Abstract
In the asynchronous flight muscles of higher insects, the lattice planes of contractile filaments are strictly preserved along the length of each myofibril, making the myofibril a millimetre-long giant single multiprotein crystal. To examine how such highly ordered structures are formed, we recorded X-ray diffraction patterns of the developing flight muscles of Drosophila pupae at various developmental stages. To evaluate the extent of long-range myofilament lattice order, end-on myofibrillar microdiffraction patterns were recorded from isolated quick-frozen dorsal longitudinal flight muscle fibres. In addition, conventional whole-thorax diffraction patterns were recorded from live pupae to assess the extent of development of flight musculature. Weak hexagonal fluctuations of scattering intensity were observed in the end-on patterns as early as approximately 15 h after myoblast fusion, and in the following 30 h, clear hexagonally arranged reflection spots became a common feature. The result suggests that the framework of the giant single-crystal structure is established in an early phase of myofibrillogenesis. Combined with published electron microscopy results, a myofibril in fused asynchronous flight muscle fibres is likely to start as a framework with fixed lattice plane orientations and fixed sarcomere numbers, to which constituent proteins are added afterwards without altering this basic configuration.
Keywords: Drosophila, flight muscle, X-ray microbeam, synchrotron radiation
1. Introduction
Insects are the most prosperous group of animals in terms of the number of species, and the key to their success is apparently their ability of flight and small body sizes. However, the combination of these features has forced insects to increase their wing-beat frequencies beyond the range manageable by the conventional scheme of contraction–relaxation cycles, which are accompanied by the energetically costly reuptake of intracellular calcium ions. This requirement is believed to have driven insects to develop the mechanism of asynchronous flight muscle operation, in which flight muscles stay activated during flight, while they oscillate at frequencies much higher than those of nerve impulses (see Pringle 1981). Although asynchronous flight muscles are likely to have occurred many times independently in the course of evolution (Pringle 1981; Josephson et al. 2000), they share many functional and structural features. Among these are the ability of stretch activation (Pringle 1978), crystal-quality arrangement of contractile proteins within a sarcomere (Worthington 1961; Holmes et al. 1980; Tregear et al. 1990, 1998; Dickinson et al. 2005) and the presence of C-filaments that connect the thick filaments to Z-lines (White 1983).
Using the technique of recording end-on X-ray diffraction patterns of single myofibrils from insect flight muscles, we have added another unique feature to the list, i.e. the long-range crystallinity of myofibrillar proteins: the crystal-quality regularity of contractile protein arrangement is not confined within a single sarcomere, but extends throughout a myofibril, so that the entire myofibril can be regarded as a giant single multiprotein crystal. This feature was first found in the bumble-bee (Hymenoptera; Iwamoto et al. 2002), and a later study has revealed that this is a universal feature of insects with asynchronous flight muscles (Iwamoto et al. 2006).
The next question to be addressed is how such a highly ordered structure is formed during the development of insect body. In holometabolous insects, flight muscles are formed during the pupal stage. Here we used pupae (and adults as references) of a fruit fly, Drosophila melanogaster (wild-type), as material to address this question and observed the course of flight muscle formation by means of X-ray diffraction. We chose this well-established experimental animal owing to its short life cycle, well-described stages of pupal development (Bainbridge & Bownes 1981) and the detailed electron microscopic (EM) observations of myofibrillogenesis (Shafiq 1963; Reedy & Beall 1993a,b).
We adopted two approaches to follow the course of myofibrillogenesis by means of X-ray diffraction. One was to irradiate conventionally sized X-ray beams (approx. 100 μm) to the lateral side of the thoraces of live pupae or adults. This gave general information about the extent of development of the two antagonistic indirect flight muscles (IFMs), i.e. dorsal longitudinal muscle (DLM) and dorsoventral muscle (DVM), and their orientations in the thorax. The other was to irradiate X-ray microbeams (approx. 2 μm) to isolated quick-frozen flight muscle fibres in an end-on configuration (Iwamoto et al. 2006). This gave information about the extent of the long-range order of myofilament lattice planes.
The combination of information from the two approaches, together with the published EM observations, has given rise to a picture of how the myofibrils in an asynchronous flight muscle are built during pupal development. In other words, the basic architecture of a myofibril (lattice plane consistency and the number of sarcomeres) is complete shortly after the formation of fused myotubes, when the earliest vestige of myofilament lattice is recognized in diffraction patterns. A brief account of the present study has been presented in an abstract form (Iwamoto et al. 2006b).
2. Material and methods
(a) Materials
The wild-type strain of D. melanogaster (Hikone-R) was provided from Drosophila Stocks of Ehime University. The flies were raised in plastic tubes on Drosophila instant medium (Formula 4-24; Carolina Biological Supply) at a room temperature of approximately 24°C. In most experiments, the last instar larvae that had crawled out of the medium and had become relatively immobilized were removed from the tubes to plastic trays, each with a thin plastic film on the bottom. The larvae were collected twice a day. Most of the larvae formed puparia on the plastic film within 6 h after collection, so that the time of puparium formation (recognized as the time of the eversion of anterior spiracles) was defined as 3 h after the time of each collection. For conventional X-ray diffraction recordings, small pieces of the plastic film were cut out with the pupae, and they served as convenient mounts for X-ray recordings. The sex of the pupae was not identified.
Fifteen individuals were selected from the group of the same age and were subjected to conventional X-ray recording. Some of the individuals used for microdiffraction (in earlier measurements) were not marked for their time of puparium formation, but their ages were estimated from their external features, such as eye colours. The nomenclature by Bainbridge & Bownes (1981) is useful in staging pupal development and will be referenced throughout the text.
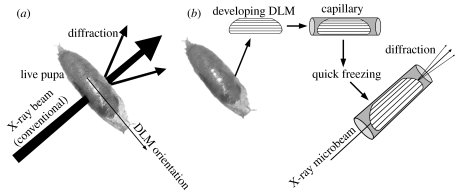
For conventional X-ray diffraction recordings from the thoraces, live pupae were used as they stayed on the pieces of plastic film (figure 1a). Adults as references were immobilized with silicone rubber glue and were subjected to X-ray recording in the same manner. For end-on microdiffraction recordings from myofibrils, the DLM was dissected from each pupa and transferred to a skinning solution which was a relaxing solution (Iwamoto 1995) containing 0.5% Triton X-100 and 7.5 μl ml−1 of protease inhibitor cocktail (Sigma-Aldrich). After skinning, the DLM was transferred to a rigor solution (Iwamoto 2000) containing 20 mM butanedione monoxime and then to a rigor solution containing 20% methyl pentanediol, which served as a cryoprotectant. The DLM processed in this way, often fragile, was placed in a thin-walled glass capillary (0.3 mm in diameter; Hampton Research; figure 1b) before it was finally quick-frozen as described (Iwamoto et al. 2005, 2006a).
Figure 1.
Methods of X-ray diffraction recordings from Drosophila pupae. (a) Conventional X-ray diffraction recording from the thorax of a live pupa. The X-ray beampath is perpendicular to the axes of both dorsal longitudinal muscle (DLM) and dorsoventral muscle (DVM). (b) Isolation of a DLM specimen, handling and end-on microdiffraction recording. The X-ray beampath is parallel to the myofibrillar axis.
(b) X-ray diffraction recording
All diffraction recordings were done at the BL40XU beamline of SPring-8 (Inoue et al. 2001). The wavelength was 0.1 nm, the specimen-to-detector distance was approximately 3.3 m and the detector was a cooled CCD camera (C-4880, Hamamatsu Photonics) used in combination with an image intensifier (V5445P-mod, Hamamatsu Photonics).
For the conventional diffraction recording from the thorax, the X-ray beam introduced to the experimental hutch was used without further trimming (beamsize, 40×250 μm in vertical and horizontal directions, respectively). The long axis of the pupal body was placed perpendicular to the beam axis, so that both DVM and DLM were nearly perpendicular to the beam axis (figure 1a). In this configuration, the strong equatorial reflections were recorded from both muscles. Very weak X-ray beams were sufficient to record these equatorial reflections, and the exposure time was typically 300 ms after attenuation to 1/100 000 by an aluminium absorber and a rotating slit shutter.
The end-on microdiffraction patterns of frozen myofibrils (figure 1b) were recorded as described (Iwamoto et al. 2006). Approximately 110 diffraction patterns were recorded from each specimen by mechanically scanning it in vertical and horizontal directions in 10 μm steps.
(c) Data processing
In the end-on diffraction patterns of the myofibrils from the pupae of early stages, the feature of long-range hexagonal lattice order was not visually recognizable. To detect such a feature from those patterns, the angular autocorrelation function (Iwamoto et al. 2006) was calculated for the scattering angle expected for the 1,0 reflection (between 1/40 and 1/45 nm−1). The angular autocorrelation function is defined as
where x and θ refer to rotary angles in the detector plane (in degrees) and I(x) is the scattering intensity (after subtraction of isotropic background scattering) at the particular spot on the pattern. If a hexagonal order exists, the function should have a peak at 60°. Thus, the criteria for the presence of long-range hexagonal lattice order are (i) for the scattering angle for the 1,0 reflection,
and (ii) outside the scattering angle for the 1,0 reflection (between 1/50 and 1/55 nm−1, and between 1/33 and 1/38 nm−1),
A relatively wide range of permissible angles (60±7°) were adopted by considering that the reflection peaks would be broad in early stages of flight muscle development. In this setting, a mixture of two hexagonal lattices will also be detected if their lattice plane angles are 30° apart.
3. Results
(a) Conventional X-ray diffraction from the thorax
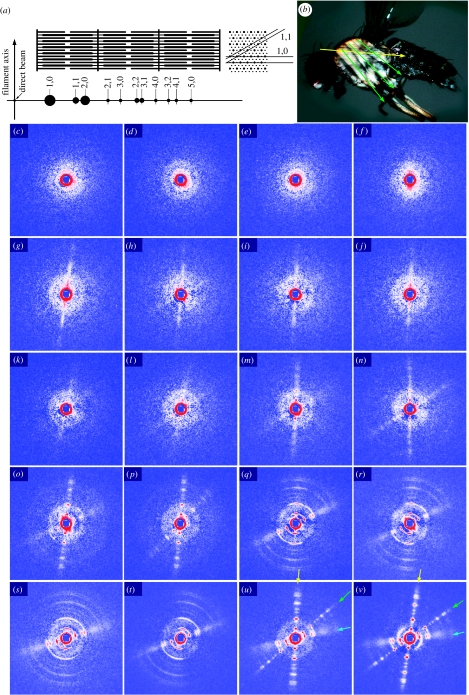
Conventionally sized X-ray beams (40×250 μm) were irradiated from the lateral side of the thoraces of live pupae as well as adult flies (figure 2). In this configuration, the X-ray beampath is perpendicular to both IFMs (DLM and DVM), so that the Bragg reflections of the hexagonal filament lattices (equatorial reflections) should appear in the direction perpendicular to the filament axis. The equatorial diffraction patterns of muscle are usually recorded as images rotary averaged around its axis. In this way of recording, information about the lattice plane orientations is lost and the reflections are reduced to a one-dimensional array. In insect flight muscle, the strongest reflections are the 1,0 and 2,0 Bragg reflections, and higher-order reflections of up to 5,0 may be observed in asynchronous flight muscles (the two numbers are the h and k crystallographic indices; figure 2a).
Figure 2.
Conventional X-ray diffraction recording from the thoraces of pupae at various developmental stages and of adult flies. (a) Schematic of the sarcomere structure in insect flight muscle and the equatorial reflections recorded from it. In the cross section, only the 1,0 and 1,1 lattice planes are indicated. (b) Muscle fibre orientations in flight musculature. Yellow arrow, DLM; green arrows, DVM. The equatorial reflections are generated in the direction perpendicular to these arrows. (c–v) Diffraction from pupae at various developmental stages and from adult flies. To describe the adult patterns first (u,v), they consist of three sets of equatorial reflections. The reflections indicated by the yellow and green arrows originate from the DLM and DVM, respectively. The near-horizontal set indicated by blue arrows is likely to originate from the tergotrochanter muscle. (c,d) Pupae at 16 h after puparium formation (APF) showing ring-like reflections with a spacing close to the 1,0 reflection. (e,f) Pupae at 26 h APF. (g,h) Pupae at 39 h APF showing a vertical streak of intensities corresponding to DLM. (i,j) Pupae at 51 h APF, (k,l) pupae at 61 h APF showing weak 1,0 peaks on the streaks. (m,n) Pupae at 74 h APF showing streaks for both DLM and DVM with weak peaks at 1,0 and 2,0 positions. (o,p) Pupae at 85 h APF, with stronger and sharper reflections, including those of higher orders. (o) The reflections from the tergotrochanter are observed for the first time (only 1,1 reflections). (q,r) Pupae at 98 h APF. (s,t) Pupae at 106 h APF (immediately before eclosion). Note that the reflections from the DLM and DVM are highly disoriented, while those from the tergotrochanter are not. (u) A freshly eclosed adult at 117 h APF. (v) An adult at 201 h APF. The patterns are after subtraction of isotropic diffuse scattering, due to various non-muscle components, by the method described by Iwamoto et al. (2003). The central blue square is due to the process of scattering subtraction. The gradation has been adjusted for each diffraction pattern for maximum visibility, so that the intensities of reflections in different panels should not be directly compared.
In adult flies, the DLM and DVM occupy most of the space of the mesothorax. Reflecting this, the diffraction pattern from the adult thorax mainly consists of two sets of equatorial reflections with different orientations (figure 2u,v). The more intense near-vertical set corresponds to the DLM, and the diagonal less-intense set corresponds to the DVM. Each of the two sets consists of sharp reflection spots characteristic of asynchronous flight muscle, and higher-order reflections of up to 5,0 can be recognized. Little fanning out (tendency of higher-order reflections to spread in arcs) is observed in each set, indicating that the myofibrils are straight and parallel to each other within each muscle fibre population.
The third near-horizontal set of equatorial reflections shows features distinct from those of DLM or DVM, i.e. much stronger 1,1 reflection and ill-defined higher-order reflections (see also the profile in figure 3c). The most likely candidate for the source of this set is the non-flight synchronous tergotrochanter muscle (or tergal depressor of the trochanter; Peckham et al. 1990; see also Reedy & Beall 1993a, for its development), which constitutes a significant presence in the thoracic musculature. In the electron micrograph of the cross section of the tergotrochanter by Peckham et al. a single thick filament seems to be surrounded by up to 12 thin filaments (twice the number for flight muscles). In this configuration, an extra thin filament is positioned at the trigonal point (the centre of gravity of the triangle formed by three neighbouring thick filaments). This is the only position of thin filaments in vertebrate skeletal muscle, besides the one in the middle of the two neighbouring thick filaments. The former would contribute to the increased intensity of the 1,1 reflection as in vertebrate skeletal muscle, in which the 1,1 reflection is much more intense than the 2,0 reflection.
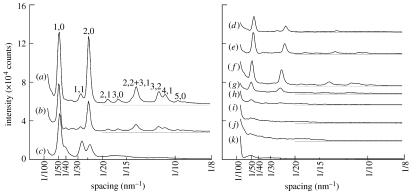
Figure 3.
Intensity profiles of equatorial reflections recorded from whole pupae and an adult fly. (a–c) Profiles from an adult fly (201 h APF). (a) DLM, (b) DVM and (c) ‘tergotrochanter’. Note the much more intense 1,1 and ill-defined higher-order reflections in (c). (d–j) DLM profiles from pupae: (d) 106 h APF, (e) 98 h APF, (f) 85 h APF, (g) 74 h APF, (h) 61 h APF, (i) 51 h APF and (j) 39 h APF. The intensities in (d,e) represent only a part of the highly curved reflections. (k) Rotary-averaged intensity profile at 26 h APF showing a peak at 1/50 nm−1. The profiles are vertically offset so that they do not overlap with each other. All the profiles were obtained from the diffraction patterns shown in figure 2, after subtraction of isotropic scattering.
In young pupae at the age of 16 h after puparium formation (APF, the stage P5 in Bainbridge and Bownes nomenclature), the patterns show no reflection or scattering with orientations corresponding to the DLM or DVM (figure 2c,d). Instead, at least 9 out of 15 pupae showed a circular reflection at a spacing of approximately 1/50 nm−1, which may be considered to originate from myofilament lattices. Since no corresponding reflection is observed in the end-on diffraction patterns, it may originate from muscle cells other than flight muscle primordia, e.g. remaining larval body-wall muscle cells. The patterns of the pupae at 26 h APF are similar (figure 2e,f). The pattern in figure 2f shows weak scattering in a vertical direction.
The earliest clear feature reminiscent of flight muscle reflection is a vertical streak with no definitive intensity peaks (figure 2g,h). This streak is observed in 7 out of 15 pupae at 39 h APF, at which the pupae are totally whitish with no discernible eye pigmentation (stage P7). The vertical streak indicates a longitudinally oriented structure (reminiscent of the DLM reflection) without definitive lattices. The pattern in figure 2h also shows a very faint diagonal streak reminiscent of the DVM reflection. At 51 (figure 2i,j) and 61 h (figure 2k,l) APF, streaks are commonly observed and they often show faint 1,0 peaks. In addition, in a few pupae, a weak and diffuse continuous ring is observed with a spacing corresponding to the 2,0 equatorial reflection. The origin of this reflection has not yet been identified. Externally, most of the pupae remain totally whitish, but at 61 h APF, their eyes become very slightly pigmented in yellow (stage P8).
At 74 h APF, many of the patterns show sharper peaks at both 1,0 and 2,0 positions on the streaks for the DLM and occasionally for the DVM, although they are still weak (figure 2m,n). No peaks are found beyond the 2,0 reflection. The continuous diffuse rings are still observed. The eyes are pigmented in orange, but the rest of the body remains whitish (stage P9).
At 85 h APF, when the eyes are darker (stages P9–11), the reflections are so sharp and well separated from each other that they can no longer be described as streaks (figure 2o,p). Higher-order reflections of up to 5,0 are recognized. In the pattern of figure 2o, the tergotrochanter reflection can be observed for the first time. Only the 1,1 reflection is clearly recognized.
At 98 (figure 2q,r) and 106 h (figure 2s,t) APF (stage P15 with darkened wings and bristles), the reflections become more intense. What is remarkable at this period is that the good orientations of the myofibrils are lost, and the reflections fan out in arcs. In this stage, increasing internal pressure could cause the muscle fibres to buckle, due to continual protein synthesis in a confined space. The relief of this pressure upon eclosion may straighten back the muscle fibres, restoring the initial good orientations of reflections. The buckling is not considered to be due to any damage but to represent a normal developmental process, because it is observed in the pupae of this stage without exception, and as described below, the irradiated pupae eclosed normally. The muscle fibres isolated from the pupae of this stage show no evidence of buckling, as judged from their end-on reflections (see below). The tergotrochanter reflections show no sign of buckling.
The course of change of reflections from whole pupae is summarized in table 1 (electronic supplementary material), together with the results from end-on diffraction recordings (see below) and EM observations (Reedy & Beall 1993a). In addition, the intensity profiles of equatorial reflections from the DLM are summarized in figure 3. For an adult fly, the profiles for the DVM and the tergotrochanter are shown along with that of the DLM (figure 3a–c).
Some of the pupae were kept after irradiation to measure the rate of eclosion. In total, 40 out of 42 irradiated pupae finally eclosed, indicating that the irradiation affected the viability of the pupae minimally. The adult flies also survived after irradiation.
(b) End-on microdiffraction from quick-frozen myofibrils
The reflections recorded in the end-on diffraction configuration (the X-ray axis coincides with the fibre axis) are similar to those in the equatorial reflections, but the differences are that the reflections are recorded in a two-dimensional plane, and that the information about the lattice plane orientations is preserved (figure 1b; see also Iwamoto et al. 2002).
The end-on microdiffraction patterns recorded from adult DLM are typical of asynchronous flight muscles (see Iwamoto et al. 2006a) showing clear signs of single hexagonal lattices of myofilaments (figure 4aa–ad). This indicates that the myofibrils of Drosophila flight muscle are of giant single-crystal type as in other insects with asynchronous flight muscles.
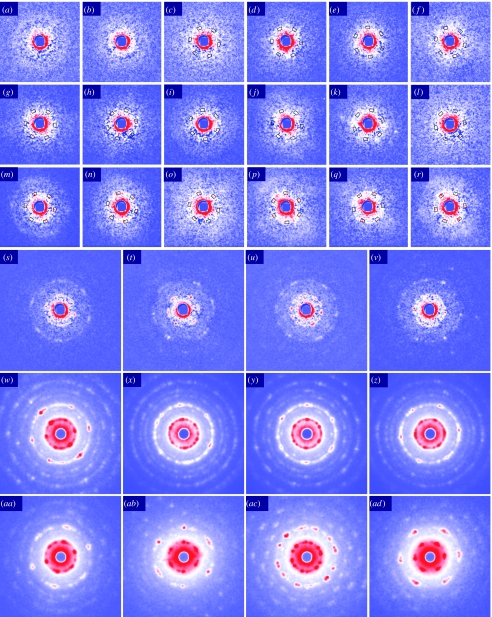
Figure 4.
End-on microdiffraction patterns of quick-frozen DLM myofibrils from pupae at various developmental stages and from adult flies. (a–z) Diffraction from myofibrils from pupae. (a–c) 23–27 h APF showing speckle-like scattering which often forms a peak at a radius smaller than at 1/50 nm−1, typically 1/57 nm−1. In (c), the periodical intensification of scattering is software detected at 60° intervals in the scattering angle expected for the 1,0 reflection (1/40–1/45 nm−1; boxes; see text for details). (d–f) 42–45 h APF. (g–l) 58–61 h APF. (m,n) 76–77 h APF. Shown are among the patterns which show software-detected intensification at 60° intervals in the scattering angle expected for the 1,0 reflection (boxes). In (m,n), the hexagonal arrangement of the 1,0 peaks are visibly clear, but the 2,0 reflections are still obscure. (s–v) 87 h APF. Both 1,0 and 2,0 reflections are clearly observed, as well as some higher-order reflections. (w–z) More than 100 h APF (about to eclose, age estimated from appearance). (aa–ad) Adults (age not recorded). In these patterns, the features of hexagonal myofilament lattice are clearly observed (see Iwamoto et al. 2006a). Isotropic scattering has been subtracted from patterns (a–v).
The youngest pupae from which the DLMs were dissected were those at 23–27 h APF, at which each DLM consisted of six fusiform primodia surrounded by a large number of small cells (presumably myoblasts), giving the primodia a granular appearance (electronic supplementary material). The end-on diffraction patterns appear as featureless diffuse scattering, but show many speckle-like fine spots after subtracting the background of isotropic scattering (figure 4a–c). The spots sometimes aggregate around a radius of approximately 1/57 nm−1, forming a peak in the rotary-averaged profile (not shown). This spacing (57 nm) is too large for the unit cell size of myofilament lattices, and may originate from the sleeve of microtubules in which nascent myofibrils would later develop (Reedy & Beall 1993a).
The DLMs isolated from pupae at 42–45 h APF were markedly shorter than the earlier primodia. Each cell was cylinder like in shape with a rather uniform diameter (electronic supplementary material). The end-on diffraction patterns of such cells are similar to those from younger specimens, consisting of many speckle-like spots, which again form a peak at scattering angles of less than 1/50 nm−1 (figure 4d–f). Very few of the patterns show a peak at the 1,0 spacing in the rotary-averaged profiles (not shown). None of the patterns show any visually recognizable sign of hexagonal lattice order. In the myofibrils at this stage, which contain only three to four thick filaments across (Reedy & Beall 1993a), the feature of hexagonal order of myofilaments would at best appear as some modulation of scattering intensities along the circumference at the 1,0 spacing with 60° periodicities. To test whether such modulation existed in any of the diffraction patterns, the angular autocorrelation function was calculated along the 1,0 circumference of each diffraction pattern and tested to see whether the pattern met the criteria for the hexagonal feature (see §2).
Out of 605 diffraction patterns of six pupae at 42–45 h APF, 11 met the criteria, but 2 were excluded because the software detection was apparently influenced by the intensity due to parasitic scattering, which probably originated from the ice interface. Three of the remaining nine patterns which met the criteria are shown in figure 4d–f. We consider that the detection of nine patterns with hexagonal ordering (1.5% of 605) is only slightly above the noise level of 1.1%, as derived from the same test applied to the pupae at 23–27 h APF. At this earlier stage, 5 out of 371 patterns of five pupae met the criteria for hexagonal ordering, and one was excluded owing to parasitic scattering. APF 23–27 is the stage before the myoblast fusion, so that myofibrils with hexagonal ordering throughout are not expected to be present. Thus, the remaining four patterns (1.1% of 371) should reflect intensity fluctuation due to noise (One of such patterns is shown in figure 4c). As mentioned earlier, the myofibrils in the pupae at 42–45 h APF have only three to four thick filaments across. Such small myofibrils would probably not provide enough intensity to judge long-range hexagonal order even with the aid of software-based recognition.
The DLMs isolated from pupae at 58–61 h APF had substantially lengthened (approx. two-third of adult length; see the electronic supplementary material), but its weak birefringence as observed with a polarizing microscope (not shown) suggests the paucity of myofilaments. Out of 612 end-on diffraction patterns, 123 patterns showed a peak at the 1,0 spacing in the rotary-averaged profiles. As in the pupae at 42–45 h APF, diffraction patterns with the visibly apparent feature of hexagonal order were not yet found at this 58–61 h stage. Out of 612 diffraction patterns, 25 met autocorrelation criteria for hexagonal ordering and 6 were excluded owing to parasitic scattering, leaving 19 patterns (3.1% of total). Out of these 19 patterns that met the criteria, six patterns are shown in figure 4g–l.
The DLMs isolated from pupae at 76–77 h APF had attained approximately five-sixths of adult length, and their birefringence was stronger. Most of the 323 patterns of three pupae showed a clear peak at the 1,0 spacing in the rotary-averaged profiles. At this stage, many of the patterns show a visibly clear feature of hexagonal order on the 1,0 reflections, although their 2,0 reflections are still obscure (figure 4m–r). Thirty-three patterns met the criteria, and none was excluded (10.2% of total).
The DLMs isolated from a pupa at 87 h APF exhibit end-on diffraction patterns in which the feature of hexagonal order is evident (figure 4s–v). Both the 1,0 and 2,0 reflections are clearly recognized as isolated spots, and occasionally higher-order reflections are observed (in figure 4v, three spots of the 3,0 reflection are recognized).
The myofibrils in the DLMs of the pupae shortly before eclosion seem to be highly disoriented as described above, but the DLMs isolated at this stage give very regular diffraction patterns with a clear feature of hexagonal order (figure 4w–z). Higher-order reflections are always observed. The occurrence of these clear patterns suggests that the DLM fibres straighten up when they are dissected from the pressurized pupal thoraces.
The course of change of myofibrillar diffraction patterns during development is also summarized in table 1 (electronic supplementary material).
4. Discussion
In this study, the course of myofibrillogenesis in Drosophila pupae was followed by means of conventional X-ray diffraction and end-on microdiffraction. Owing to the high transmission of X-ray beams, the diffraction technique is suitable for detecting overall regularity in molecular arrangement in bulk specimens such as the thoraces of insects. With the wavelength of the X-ray used here (0.1 nm), this technique will be applicable to larger insects, including honeybees and silkworm moths. In the conventional X-ray diffraction recordings, in which the beamsize is comparable to the size of a Drosophila thorax (approx. 1 mm), the extent of development of thoracic musculature can be diagnosed with a single shot of ‘chest X-ray’. This is a non-invasive method requiring relatively low doses of X-rays and may be incorporated for routine checks of developmental status and/or quick assessment of effect of mutations on flight muscle-related genes. On the other hand, the end-on microdiffraction recording is the only technique that enables a single-shot test of the long-range crystallinity of myofibrils (whether the sarcomeres within a myofibril have common lattice planes), and the only alternative would be the more labour-intensive serial sectioning in EM. Here we applied this technique to isolated flight muscle fibres, but it would also be applicable to whole pupae if a reduced signal-to-noise ratio is tolerable.
The process of formation of IFMs in Drosophila (especially in its early stages) has been extensively studied (e.g. Fernandes et al. 1991; Farrell et al. 1996; Roy & VijayRaghavan 1998). The process of early myogenesis in the DLM as described in these studies involves the survival of three larval body-wall muscle fibres, which are later longitudinally split and serve as six templates of DLM fibres. Myoblasts eventually fuse to these templates to form myotubes by 30 h APF (summarized in the first column of table 1, electronic supplementary material). Shortly after this, high-level expression of adult-specific proteins commences, at 32–36 h APF (Nongthomba et al. 2004), i.e. a few hours before the appearance of the initial fibrils (Reedy & Beall 1993a) and the streak in the present study (figure 2g–h).
During the following course of myofibrillogenesis, the present X-ray observations can be correlated with the published EM observations (Reedy & Beall 1993a), and these are also summarized in table 1(electronic supplementary material). In EM, initial fibrils with irregular Z-bodies are formed by 42 h APF. These initial myofibrils have only three to four thick filaments across (or 7–12 thick filaments in cross section). Their arrangement in the lattice is also likely to be more disordered than in the adult IFM. This period corresponds to the appearance of a vertical streak at this stage (figure 2g,h), suggesting that the myofibrils already extend along the DLM fibres. At 60 h APF, clear sarcomere structures are seen in the EM images, and in these images the sarcomeres do not increase in number but increase only in their length (from approx. 1.7 to 3 μm) and diameter (Reedy & Beall 1993a). This period coincides with the first appearance of lattice reflections in the conventional X-ray diagram (figure 2k,l), At this stage, the features of the hexagonal myofilament lattice are not visibly evident in the end-on diffraction patterns, but the software-based detection reports an increasing number of patterns with hexagonal features (figure 4g–l). Within the next approximately 16 h, pupae seem to experience a rapid growth of myofibrils, and the hexagonal features of the 1,0 reflections become visibly recognizable in the end-on diffraction patterns (figure 4m–r). At this stage, the 1,0 reflection appears as an assembly of isolated spots and never takes the form of a diffuse ring as observed in synchronous IFMs (see Iwamoto et al. 2006a). This indicates that the structure with a fixed lattice orientation has been firmly established by this stage.
The present study shows that the long-range hexagonal order may already exist at as early as 42–45 h APF, and the rate of detection increases through 77 h APF. This does not necessarily mean that the long-range hexagonal order of myofilament lattice is gradually formed in this period. Rather, it is possible that the long-range hexagonal arrangement is already in place at this early stage (42–45 h APF), when each myofibril has only a few thick filaments across. We consider this way because: (i) the signal is close to the detection limit, with the current technique, it would be natural to consider that many myofibrils with a long-range hexagonal order elude detection, (ii) our procedure is to mechanically scan the specimen at fixed intervals to exclude subjective selection of data, and (iii) it is technically very difficult to perfectly align the myofibrillar axis along the beampath, especially when the specimen is fragile at early stages. After the establishment of the long-range hexagonal lattice order, component proteins of sarcomeres would be simply added while the basic myofibrillar structure remains unchanged.
To further corner the moment of establishment of the long-range hexagonal order, a more sensitive detection system would be required. This includes the use of a reduced amount of scattering materials in the beampath, such as Kapton windows. The beamsize should be reduced further, because the nascent myofibrils are much thinner than the current beam diameter (approx. 2 μm). This beamsize is the practical limit attained by aperture optics, and the beamsize smaller than this would result in more pronounced Fraunhofer diffraction, which could severely compromise the quality of diffraction patterns. As alternatives, Fresnel zone plate optics or a pair of mirrors in the K–B (Kirkpatric–Baez) configuration may be used, provided they do not generate significant parasitic scattering.
To establish the probable scenarios for the generation of the giant single-crystal structure, a number of questions are to be addressed: does the process involve spontaneous assembly of newly synthesized component proteins, or does it need a kind of ruler which defines the periodicity and the path along which the proteins are assembled? Is the structure built de novo or to some extent inherit the structure already present in myoblasts? How is this construction related to the synthesis of adult IFM-specific protein isoforms? Answers to these questions would not be obtained by X-ray diffraction experiments alone, and coordinated approaches using molecular and cell biological techniques would be indispensable.
As for the origin of myofibrils, the earlier EM images recorded by Shafiq (1963) have shown some fragmentary myofibrils in myoblasts before fusion (the age of the pupae used is not specified). The presence of such myofibrils has not been confirmed since then, and the nature of isoforms involved in these myofibrils is also unknown. In the light of observations by Fernandes et al. (1991), the isoforms may not be of adult types. Currently, it is generally believed that the myofibrils are formed de novo in fused myotubes.
Thus, the picture of the myofibrillogenesis in Drosophila IFM is still incomplete, and future microdiffraction studies are expected to shed light on these unsolved questions.
The origin of the ring-like reflection with spacing of the 2,0 reflection, often observed in the pupae of age 90 h or younger, is also unknown. Its ring-like appearance suggests that the source of this reflection is highly disoriented, but the close coincidence of its spacing with that of DLM suggests that it is also of flight muscle origin (non-flight muscles are expected to have greater lattice constants; see Iwamoto et al. 2006a). In this period, the reflections from the DVM are not as clear as those from the DLM, so that it could reflect the early disordered state of the DVM. The origin of the DVM is reported to be different from that of the DLM, i.e. it is generated by the direct fusion of myoblasts (Fernandes et al. 1991). On the other hand, it has also been shown that the DLM can be generated by the direct fusion of myoblasts if the larval precursor is ablated (Fernandes & Keshishian 1996). To what extent the differences in the origin affect the later development of the IFMs is another unsolved question that could be addressed by microdiffraction techniques.
In summary, the present study demonstrates that the X-ray diffraction technique, conventional or microdiffraction, can serve as a useful tool for developmental biology. The merit of using the X-ray diffraction technique is that information about wide-range integrity is readily obtained by taking advantage of the high penetrability of X-rays. To further pinpoint the moment of generation of the long-range lattice order, however, a number of difficulties are expected. These include the paucity of myofibrillar content, unwanted parts of the pupal body which would strongly scatter X-rays and reduce the signal-to-noise ratio, as well as the fragility of the pupal body. However, the difficulties will be overcome if one uses finer X-ray beams by using improved optics, in combination with the technique of freezing/trimming of the entire pupa, after penetration of proper cryoprotectants, to remove unnecessary parts and also to stabilize the structure of interest (see Iwamoto et al. 2006a).
Acknowledgments
This work was performed under the approval of the SPring-8 Proposal Review Committee (proposals no. 2006A1413 and 2007A1191).
We thank Drosophila Stocks of Ehime University that provided the wild-type flies. This work was supported by Grant-in-Aid, grant no. 15500294, Ministry of Education, Culture, Sports, Science and Technology, Japan.
Supplementary Material
Time course of change of DLM length, with some micrographs of isolated DLM (scale bar, 0.5<ce:hsp sp="0.25"/>mm)
Summary of the process of myofibrillogenesis in Drosophila pupa
References
- Bainbridge S.P, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J. Embryol. Exp. Morph. 1981;66:57–80. [PubMed] [Google Scholar]
- Dickinson M, Farman G, Frye M, Bekyarova T, Gore D, Maughan D, Irving T. Molecular dynamics of cyclically contracting insect flight muscle in vivo. Nature. 2005;433:330–333. doi: 10.1038/nature03230. doi:10.1038/nature03230 [DOI] [PubMed] [Google Scholar]
- Farrell E.R, Fernandes J, Keshishian H. Muscle organizers in Drosophila: the role of persistent larval fibers in adult flight muscle development. Dev. Biol. 1996;176:220–229. doi: 10.1006/dbio.1996.0129. doi:10.1006/dbio.1996.0129 [DOI] [PubMed] [Google Scholar]
- Fernandes J.J, Keshishian H. Patterning the dorsal longitudinal flight muscle (DLM) of Drosophila: insights from the ablation of larval scaffolds. Development. 1996;122:3755–3763. doi: 10.1242/dev.122.12.3755. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Bate M, VijayRaghavan K. Development of the indirect flight muscle of Drosophila. Development. 1991;113:67–77. doi: 10.1242/dev.113.1.67. [DOI] [PubMed] [Google Scholar]
- Holmes K.C, Tregear R.T, Barrington-Leigh J. Interpretation of the low angle X-ray diffraction from insect flight muscle in rigor. Proc. R. Soc. B. 1980;207:13–33. doi:10.1098/rspb.1980.0012 [Google Scholar]
- Inoue K, Oka T, Suzuki T, Yagi N, Takeshita K, Goto S, Ishikawa T. Present status of high flux beamline (BL40XU) at SPring-8. Nucl. Instr. Methods Phys. Res. A. 2001;467/8:674–677. doi:10.1016/S0168-9002(01)00443-0 [Google Scholar]
- Iwamoto H. Strain sensitivity and turnover rate of low force cross-bridges in contracting skeletal muscle fibers in the presence of phosphate. Biophys. J. 1995;68:243–250. doi: 10.1016/S0006-3495(95)80180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto H. Influence of ionic strength on the actomyosin reaction steps in contracting skeletal muscle fibers. Biophys. J. 2000;78:3138–3149. doi: 10.1016/S0006-3495(00)76850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto H, Nishikawa Y, Wakayama J, Fujisawa T. Direct X-ray observation of a single hexagonal myofilament lattice in native myofibrils of striated muscle. Biophys. J. 2002;83:1074–1081. doi: 10.1016/S0006-3495(02)75231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto H, Wakayama J, Fujisawa T, Yagi N. Static and dynamic X-ray diffraction recordings from living mammalian and amphibian skeletal muscles. Biophys. J. 2003;85:2492–2506. doi: 10.1016/s0006-3495(03)74672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto H, Inoue K, Fujisawa T, Yagi N. X-ray microdiffraction and conventional diffraction from frozen-hydrated biological specimens. J. Synchrotron Rad. 2005;12:479–483. doi: 10.1107/S090904950501352X. doi:10.1107/S090904950501352X [DOI] [PubMed] [Google Scholar]
- Iwamoto H, Inoue K, Yagi N. Evolution of long-range myofibrillar crystallinity in insect flight muscle as examined by X-ray cryomicrodiffraction. Proc. R. Soc. B. 2006a;273:677–685. doi: 10.1098/rspb.2005.3389. doi:10.1098/rspb.2005.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto H, Inoue K, Yagi N. Development of myofibrillar structure in the pupal stage of Drosophila as examined by X-ray diffraction. Zool Sci. (Abstr.) 2006b;23:1165. [Google Scholar]
- Josephson R.K, Malamud J.G, Stokes D.R. Asynchronous muscle: a primer. J. Exp. Biol. 2000;203:2713–2722. doi: 10.1242/jeb.203.18.2713. [DOI] [PubMed] [Google Scholar]
- Nongthomba U, Clark S, Cummins M, Ansari M, Stark M, Sparrow J.C. Troponin I is required for myofibrillogenesis and sarcomere formation in Drosophila flight muscle. J. Cell Sci. 2004;117:1795–1805. doi: 10.1242/jcs.01024. doi:10.1242/jcs.01024 [DOI] [PubMed] [Google Scholar]
- Peckham M, Molloy J.E, Sparrow J.C, White D.C.S. Physiological properties of the dorsal longitudinal flight muscle and the tergal depressor of the trochanter muscle of Drosophila melanogaster. J. Muscle Res. Cell Motil. 1990;11:203–215. doi: 10.1007/BF01843574. doi:10.1007/BF01843574 [DOI] [PubMed] [Google Scholar]
- Pringle J.W.S. The Croonean lecture, 1977: Stretch activation of muscle: function and mechanism. Proc. R. Soc. B. 1978;201:107–130. doi: 10.1098/rspb.1978.0035. doi:10.1098/rspb.1978.0035 [DOI] [PubMed] [Google Scholar]
- Pringle J.W.S. The Bidder lecture, 1980: The evolution of fibrillar muscle in insects. J. Exp. Biol. 1981;94:1–14. [Google Scholar]
- Reedy M.C, Beall C. Ultrastructure of developing flight muscle in Drosophila I. Assembly of myofibrils. Dev. Biol. 1993a;160:443–465. doi: 10.1006/dbio.1993.1320. doi:10.1006/dbio.1993.1320 [DOI] [PubMed] [Google Scholar]
- Reedy M.C, Beall C. Ultrastructure of developing flight muscle in Drosophila II. Formation of the myotendon junction. Dev. Biol. 1993b;160:466–479. doi: 10.1006/dbio.1993.1321. doi:10.1006/dbio.1993.1321 [DOI] [PubMed] [Google Scholar]
- Roy S, VijayRaghavan K. Patterning muscles using organizers: larval muscle templates and adult myoblasts actively interact to pattern the dorsal longitudinal flight muscles of Drosophila. J. Cell Biol. 1998;141:1135–1145. doi: 10.1083/jcb.141.5.1135. doi:10.1083/jcb.141.5.1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiq S.A. Electron microscopic studies on the indirect flight muscles of Drosophila melanogaster II. Differentiation of myofibrils. J. Cell Biol. 1963;17:363–373. doi: 10.1083/jcb.17.2.363. doi:10.1083/jcb.17.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregear R.T, Wakabayashi K, Tanaka H, Iwamoto H, Reedy M.C, Reedy M.K, Sugi H, Amemiya Y. X-ray diffraction and electron microscopy from Lethocerus flight muscle partially relaxed by adenylylimidodiphosphate and ethylene glycol. J. Mol. Biol. 1990;214:129–141. doi: 10.1016/0022-2836(90)90152-C. doi:10.1016/0022-2836(90)90152-C [DOI] [PubMed] [Google Scholar]
- Tregear R.T, Edwards R.J, Irving T.C, Poole K.J.V, Reedy M.C, Schmitz H, Towns-Andrews E, Reedy M.K. X-ray diffraction indicates that active cross-bridges bind to actin target zones in insect flight muscle. Biophys. J. 1998;74:1439–1451. doi: 10.1016/S0006-3495(98)77856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D.C.S. The elasticity of relaxed insect fibrillar flight muscle. J. Physiol. 1983;343:31–57. doi: 10.1113/jphysiol.1983.sp014880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington C.R. X-ray diffraction studies on the large-scale molecular structure of insect muscle. J. Mol. Biol. 1961;3:618–633. doi: 10.1016/s0022-2836(61)80025-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time course of change of DLM length, with some micrographs of isolated DLM (scale bar, 0.5<ce:hsp sp="0.25"/>mm)
Summary of the process of myofibrillogenesis in Drosophila pupa