Abstract
For a complete adult spinal rat to regain some weight-bearing stepping capability, it appears that a sequence of specific proprioceptive inputs that are similar, but not identical, from step to step must be generated over repetitive step cycles. Furthermore, these cycles must include the activation of specific neural circuits that are intrinsic to the lumbosacral spinal cord segments. For these sensorimotor pathways to be effective in generating stepping, the spinal circuitry must be modulated to an appropriate excitability level. This level of modulation is sustained from supraspinal input in intact, but not spinal, rats. In a series of experiments with complete spinal rats, we have shown that an appropriate level of excitability of the spinal circuitry can be achieved using widely different means. For example, this modulation level can be acquired pharmacologically, via epidural electrical stimulation over specific lumbosacral spinal cord segments, and/or by use-dependent mechanisms such as step or stand training. Evidence as to how each of these treatments can “tune” the spinal circuitry to a “physiological state” that enables it to respond appropriately to proprioceptive input will be presented. We have found that each of these interventions can enable the proprioceptive input to actually control extensive details that define the dynamics of stepping over a range of speeds, loads, and directions. A series of experiments will be described that illustrate sensory control of stepping and standing after a spinal cord injury and the necessity for the “physiological state” of the spinal circuitry to be modulated within a critical window of excitability for this control to be manifested. The present findings have important consequences not only for our understanding of how the motor pattern for stepping is formed, but also for the design of rehabilitation intervention to restore lumbosacral circuit function in humans following a spinal cord injury.
Keywords: spinal cord injury, use-dependent plasticity, epidural stimulation, afferent control
Introduction
Under very specific conditions the lumbosacral spinal cord circuitry of a chronic low spinal cat can generate full weight-bearing stepping of the hindlimbs on a treadmill (Edgerton and Roy, 2002; Rossignol et al. 2002). Furthermore, the stepping of the hindlimbs can be modulated to accommodate different treadmill belt speeds, levels of loading, directions of movement of the treadmill belt (Edgerton et al. 2004), and spontaneous perturbations of the steps in order to continue stepping successfully (Rossignol et al., 2006). A key phrase here is “under very specific conditions”, with these conditions varying from specie to specie. For example, the adult chronic spinal cat can generate full weight-bearing stepping with sufficient step training without pharmacological intervention (de Leon et al., 1998a, b). In adult spinal mice, rats, and humans similar success in regaining stepping-like activity can be achieved, but the specific conditions differ from those observed in the cat (Edgerton and Roy, 2002). For example, unlike in the adult spinal cat that have received some step training, the execution of a consistent series of full weight-bearing steps without any assistance to guide the kinematics of the lower limbs has not been demonstrated in the adult complete spinal mouse, rat, or human even following extensive training. There is one exception to this generalization. Leblond et al. (2003) reported that adult spinal mice “can express hindlimb locomotion within 14 days of spinalization” and have “similar angular excursions of the hip, knee and ankle as intact mice”. However, full weight-bearing on the hindlimbs was not observed in that the authors stated “the animal has to be supported and a lateral stability has to be provided by the experimenters”. This was also evident from the more limited ankle excursions in the spinal vs. intact mice. With the use of additional state-dependent modulatory techniques besides step training, level of success close to that observed in cats has been achieved in rat and mice as will be discussed below. All of these observations have important applications with respect to the potential mechanisms for the recovery of locomotion following incomplete and complete spinal cord injuries in humans.
These observations lead to two different types of questions. How does the spinal circuitry generate full weight-bearing stepping while also modulating speed, load and direction without any neural input from the brain? Upon the loss of input from the brain, there is an immediate loss of the ability to generate full weight-bearing stepping in all animal models studied. Some step-like neural and movement patterns, however, can be generated by the motor pools within hours of a complete spinal cord transection via spinal cord stimulation, sensory stimulation, and/or pharmacological interventions.
A general concept that will be presented in this paper is that the spinal circuitry capable of generating full weight-bearing stepping is present within the lumbosacral spinal cord of the mouse, rat, and human as well as in the cat and that it persists after a complete spinal cord transection at a mid-thoracic level. The detailed strategies needed to access this circuitry, however, differ between species. The second general concept is that successful weight-bearing stepping can be achieved by using presently available techniques to bring the spinal circuitry to a physiological state under which the afferent information can drive the locomotor activity.
2. Modulation of the physiological state and afferent control of locomotion
What is the logic and experimental evidence that the generation of successful locomotion is a reflection of state-dependent modulation and afferent control (Fig. 1)? The concept of state-dependence implies that the spinal circuitry responds to a given stimulus differently under different conditions or “states”. The classic example is the phase-dependent modulation of the response to touching the dorsum of the paw, which induces a flexor action in the ipsilateral limb during the swing phase, but extension during the stance phase of stepping (Forssberg, 1979). Examples of a more prolonged modulation of state dependence have been demonstrated using pharmacological agonists and antagonists of selected neurotransmitter systems known to be important in the generation of locomotion (Rossignol and Barbeau, 1993). We also have demonstrated that the state dependence of the spinal circuitry can be modulated by epidural electrical stimulation of the dorsum of the lumbosacral spinal cord of the adult spinal rat, i.e., epidural stimulation at specific segments of the spinal cord can elicit and/or enable reflex and locomotor-like activity in rats having a complete spinal cord transection at a mid-thoracic level (Gerasimenko et al., 2005; Ichiyama et al., 2005; Lavrov et al., 2006). Essentially, the early experiments of Shik and colleagues (1966) demonstrated that tonic stimulation of the mesencephalic locomotor region modulates the state dependence of the spinal cord in the sense that the gait and speed of stepping at a given treadmill speed was dependent on the intensity of the stimulation.
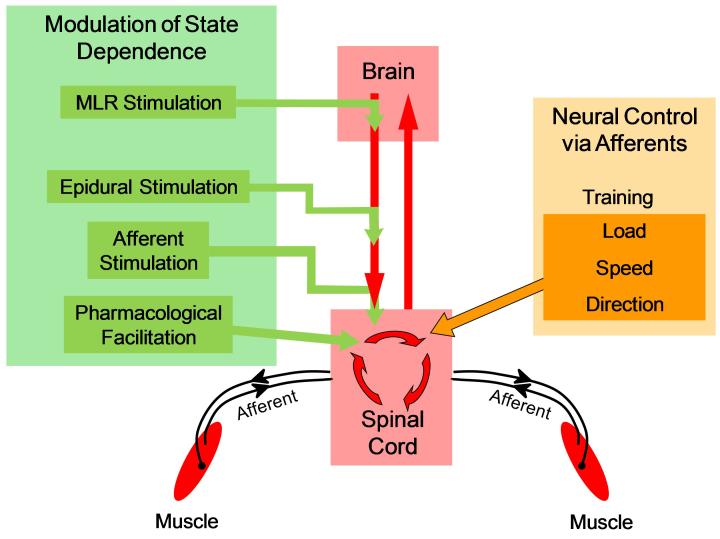
Figure 1.
This illustration provides a global perspective on the sources of neural control of posture and locomotion which normally includes the exchange of information between the brain and the spinal cord and between the sensory receptors within the muscles, joints, and skin and the spinal cord. This chapter emphasizes the importance of the afferent information from the periphery as a source of control of posture or locomotor tasks, in conjunction with the spinal circuitry to which central pattern generation is routinely attributed, when there can be no exchange of information between the brain and spinal cord, i.e., following a complete spinal cord injury. Given that it is possible to generate locomotor movements with a tonic stimulation of the mesencephalic locomotor region (MLR), details of the control of posture and locomotion are not likely to be derived from a tonic signal from the brainstem. However, this tonic stimulation can be an important source of modulatory control of the spinal circuitry, as can be epidural stimulation, afferent stimulation, and pharmacological modulation. Factors listed on the right side of the Figure point out details of the physical environment that can be detected by the spinal cord and used to instruct the spinal circuitry to activate the appropriate motor pools at the appropriate time. The sensory perception of the factors listed in the box on the right side can play an important role in shaping the physiology of the spinal circuitry through repetitive activity, i.e., training. Further evidence of the importance of the sensory information is demonstrated by the acute effects of unilateral deafferentation after which epidural stimulation will induce locomotor movements only on the intact side.
It appears that the general perception is that the role of afferents in locomotion, and in other motor tasks, is to modulate and correct movements that are initiated and sustained by the multiple supraspinal motor systems. This is almost certainly true on some occasions, but is not necessarily the case even when the neuromotor system is intact. For example, there is no clear evidence to exclude the possibility that the afferent systems play an important, and maybe even a lead role, in controlling movement (Courtine et al., 2007a), while the supraspinal centers correct or modulate the details of the motor event (Nielsen and Sinkjaer, 2002). In situations where there is no supraspinal input to the lumbosacral spinal cord, (Fig. 2B) theoretically the control could come from the specialized spinal networks responsible for generating the rhythm for locomotion. We suggest, however, that the essential control is provided by the afferents from the moving lower limbs. We propose that those spinal circuits responsible for central pattern generation receive the general, tonic control signals, and then carry out the specific details of the task with the availability of the continuously projected profiles of dynamic sensory input (Fig. 2A). This input seems to reflect the kinematics and kinetics of the lower limbs and subsequently works in concert with the spinal pre-motor circuits capable of central pattern generation to control and sustain stepping or standing.
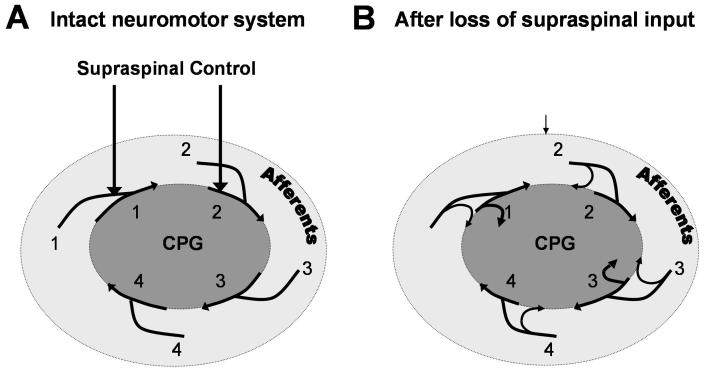
Figure 2.
This illustration emphasizes several important features of the spinal circuitry in addition its ability to generate repetitive rhythmic cycles of flexion and extension. We propose that an important feature of the spinal circuitry is its intrinsic order of activation of selected groups of neurons and that this order is reinforced by the expectation of the synchronization of order being derived from the sensory fibers from the periphery as well as being synchronized with the supraspinal input. In other words, the supraspinal input, the intrinsic circuitry of the spinal cord, and the peripheral information projecting to the spinal circuitry occur in an orderly fashion normally (A). On the other hand, when there is loss of input from the brain, the disorder evolves in the spinal circuitry and there is a loss of the routine orderly sensory information projecting to the spinal cord due to the lack of routine weight-bearing and postural tasks. All of these events contribute to a loss of order and specificity of the activation patterns to specific groups of neurons in a timely fashion (B).
Numerous experiments support our interpretation that the afferents can actually be the source of control of locomotion, at least as it occurs in vivo in the spinal animal. A complete spinal animal readily adjusts the temporal pattern of stepping in accordance with the speed of a treadmill belt (Rossignol et al., 2006). There are multiple examples of the recruitment of motor pools accommodating the level of loading placed on the hindlimb of cats (Edgerton et al., 1991) and of the lower limbs in humans (Harkema et al., 1997) with complete spinal cord injuries. Even more impressive evidence that the afferent system can be the controller is demonstrated by the ability of the hindlimbs of a complete spinal animal to walk backwards when the treadmill belt is moving in a forward direction (Gerasimenko et al., 2005). Although locomotor-like rhythms can be induced by the spinal circuitry in the absence of alternating sensory input, inhibitory or excitatory neurotransmitter agonists/antagonists can be given at levels that will not induce, but will enable, oscillatory movements of the limbs. However, when the animal’s limbs are placed on a moving treadmill belt they will begin stepping at a rate and with kinematics features appropriate for the speed of the belt. A similar effect of the motor responses to sensory input can be routinely observed during tonic epidural stimulation in a complete spinal cat or rat (Gerasimenko et al., 2005; Ichiyama et al., 2005).
A level of epidural stimulation that induces no motor response when the hindlimbs are suspended will readily generate effective, coordinated locomotor movements when the limbs are placed on the moving treadmill belt, presumably because it provides systematic and comprehensive afferent information as a source of control. Further evidence for the role of sensory information in controlling movement is provided by the observation that several weeks after unilateral deafferentation (intradural dorsal rhizotomy) in spinal rats, epidural stimulation can elicit hindlimb stepping only on the intact and not the deafferented side. Other striking evidence that peripheral afferents can initiate locomotor movements is that involuntary step-like movements can be generated in intact humans when they are placed horizontally on their side and the quadriceps muscles are stimulated continuously by vibration (Gurfinkel et al., 1998). The type of vibration used has been demonstrated to activate Group Ia muscle spindle afferents with the probability that some Group Ib fibers also are being activated. All of these experimental observations are consistent with our proposal that afferent input to the spinal cord is capable of initiating and controlling locomotion in the complete spinal mammal.
Is there a role for state-dependent modulation in the initiation, induction, and correction of locomotor tasks? Stimulation of the mesencephalic locomotor region can clearly change the state dependence of the spinal cord, e.g., change its responsiveness to sensory input. Although the brainstem can be stimulated at an intensity that does not generate locomotor movements until the limbs are placed on a moving belt, there is evidence that brainstem simulation can induce a stepping pattern in the limbs even in the absence of a moving treadmill belt (Liu and Jordan, 2005). There is little evidence, however, that such stepping-like motions are completely independent and fully weight bearing in response to stimulation of the mesencephalic locomotor region. Epidural stimulation of the dorsal region of the lumbosacral spinal cord has similar effects, except that sub-threshold stimulation rarely generates full weight-bearing stepping movements until the limbs are placed on a moving belt. Each of these cases are consistent with the concept that the state-dependence of the spinal circuitry can be modulated by the mesencephalic locomotor region or epidural stimulation of the lumbosacral spinal cord, thereby enabling spinal locomotor networks to process the sensory information for generating effective weight-bearing locomotion.
Pharmacological modulation of the state-dependency of the spinal cord also seems to be an effective way to enable locomotion in the presence of weight-bearing associated proprioceptive input. For example, either strychnine (a glycine receptor blocker) or bicuculline (a GABA receptor agonist) can significantly facilitate and enable locomotion without inducing locomotor-like movements in the absence of weight-bearing associated proprioception (de Leon et al., 1999). Relatively modest dosages of the serotonergic agonist, quipazine, have a similar effect. Although higher doses of quipazine and other excitatory drugs can induce locomotor-like movements, quipazine in itself does not induce and control stepping at lesser dosages (Fong et al., 2005; Guertin et al., 2004) Thus, it can modulate the inhibitory and excitatory properties of spinal neural circuits to a net excitable state that step-related proprioception can generate successful movements.
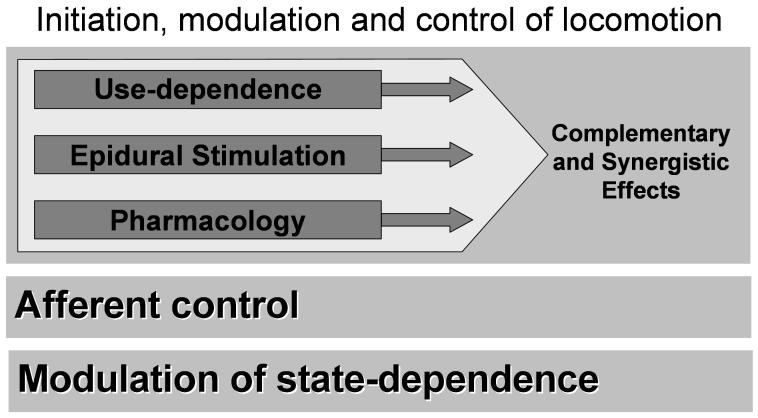
This physiological state can be controlled readily via brainstem simulation, epidural stimulation, pharmacological facilitation, the level of activity of a given synaptic pathway, and to some degree tonic stimulation of afferents (Fig. 3). Indications that these modulatory procedures are not controlling, but enabling, locomotion is that all of these stimuli are tonic in nature and have minimal spatial and temporal meaning, as is essential for controlling the precise sequence of muscle synergies that underlies locomotion, i.e., there is little detail needed to activate all of the motor pools in a coordinated fashion as occurs during weight-bearing locomotion.
Figure 3.
This chapter centers around the concept that the spinal cord circuitry changes its functional properties readily and routinely and that some of these functional properties can be modulated, with the principal modulators being related to how the spinal circuitry is being used, how it responds to a given pharmacological agonist or antagonist, and how it responds to tonic epidural stimulation. Each of these factors can readily modulate the state-dependence of the spinal cord. Modulation of this circuitry in combination with “control” from the sensory input can result in effective weight-bearing stepping.
3. Sources of control of locomotion before and after a complete spinal cord injury
Although the neural control of locomotion involves multiple supraspinal centers, spinal networks, and sensory information derived from the periphery (Fig. 1), the specific roles of each of these sources are not well defined. As noted earlier, effective weight-bearing stepping can be generated in the absence of input from supraspinal centers. Often this is presumed to be possible because of the presence of central pattern generators in the spinal cord (Grillner, 2003). Since it is often assumed that without central pattern generation capability humans would be unable to generate locomotion without supraspinal input, i.e., following a brain or spinal cord injury, there has been considerable focus on the issue of whether such central pattern generators are present in the human spinal cord (Duysens and Van de Crommert, 1998). The defining feature of central pattern generation considered to be most critical is its ability to generate repetitive patterns of reciprocal activity between extensor and flexor muscles consistently in the absence of any supraspinal or oscillating afferent input. We suggest that a critical feature of central pattern generators is their ability to receive, interpret, and process sensory information that can in turn generate the appropriate motor output. We propose, therefore, that rather than focusing on a conceptual dichotomy between central pattern generation and sensory control of locomotion, the important feature of these two neural systems is their ability to interact and function as a highly integrative system. The spinal circuitry associated with locomotion must receive and interpret very complex, highly ordered continuous proprioceptive information such that the evolving motor output is generated and adjusted accordingly (Fig 2B). Therefore, as illustrated in Figure 2A and B the important point of focus should be the interaction between the afferents and the spinal circuitry that receives and processes the proprioceptive and cutaneous inputs linked to weight-bearing stepping more than the capacity to generate repetitive cycles.
The ability to carry out this integrative function between central pattern generators and the sensory system becomes essential following a complete spinal cord injury, if sustained stepping is to occur. Following the loss of supraspinal control, the spinal circuitry will have changed significantly. The most obvious change will be the withdrawal of synapses associated with supraspinal input. In addition, even assuming no direct neurotrauma to the neural circuitry that generates stepping, there will be an extensive number of adaptive processes. Following severe, but incomplete, spinal cord injury the ability to selectively activate specific muscle groups remains but is very limited, as illustrated by the generation of complex and uncoordinated movements of multiple joints when the subject is trying to move only one joint. In other words, the projection of the motor signal to the periphery becomes highly divergent (Maegele et al., 2002). This loss of specificity of projection of the motor commands must reflect changes in functional connectivity, both supraspinally and spinally (Raineteau and Schwab, 2001). Figure 2B illustrates a conceptual schematic of the changes in the spinal circuitry that has lost its specificity, order, and directionality. This dysfunctional divergence could be located directly within the afferent projections and/or within the circuitry responsible for central pattern generation.
The expanded divergence of the command signals also is manifested physiologically as poor coordination among motor pools during locomotion. For example, although flexor and extensor motor pools could be activated readily in chronic spinal cats that were unable to step immediately following a period of stand training, there was very poor coordination among flexor and extensor motor pools (de Leon et al., 1998b). This lack of coordination reflects a low probability of predicting the level of excitation of a given muscle relative to some specific phase of the step cycle. If there is a random chance of a motor pool being activated at any time during the step cycle, then the probability of excitation would be similar throughout the step cycle. On the other hand, if there was a highly regular and predictive onset and termination of activation of a given motor pool at a specific time during the step cycle, then the motor pools can be activated in an orderly and coordinated manner. Figure 4 shows a clear example of the unpredictability and lack of order of motor pool activation and complete inability to step in a complete spinal cat prior to the administration of strychnine compared with the highly predictable patterns and full weight-bearing stepping within 30 minutes after strychnine administration. This rapid onset of motor pool coordination following the pharmacological depression of glycinergic inhibition occurred in chronic spinal animals that previously were not capable of weight-bearing stepping (de Leon et al., 1999).
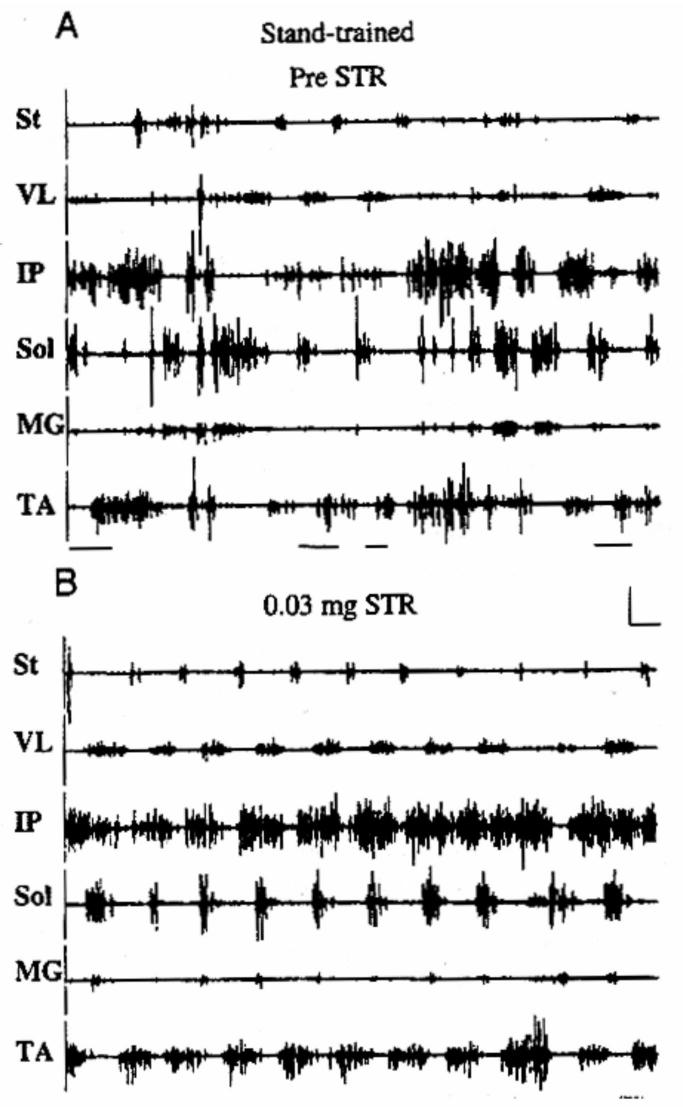
Figure 4.
EMG recordings from the hindlimbs of an adult spinal cat that had been trained to stand for 12 weeks after a complete spinal cord injury. The animal was incapable of generating any weight-supporting stepping following this stand training. The cat was then given a small dosage of strychnine (STR), intraperitoneally, a glycinergic blocker, and 30 minutes later could walk on the treadmill with full weight-bearing at speeds of up to 0.6 m/sec. The Figure shows the erratic bursting of activity of flexors and extensors prior to receiving strychnine, but a highly ordered and predictable bursting pattern of flexor and extensor muscles following its administration. St, semitendinosus; VL, vastus lateralis; IP, iliopsoas; Sol, soleus; MG, medial gastrocnemius; and TA, tibialis anterior. Horizontal calibration, 1 s; vertical calibration is 1 mV for all muscles except for the Sol, which is 2 mV. (Taken from de Leon et al., 1999).
4. The enabling and synergistic effects of pharmacological interventions, epidural stimulation, and training on stepping ability after a spinal cord injury
4.1 Pharmacology
Locomotion can be facilitated by enhancing the level of excitation via serotonergic, noradrenergic, and glutamatergic systems (Rossignol and Barbeau, 1993). Another approach has been to potentiate disinhibition by blocking the inhibitory neurotransmitters glycine and GABA, which has been briefly described above. There are numerous examples of pharmacological manipulations changing the efferent patterns associated with fictive locomotion using the neonatal isolated spinal cord as an experimental model (Cazalets et al., 1992; Cowley and Schmidt, 1995; Kiehn, 2006). Fewer studies have examined the role of these pharmacological manipulations on the locomotor output under in vivo conditions. There are reasonably clear examples that administration of α-2 adrenergic agonists (e.g., clonidine) (Giroux et al., 1998), serotonergic agonists (e.g., quipazine) (Barbeau and Rossignol, 1990; Fong et al., 2005), glycinergic agonists (e.g., strychnine) de Leon et al., 1999, and GABAergic agonists (e.g., Robinson and Goldberger, 1986) can improve the locomotion of complete spinal animals, particularly when the locomotor capability is very poor prior to the pharmacological treatment. In most of these studies, however, a distinction is not made as to whether the pharmacological agent is actually inducing locomotion vs. changing the physiological state of the spinal cord so that it can generate locomotion when the afferent systems are allowed to control the motor output. In reports of the facilitation of locomotion in response to either strychnine or quipazine, it was clear that the drug itself, at least for the dosages used, did not induce stepping (Cai et al., 2006b; de Leon et al., 1999; Fong et al., 2005). When the hindlimbs were placed on a moving treadmill belt after administration of the drug, however, effective weight-bearing locomotion could be generated. The observation that the stepping rate readily accommodated the speed of the treadmill belt provided further evidence that the afferent control played a crucial role in defining the locomotor output (Fig. 5).
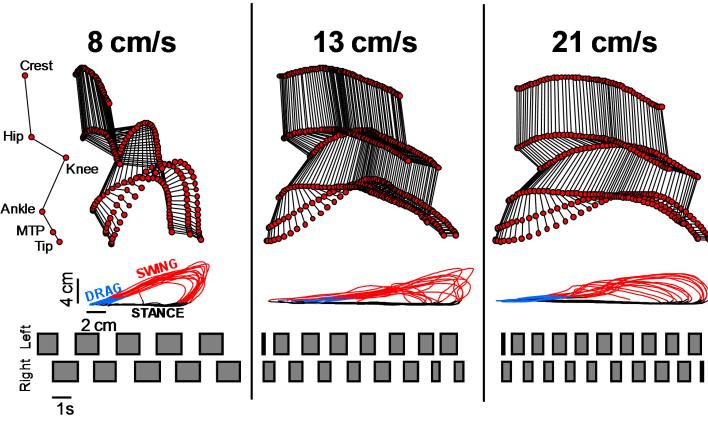
Figure 5.
Modulation of the kinematic features with changes in treadmill belt speed in a complete spinal rat walking bipedally under epidural stimulation and quipazine administration. The stick decompositions of the hindlimb movements during the swing phase of gait as well as successive (n = 10 steps) trajectories of the hindlimb endpoint during both the stance and swing phase of gait show the progressive adaptation of hindlimb kinematics with increasing treadmill belt speed. Likewise, increasing the treadmill belt speed resulted in a decrease in stance duration and increase in step length while interlimb coordination remains preserved. The boxes at the bottom of the Figure indicate the period of stance.
4.2. Epidural stimulation
Tonically stimulating the dorsal surface of the lumbosacral spinal cord via electrodes placed on top of the dura can induce locomotor-like movements in vivo in complete spinal rats (Ichiyama, 2005) and cats Gerasimenko et al., 2003) and in humans that are classified as clinically complete (Dimitrijevic et al., 1998). The intensity, frequency, and site of simulation are important parameters to control in defining the nature of the locomotor movements that are generated. In rats and cats, epidural stimulation alone can generate locomotor movements that can bear some, although very limited, weight bearing. In humans that receive a similar type of stimulation, locomotor-like movements can be generated when there is no weight bearing and there is a recent report that some weight-bearing steps can be generated when some weight -bearing is permitted (Huang et al., 2006). Epidural stimulation in combination with step training also has been reported to improve locomotor ability in clinically incomplete spinal cord subjects. The data presented to support this conclusion, however, are equivocal and complicated by the fact that the subject’s injury was incomplete and had some stepping capability without the epidural stimulation/step training intervention (Carhart et al., 2004).
On the other hand, epidural stimulation and pharmacological facilitation, each of which modulates the physiological state of the spinal cord toward one that can more readily generate locomotion, can act synergistically on locomotor performance. In the presence of both treatments the quantity and quality of stepping of adult complete spinal rats exceeds that which is observed when either intervention is used alone (Fig. 6). As noted above, our interpretation of these results is that the two modulatory events do not induce stepping, but enable stepping to occur when the sensory input is used as the source of control. Although we do not know the mechanism for the synergistic effect of these two modulatory interventions, it seems reasonably apparent that the mechanisms via which they induce their effects differ and, therefore, the benefits of each approach can be complementary.
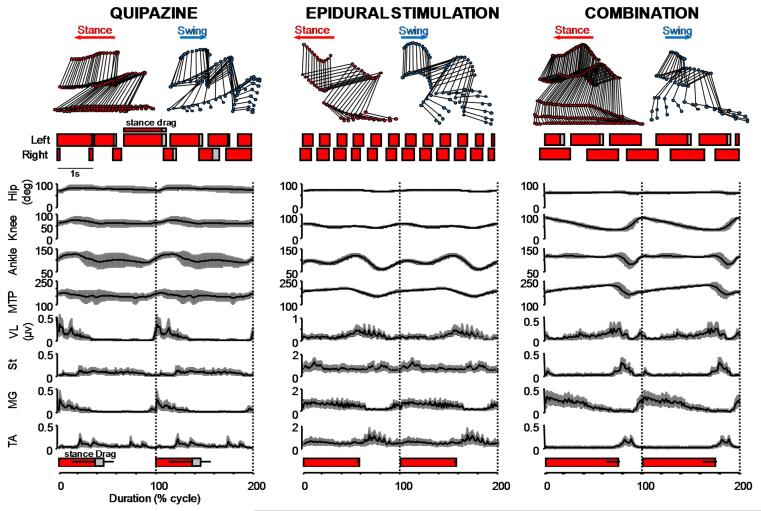
Figure 6.
Average (n = 15 steps) trajectory of hindlimb joint angles (±SD) and associated patterns of EMG activity during bipedal stepping enabled by quipazine, epidural stimulation, and the combination of both. The gait diagram of the left and right hindlimb movements is also displayed for each condition. Dark (red) and light (gray) boxes represent the duration of the stance and drag phases. Quipazine facilitates the flexion phase, whereas epidural stimulation applied over the S1 spinal segment promotes the extension phase. Applying both quipazine and epidural stimulation results in improved locomotion whose characteristics combine the individual effect of either treatment. Muscle abbreviations, same as in Fig. 4; marker positioning, same as Fig. 5. The mean duration (+ SD) of the stance and dragging phases is reported at the bottom of each panel.
4.3. Use-dependent plasticity
Repetitive patterns of activation of a synapse changes the properties of that synapse within a timeframe of milliseconds to months. At the systems level it is equally clear that the synaptic efficacy of sensorimotor pathways can be modulated by repetitive activity. Furthermore, some of the specific pathways that are modified are most likely those that are being activated, but inhibition can be enhanced as well. For example, when a spinal cat is trained to step it learns to step, and when the same cat is trained to stand it learns to stand, but steps very poorly (de Leon et al., 1998a). Following a complete spinal cord transection in the adult cat, some weight-bearing locomotion generally will begin to occur, particularly with step training, within a matter of a few weeks. Rossignol and colleagues (Chau et al., 1998) demonstrated that administration of clonidine immediately before training and beginning within 3 days after a complete spinal transection in adult cats, resulted in the recovery of weight-bearing stepping within 11 days. Although it was noted that the “spinal locomotor network might be further consolidated or molded through the peripheral afferent inflow during locomotor training”, there are two possible explanations for the improved locomotion. One explanation is that the repetitive administration of clonidine directly induced alterations in the spinal circuitry so that it could generate stepping. A second possibility is that the clonidine changed the physiological state of the spinal cord, thus enabling the afferent systems to assume control and activate the appropriate neural pathways, i.e., a use-dependent mechanism, and not a direct pharmacologically-induced mechanism. In either case, the results are consistent with the interpretation that neural pathways that can generate stepping persisted after spinal cord transection, and it was a matter of gaining access to that network to induce stepping.
We hypothesized that it was the repeated use of the sensorimotor pathways associated with weight-bearing stepping, and not the direct pharmacological facilitation, that increased the efficacy of sensorimotor pathways involved in stepping (Edgerton et al., 2004). A similar concept was proposed by Chau et al. (1998) based the results of repeated injections of clonidine and step training immediately after a spinal cord transection. It cannot be determined from this study, however, whether the improved stepping was due to the pharmacological effect itself or by the combined or interactive effect of training and clonidine.
To address the question of whether there was an important interactive effect of the pharmacological intervention and the training, we administered the 5-HT receptor agonist quipazine daily to a group of spinal mice that were step trained and to a group that were not trained (Cai et al., 2006b; Fong et al., 2005). The non-trained mice receiving quipazine showed no sustained improvement in stepping over a period of several weeks, whereas the trained mice receiving quipazine showed a significant improvement in stepping ability. Furthermore, although there was some improvement in stepping in the trained mice in the absence of quipazine administration, there was a marked synergistic effect between the two interventions. These results demonstrate that the improved stepping was a function of the repetitive activation of the sensorimotor pathways that was enabled by quipazine treatment. Further evidence that the serotonergic system can be used to facilitate the recovery of locomotion in complete spinal rats has been demonstrated by Orsal and colleagues (Feraboli-Lohnherr et al., 1997; Ribotta et al., 2000). They transplanted embryonic raphe cells secreting 5-HT in the spinal cord distal (T11) to a complete lesion at T8. These rats, demonstrated improved locomotion over a period of 9 weeks compared to non-transplanted and T9-transplanted animals.
5. Strategies to achieve more optimal physiological states for locomotion
We have initiated a series of experiments designed to characterize optimal physiological states of the lumbosacral spinal cord for stepping in chronic spinal rats. Our strategy has been to acutely, i.e., on a daily basis, modulate state dependence pharmacologically combined with epidural stimulation, in an attempt to achieve a more prolonged or chronic modulation based on use-dependent plasticity (step training). These three modulatory approaches used in concert have proven to be highly successful. We have been able to achieve full weight-bearing bipedal stepping over a wide range of treadmill speeds in rats spinalized as adults that have received daily step training in the presence of quipazine and epidural stimulation (Gerasimenko, 2006). As emphasized previously, the combination of quipazine and epidural stimulation does not directly induce weight-bearing stepping. Locomotion, however, can be enabled by each of these modulators when administered at levels that do not initiate locomotor-like movements in the absence of any sensory input associated with weight bearing stepping. When weight-bearing, step-related proprioceptive information is provided to the spinal circuitry, however, it can generate full weight-bearing stepping over a range of speeds and loads.
Although we have achieved significantly improved “physiological states” for generating stepping, it is unlikely that these states are optimal. For example, our selection of quipazine as a single pharmacological intervention, and the dosages and method of administration (intraperitoneally) are almost certainly not producing an optimal “pharmacological state”. Rather it seems that some combination of several different pharmacological agonists and antagonists will ultimately prove to be most successful. For example, the combination of 5-HT1A, 5-HT7 and 5-HT2 agonists improves stepping ability of spinal rats beyond the action of either serotonin agonist (Antri et al., 2003). Furthermore, it is almost certain that the optimum combination and dosage of the pharmacological agents will change over time following an injury due to the modulation of chronic levels of activity, diet, psychological state, immunological status, etc. Although the challenge here is indeed intimidating, it should be possible to sustain and improved functional state of the spinal circuitry following spinal cord injury. We are, however, only at the beginning stages of assessing the efficacy of epidural stimulation in modulating the physiological state of the spinal cord. Electrode design and the ability to selectively activate specific regions of the spinal cord should improve with time.
5.1. Biochemical plasticity in response to training
We have considered multiple experimental approaches in attempting to understand the neurophysiological and biochemical mechanisms involved in spinal learning, one manifestation of specific use-dependent plasticity. We have attempted to identify biochemical adaptations that occur in response to learning to step or to stand and have compared these responses to those associated with learning in the hippocampus. To date, the levels of several learning-related molecules have been found to change in the spinal cord following step training. For example, cyclic AMP responsive element binding protein (CREB) has been shown to be phosphorylated (pCREB) during learning. We have observed that the number of motoneurons expressing pCREB in specific lumbar motor pools is higher in quipazine-treated, step-trained than non-trained spinal mice. Similarly, only the trained spinal mice contained a comparable number of immunopositive cells for the phosphorylated GluR1 subunit of the AMPA receptor in motor pools to levels observed in intact mice, suggesting a facilitation of glutamate receptor signaling in the trained mice. A significant increase in stepping performance was observed only in the trained mice. Administration of quipazine alone did not affect stepping ability, consistent with an absence of cellular changes observed in the non-trained mice. These data suggest that the repetitive activation of spinal sensorimotor pathways during quipazine-enabled step training could mediate the facilitation of glutamate receptor function and activation of the transcription factor CREB in the lumbar spinal cord, and that these alterations were associated with an improvement in stepping ability after a spinal cord injury.
We also have assessed the roles of supraspinal and afferent input in defining the dynamics of the 5-HT1A receptor in specific laminae of the spinal cord. We found an increase in 5-HT1A receptor immunopositive cells and/or axon hillocks following a complete spinal cord transection in adult rats. However, these increases were no longer apparent when the afferents were removed via dorsal rhizotomies, suggesting a conditional afferent-related phenomenon that may or may not be activity dependent. Changes in receptor distribution could be important due to the intracellular consequences associated with 5-HT1A receptor activation, i.e., increases or decreases can be detrimental to the delicate electrophysiological balance in the spinal locomotor networks. Ultimately, a comprehensive understanding of the mechanisms underlying the receptor dynamics in the spinal cord may improve the efficacy of pharmacological interventions following a spinal cord injury.
5.2 Neurophysiological plasticity in response to training
A range of synaptic changes following a complete transection of several sensorimotor pathways has been reported. For example, the amplitude of Group Ia fiber-evoked monosynaptic EPSPs (sEPSPs) was higher in trained than non-trained rats that were spinally transected as neonates. Cote and Gossard (2004) reported a reduction in Ia-evoked EPSPs in trained adult spinal cats, but enhanced cutaneous reflexes in trained compared to non-trained spinal cats when stimulating afferents that were thought to be activated during weight-bearing stepping. They also observed that the Group Ib (Golgi tendon organ) disynaptic input to homonymous motoneurons was reversed from inhibition to excitation more frequently in trained than in non-trained cats (Cote et al., 2003).
Synaptic input to motoneurons from propriospinal pathways in step-trained spinal rats has been examined. Petruska et al. (in press) observed that the efficacy of propriospinal pathways was closely linked to stepping ability in adult rats that had been spinalized at five days of age. For example, stimulation of the ventrolateral funiculus (central-EPSP, cEPSP) induced higher amplitude polysynaptic cEPSPs recorded from motoneurons in trained than non-trained or poorly stepping spinal rats. Passive exercise (motorized bicycle training) in spinally transected rats also restored the frequency-dependent depression of the H-reflex to the level of intact animals (Reese et al., 2006). One of proposed mechanisms of training is a restoration of presynaptic inhibition. There seems to be some activity-dependent factor that affects the efficacy of a range of synergistic pathways, i.e., a decrease in presynaptic inhibition after a spinal cord injury and a concomitant facilitation of the monosynaptic H-reflex and less H-reflex frequency-dependent depression. The progressive nature of the training effect was evident. For example, after 15 or 30 days of training the H-reflex inhibition was only partially restored, whereas at 90 days the H-reflex frequency-dependent depression had normalized (Reese et al., 2006). A similar effect has been described in humans where motorized bicycle training provided recovery of H-reflex frequency-dependent depression within 10 weeks. The normalization of the monosynaptic reflex by training returned to pre-training level within a few weeks after training was stopped (Kiser et al., 2005).
Some changes in the intrinsic electrophysiological properties of motoneurons also occur after a spinal cord injury with and without step training. Petruska et al. (in press) found a significant relationship between the locomotor capacity of rats spinalized as neonates and the depth of the after-hyperpolarization (AHPd) of action potentials generated in the motoneurons. “Passive” cyclic exercise in rats spinally injured as adults also can return AHPd toward values observed in control rats (Beaumont et al., 2004). The fact that imposing cyclic activity on the hindlimbs induces considerable activation of the spinal circuitry and the associated muscles suggests that the mechanism of this intervention is an activity-related event.
Given that the morphology of a motoneuron has a predominant effect on its electrophysiological properties, it is interesting that motoneuron structure is affected by training after spinal transection (Gazula et al., 2004). The size of the dendritic tree of motoneurons following spinal cord transection decreases, but these morphological features can be returned to normal values by training on a cycle. This same study, however, showed no morphological effects of spinal cord transection or training on the size of the soma. Previous data are consistent with stability of the motoneuron morphology following either spinal cord transection or spinal cord isolation (Chalmers et al., 1992).
Although a number of electrophysiological changes of the spinal circuitry have been reported in in situ preparations and some of these changes are use-dependent, it remains difficult to determine whether, and which, of these effects might be responsible for improved performance in vivo. The ability to test reflex responses during recovery from a spinal cord injury under in vivo conditions may provide a means of determining more clearly any cause-and-effect relationships. For example, we (Gerasimenko et al., 2006; Lavrov et al., 2006) have demonstrated that chronic electrodes implanted epidurally over specific spinal cord segments can be used to evoke and track monosynaptic and polysynaptic responses in multiple flexor and extensor muscles of the hindlimb over a prolonged period of time after a spinal cord injury (Fig. 7A and B). We also have reported that the progressive reappearance of polysynaptic reflex responses in hindlimb muscles (Fig. 7B) are correlated with the gradual recovery of locomotor capacity of adult rats with a complete thoracic transection, i.e., about 3 weeks after the injury (Fig. 7A).
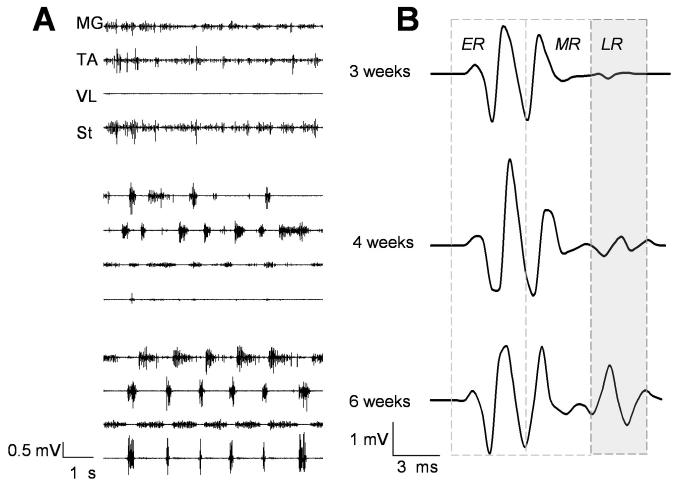
Figure 7.
A: EMG patterns of the MG, TA, VL, and St muscles at 3, 4 and 6 weeks post-surgery during stepping induced by epidural stimulation (40 Hz) at S1. Note the more robust and consistent EMG burst patterns at 6 compared to 3 and 4 weeks. B: Representative ER (early response, direct activation), MR (middle response, a monosynaptic response), and LR (late response, a polysynaptic response) from the TA muscle at 4.5 V stimulation 3, 4, and 6 weeks after ST. Note that the LR is minimal at 3 weeks and progressively increases from 4 to 6 weeks. Muscle abbreviations are the same as in Fig. 4. (Taken from Lavrov et al., 2006).
Examining the dynamics of these monosynaptic and polysynaptic responses to epidural stimulation during stepping should be even more useful than stimulation in a static and/or anesthetized state (Gerasimenko et al., 2006). The amplitude of spinal cord evoked motor potentials in hindlimb muscles during continuous bipedal stepping in non-disabled rats varies as a function of the phase of the step cycle (Fig. 8A). In response to L2 stimulation, the amplitude of the epidurally induced monosynaptic motor response in vivo in normal rats was strongly facilitated during the stance and swing phases of gait in extensors and flexor muscles, respectively. In general, there was a strong co-variation between the output of motoneurons, as seen in the recorded EMG activity (Fig. 8A, left traces), and the modulation of monosynaptic reflex responses. Courtine et al. (2007b) observed similar modulation pattern of monosynaptic reflex responses from lower limb muscles during human locomotion (Fig. 8B). These studies demonstrate the highly dynamic nature of the activity in the spinal neural circuitry during stepping, i.e., sensory information provided to the spinal cord is interpreted in a state-dependent manner.
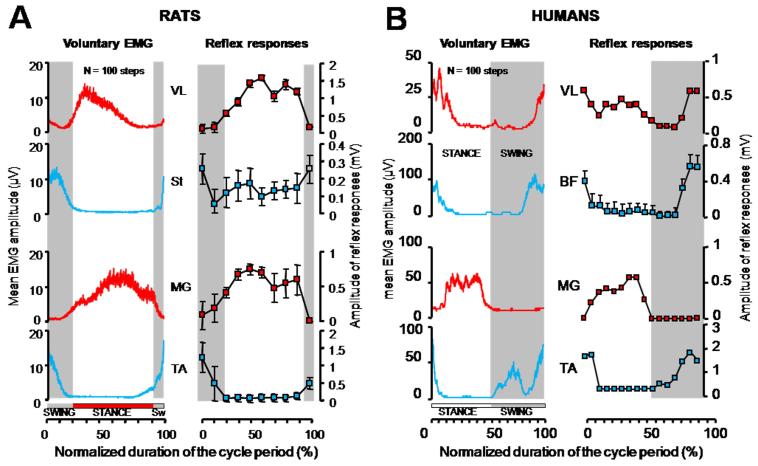
Figure 8.
Monosynaptic reflex responses were elicited by epidural stimulation at L2 in rats (A) and by transcutaneous electrical stimulation between the T12-L1 spinal processes in humans (B). Stimulations were delivered at different times of the stride period while both non-disabled rat and human subjects were stepping bipedally on a treadmill. The EMG activity recorded during all the steps preceding stimulation (n > 100 steps) were averaged and are displayed in left panels. The mean amplitude (±SD) of reflex responses is shown in right panels. There was a strong correlation between the ongoing motor output and the size of the reflex responses, both in rats and humans. BF, biceps femoris, all other muscle abbreviations, same as in Fig. 4. (A, modified from Gerasimenko et al., 2006: B, modified from Courtine et al., 2007b).
5.3. Are the variations in the pathways activated from step to step an important feature in spinal learning?
The limitation with the kinds of biochemical and physiological observations noted above is that they give little insight into how complex networks of neurons learn. Although it is difficult to address the cellular mechanisms of learning in such a complex spinal neural network, system-level mechanisms may provide important insight into how spinal animals relearn to step. There are many examples of even the simplest task having some intrinsic level of variation in both the biomechanics and the timing of the neurons recorded during the repetitive performance of the task. Stepping is an excellent example of a motor task that is performed routinely and repetitively, but even under the most controlled conditions on a treadmill, no two steps are identical (Fig. 9). Regardless of how much even a professional athlete practices a task, there will be variation in the success of the performance. This intrinsic variation in all movements is highly suggestive of a fundamental feature of the neural control of movement. We addressed this issue by studying the ability of spinal mice to learn to step. We hypothesized that controlling the kinematics of the lower limbs during bipedal stepping such that there was minimal variation would result in poorer stepping ability then if some level of variation was allowed during the stepping (Fong et al., 2005). The variation was permitted by applying an “assist-as-needed” mode of control of a robotic arm attached to the ankle of each hindlimb during a 10-min training session, five days a week. Two “assist-as-needed” modes were used on separate groups of mice, where the robotic device permits the ankle position of the animal to step within a predefined level of variance from the desired training trajectory. This way, only when the trajectory deviates from the desired training trajectory greatly, does the robotic device assist the animal back to the target training trajectory. In this experiment (Cai et al., 2006b), one group was assisted such that each leg could operate independently. A second group was assisted with the additional criterion being that the two limbs must function in an alternating, out of phase fashion. An additional group of mice were trained using a rigid training paradigm in which no variation was allowed during stepping. Based on several measures of step performance, the mice trained with the assist-as-needed mode and some degree of alternating interlimb coordination performed better than the other two groups. These data suggest that some toleration of variance in the stepping trajectory is beneficial, in particular when interlimb coordination is imposed. This conclusion is consistent with previous evidence suggesting that one of the more important features of successful stepping in the spinal cats was the ability to sustain the appropriate interlimb coordination (de Leon et al., 1998b).
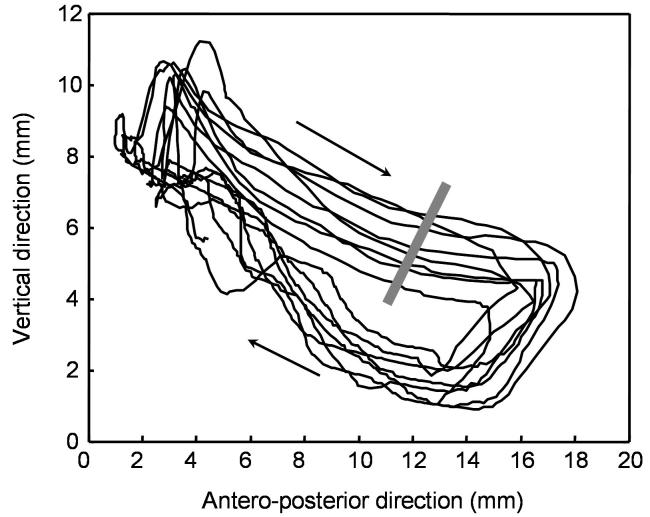
Figure 9.
Trajectory plot of the ankle of an adult transected mouse successfully stepping on a moving treadmill for 10 s at a rate of 3 cm/s after 4 weeks of quipazine-enabled step training. Arrows are showing the direction of ankle movements. Note the variability in the ankle trajectory in spite of the successful plantar stepping, weight-bearing execution of locomotion.
These data imply that the most effective learning paradigm for spinal cord injured subjects would allow some critical level of variation in stepping kinematics and kinetics (Cai et al., 2006a). We propose that this could be an important feature of the software control that is applied to robotics designed to retrain spinal cord injured subjects to step. While these data raise the issue as to what the ideal pattern of variation might be for a given subject, it is impossible to test every permutation experimentally. Thus we are currently using a computational learning model to test our hypothesis that there is an optimal level of variation in the kinematics and dynamics of stepping. We predict that step training related learning will not occur if the pattern is too fixed or too variable (Cai et al., 2006b).
6. Gaining access to the spinal circuitry that can generate locomotion in humans: taking advantage of state-dependent modulation of the spinal circuitry while using afferents as a source of control
The clinical question that these experimental observations in animal models raise is: How do we gain access to the circuits that have the ability to generate and control full weight-bearing stepping? All of the results from the animal models suggest that any strategy for recovering locomotor ability should include modulation of the physiological state of the spinal cord while using the sensory system as the “controller” for motor function. In fact, state-dependent processing of sensory input has been demonstrated in humans as well. Courtine et al. (2007b) showed that the gain in the monosynaptic neural pathway is modulated in virtually all lower limb muscles of normal subjects during walking in a manner similar to the patterns observed in the bipedally walking rat (Fig. 8B) (Gerasimenko et al., 2006).
For the interventions noted above to be practical for a spinal cord injured subject a number of factors must be considered. We have presented an “enabling” concept as opposed to an “inducing” concept to describe the responses to pharmacological agents, to epidural stimulation, and to some degree use-dependent plasticity such as step training. In other words, it is technically feasible, at least from an engineering perspective, to administer some “pharmacological cocktail” directly to the spinal cord at a dosage that is needed at any given time point. It also is technically feasible to apply electrical stimulation to the dorsum of the spinal cord at specific segmental levels in a manner that can induce step-like patterns (Dimitrijevic et al., 1998).
These new interventions (Fig. 3) may make it feasible for a patient with a complete lesion of the spinal cord to stand and to step when the individual chooses. If the spinal cord in the absence of any input from the brain is “ready”, i.e., the physiological state of the spinal neural circuits is appropriately tuned, it can initiate standing or stepping by manipulating the position of the body so that pressure is placed on the bottom of the feet to initiate an extensor thrust. With appropriate training this extensor thrust can be used to sustain standing in complete spinal cord injured subjects (Wernig et al., 1998). Once the individual is standing, manipulations of loading the limbs and joint angles in the legs also can be used to initiate stepping. This approach is more feasible for paraplegics than quadriplegics, because they can use their upper limb and trunk muscles to aid in balance and support as is necessary when using a walker or cane.
7. Conclusions
In the present chapter we have shown that electrical stimulation of the spinal cord and/or pharmacological administration such as serotonin agonists can enable stepping in adult mammals with complete spinal cord transection. We propose that these stepping-enabling strategies can bring the spinal cord to a physiological state at which the motor circuits can process the sensory information in a manner sufficient for generating coordinated, weight-bearing stepping. We emphasize, however, that sensory information can be used by the spinal circuitry to actually control, and not just modulate, locomotion. A combination of stepping-enabling agents and taking advantage of the capacity of afferent input to drive stepping activity can be used to improve locomotor function in humans with a spinal cord injury.
Acknowledgments
The work presented in this paper was supported by NIH NS16333, NIH NS42291, RFBR-CRDF 07-04-91106, the Christopher and Diana Reeve Foundation, and the California Roman Reed Bill.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antri M, Mouffle C, Orsal D, Barthe JY. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur. J. Neurosci. 2003;18:1963–1972. doi: 10.1046/j.1460-9568.2003.02916.x. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990;514:55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Houle JD, Peterson CA, Gardiner PF. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve. 2004;29:234–242. doi: 10.1002/mus.10539. [DOI] [PubMed] [Google Scholar]
- Cai LL, Courtine G, Fong AJ, Burdick JW, Roy RR, Edgerton VR. Plasticity of functional connectivity in the adult spinal cord. Phil. Trans. R. Soc. Lond. B Biol. Sci. 2006a;361:1635–1646. doi: 10.1098/rstb.2006.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai LL, Fong AJ, Otoshi CK, Liang Y, Burdick JW, Roy RR, Edgerton VR. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J. Neurosci. 2006b;26:10564–10568. doi: 10.1523/JNEUROSCI.2266-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart MR, He J, Herman R, D’Luzansky S, Willis WT. Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans. Neural. Syst. Rehabil. Eng. 2004;12:32–42. doi: 10.1109/TNSRE.2003.822763. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J. Physiol. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers GR, Roy RR, Edgerton VR. Adaptability of the oxidative capacity of motoneurons. Brain Res. 1992;570:1–10. doi: 10.1016/0006-8993(92)90556-o. [DOI] [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Early locomotor training with clonidine in spinal cats. J. Neurophysiol. 1998;79:392–409. doi: 10.1152/jn.1998.79.1.392. [DOI] [PubMed] [Google Scholar]
- Cote MP, Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J. Neurosci. 2004;24:11317–11327. doi: 10.1523/JNEUROSCI.1486-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, Menard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J. Neurosci. 2003;23:2789–2796. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, De Nunzio AM, Schmid M, Beretta MV, Schieppati M. Stance- and locomotion-dependent processing of vibration-induced proprioceptive inflow from multiple muscles in humans. J. Neurophysiol. 2007a;97:772–779. doi: 10.1152/jn.00764.2006. [DOI] [PubMed] [Google Scholar]
- Courtine G, Harkema SJ, Dy CJ, Gerasimenko YP, Dyhre-Poulsen P. Modulation of Multisegmental Monosynaptic Responses (MMR) in a variety of leg muscles during walking and running in humans. J Physiol. 2007b doi: 10.1113/jphysiol.2007.128447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Effects of inhibitory amino acid antagonists on reciprocal inhibitory interactions during rhythmic motor activity in the in vitro neonatal rat spinal cord. J. Neurophysiol. 1995;74:1109–1117. doi: 10.1152/jn.1995.74.3.1109. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 1998a;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J. Neurophysiol. 1998b;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J. Neurophysiol. 1999;82:359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Gerasimenko Y, Pinter M. Evidence for a spinal central pattern generator in humans. Ann. N. Y. Acad. Sci. 1998;860:360–376. doi: 10.1111/j.1749-6632.1998.tb09062.x. [DOI] [PubMed] [Google Scholar]
- Duysens J, Van de Crommert HW. Neural control of locomotion; The central pattern generator from cats to humans. Gait Posture. 1998;7:131–141. doi: 10.1016/s0966-6362(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, de Guzman CP, Gregor RJ, Roy RR, Hodgson JA, Lovely RG. Trainability of the spinal cord to generate hindlimb stepping patterns in adult spinalized cats. In: Shimamura TM, Grillner S, Edgerton VR, editors. Neurobiological Basis of Human Locomotion. Japan Scientific Societies Press; Tokyo: 1991. pp. 411–423. [Google Scholar]
- Edgerton VR, Roy RR. Paralysis recovery in humans and model systems. Curr. Opin. Neurobiol. 2002;12:658–667. doi: 10.1016/s0959-4388(02)00379-3. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci . 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Feraboli-Lohnherr D, Orsal D, Yakovleff A, Gimenez y Ribotta M, Privat A. Recovery of locomotor activity in the adult chronic spinal rat after sublesional transplantation of embryonic nervous cells: specific role of serotonergic neurons. Exp. Brain Res. 1997;113:443–454. doi: 10.1007/pl00005597. [DOI] [PubMed] [Google Scholar]
- Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J. Neurosci. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J. Neurophysiol. 1979;42:936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Gazula VR, Roberts M, Luzzio C, Jawad AF, Kalb RG. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J. Comp. Neurol. 2004;476:130–145. doi: 10.1002/cne.20204. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Avelev VD, Nikitin OA, Lavrov IA. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci. Behav. Physiol. 2003;33:247–254. doi: 10.1023/a:1022199214515. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Lavrov IA, Bogacheva IN, Shcherbakova NA, Kucher VI, Musienko PE. Formation of locomotor patterns in decerebrate cats in conditions of epidural stimulation of the spinal cord. Neurosci. Behav. Physiol. 2005;35:291–298. [PubMed] [Google Scholar]
- Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, Roy RR, Edgerton VR. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J. Neurosci. Meth. 2006;157:253–263. doi: 10.1016/j.jneumeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Giroux N, Brustein E, Chau C, Barbeau H, Reader TA, Rossignol S. Differential effects of the noradrenergic agonist clonidine on the locomotion of intact, partially and completely spinalized adult cats. Ann. N. Y. Acad. Sci. 1998;860:517–520. doi: 10.1111/j.1749-6632.1998.tb09092.x. [DOI] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Guertin PA. Synergistic activation of the central pattern generator for locomotion by l-beta-3,4-dihydroxyphenylalanine and quipazine in adult paraplegic mice. Neurosci. Lett. 2004;358:71–74. doi: 10.1016/j.neulet.2003.12.120. [DOI] [PubMed] [Google Scholar]
- Gurfinkel VS, Levik YS, Kazennikov OV, Selionov VA. Locomotor-like movements evoked by leg muscle vibration in humans. Eur. J. Neurosci. 1998;10:1608–1612. doi: 10.1046/j.1460-9568.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Huang H, He J, Herman R, Carhart MR. Modulation effects of epidural spinal cord stimulation on muscle activities during walking. IEEE Trans. Neural. Syst. Rehabil. Eng. 2006;14:14–23. doi: 10.1109/TNSRE.2005.862694. [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 2005;383:339–344. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiser TS, Reese NB, Maresh T, Hearn S, Yates C, Skinner RD, Pait TG, Garcia-Rill E. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J. Spinal Cord Med. 2005;28:241–245. doi: 10.1080/10790268.2005.11753818. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J. Neurophysiol. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- Leblond H, L’Esperance M, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. J. Neurosci. 2003;23:11411–11419. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J. Neurophysiol. 2005;94:1392–1404. doi: 10.1152/jn.00136.2005. [DOI] [PubMed] [Google Scholar]
- Maegele M, Muller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J. Neurotrauma. 2002;19:1217–1229. doi: 10.1089/08977150260338010. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Sinkjaer T. Afferent feedback in the control of human gait. J. Electromyogr. Kinesiol. 2002;12:213–217. doi: 10.1016/s1050-6411(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Ichiyama RM, Crown ED, Tansey KE, Roy RR, Edgerton VR, Mendell LM. Changes in motoneuron properties and synaptic inputs related to step training following spinal cord transection in rats. J. Neurosci. doi: 10.1523/JNEUROSCI.2302-06.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Reese NB, Skinner RD, Mitchell D, Yates C, Barnes CN, Kiser TS, Garcia-Rill E. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord. 2006;44:28–34. doi: 10.1038/sj.sc.3101810. [DOI] [PubMed] [Google Scholar]
- Ribotta MG, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J. Neurosci. 2000;20:5144–5152. doi: 10.1523/JNEUROSCI.20-13-05144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GA, Goldberger ME. The development and recovery of motor function in spinal cats. II. Pharmacological enhancement of recovery. Exp. Brain Res. 1986;62:387–400. doi: 10.1007/BF00238858. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Phil. Trans. R Soc. Lond. B Biol. Sci. 2006;361:1647–1671. doi: 10.1098/rstb.2006.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Barbeau H. Pharmacology of locomotion: an account of studies in spinal cats and spinal cord injured subjects. J. Am. Paraplegia Soc. 1993;16:190–196. doi: 10.1080/01952307.1993.11735900. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Bouyer L, Barthelemy D, Langlet C, Leblond H. Recovery of locomotion in the cat following spinal cord lesions. Brain Res. Brain Res. Rev. 2002;40:257–266. doi: 10.1016/s0165-0173(02)00208-4. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN. [Control of walking and running by means of electric stimulation of the midbrain] Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Wernig A, Nanassy A, Muller S. Maintenance of locomotor abilities following Laufband (treadmill) therapy in para- and tetraplegic persons: follow-up studies. Spinal Cord. 1998;36:744–749. doi: 10.1038/sj.sc.3100670. [DOI] [PubMed] [Google Scholar]