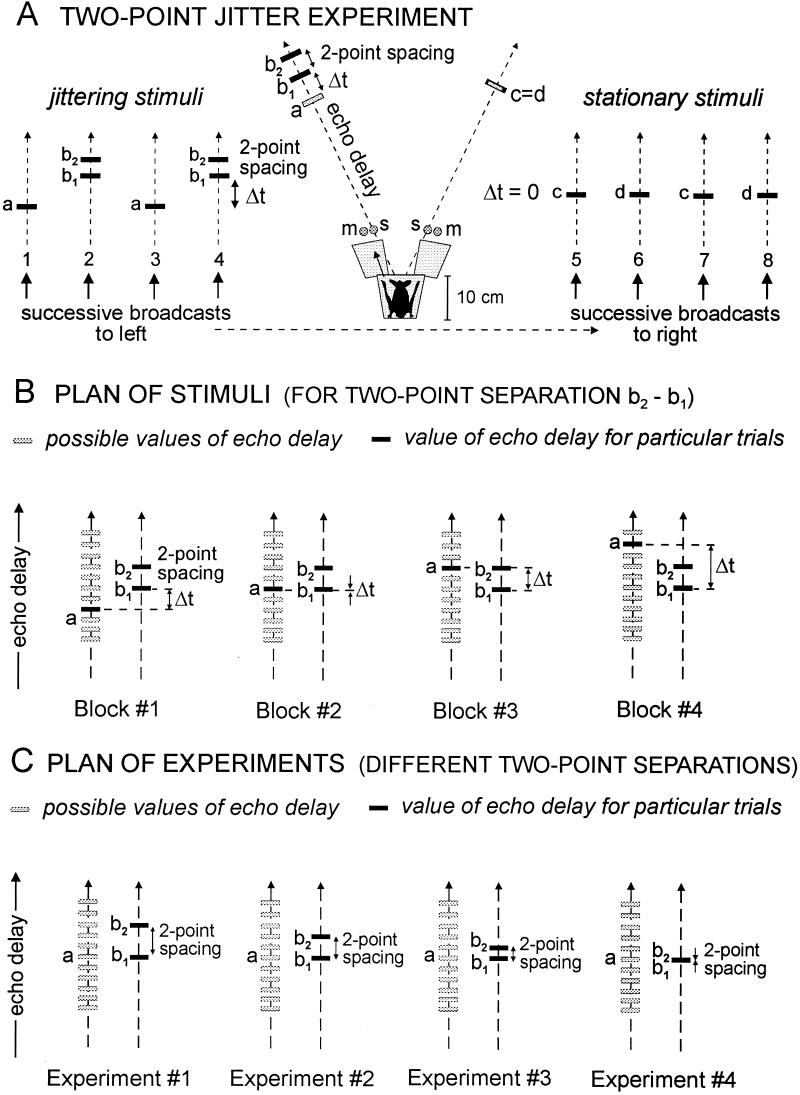
Fig. 1A shows the behavioral procedure (“two-point jitter experiment”) we used for studying the acuity of echo-delay resolution (13, 15). The bat is trained to sit on an elevated Y-shaped platform (shaded in Fig. 1A) and broadcast sonar sounds into two Bruel & Kjaer Model 4138 condenser microphones (m) located 10–12 cm away, one on the bat’s left and one on the right, separated by about 40°. The signals from the left and right microphones are delayed electronically and replayed to the bat as artificial echoes from the corresponding RCA type 112343 electrostatic loudspeakers (s, located next to the microphones). The frequency response of the simulator extends from 20 kHz to about 85 kHz (±8 dB), and pairs of these loudspeakers are similar in their frequency response (±4 dB differences from 20 to 85 kHz) (13); interchanging or replacing them does not affect the bat’s performance. Taken as a whole, the apparatus shown in Fig. 1A is a two-channel electronic target simulator which picks up each of the bat’s broadcasts, delays the signal, and returns echoes at specified times. These echoes consist either of single replicas of the broadcast sound (a, c, d) or a two-point double replica (b1 + b2) with various two-point spacings. The bat’s task is to decide whether the electronic echoes vary in delay from one broadcast to the next (jitter between two delay values) or are stationary in delay from one broadcast to the next. Our method for measuring resolution is to introduce the two-point stimulus and determine how its presence affects the bat’s ability to detect the jitter. The jittering stimuli consist of the single echo a alternating with the double or two-point echo b1 + b2. The stationary stimuli consist of the single echoes c and d. In Fig. 1A, the two-point spacing between b1 and b2 is the stimulus characteristic we investigate as the independent variable. This time interval should not be confused with the jitter interval, Δt, which, for convenience, is the interval between the first of the two points (b1) and single-point echo (a). For the jittering stimuli Δt varies from 18.6 μs down to 0 μs in different experiments, whereas for the stationary echoes Δt is always 0 μs. The jittering and stationary echoes are created by identical apparatus incorporating electronically switched delay lines that alternate from one bat broadcast to the next; the stationary echoes just have the same delay-line settings while the jittering echoes have different settings. We describe the stationary echoes as c and d to emphasize that no incidental characteristics of any part of the apparatus are different for jittering and stationary echoes.
Figure 1.
(A) Diagram of two-point jittering-echo experiment (details in text) for measuring the smallest two-point spacing between two concurrent echoes (b1, b2) where each echo is perceived separately, showing examples of jittering stimuli (left) and stationary stimuli (right). Jitter interval, Δt, is defined from echo b1 to echo a. (B) Diagram of stimuli on successive blocks of trials for a given experiment with different jitter intervals, Δt, and a fixed two-point spacing of b1 and b2. (C) Diagram of stimuli for successive experiments, each with a different value for the two-point spacing of b1 and b2. The essential procedure is to reduce the size of the two-point spacing from 10 μs to 0 μs in small steps to determine the smallest spacing for which the bat’s jitter performance curve in percentage errors still contains two peaks—one for b1 and another for b2—rather than one peak for b1 and b2 together.
Our goal is to study the bat’s image of b1 and b2 as a pair. To do so, the two-point stimulus has to be embedded within a procedure that allows us to use the single echo a as a probe stimulus for examining perception of each point separately. Thus, the critical psychophysical comparison made by the bat is between echo a and echo b1 + b2, not between c or d and b1 + b2. The experimental procedure (described in Fig. 1B) is to vary the size of the jitter interval (Δt) by moving this probe echo a to different points in time relative to each point (b1, b2) in the two-point echo. In Fig. 1, small gray rectangles show different possible locations of echo a, while black rectangles show the actual values of a and b1 + b2 during any particular trial. The rationale for this procedure is that the bat will make many errors in its jitter-detection task (Fig. 1A) when echo a is aligned at the same delay as either echo b1 or echo b2. The occurrence of these excess errors reveals whether it perceives b1 and b2 as having discrete delays. This process requires that we carry out a series of different jitter-detection experiments (described in Fig. 1C), one for each time separation of the two-point echo b1 + b2, to determine how these different separations affect the bat’s ability to perceive the jitter. We used two-point separations of 10.6 μs, 5.3 μs, 4.0 μs, 2.7 μs, 1.3 μs, and 0 μs. Each experiment (e.g., experiments 1–4, Fig. 1C) involves presentation of a whole series of jitter intervals (Δt) on different blocks of trials (e.g., blocks 1–4, Fig. 1B).
Jitter-Detection Task.
To receive a reward on any given trial, the bat has to determine which side (left, right) delivers echoes which jitter in delay (a and b1). It is trained to move forward (arrow in Fig. 1A) onto the left or right arm of the Y-shaped platform toward the correct loudspeaker producing jittering echoes a and b1 (for which Δt ≠ 0), where it is given a piece of mealworm (Tenebrio larva) offered with forceps (13). If it moves toward the wrong loudspeaker producing stationary echoes c and d (for which Δt = 0), it receives no reward, while the experiment halts for a brief time-out period. The jitter to be detected is between echo a and echo b1; the second part of the two-point echo, b2, is not introduced into the procedure until after the bat has learned to determine which side of the apparatus delivers the jittering echoes. Once trained to the jitter itself, the bats did not subsequently relearn simply to go to whichever side produced the double echo b1 + b2.
A crucial feature of the procedure in Fig. 1A is that each of the bat’s sonar broadcasts leads to the delivery of only one of the electronic echoes—single echo a or double echo b1 + b2 from the jittering side and single echo c or single echo d from the stationary side. The next sonar broadcast then leads to delivery of the other electronic echo from that side. The bat thus has to remember the delay of one echo and compare it with the delay of the next echo to determine whether there is any jitter present. At a minimum, the trace of each echo has to persist until after broadcast of the next sonar sound and reception of the corresponding next echo, an interval typically of 30–50 ms depending on the repetition rate of the bat’s sounds, which was roughly 20–30 sounds/s during individual trials. In contrast, the size of the jitter interval and the spacing between the two-point echoes are only a few microseconds. For each trial the bat is presented with both the correct (jittering) and the incorrect (stationary) stimuli in a two-alternative forced-choice procedure, but the apparatus prevents it from actually receiving echoes through both the left and the right channels at the same time because only one of the two simulator channels is activated for each broadcast sound (13, 15). After being placed on the Y-shaped platform at the start of each trial, the bat learns to scan to its left and right by moving its head while emitting echolocation sounds. The moderately directional sonar sounds (17) are steered by these left-right head movements and thus impinge on the left and right microphones with different amplitudes. An electronic comparator activates only the channel for that microphone which receives the stronger version of each emitted sound, and only the loudspeaker for that channel returns an electronic echo. If the bat aims its broadcast sounds to the left, the left channel is activated and it receives echoes from the left; if the bat aims its broadcasts to the right, the right channel is activated and it receives echoes from the right. The comparator explicitly prevents simultaneous activation of both channels on any one sound, and a narrow “dead zone” in the comparator’s response keeps both channels off when the bat aims its sounds exactly half-way between the two microphones (15).
During a typical trial, the bat emits roughly 5–20 sounds at one channel before its head movements shift the activation to the other channel, so it receives a series of approximately 5–20 echoes from each channel in succession. On some trials, it scans each channel only once, but often it scans one or both channels several times before making its choice—each scan still consisting of about 5–20 broadcast sounds (13). The correct (jittering) stimulus appears either on the left or on the right from one trial to the next according to a pseudorandom schedule. At each value of Δt, 40–60 trials are conducted in a block over 1 or 2 days (usually 30–60 trials/day), and then the value of Δt is changed. Perfect performance is 100% correct choices (0% errors), and chance performance is 50% correct choices (50% errors). The data are presented as plots showing percentage errors achieved by each bat at different values of the jitter interval (Δt) for each separate experiment involving a different two-point separation.
Presentation of Stimuli for Blocks of Trials in Different Experiments.
Fig. 1A illustrates the pattern of the two-point and one-point jittering stimuli. (In Fig. 1, gray rectangles show possible delay values; black rectangles show delays actually used in a given trial of any one experiment.) First, the bat aims its head and sonar sounds to the left to receive the jittering echoes a and b1 + b2, which alternate back and forth from one sonar broadcast to the next (on successive broadcasts numbered 1, 2, 3, and 4 in Fig. 1A). Then the bat shifts the aim of its head and sounds to the right to receive the stationary echoes c or d (on successive broadcasts numbered 5, 6, 7, and 8 in Fig. 1A). The bat usually scans both left and right channels in less than 1–2 s. Fig. 1B shows the plan of stimuli over successive blocks of trials for any particular two-point separation between b1 and b2. With the delay of echo b1 used as reference for the jitter, echo a is varied over a series of delays relative to b1 and b2 on successive blocks of trials. (Possible delay values for echo a are shown in gray, while the value actually used on one particular block of trials is shown in black.) When the perceived delay of echo a matches the perceived delay of echo b1 (shown as block 2 in Fig. 1B), the jitter is hard to detect and the bat makes many errors on that block of trials compared with other blocks of trials where echoes a and b1 have different delays (14, 15). The key to using this method for measuring the bat’s two-point resolution is that the bat also makes many errors when the delay of echo a matches the delay of echo b2. Specifically, the occurrence of many errors when echo a matches either echo b1 or echo b2 indicates that the bat has remembered a value for the delay of echo b2 as well as a value for the delay for echo b1. The procedure for measuring the bat’s two-point threshold is to reduce the size of the two-point separation in a series of separate experiments until b1 and b2 no longer produce separate peaks of errors in the bat’s jitter-detection performance curve (until there is only one error peak for b1 and b2 together, not two peaks for b1 and b2 separately).
For any given block of experimental trials at a specific two-point separation (e.g., blocks 1–4, Fig. 1B), the delay of a (black rectangle) is fixed, while a different delay of a is chosen for the next block of trials. Fig. 1B shows four such blocks of trials at one value of the two-point separation, while Fig. 1C shows a series of four different experiments with other two-point separations, each experiment with its own corresponding blocks of trials. When all of the possible values for the delay of a (gray rectangles in Fig. 1B) have been covered by different blocks of trials, the experiment (e.g., experiment 2, Fig. 1C) is finished for that particular two-point separation. The next experiments have different values for the two-point separation (experiments 3 and 4). The size of the jitter (Δt) on successive blocks of trials ranges from 18.6 μs down to 0 around a mean overall delay of 3.275 ms (equivalent to a simulated target range of about 56 cm). Because of the design of our digital delay lines (13), the smallest increment of delay available was 1.33 μs over this range. We used analog delay lines to supplement the digital delay lines so that delay steps smaller than 1.33 μs were possible, but these results are not needed to determine the two-point threshold (see below). The stationary echoes had a delay of 3.275 ms, which equals the mean of the jittering echoes ([a + b1]/2 = c = d) for each jitter condition. Thus, Fig. 1 B and C only shows the stimuli in relation to the delay of b1 as a reference; the absolute delays of both b1 and a were adjusted from one value of Δt to another to keep the mean delay of these jittering echoes at 3.275 ms. The delay of echo b2 was kept at a fixed separation from echo b1 and therefore moved with echo b1 as it was changed to keep the mean of the jittering echoes at the required fixed value. The overall acoustic delay of each simulated echo (a, b1, b2, c, d) is compounded from the travel time of the bat’s sound to the microphone (290 μs for a nominal 10-cm path length) plus an adjustable electronic delay (about 2695 μs, but varied according to Δt; ref. 13) plus the travel time from the loudspeaker back to the bat (again, 290 μs for a nominal 10 cm).
Acoustic Characteristics of Stimuli.
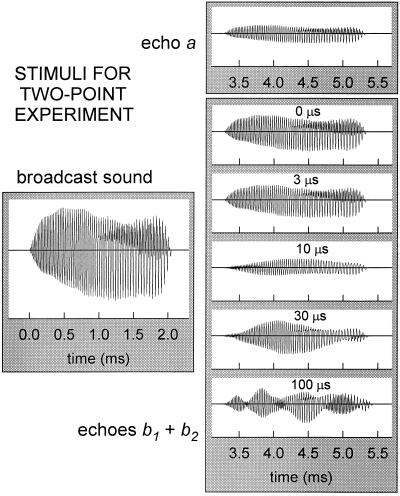
The delay increments for the jittering stimuli are only a few microseconds (Fig. 1B), as are the time separations of the two-point echoes (Fig. 1C), but the bat’s sonar broadcasts are sounds with durations of 2–4 ms emitted at intervals of 20–30 ms. Consequently, the tiny offsets in delay which characterize the two-point stimuli are obscured by the relatively long overlapping waveforms of the sounds themselves. Fig. 2 illustrates a representative biosonar emission from Eptesicus (broadcast sound) and the various stimuli (echo a; echoes b1 + b2) that the apparatus would deliver to the bat. (Echoes c or d are the same as echo a.) The emitted signal is approximately 2 ms long, and each individual electronic echo (a, b1, b2, c, d) is effectively just a reduced-sized replica of this sound returned to the bat on the platform. Each such electronic echo was presented to the bat at an amplitude of −34 dB relative to the bat’s broadcast sound at the microphone (13). The bat’s broadcasts were approximately 95–102 dB SPL (peak-to-peak) at 10 cm, which places individual echoes at about 61–68 dB SPL (peak-to-peak), or about 52–59 dB SPL RMS. An ideal point-target located 56 cm away would return echoes that are 30 dB weaker due to spreading losses alone, and the additional 4 dB attenuation of the stimuli corresponds to a somewhat reduced target strength. At certain orientations, body parts of fluttering moths and other targets that have been used in studies of sonar-guided interception produce echoes of comparable strength (18–20), so the stimuli are inside the range of biologically reasonable amplitudes.
Figure 2.
Sound-pressure waveforms for a representative echolocation sound emitted by Eptesicus and for echoes a or b1 + b2 delivered to the bat by the apparatus in Fig. 1A. Overlap and interference of b1 and b2 alters the waveform of two-point echoes according to their time separation (0, 3, 10, 30, 100 μs). The stimuli reaching the bat are not “points” as implied schematically by Fig. 1 but sounds several milliseconds long, and the apparent separation of b1 and b2 in Fig. 1 is obscured when the echoes overlap and interfere with each other.
Whereas echoes a, c, and d are single replicas (e.g., echo a, Fig. 2), the two-point echo b1 + b2 consists of two replicas of the broadcast delivered to the bat at almost the same instant. The waveforms of two-point echoes consequently differ from the waveform of a single replica as a result of mutual interference because there is reinforcement and cancellation of amplitude at different frequencies according to the size of the two-point separation. Fig. 2 shows a series of two-point echoes at delay separations of 0, 3, 10, 30, and 100 μs. Two replicas added together at identical delays (0 μs separation) merely form an echo for b1 + b2 that is twice as strong (+6 dB) as the single replica a. However, as the size of the two-point separation increases, echo b1 + b2 differs by progressively greater amounts from the single replica. Depending on the size of the two-point separation, mutual interference is manifested first as a reduction in overall amplitude (for two-point separations smaller than 5–10 μs) and then as a series of peaks and notches in the echo envelope (for two-point separations larger than 5–10 μs). The complexity of the two-point waveforms in Fig. 2 created by interference translates into a series of peaks and notches in the two-point echo’s spectrum. For delay separations greater than 10 μs, there are multiple notches which can easily be discerned in the spectrum; for separations from 5 to 10 μs, there is a single, easily discerned notch; however, for separations of 5 μs or less, there no longer is a well-defined notch in the spectrum. The reason is that the bat’s sonar broadcasts contain frequencies from a minimum of about 20 kHz to a maximum of 100–110 kHz, whereas the spectral notch would be located at frequencies above 100 kHz if the two-point separation is shorter than 5 μs. Not only do the echoes contain no frequencies high enough to depict the interference notch above 100–110 kHz, but the bat’s hearing for echoes also is restricted to frequencies from 20 kHz to no more than 100–110 kHz (21). Consequently, the spacing of the two-point echoes can be “read” from the pattern of peaks and notches in the interference spectrum only for delay separations larger than 5 μs.
RESULTS
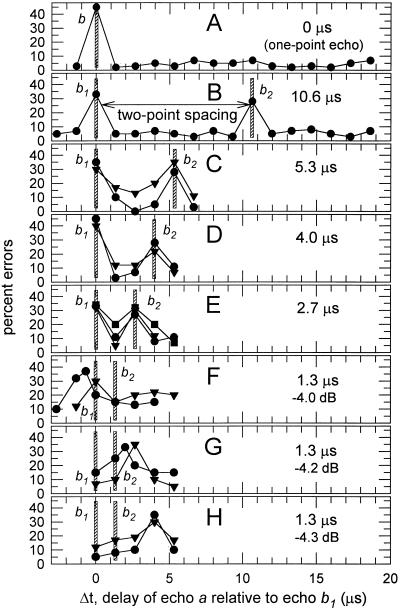
Fig. 3 shows results (percentage errors) from the series of two-point jitter experiments diagrammed in Fig. 1. Two big brown bats, bat 3 (filled circles in Fig. 3) and bat 5 (filled triangles in Fig. 3), completed blocks of 40–60 trials on two-point spacings of 5.3, 4.0, 2.7, 1.3, and 0 μs. Subsequently, a third bat, bat 4 (filled squares in Fig. 3), was diverted from another experiment and tested on what proved to be the most critical delay spacing of 2.7 μs. [The trial-by-trial behavior of big brown bats in jitter experiments is described in detail elsewhere (13, 15).] The curves in Fig. 3 show the bats’ performance at detecting the jitter for different delays of echo a relative to echo b1 (horizontal axis of each plot) and also for different two-point delay separations of b1 and b2 (B–H). Vertical crosshatched bars on each plot in Fig. 3 mark the locations of echo b1 and echo b2 in relation to the various experimental delays of echo a (echo b1 is at 0 μs).
Figure 3.
(A–H) Performance of bats in a series of different jitter experiments with two-point spacings of 0 μs, 10.6 μs, 5.3 μs, 4.0 μs, 2.7 μs, and 1.3 μs. (Each experiment as in Fig. 1B; successive experiments as in Fig. 1C.) Curves in each plot trace percentage errors in 40–60 trials as function of delay of single jittering echo a relative to delay of echo b1 (i.e., Δt) regardless of spacing between b1 and b2. Data points are filled circles for bat 3, filled squares for bat 4, and filled triangles for bat 5 (13). Vertical crosshatched bars mark locations of echoes b1 and b2 relative to delays of a in each experiment. Curves contain separate error peaks for b1 and b2 until two-point spacing declines to 1.3 μs, where only one error peak is present (see text). Three different plots show 1.3-μs performance curves for different relative amplitudes of b1 + b2 (F, −4.0 dB; G, −4.2 dB; H, −4.3 dB).
To serve as a reference, Fig. 3A shows representative results for the case of a one-point echo (single echo b by itself). The bat in Fig. 3A made about 3–7% errors at all relative delays of a from 1.33 to 18.6 μs. However, at 0 μs, where the delay of a matches that of b, the bat’s performance was 46% errors or chance (13). When echoes a and b had 0 delay difference, there was no jitter to be detected and the bat performed randomly, but when the delay difference was 1.33 μs or larger, it uniformly made few errors. This curve illustrates the rationale for the jitter procedure—the bat made a single error peak when the delays to be remembered are the same. The question is whether there will be an additional error peak for echo b2 when the one-point echo b is replaced with a two-point echo b1 + b2.
Fig. 3B shows representative results in the two-point experiment at a relatively large delay spacing of 10.6 μs (14, 15). Here, the bat made only 2–8% errors at all delays of a except those delay values corresponding to echo b1 at 0 μs and echo b2 at 10.6 μs, where the bat’s performance was much poorer, at 28–33% errors. These error peaks indicate that the bat’s perception of the jitter was impaired when the delay of echo a matched the delay of either echo b1 or echo b2. The peak error rate for a single-point target is around 50% errors as in Fig. 3A, but it typically declines to about 28–35% for each point in a two-point target (14, 15). This effect is not peculiar to the jitter task; it occurs even in an ordinary echo-delay discrimination task with two-point echoes (12). Next, Fig. 3 C–E shows the performance of big brown bats for smaller two-point separations of 5.3 μs, 4.0 μs, and 2.7 μs. These performance curves also contain two discrete, well-defined error peaks at the delays of b1 and b2. However, in contrast to the results for spacings of 2.7, 4.0, and 5.3 μs, the performance curves in Fig. 3F for a delay spacing of only 1.3 μs contain just one error peak. These results indicate that the bat’s threshold for two-point resolution (separate error peaks for each point) must be between 1.3 and 2.7 μs, or about 2 μs.
Before accepting 2 μs as the bat’s limit for delay resolution, we need to be certain that there really is only one error peak for a 1.3-μs delay spacing. Fig. 3 F–H shows three separate sets of 1.3-μs data for experiments in which echoes b1 and b2 were presented at slightly different amplitudes relative to echo a (−4.0 dB in Fig. 3F, −4.2 dB in Fig. 3G, −4.3 dB in Fig. 3H). Decreasing the amplitude by small amounts causes a slight retardation in the latencies of neural responses representing b1 + b2 and thus causes a slight increase in their perceived delay. This effect is called amplitude/latency trading (22); in Eptesicus it is −13 to −17 μs/dB (12, 13, 23). In Fig. 3 F–H, the location of the error peak for each bat is shifted rightward to slightly longer delays as echo amplitude is decreased. This shift is about 1.3–1.5 μs for each 0.1-dB reduction in echo amplitude, as would be expected from previous measurements of 13–17 μs for each full decibel of attenuation. (Note that the locations of the error peaks in Fig. 3 F–H do not match the delays of echo b1 or echo b2 as they did in Fig. 3 A–E. The merging of both b1 and b2 into a single perceived delay alters the relation between amplitude and perceived delay that prevailed for all the two-point echoes which were perceived as containing two points. On top of this, the use of amplitude/latency trading detaches the perceived delays of these echoes from the scale in Fig. 3 A–E.) Here, we use amplitude/latency trading to slide the apparent delay of the two-point echo to slightly different locations along the delay axis relative to the 1.33-μs delay-line steps so that we can obtain slightly different views of the same peak. (Curves for bat 3 in Fig. 3 F and G each contain one data point interpolated between points with 1.33-μs delay steps due to the use of analog delay line; other data from analog delay steps are not needed to show where the two-point threshold lies.) By comparing the shapes of the peaks in all three experiments (Fig. 3 F–H), we see that there is only one peak, not two peaks that might have been missed because the size of the smallest digital delay step allowed by the apparatus (1.33 μs) happens to be very close to the bat’s own two-point limit (2 μs).
DISCUSSION
From Fig. 3, using the jitter experiment’s criterion of one error peak for each part of the two-point stimulus, big brown bats can separately perceive the delays of two-point biosonar echoes arriving as close together as 2 μs. This delay resolution is equivalent to a difference in distance of about 0.3 mm between two reflecting surfaces or points within the same object. Although this is indeed fine range resolution, it is only two or three times sharper than the resolution of less than a millimeter already measured using complex target surfaces (5–7), and it is about the same as the limit measured in two-point delay discrimination tests (8–11), which, however, do not show whether the bat actually perceives each reflecting point separately. A two-point limit no worse than 5–10 μs has been predicted from the performance of bats in a variety of naturalistic tasks such as interception and discrimination of airborne targets or obstacle avoidance (3). We used a stable psychophysical condition of low uncertainty to measure two-point resolution as sensitively as possible—echoes from one side of the target simulator (Fig. 1A) either jitter in delay or are stationary, and the bat’s jitter-detection performance in this easily learned task is affected by the relative timing of a probe echo (a) in relation to the two-point echo (b1 + b2) at different delay separations.
For the bat to make separate error peaks at the delays of echo b1 and echo b2, each delay comprising the two-point stimulus has to be perceived in a form that can be confused with the delay of echo a in the jitter task. From the waveforms shown in Fig. 2, individual reflections comprising the two-point echoes tested in Fig. 3 are so close together that they overlap almost completely. Previous echo-detection experiments (11) have established that Eptesicus treats two-point echoes at separations shorter than 300–400 μs as being one single, overlapping sound with a single delay and a single interference spectrum, and all of the two-point stimuli used here are no more than 10.6 μs apart. Furthermore, Eptesicus can readily discriminate the spectrum of (for example) the 10.6-μs two-point echo from the spectrum of a single-point echo (9–11), so the interference spectrum is available to the bat. If the bats use spectral cues for detecting the jitter directly (7–10), they should be able to determine that the jittering echoes always contain a spectrally distinct double echo and thus they should always perform the task with few errors. Instead, the bats experience errors whenever the single-point echo, a, coincides in delay with either of the two-point echoes, b1 or b2, indicating that the two-point echo is perceived in terms of its delay structure, not merely its spectrum (14).
For the delay of the second of the two-point echoes to be perceived, information in the interference spectrum has been moved into the time domain to create an estimate of delay. Transformation of echo interference spectra into delay estimates has been demonstrated explicitly for large delay separations of 10–100 μs (12, 14, 15); it is a type of deconvolution that is computationally realizable (14), and the present experiments show that it extends to the shortest two-point separations the bats can resolve. However, for the smaller delay separations in Fig. 3, the spectral pattern the bats use cannot simply be the locations of interference peaks and notches. For separations as small as 2–3 μs, the lowest frequency notch would be located at 167–250 kHz—frequencies too high for the bat to hear (21) and frequencies not contained in the bat’s broadcasts or in the echoes it received in these experiments. To assign a delay value to both echoes, the bats have to reconstruct the time separation of the two points from the very slight low-pass effect associated with interference at separations of less than 5 μs.
Using the fastest neuronal recovery times in the bat’s auditory system as a guide, two-point resolution should be about 300–400 μs (24), not 2 μs. More generally, variability in response latency in conjunction with amplitude/latency trading in frequency-modulated bats has been taken as evidence that echo delay cannot usefully be perceived with an accuracy significantly better than 100 μs (22), which would restrict resolution to comparably large values. Nevertheless, when “asked” to detect changes in echo delay from one broadcast to another, Eptesicus assigns separate delay values to each of the overlapping echoes at spacings as small as 2–3 μs. The crude delay accuracy of 100 μs and delay resolution of 300–400 μs predicted physiologically seems adequate to explain the bat’s ability to intercept flying prey when no other objects are near enough to return competing echoes (2, 3), and certainly the 2-μs resolution limit measured here is far in excess of what would be needed just to capture individual flying insects. However, fine delay resolution is necessary to determine the locations of multiple reflecting surfaces and to perceive the shape of a complex target in spatial terms—all of which might be necessary in more complicated acoustic surroundings (3). Our results suggest that echolocation has evolved to serve the bat in a wider variety of orientation tasks than just capture of isolated flying insects. The bat’s images are richer in content than has been thought from physiological evidence alone, which means that its echo-processing mechanisms are more sophisticated than has been revealed so far in most physiological studies (25).
Acknowledgments
We are grateful for the inexhaustible patience of reviewers for this paper. This research was supported by Office of Naval Research Grants N00014-89-J-3055 and N00014-95-l-1123, National Institute of Mental Health Grant MH00521 (RSDA), National Science Foundation Grants BCS-9216718 and BES-9622297, National Institute of Mental Health Training Grant MH19118, McDonnell-Pew Grant T89-01245-023, and Deafness Research Foundation funds.
References
- 1. Griffin D R. Listening in the Dark. New Haven, CT; reprinted 1986 Cornell Univ. Press, Ithaca, NY: Yale Univ. Press; 1958. [Google Scholar]
- 2.Simmons J A. Cognition. 1989;33:155–199. doi: 10.1016/0010-0277(89)90009-7. [DOI] [PubMed] [Google Scholar]
- 3.Simmons J A, Ferragamo M J, Saillant P A, Haresign T, Wotton J M, Dear S P, Lee D N. In: Hearing by Bats. Popper A N, Fay R R, editors. New York: Springer; 1995. pp. 146–190. [Google Scholar]
- 4.Moss C F, Schnitzler H-U. In: Hearing by Bats. Popper A N, Fay R R, editors. New York: Springer; 1995. pp. 87–145. [Google Scholar]
- 5.Habersetzer J, Vogler B. J Comp Physiol. 1983;152:275–282. [Google Scholar]
- 6.Simmons J A, Lavender W A, Lavender B A, Doroshow C A, Kiefer S W, Livingston R, Scallet A D, Crowley D E. Science. 1974;186:1130–1132. doi: 10.1126/science.186.4169.1130. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt S. Nature (London) 1988;331:617–619. doi: 10.1038/331617a0. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt S. J Acoust Soc Am. 1992;91:2203–2223. doi: 10.1121/1.403654. [DOI] [PubMed] [Google Scholar]
- 9.Mogdans J, Schnitzler H-U. J Acoust Soc Am. 1990;88:754–757. doi: 10.1121/1.399724. [DOI] [PubMed] [Google Scholar]
- 10.Mogdans J, Schnitzler H-U, Ostwald J. J Comp Physiol A. 1993;172:309–323. doi: 10.1007/BF00216613. [DOI] [PubMed] [Google Scholar]
- 11.Simmons J A, Freedman E G, Stevenson S B, Chen L, Wohlgenant T J. J Acoust Soc Am. 1989;86:1318–1332. doi: 10.1121/1.398693. [DOI] [PubMed] [Google Scholar]
- 12.Simmons J A, Moss C F, Ferragamo M. J Comp Physiol A. 1990;166:449–470. doi: 10.1007/BF00192016. [DOI] [PubMed] [Google Scholar]
- 13.Simmons J A, Ferragamo M, Moss C F, Stevenson S B, Altes R A. J Comp Physiol A. 1990;167:589–616. doi: 10.1007/BF00192654. [DOI] [PubMed] [Google Scholar]
- 14.Saillant P A, Simmons J A, Dear S P, McMullen T A. J Acoust Soc Am. 1993;94:2691–2712. doi: 10.1121/1.407353. [DOI] [PubMed] [Google Scholar]
- 15.Simmons J A. J Comp Physiol A. 1993;172:533–547. doi: 10.1007/BF00213677. [DOI] [PubMed] [Google Scholar]
- 16.Kurta A, Baker R H. Mammalian Species. 1990;356:1–10. [Google Scholar]
- 17.Hartley D J, Suthers R A. J Acoust Soc Am. 1989;85:1348–1351. [Google Scholar]
- 18.Kober R, Schnitzler H-U. J Acoust Soc Am. 1990;87:882–896. [Google Scholar]
- 19.Moss C F, Zagaeski M. J Acoust Soc Am. 1994;95:2745–2756. doi: 10.1121/1.409843. [DOI] [PubMed] [Google Scholar]
- 20.Simmons J A, Chen L. J Acoust Soc Am. 1989;86:1333–1350. doi: 10.1121/1.398694. [DOI] [PubMed] [Google Scholar]
- 21.Koay G, Heffner H E, Heffner R S. Hear Res. 1997;105:202–210. doi: 10.1016/s0378-5955(96)00208-0. [DOI] [PubMed] [Google Scholar]
- 22.Pollak G D, Marsh D S, Bodenhamer R, Souther A. J Neurophysiol. 1977;40:926–942. doi: 10.1152/jn.1977.40.4.926. [DOI] [PubMed] [Google Scholar]
- 23.Burkard R, Moss C F. J Acoust Soc Am. 1994;96:801–810. doi: 10.1121/1.410318. [DOI] [PubMed] [Google Scholar]
- 24.Casseday J H, Covey E. Brain Behav Evol. 1996;47:311–336. doi: 10.1159/000113249. [DOI] [PubMed] [Google Scholar]
- 25.Simmons J A, Saillant P A, Ferragamo M J, Haresign T, Dear S P, Fritz J, McMullen T A. In: Auditory Computation. Hawkins H L, McMullen T A, Popper A N, Fay R R, editors. New York: Springer; 1996. pp. 401–468. [Google Scholar]