Abstract
The identification of H5N1 in domestic poultry in Europe has increased the risk of infection reaching most industrialized poultry populations. Here, using detailed data on the poultry population in Great Britain (GB), we show that currently planned interventions based on movement restrictions can be expected to control the majority of outbreaks. The probability that controls fail to keep an outbreak small only rises to significant levels if most transmission occurs via mechanisms which are both untraceable and largely independent of the local density of premises. We show that a predictor of the need to intensify control efforts in GB is whether an outbreak exceeds 20 infected premises. In such a scenario neither localized reactive vaccination nor localized culling are likely to have a substantial impact. The most effective of these contingent interventions are large radius (10 km) localized culling and national vaccination. However, the modest impact of these approaches must be balanced against their substantial inconvenience and cost.
Keywords: avian influenza, control strategies, modelling
1. Introduction
Since the beginning of 2006, the H5N1 subtype of the highly pathogenic avian influenza (HPAI) virus has spread from Asia to Europe (Domenech et al. 2006) with outbreaks in industrialized nations becoming increasingly probable. Globally, over 140 million birds have been slaughtered in an attempt to control H5N1 transmission (Webster et al. 2006a). Current EU recommendations to control a new incursion of H5N1 are that movement restrictions are put in place as rapidly as possible (European Commission 2006a). Although the exact implementation of such restrictions varies by country, most of them involve isolation of the infected premises (IP) and implementation of restricted geographical zones around the IP. In Great Britain (GB) current plans for control of an outbreak incorporate isolation of the IP, implementation of protection and surveillance zones (SZs) within 3 and 10 km, respectively, of each IP in which the movement of poultry and people between farms is restricted; and tracing and isolation of farms that have recently been in contact with the IPs (termed ‘dangerous contacts’, DC; Department for the Environment Food and Rural Affairs 2006a). This policy was associated with success in restricting the recent incursion of H5N1 HPAI on a commercial turkey farm in Suffolk (Department for the Environment Food and Rural Affairs (Defra) 2007) to a single premises.
The main factor determining the potential scale of an outbreak is the between-premise reproduction number R0, defined as the average number of premises infected by an initial IP at the start of an outbreak. Detailed analysis of data from a Dutch outbreak of HPAI suggested R0 of up to 6 in the densest areas of poultry farming (Stegeman et al. 2004) while analysis of summary data from outbreaks in The Netherlands, British Columbia and Italy suggest R0 is in the range 1.5–3 (Garske et al. 2007). An outbreak can be considered to be under control once this value is reduced to below 1 although a substantial number of premises may continue to be affected in the tail of the epidemic even once control is achieved.
The transmission of HPAI is likely to vary geographically between and within countries due to regional variation in parameters which affect the susceptibility of farms to infection and their infectiousness to other farms. These include the mix of poultry species (with turkeys most susceptible to HPAI and asymptomatic infection common in ducks; Capua & Marangon 2004; Ellis et al. 2004; Sturm-Ramirez et al. 2004, 2005; Tumpey et al. 2004; Hulse-Post et al. 2005; McNally et al. 2006), whether poultry are kept indoors or outdoors and therefore are potentially in contact with wild birds, the number of birds on a premises and the biosecurity operated on the premises. Using data on these and on the natural history of infection in birds, we constructed a simulation model of HPAI transmission to evaluate how well current AI contingency plans could contain an incursion of H5N1 HPAI into the GB poultry flock. We considered two potential routes for transmission: (i) via connections between premises that occur owing to the movement of birds or shared resources (slaughter house or integrated company connections) and thus reflect the industry network structures and (ii) spatially localized random transmission between premises, intended to represent occasional ‘non-network’ contacts between premises, infection mediated by wild birds or air-borne spread.
2. Material and methods
(a) Population data
Data on all poultry premises holding more than 50 birds were obtained from the Poultry Register Database collated by Defra in February 2006. This contains 23 516 premises in total of which 11 967 kept less than 500 birds. The models were parametrized using data on the location, number of birds, species and husbandry purposes and company status (integrated/independent) of premises (see §1 in the electronic supplementary material for further details). Additionally, sample surveys of multi-site and single-site companies (3989 premises), 95 slaughterhouses and 45 catching companies by L.S. and J.W. was used to derive the distribution of number of contacts and the distance between these contacts.
(b) Mathematical model
We developed a simulation model based on individual flocks. Each flock can be in one of the following disease states: susceptible, latent, infectious, detected, isolated, restricted or culled. The durations and properties of these states are determined by the flock type and the control policy it is subject to (see §2.6 in the electronic supplementary material for details). Disease can be transmitted spatially or through direct contact between premises.
Spatial transmission was mediated by the spatial kernel where d is the distance between two premises, α=1.2 and γ=2.6 (see §3.1 in the electronic supplementary material). Two modes of spatial transmission were considered: (i) a constant, density-independent contact rate, which might describe contact through equipment or people and (ii) a density-dependent contact rate which might better represent air-borne transmission. Premises with less than 500 birds were subject only to spatial transmission, and not part of the transmission mediated through the industry networks described below, as these premises are unlikely to participate in the industry movements. Technical details for the spatial transmission are given in the electronic supplementary material, §3.1.
In order to cover a range of possible contact structures, we implemented two different contact network structures. In the first, contact between premises is through an overlapping mixing group structure, where premises belong to the same group if they were associated with the same company or shared a bird supplier or slaughter house. This is meant to mimic the indirect contact between premises due to shared facilities, and therefore transmission between any two premises could occur in either direction. Groups are implicitly spatially localized (via the distributions in figure 1d), and incorporate industry and company structure, species, husbandry purpose and distance. Potential contact between two group members can occur when both are using the common service at the same time. To match industry patterns, bird movements for slaughter are modelled as being periodic—occurring every 40 days and taking up to 3 days to complete. Similarly, visits from feed suppliers occur every 7 days. As a result, each group member is connected to only a proportion of fellow members at any time. Connections within these smaller groupings are not directional as they model the contacts between premises of similar types rather than the typical directional movement of birds from the hatchery to the rearing premises and finally the slaughterhouse.
Figure 1.
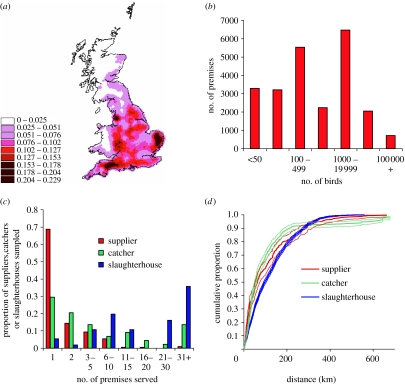
Structure of the GB poultry flock based on the 23 516 premises holding 271 million birds registered with Defra on 28 February 2006. (a) Spatial distribution of poultry premises, (b) distribution of total number of birds at each premise, (c) distribution of number of premises served by a slaughterhouse, catching company and integrated company and (d) cumulative distribution and 2.5th and 97.5th percentiles for the distance between premises and their slaughterhouse, catching company headquarters and bird suppliers (for non-company premises).
In addition, a fixed-link network transmission model was developed for comparison, where each premises is linked to a small number of other premises through commercial contacts rather than through the larger group structures with periodic contacts. The number of links (node degree) was drawn from a negative binomial distribution, parametrized to match the expected number of connections premises would make over the course of infectiousness, as indicated by the network contact data (see §3.3 in the electronic supplementary material). The mixing patterns between premises were based on a classification of premises according to species and husbandry purpose and company affiliations. Data indicated mixing to be moderately assortative (see §3.3 in the electronic supplementary material). The model incorporates a weak spatial structure by matching the distribution of distances between linked premises to that expected according to the use of slaughterhouses and other suppliers. For the epidemic simulations, different network structures were generated and five outbreaks per network were simulated. For the fixed network transmission model, no spatial transmission was considered.
In the group structure and fixed network, only premises with more than 500 birds were included, as smaller premises are clearly not commercial and are unlikely to participate in the industry movements. This exclusion of the smaller premises also accounts for the positive correlation that was observed between contact frequency and premise size (see §1.2 in the electronic supplementary material).
In both models, we additionally explore the impact of infectiousness scaling with the number of birds on premises. We consider infectiousness independent of flock size and also as a saturating function of population (using the function where n is the number of birds on a premises and NC=1000 is a constant). We choose this value of NC as it serves to discriminate between commercial holdings and smallholdings and hence between distinct methods of husbandry. Further details, including mathematical derivations of parameters, for both models are given in §3 in the electronic supplementary material.
We considered five different incursion scenarios representing single or multiple incursions in either fixed locations or at randomly selected premises. Unless otherwise stated, the results presented here assume a single incursion occurs in a premises chosen randomly from the population. For each scenario, between 1000 and 5000 epidemic realizations were generated.
(c) Natural history parameters
Experimental data for HPAI from individual chickens and turkeys suggest a period from inoculation to death of between 3 and 5 days with viral shedding occurring by 3 days post-infection (Sturm-Ramirez et al. 2004, 2005; Tian et al. 2005; van der Goot et al. 2005; Webster et al. 2006b) with one study reporting viral shedding in the buccal cavity 24 hours post inoculation in chickens and 8 hours post inoculation in turkeys (Essen et al. 2006). To match these data, we assume a fixed latent period of 12 hours and infectious period of 2 days following which the birds die. A simple compartmental within-flock model was used to translate data on individual bird disease parameters to those that are plausible at a flock level. Assuming a high within-premises R0 of 40, on the premises level, this translates to a latent period of 1.5 days and an infectious period for between-premises transmission of 4 days (see §2.5 and figures S2–S7 in the electronic supplementary material for further information and sensitivity analyses). Reducing the within-premise R0 increases the latent and infectious periods by only 0.5 day each.
In the between-flock models, we assume fixed waiting times in each state, however, exponentially distributed waiting times yielded essentially identical results. For scenarios with interventions, we assume that it takes 12 hours from the onset of infectiousness (which we assume is coincident with the onset of clinical signs) to detection and a further 24 hours for the premise to be isolated. From detection we assume it takes 36 hours for any restriction zone to be implemented and 2.5 days for the birds to be culled. These timings are similar, albeit a little faster than the response achieved in the recent outbreak of H5N1 in Suffolk (Department for the Environment Food and Rural Affairs 2007). Sensitivity to these natural history parameters is presented in §5.4 in the electronic supplementary material. Based on analysis of previous HPAI outbreaks in commercialized settings, we consider two values for reproduction number in our simulations: R0=1.5 and 3.0. Within these constraints, group and spatial transmission parameters were tuned to give various fixed proportions of transmissions to be spatial, averaged over all holdings and in a naive population, thus determining the transmission parameters βG and βS (see §3.4 in the electronic supplementary material for further details).
(d) Interventions
Planned interventions (Department for the Environment Food and Rural Affairs 2006b) and feasible alternatives (Capua & Marangon 2003; Capua & Alexander 2006) for the control of an avian influenza outbreak in the UK are well known. In the absence of a significant outbreak, however, little is known about the effectiveness of these measures and the efficiency with which they will be put in place. The interventions described below are felt to be optimistic but achievable and hence test effectiveness of the strategies themselves rather than the consequences of partial or inefficient implementation. Nevertheless, we also include some sensitivity analysis with respect to key intervention parameters in §5 in the electronic supplementary material.
We consider currently planned interventions which include isolation of the IP (90% reduction in the transmission probability of network contacts but no effect on local spatial transmission), implementation of 3 and 10 km protection and surveillance zones (PS/SZ), and tracing and isolation of DC. We assume that the protection and SZ are similar and responses to IPs within the SZ occur faster (12 hours from onset of infectiousness to isolation) than those outside (36 hours). In addition, for all premises within the PZ/SZ, the susceptibility and onward infectiousness is reduced by approximately 70% for network and spatial transmission. DC are taken to be members of the same mixing groups as an IP in the simulation model and the direct network neighbours in the network model. The impact of DC tracing is assumed to be a 75% reduction in susceptibility of uninfected DC premises and 75% reduction in infectiousness for infected DCs. For more information, see §2.6 and in particular tables S5 and S6 in the electronic supplementary material. Sensitivity analyses to these assumptions are presented in §§5.5 and 5.6 in the electronic supplementary material.
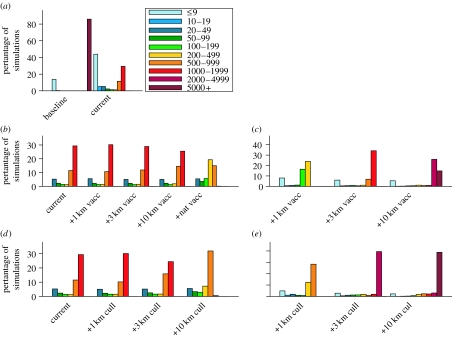
In addition, we examine a range of contingency interventions that might be employed in the event of currently planned approaches failing. Results for uncontrolled epidemics and current control plan (figure 4a) show the typical bimodal outbreak size distribution, where most outbreaks either die out in the early stages or develop into a major epidemic, but very few epidemics cease at an intermediate outbreak size. Therefore, if an outbreak has reached a value within this intermediate regime, it is likely to grow large. Here, we chose 20 IPs at the lower end of this trough in the outbreak size distribution as an indicator for a large epidemic, and use this value as a trigger for these reactive interventions. We test both global and local vaccination and local ring culling policies (both at various radii). It is not practical to vaccinate broiler chicks due to their large numbers and short lifespan. We assume that it is feasible to vaccinate all chicken layers and turkeys which constitute approximately 35% of the premises. Based on experimental data, vaccination at a population level is assumed effective after 21 days (see §2.4 in the electronic supplementary material). In the model, vaccination reduces susceptibility and infectiousness by 75% but the premises-level latent and infectious-to-detection periods are increased to 3 and 1 days, respectively. This choice assumes the use of sentinel birds (European Commission 2006b; Savill et al. 2006, see also §2.5 in the electronic supplementary material). Culling is assumed to be completed 7 days after implementation. Further sensitivity analyses to these assumptions are presented in §5.6 in the electronic supplementary material.
Figure 4.
Final outbreak size distributions. (a) Distribution of the number of IPs in the absence of any controls (baseline) and for currently planned interventions (current), (b) as (a) but only showing model runs with more than 20 IPs and also showing the effect of radial and national vaccination policies which are implemented once 20 IPs have been reached, (c) as (b) but showing the number of premises that are vaccinated during the course of the epidemic, (d) as (b) but showing the impact of radial culling policies which are implemented once 20 IPs have been reached, (e) as (d) but showing the number of premises that are pre-emptively culled (additional to the IPs). Bars show the percentage of simulations that fall into the various size categories. All runs are for the purely spatial model with R0=3, for the worst case of density-independent transmission and size-independent contact rate. All epidemics were seeded at a single randomly drawn premise and 1000 simulations were undertaken to calculate each distribution.
3. Results
(a) Poultry industry structure
Data on the structure of the British poultry population collated by the GB Department for the Environment, Food and Rural Affairs in February 2006 records data on 23 516 premises holding 271 million birds (figure 1 and electronic supplementary material). These show that the poultry industry is spatially clustered with a high density of farms in East Anglia, the Welsh borders region and the southwest (figure 1a). This clustering reflects the geographical distribution of the chicken industry in which many birds are kept in large premises (figure 1b). In contrast, other species such as ducks, geese and pheasants are kept in smaller holdings and distributed more uniformly across the country. Approximately 40% of all premises report holding some free-range birds.
The potential for transmission of HPAI will, among other factors, depend on the contact network between premises. Surveys carried out by L.S. and J.W. of connections between premises and slaughterhouses, as a result of movement of vehicles and personnel including those made by catching companies and within integrated companies, revealed that most premises were connected to relatively few other premises but that a small number (usually those part of large integrated companies) were connected to many more premises (figure 1c). The wide range of distances over which these connections operate is illustrated by the distributions shown in figure 1d.
(b) Baseline scenarios—outbreaks in the absence of interventions
Prior to evaluating the impact of interventions, we explored the ‘natural’ dynamics of the epidemic in the absence of controls. Given the uncertainties in the epidemiological parameters, this allowed us to identify which possible combinations presented the greatest risk against which control policies would need to be effective. Here, we highlight some of the results discussed in detail in §4 in the electronic supplementary material.
Probabilities of early extinction were found to be very strongly influenced by geographical position of the initial seeding event, particularly when disease transmission was density- or size dependent. At R0=1.5, a single incursion into a sparse region (Fife) leads to 97% of outbreaks extinct in two weeks, while an incursion into a dense region (Norfolk) leads to extinctions in only 5% of cases. To offset this effect, simulations in the remainder of this paper are initiated with a randomly chosen infected premises.
Increasing the proportion of infectious contacts taking place through the commercial sector was found to have a strong influence on an epidemic, decreasing the final size and increasing the probability of early extinction. Commercial sector transmission might be expected to increase transmission by linking premises over longer distances, but this mechanism is dominated by the daily substructure of infectious contacts made within groups. This mechanism means that there is a high probability that a group member will not be in infectious contact with other members while infectious, resulting in extinction in the chains of transmission. More generally, we can say that for a given mean number of secondary infections (R0) for an index case, the group transmission mechanism has a high variance compared to spatial contact and this is known to increase the probability of early extinction of an epidemic. In contrast, the network model, which lacks the daily substructure has much lower rates of extinction reflecting a low proportion of premises with no or very few connections.
The group and network models can be thought of as two extremes in terms of the potential for transmission in the commercial sector. Some mechanisms of contact (such as bird movements and feed deliveries) appear to be highly periodic favouring the type of structure represented in the group model. Other contact mechanisms (such as cleaners, building maintenance, egg collections, veterinary staff and farm workers) are likely to be more regular and less episodic, favouring the type of structure represented in the network model. To predict the potential scale of an outbreak therefore requires further understanding of the more detailed nature and frequency of these contacts than it was possible to obtain from the current network data.
(c) Control of outbreaks of HPAI using currently planned measures
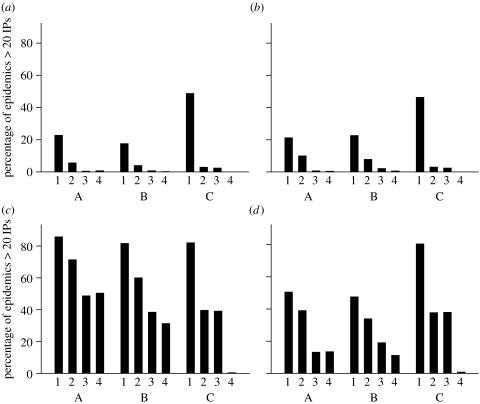
Figure 2 shows the proportion of outbreaks that are controlled under the different intervention scenarios for two values of R0 (1.5 and 3). As would be expected, controls were more effective at lower values of R0. For the lower value of R0, most outbreaks are easily controlled even with IP isolation alone. For the higher value of R0, most of the currently planned measures are required. If transmission occurs predominantly through the industry network (model C in figure 2), isolation of the IP and tracing and isolation of DC such as those farms within the same company is effective in preventing onward infection. For such scenarios, the PZ/SZs which form part of the GB contingency plan have little effect, as the majority of industry contacts (approx. 90%) are made at distances over 20 km (figure 1d). In contrast, if most transmission is not via known industry contacts but via spatially localized random contacts, then the PZ/SZs have a large impact on reducing onward transmission.
Figure 2.
Impact of current HPAI contingency plans on the epidemic size (total number of IPs). Bars show the proportion of simulated epidemics with over 20 IPs for three model structures and four intervention scenarios. (a,b) R0=1.5 and (c,d) R0=3.0. (a,c) assume density-dependent spatial transmission and a premise size-dependent transmission rate while (b,d) assume density-independent spatial transmission and a premise size-independent transmission rate. Bottom legend: A, 100% spatial transmission; B, 50% spatial transmission and 50% periodic network contact; C, pure network transmission. Interventions are denoted by the numbers on the x-axis: 1, no intervention; 2, isolation of IP; 3, isolation of IP and implementation of protection and surveillance zones (PZ/SZ); 4, isolation of IP, implementation of PZ/SZ and tracing of dangerous contacts (DC). All epidemics were seeded at a single randomly drawn premise and1000 simulations per scenario were performed.
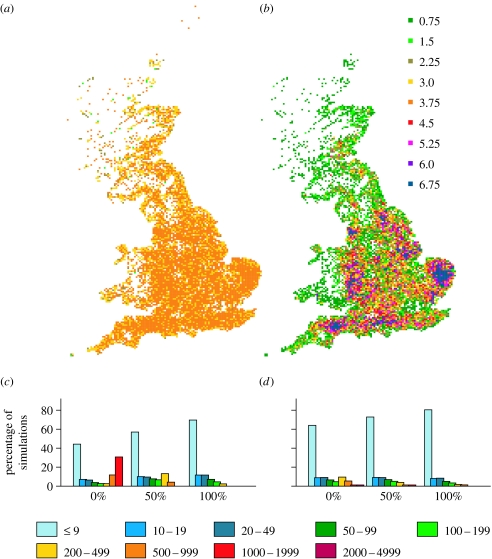
The outcome of epidemics is strongly influenced by the nature of the spatial transmission process. Density dependence in spatial transmission leads to large variations in R0 across different regions with dense areas (e.g. Norfolk) having local R0 in excess of 10 while sparse regions may have R0 well below 1 (figure 3b). Since high-density areas are strongly correlated with large premises (through industry), size dependence increases this heterogeneity. With random seeding, most epidemics will start in dense regions. Their ability to spread through sparser regions will depend on the local R0, which will be lower for density-dependent scenarios than for density independent (figure 3a,b). As a result, local control policies like PZ/SZ will be better able to prevent outbreaks escaping from high-density regions. Under a density-independent scheme, R0 is relatively homogeneous across the population and an established outbreak can spread easily across sparser regions. The effect of the increasing density dependence can be seen in figure 3c,d.
Figure 3.
(a) Risk maps with density-independent spatial transmission and a premise size-independent transmission rate. Colours denote the local R0. (b) as (a) but with density-dependent spatial transmission and a premise size-dependent transmission rate. (c) Final size of simulated epidemics with all current planned interventions for various proportions of density-dependent spatial transmission (indicated on the x-axis) with premise size-independent transmission rate. (d) as (c) but with premise size-dependent transmission rate. Bars show the percentage of simulations that fall into the various size categories. All epidemics were seeded at a single randomly drawn premise. All runs are for mean R0=3 and for purely spatial transmission.
(d) Use of vaccination or additional culling strategies
Figure 4 illustrates the effect of the contingent interventions on outbreak size and their expense in terms of resources. Ring vaccination has a negligible effect for radii less than 10 km. Only national vaccination is significant, reducing final size to between 200 and 1000 IPs in established outbreaks. Even this moderate effect is only achieved by vaccinating upwards of 2000 premises. Efficacy of vaccination is undermined as a local intervention by the long period (21 days) necessary for it to take effect and as a national strategy by the fact that only 35% of the poultry population (chicken layers and turkeys) are being vaccinated. For a given radius, ring culling is marginally more effective than ring vaccination (figure 4d) as it is more readily applied to all birds, not just chicken layers and turkeys. Consequently, however, the expense in terms of the number of premises subject to intervention is much higher than for ring vaccination.
4. Discussion
Based on our assumptions, our results demonstrate that the great majority of outbreaks are likely to be rapidly controlled, lasting less than 14 days and with fewer than 20 IPs, if the current contingency plans are implemented at a reasonably high, though achievable, level of effectiveness. These results hold across a wide range of scenarios including whether transmission occurs mainly through industry contacts or if spatial transmission is the dominant mode. If transmission occurs mostly through industry contacts, longer distance contacts mean that the proposed localized PZ/SZs are likely to have relatively little impact when compared with rapid isolation of the IPs and tracing. In contrast, if transmission occurs spatially, the PZ/SZs are predicted to have a greater impact.
If some transmission does occur spatially, then the mechanisms driving this spatial transmission are also important in determining which controls will be effective, as was demonstrated for the outbreak of foot-and-mouth disease in 2001 (Chis-Ster & Ferguson 2007). Our results demonstrate that if spatial transmission is density dependent then we can expect a higher degree of variation in R0 with some areas of high poultry density (such as East Anglia) experiencing intense transmission while others are unable to sustain transmission (R0<1).
The protection and SZs and most spatial transmission are at a similar or smaller spatial scale to density-related R0 clustering and hence these local interventions are particularly successful in regions of low R0. This helps to confine outbreaks. In contrast, if spatial transmission occurs under a density-independent process, R0 is much more homogeneous and PZ/SZ control is less effective. It is impossible to say prior to an outbreak how important these different roles of transmission will be and hence an ‘all-embracing’ control policy such as that currently planned is most appropriate. Analysis of contact data (see §1.2 in the electronic supplementary material) indicates a positive correlation between number of contacts and size of holding, but this does not take into account differences in biosecurity between smallholdings and large-scale commercial operations. Evidence from the H7N7 outbreak in The Netherlands suggests that density-dependent transmission accounts well for the distribution of IPs (Boender et al. 2007); however, industry structure can be expected to vary between countries. If the nature of the spatial transmission process could be better evaluated prior to an outbreak, the effectiveness and efficiency of control policies could be greatly enhanced.
Vaccination of some birds (such as valuable species or those particularly susceptible to HPAI) has been extensively debated and is being undertaken in other European countries. The main criticism of a vaccination-based strategy is that it could reduce the severity of disease in birds, resulting in slower detection within a farm and thereby increasing the scope for onward transmission (European Commission 2006b; Savill et al. 2006). However, vaccination in combination with sentinel birds and consistent surveillance has been demonstrated to be effective in controlling outbreaks in northern Italy using a strategy in which naturally acquired infection can be differentiated from antibodies produced in response to vaccination (Capua et al. 2003; Marangon et al. 2004). In the GB context, we do not consider this as a feasible or cost-effective option given that the majority of scenarios could be controlled without prior vaccination of the national flock. Rather, we considered whether vaccination initiated in the event of an uncontained outbreak would be an effective control strategy or whether in such scenarios additional culling of birds within the locality of IPs (as occurred in the H7N7 outbreak in The Netherlands in 2003 and the H7N3 outbreak in British Columbia in 2003; Canadian Food Inspection Agency 2004; Stegeman et al. 2004) would be more effective.
Our model predicts that a threshold of 20 IPs is an appropriate cut-off at which to initiate further interventions. If this was reached and no additional controls were implemented, a large outbreak would be probable. The impact of reactive vaccination is limited by the fact that in individual birds, the effectiveness of vaccines does not peak until approximately 18–21 days post-inoculation (see §2.4 in the electronic supplementary material). Firstly, combining this with even the most optimistic vaccination schedule therefore means that we cannot expect vaccination at a national level to become effective for at least three weeks. Secondly, owing to their large numbers and short lifespan, it is not feasible to vaccinate broiler chickens. Thus, a large pool of birds will remain unvaccinated and will sustain onward transmission of the virus and hence reactive vaccination is predicted to have little impact. In contrast, culling of birds within radii of IPs is predicted to reduce the number of IPs more than vaccination, particularly if implemented at a radius of 10 km. However, the total number of premises culled is then typically much higher than the total number of IPs predicted by the models in the absence of the culling policy. In addition, the rapid culling of large numbers of holdings in high-density regions while maintaining biosecurity will clearly present considerable logistical problems. Such a policy therefore does not appear to be a profitable control strategy unless earlier regaining of disease-free status is deemed more valuable than the total number of birds lost. On balance, vaccination of the national chicken layer and turkey flock might therefore be the intensification policy option of choice if default interventions are shown to have failed. However, the use of such a challenging strategy—in terms of rapid availability of sufficient doses of vaccine and of staff and equipment to vaccinate large numbers of birds on a short time scale—means that the costs of any large-scale vaccination strategy would have to be carefully weighed against the limited benefits.
Our results are unavoidably predicated on a number of assumptions, principally on the possible nature of disease transmission within the poultry industry and the effectiveness and efficiency of intervention strategies as well as the duration of infectiousness at the flock level and the speed of implementation of controls. To offset this uncertainty, we have carried out extensive sensitivity analysis as detailed in the electronic supplementary material. We believe that our conclusions with respect to the effect of interventions are robust but that the distributions of outbreak sizes are highly sensitive to the nature and proportion of transmission within the commercial sector and the density dependence or independence of the spatial transmission process. These uncertainties could be reduced only with considerably more data on the structure and movements within the UK poultry industry.
5. Author contributions
J.T. designed, programmed and analysed the simulation model. T.G. designed, implemented and analysed the network model and assisted in analysing the simulation model. I.C.T. assisted with data preparation and model fitting. N.M.F. wrote the within-farm model, helped design the simulation model and coordinated the research at Imperial College. A.C.G. undertook the analysis of premise contact and demographic data, helped design the network model, coordinated research at LSHTM and wrote the first draft of the paper. L.S. and J.W. undertook the survey of poultry industry contact networks. J.G. and D.P. provided input on the biological and epidemiological characteristics of HPAI outbreaks. All authors edited and approved the final text.
Acknowledgments
This work was supported by research funding from DEFRA, BBSRC and the European Union FP6 INFTRANS project (contract 513715). The views expressed here are those of the authors.
Supplementary Material
The supplementary information contains background, supporting information and details of the models used as well as results and sensitivity analyses for outbreak scenarios and control measures not covered in the main text
References
- Boender G.J, Hagenaars T.J, Bouma A, Nodelijk G, Elbers A.R.W, de Jong M.C.M, van Boven M. Risk maps for the spread of highly pathogenic avian influenza in poultry. PLoS Comput. Biol. 2007;3:e71. doi: 10.1371/journal.pcbi.0030071. doi:10.1371/journal.pcbi.0030071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Food Inspection Agency 2004 A short summary of the 2004 outbreak of high pathogenicity avian influenza (H7N3) in British Columbia, Canada.
- Capua I, Alexander D.J. The challenge of avian influenza to the veterinary community. Avian Pathol. 2006;35:189–205. doi: 10.1080/03079450600717174. doi:10.1080/03079450600717174 [DOI] [PubMed] [Google Scholar]
- Capua I, Marangon S. The use of vaccination as an option for the control of avian influenza. Avian Pathol. 2003;32:335–343. doi: 10.1080/0307945031000121077. doi:10.1080/0307945031000121077 [DOI] [PubMed] [Google Scholar]
- Capua I, Marangon S. Vaccination for avian influenza in Asia. Vaccine. 2004;22:4137–4138. doi: 10.1016/j.vaccine.2004.04.017. doi:10.1016/j.vaccine.2004.04.017 [DOI] [PubMed] [Google Scholar]
- Capua I, Terregino C, Cattoli G, Mutinelli F, Rodriguez J.F. Development of a DIVA (differentiating infected from vaccinated animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza. Avian Pathol. 2003;32:47–55. doi: 10.1080/0307945021000070714. doi:10.1080/0307945021000070714 [DOI] [PubMed] [Google Scholar]
- Chis-Ster I, Ferguson N.M. Estimating key epidemiological parameters for the 2001 British FMD epidemic. PLoS ONE. 2007;2:e502. doi: 10.1371/journal.pone.0000502. doi:10.1371/journal.pone.0000502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department for the Environment Food and Rural Affairs 2006a The Avian Influenza and Influenza of Avian Origin in Mammals (England) Order 2006.
- Department for the Environment Food and Rural Affairs 2006b Exotic Animal Generic Contingency Plan: 2006 Draft Consultation Document July 2006.
- Department for the Environment Food and Rural Affairs 2007 Avian influenza (bird flu): news archive.
- Domenech, J., Lubroth, J. & Martin, V. 2006 Avian influenza: global situation. In FAO/OIE Int. Scientific Conf. on Avian Influenza and Wild Birds, Rome, Italy
- Ellis T.M, Leung C, Chow M.K.W, Bissett L.A, Wong W, Guan Y, Peiris J.S.M. Vaccination of chickens against H5N1 avian influenza in the face of an outbreak interrupts virus transmission. Avian Pathol. 2004;33:405–412. doi: 10.1080/03079450410001724012. doi:10.1080/03079450410001724012 [DOI] [PubMed] [Google Scholar]
- Essen, S., Gardner, R., Outtrim, L., Koylass, M., McCauley, J., Iqbal, M. & Brown, I. 2006 Comparison of infectivity, duration and levels of shedding of low and high-pathogenicity avian influenza A H7N1 viruses in chickens and turkeys. In 10th Conf. on Avian Influenza, Cambridge, UK
- European Commission 2006a EU Directives 2005/94/EC, 2006/415/EC and 2006/416/EC.
- European Commission 2006b Vaccination of Poultry against Highly Pathogenic Avian Influenza H5N1 (DIVA Strategy).
- Garske T, Clarke P, Ghani A.C. The transmissibility of highly pathogenic avian influenza in commercial poultry in industrialised countries. PLoS ONE. 2007;2:e349. doi: 10.1371/journal.pone.0000349. doi:10.1371/journal.pone.0000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse-Post D.J, et al. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc. Natl Acad. Sci. USA. 2005;102:10 682–10 687. doi: 10.1073/pnas.0504662102. doi:10.1073/pnas.0504662102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangon S, Capua I, Pozza G, Santucci U. Field experiences in the control of avian influenza outbreaks in densely populated poultry areas. Dev. Biol. (Basel) 2004;119:155–164. [PubMed] [Google Scholar]
- McNally, A., Aldous, E., Alexander, D. & Brown, I. 2006 Pathogenesis of the current European H5N1 virus A.tylTurkey/l194/05. In 10th Conf. on Avian Influenza, Cambridge, UK
- Savill N.J, St Rose S.G, Keeling M.J, Woolhouse M.E.J. Silent spread of H5N1 in vaccinated poultry. Nature. 2006;442:757–757. doi: 10.1038/442757a. doi:10.1038/442757a [DOI] [PubMed] [Google Scholar]
- Stegeman A, Bouma A, Elbers A.R.W, de Jong M.C.M, Nodelijk G, de Klerk F, Koch G, van Boven M. Avian influenza A virus (H7N7) epidemic in The Netherlands in 2003: course of the epidemic and effectiveness of control measures. J. Infect. Dis. 2004;190:2088–2095. doi: 10.1086/425583. doi:10.1086/425583 [DOI] [PubMed] [Google Scholar]
- Sturm-Ramirez K.M, et al. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J. Virol. 2004;78:4892–4901. doi: 10.1128/JVI.78.9.4892-4901.2004. doi:10.1128/JVI.78.9.4892-4901.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm-Ramirez K.M, et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J. Virol. 2005;79:11 269–11 279. doi: 10.1128/JVI.79.17.11269-11279.2005. doi:10.1128/JVI.79.17.11269-11279.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G.B, et al. Protective efficacy in chickens, geese and ducks of an H5N1-inactivated vaccine developed by reverse genetics. Virology. 2005;341:153–162. doi: 10.1016/j.virol.2005.07.011. doi:10.1016/j.virol.2005.07.011 [DOI] [PubMed] [Google Scholar]
- Tumpey T.M, Kapczynski D.R, Swayne D.E. Comparative susceptibility of chickens and turkeys to avian influenza A H7N2 virus infection and protective efficacy of a commercial avian influenza H7N2 virus vaccine. Avian Dis. 2004;48:167–176. doi: 10.1637/7103. doi:10.1637/7103 [DOI] [PubMed] [Google Scholar]
- van der Goot J.A, Koch G, de Jong M.C.M, van Boven M. Quantification of the effect of vaccination on transmission of avian influenza (H7N7) in chickens. Proc. Natl Acad. Sci. USA. 2005;102:18 141–18 146. doi: 10.1073/pnas.0505098102. doi:10.1073/pnas.0505098102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R.G, Peiris M, Chen H.L, Guan Y. H5N1 outbreaks and enzootic influenza. Emerg. Infect. Dis. 2006a;12:3–8. doi: 10.3201/eid1201.051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R.G, Webby R.J, Hoffmann E, Rodenberg J, Kumar M, Chu H.J, Seiler P, Krauss S, Songserm T. The immunogenicity and efficacy against H5N1 challenge of reverse genetics-derived H5N3 influenza vaccine in ducks and chickens. Virology. 2006b;351:303–311. doi: 10.1016/j.virol.2006.01.044. doi:10.1016/j.virol.2006.01.044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary information contains background, supporting information and details of the models used as well as results and sensitivity analyses for outbreak scenarios and control measures not covered in the main text