Abstract
Midkine (MDK) is a heparin-binding growth factor involved in growth, survival, migration, and differentiation of various target cells and dysregulation of MDK signaling is implicated in a variety of inflammatory diseases and cancers. Although MDK has been reported to act on endothelial cells and to have proangiogenic effects, the exact role of MDK in angiogenesis is poorly defined. Here, we report that MDK is actually a modulator of angiogenesis and that it can abrogate the vascular endothelial growth factor A (VEGF-A)-induced proliferation of human microvascular endothelial cells in vitro through the downregulation of proangiogenic cytokines and through the upregulation of the antiangiogenic factor, tissue inhibitor of metalloproteinase 2. Phosphorylation of vascular endothelial growth factor receptor 2 (VEGFR-2) and of downstream signaling molecules, such as phosphatidylinositol-3-kinase and mitogen-activated protein kinases, is also impaired. Moreover, MDK downregulates VEGF-A-induced neovascularization and vascular permeability in vivo. We propose a model in which MDK is a new modulator of the VEGF-A-VEGFR-2 axis.
Introduction
Angiogenesis is the generation of new blood vessels from preexisting microvasculature and is regulated by a number of growth factors, among which the vascular endothelial growth factor (VEGF) family of proteins is most prominent [1]. Because tumor growth and metastasis depend on new blood vessel formation, angiogenesis has become an attractive target for cancer therapy [2]. Vascular endothelial growth factor family members exert their activities by binding to three structurally related receptor tyrosine kinases, VEGF receptor (VEGFR)-1, -2, and -3. The prominent VEGF-A isoform exerts most of its biologic activities, such as endothelial cell (EC) proliferation, migration, survival, and permeability, by activating VEGFR-2 [1,3,4]. Although EC proliferation is an important component of angiogenesis, VEGF has relatively weak mitogenic activity compared to other growth factors, such as basic fibroblast growth factor (bFGF) [1,3]. It is thought that at least part of the success of anti-VEGF strategies in the clinic can be attributed to the potent activity of VEGF on vascular permeability [4,5].
The growth factor Midkine (MDK), also known as Neurite growth.promoting factor 2, is a 13-kDa heparin-binding protein that promotes the growth, survival, migration, and differentiation of various cell types [6–8]. Midkine shares 50% identity in amino acid sequence with Pleiotrophin, and together, they represent the two members of a distinct family of heparin-binding growth factors [7,8]. Several reports suggest that MDK is a ligand for Syndecan-3, anaplastic lymphoma kinase, low-density lipoprotein receptor.related protein, α4β1- and α6β1-integrin, and the receptor-like protein-tyrosine phosphatase-zeta [6,8]. Clinical reports indicate that MDK is overexpressed in a variety of human carcinomas and that high levels of MDK in patient serum correlate with poor clinical outcome [9–11]. Furthermore, high MDK expression levels in primary human tumors were shown to correlate with increased tumor angiogenesis [12]. It has also been reported that in xenografts from two cancer cell lines of epithelial origin, overexpression of the MDK gene can cause increased angiogenicity, resulting in enhanced malignant proliferation of cancer cells [13,14]. Taken together, an increasing number of studies suggest that MDK is a candidate molecular target for the therapy for carcinomas. However, unlike its close homolog Pleiotrophin, MDK's biologic significance in angiogenesis in general and its mechanism of action in ECs in particular still remains to be clarified.
Our study addresses the complex role of MDK in VEGF signaling, using primary human microvascular ECs (HMVECs) as a model system. In contrast to previously published results, which are primarily based on overexpression experiments in epithelial cancer cell lines, our data show that MDK can be a potent negative modulator of VEGF-A-induced proangiogenic activities in vitro and in vivo and shed new light on the role of MDK in angiogenesis.
Materials and Methods
Cell Lines and Reagents
Primary HMVECs were obtained from Lonza Inc. (Allendale, NJ) and were cultured according to the suppliers' protocol. The human angiogenesis antibody array (TranSignal Angiogenesis Antibody Array) was purchased from Panomics Inc. (Fremont, CA). BrdU Incorporation and Cell Death Detection ELISAplus assay kits were purchased from Roche Applied Science (Indianapolis, IN). Antibodies raised against the following proteins were used: phosphotyrosine-specific antibody [mouse monoclonal antibody (mAb): 4G10 (PY); UBI (now Millipore), Temecula, CA], phospho-VEGFR-2 antibodies [rabbit mAb (rmAb): Y1175 (19A10); Cell Signaling Technology Inc., Danvers, MA], VEGFR-2 antibody [rabbit polyclonal antibody (pAb); Santa Cruz Biotechnology, Santa Cruz, CA], p85 antibody (ascitic fluid; UBI), phospho-specific extracellular-regulated kinase (ERK) 1/2 antibody (pAb; Cell Signaling), β-actin (ascitic fluid; Sigma, St. Louis, MO). Horseradish peroxidase-coupled secondary antibodies were purchased from Jackson Laboratories Inc. (West Grove, PA). Bovine serum albumin (BSA) and Evans blue were from Sigma. Fertilized special pathogen-free eggs were purchased from Charles River (St. Louis, MO). Recombinant human VEGF-A, MDK, tissue inhibitor of metalloproteinase 2 (TIMP-2), bFGF, and human transforming growth factor alpha (TGF-α), tumor necrosis factor alpha (TNF-α), and TIMP-2 ELISA kits (HS Quantikine) were purchased from R&D Systems Inc. (Minneapolis, MN). We also expressed MDK in Escherichia coli and Pichia pastoris following published protocols. The MDK preparations were purified to homogeneity without detectable contaminants using affinity chromatography, ion exchange, and gel filtration chromatography. The biologic activity of MDK preparations was confirmed in neurite outgrowth assays. All MDK preparations demonstrated similar purities and activities, and we used the commercially available MDK for all assays presented here. Collagen I-coated culture dishes were from BD Biosciences (Bedford, MA).
Western Immunoblot Analysis and Cytokine Analysis
Western blot analysis was done as described previously [15]. Briefly, HMVECs were plated onto collagen I-coated culture plates and serum-starved (0.1% fetal bovine serum in endothelial cell basal medium 2 for 24 hours containing 10 ng/ml heparin). Midkine, VEGF-A, or both were added in the indicated time points and maintained at 37°C. After growth factor stimulation, cells were lysed; lysates were cleared before protein concentrations were determined.
To analyze the cytokines secreted by HMVECs, cells were treated as follows: 2 ml of conditioned medium were collected after 72 hours and cytokines were detected either by probing the angiogenesis antibody array or by performing the corresponding ELISAs following the respective supplier's protocols.
Proliferation, Bromodeoxyuridine Incorporation, and Apoptosis Assay
Human microvascular ECs were plated at a density of 8 x 104/ml onto collagen I-coated 96-well culture plates in complete media (serum and growth factors: microvascular endothelial growth media 2), incubated for 24 hours, and subsequently serum-starved for 24 hours in endothelial cell basal medium 2 supplemented with 5% fetal bovine serum. Human microvascular ECs were either left untreated or challenged with 25 ng/ml VEGF-A, 10 ng/ml MDK, or both growth factors as indicated. As a positive control, complete media was added. All media contained 10 ng/ml heparin. Cells were incubated for 5 days, and cell number was quantified using a reagent (Cell Titer Glo; Promega, Madison, WI) according to the manufacturer's protocol.
Bromodeoxyuridine (BrdU) Incorporation and apoptosis assays were performed concurrently under similar conditions as previously mentioned, with the exception that cells were challenged with the indicated proteins in the presence of BrdU for 48 hours. The extent of the incorporated BrdU and the increase of apoptotic events were quantified according to the manufacturer's protocols.
Chick Chorioallantoic Membrane Assay
The chick chorioallantoic membrane assay (CAM assay) was performed as described elsewhere [16]. Briefly, fertilized eggs (n = 10 per group) were incubated at 38°C in an egg incubator for 9 days. Eggs were candled to verify the viability of each embryo and to localize blood vessels. The growth factors were applied directly on the membrane in a relatively avascular region. Phosphate-buffered saline (PBS) and VEGF-A served as negative and positive controls. Midkine was tested in the absence or presence of VEGF. Twenty nanograms of each growth factor was used at a concentration of 2 µg/ml. After the injection, eggs were placed in the incubator for 48 hours, and the angiogenic response was visually scored using a dissecting microscope. This study was performed double-blinded, and representative micrographs are shown.
Miles Vascular Permeability Assay
The Miles vascular permeability assay (Miles assay) was performed as described previously [17]. Briefly, the back skin of Balb/c mice was depilated 24 hours before the experiment, and each treatment group consisted of three mice. Evans blue dye (100 µl of a 1% solution in 0.9% NaCl) was injected intravenously into mice. After 10 minutes, 50 µl of human VEGF-A (1 ng/µl), MDK (1 ng/µl), VEGF-A and MDK (2 ng/µl), or PBS were injected intradermally into the depilated back skin. Animals were euthanized, and an area of skin that included the entire injection site was removed. Extravasated Evans blue dye bound to plasma proteins was extracted from the skin by incubation in formamide for 24 hours at 60°C, and the fluorescence of the extracted dye was measured in a plate reader at 620 nm/590 nm.
Rat Corneal Angiogenesis Assay
All in vivo experiments were conducted in accordance with the guidelines of the Amgen Animal Care and Use Committee. Corneal angiogenesis was induced in female CD rats (Charles River Laboratories, Inc., Wilmington, MA) as described [18]. Briefly, 0.6-mmdiameter circular discs of Nylaflo membrane (Pall Corp., Ann Arbor, MI) were incubated in PBS containing 0.1% BSA, 0.1% BSA with 10 µM VEGF (R&D Systems Inc.), or 0.1% BSA with 10, 30, or 100 µM MDK [Chemicon (now Millipore)] for 1 hour at 4°C. A single disc was inserted into a pocket in the corneal stroma of anesthetized rats. After 7 days, the corneas were photographed using an ophthalmic slit lamp (Nikon Instruments, Melville, NY), and numerical data were generated from the digital images using an image analysis software (MetaMorph v4.5; Universal Imaging, Downingtown, PA). For each corneal image, the number of blood vessels intersecting the midpoint between the disc and the limbus was counted. Values represent the group mean ± SEM (n = 8 per group). Statistical significance was assessed by analysis of variance followed by Fisher's post hoc test.
Results and Discussion
To elucidate the physiological effect of MDK in angiogenesis, we used HMVECs as a model system. Because α4β1- and α6β1-integrins were described as functional receptors for MDK and β1-integrins mediate adhesion of HMVEC to collagen I matrices [19], we initially performed an in vitro proliferation assay on collagen I-coated plates (Figure 1). Cells were serum-starved for 24 hours and either left untreated or challenged with VEGF-A, MDK, or both growth factors together. As a positive control, cells were stimulated in the presence of complete growth medium (CM group). We observed almost a three- and six-fold growth induction of HMVECs in the presence of VEGF-A and in the positive control CM group, respectively. However, MDK alone suppressed proliferation and even abolished VEGF-A-induced proliferation of HMVECs. We also performed proliferation experiments with human umbilical vein ECs under identical conditions and obtained similar results (data not shown). These results are unexpected, because MDK has been reported to act as a mitogen on other cell types, e.g. epithelial cells or fibroblasts (reviewed in the study of Muramatsu [20]), and suggest a distinct role of MDK in ECs. To elucidate the molecular basis of our findings, we investigated whether MDK stimulation induces the secretion of antiangiogenic factors. We used an array with immobilized antibodies against 19 selected soluble proteins related to angiogenesis and performed the same experiment under identical conditions. After 72 hours, the conditioned media of treated HMVECs were collected and analyzed for secreted proteins (Figure 1B). After normalization, untreated and MDK-treated HMVECs showed no difference in cytokine secretion (Figure 1B, left and middle right panels), whereas VEGF-A treatment alone led to increased secretion of the proangiogenic factors TGF-α and TNF-α [21] and the antiangiogenic factor TIMP-2 [21] (Figure 1B, middle left panel). However, in the presence of MDK and VEGF-A, the secretion of TGF-α and TNF-α was downregulated and the expression of TIMP-2 was significantly increased (Figure 1B, right panel). To further substantiate our finding, we also quantified the amount of secreted TGF-α, TNF-α, and TIMP-2 by performing ELISAs, where we observed the same effect (Table 1). It has been shown that TIMP-2 abrogates VEGF-A-induced EC proliferation in vitro and angiogenesis in vivo through binding to α3β1-integrins, leading to enhanced protein-tyrosine phosphatase activity and downregulation of tyrosylphosphorylated VEGFR-2 [22].
Figure 1.
Midkine-induced upregulation of TIMP-2 and downregulation of TGF-α and TNF-α inhibits VEGF-A-mediated proliferation and BrdU incorporation of HMVECs. (A) Midkine suppresses the proliferation of ECs. Cell number was determined by the measurement of intracellular ATP content (Cell Titer Glo) every 24 hours for 3 days in octuplicates. (B) Midkine elevates TIMP-2 secretion and downregulates expression of TGF-α and TGF-α in HMVECs. Conditioned medium of stimulated HMVECs was analyzed after 72 hours using an antibody array (see Materials and Methods). (C) Midkine abrogates VEGF-A-induced BrdU incorporation in HMVECs. Bromodeoxyuridine incorporation assay with HMVECs was performed similarly in panel (A) for 48 hours in hexaduplicates. Midkine and TIMP-2 were used in two different concentrations as indicated. Symbols indicate mean; bars, SD.
Table 1.
Quantification of MDK-Induced Secretion of TGF-α, TNF-α, and TIMP-2 by ELISA.
| TGF-α (pg/ml) | TNF-α (pg/ml) | TIMP-2 (pg/ml) | |
| NS | 1069 ± 227 | 1.019 ± 0.825 | 358.0 ± 223.0 |
| VEGF | 2234 α 610 | 3.738 α 0.703 | 4311 α 1150 |
| MDK | 923.3 α 36.0 | 0.673 α 0.679 | 388.0 α 140.0 |
| VEGF + MDK | 1222 α 383 | 0.832 α 0.428 | 6217 α 637 |
Values represent the group mean α SEM.
Quantification has been measured by ELISA using human-specific antibodies against indicated antigens. Qualitative trends correspond to previously observed results in Figure 1B.
NS indicates not stimulated.
Therefore, we speculated that MDK-induced upregulation of TIMP-2 downregulates VEGF-A-mediated proliferation. To test our hypothesis and confirm our previous result, we performed BrdU incorporation assays under similar conditions for 48 hours. We included MDK and TIMP-2 as VEGF-A antagonistic factors in two different concentrations, respectively (Figure 1C). Midkine abrogated VEGF-A-induced S-phase entry in a dose-dependent manner, indicating that MDK antagonizes VEGF-A signaling. Exogenously added TIMP-2 also diminished VEGF-A-mediated S-phase entry similar to MDK, consistent with the postulated mechanism. DNA fragmentation assays demonstrate that the observed growth-inhibitory effects are a direct result of growth inhibition, as opposed to an increase in apoptosis (see Figure W1).
To see if MDK treatment had an antagonistic effect on VEGFR-2 tyrosine phosphorylation, serum-starved HMVECs were either left untreated, challenged with MDK or VEGF-A, or pretreated with MDK or TIMP-2 for 20 minutes before stimulation with VEGF-A (Figure 2A). VEGFR-2 was immunoprecipitated, and its tyrosine phosphorylation status was detected with the phosphotyrosine antibody 4G10 (PY). We observed that VEGF-A stimulation increased tyrosine phosphorylation of VEGFR-2, whereas pretreatment with MDK inhibited the VEGF-induced phosphorylation of VEGFR-2 (Figure 2A, lanes 2 and 4). Additionally, pretreatment with TIMP-2 abrogated VEGF-A-induced phosphorylation of VEGFR-2 (Figure 2A, lane 6). We then analyzed one of two major autophosphorylated tyrosines 1175 (Y1175) of VEGFR-2 in the presence of MDK and TIMP-2 using a phosphospecific antibody (Figure W1). We observed that MDK and TIMP-2 similarly abrogated VEGF-A-induced phosphorylation of VEGFR-2 at Y1175, which corroborates our previous observation. Because VEGF-A-induced survival and mitogenicity is thought to be mainly regulated through recruitment of the signal-transducer phosphatidylinositol-3-OH-kinase (PI3K) to tyrosyl-phosphorylated VEGFR-2 [21], we also investigated the phosphorylation of the PI3K p85 subunit under similar experimental conditions (Figure 2B). As expected, p85 phosphorylation was enhanced on VEGF-A stimulation. However, pretreatment with MDK before VEGF-A stimulation abolished p85 tyrosine phosphorylation to levels indistinguishable from untreated cells (Figure 2B, left panel). ErbB2 phosphorylation, which is not regulated by VEGF or MDK, was used as a specificity control (Figure 2B, right panel). Taken together, these data show that MDK can modulate the VEGF-A signal at the plasma membrane, altering the phosphorylation status of the critical p85 binding sites, thereby impairing the activation of PI3K by VEGFR-2. Consequently, signaling events downstream of PI3K, such as mitogen-activated protein kinase and AKT activation, should also be impaired after MDK pretreatment in HMVECs. To test this hypothesis, we performed a time-course experiment (Figure 2C). Human microvascular ECs were plated onto collagen I-coated plates, serum-starved, and either left untreated, challenged with VEGF-A and MDK, or pretreated with MDK before VEGF-A stimulation for the indicated periods of time. Subsequently, cells were lysed and ERK1/2 phosphorylation levels were analyzed using phospho-specific ERK1/2 antibodies (Figure 2B). Untreated or MDK-treated cells were indistinguishable and showed no elevated ERK1/2 phosphorylation. Consistent with the literature, VEGF-A treatment led to increased ERK1/2 phosphorylation at all time points (10, 30, and 60 minutes). Pretreatment with MDK decreased the VEGF-induced ERK1/2 phosphorylation at all time points. We then tested if MDK influences VEGF-A-mediated AKT phosphorylation (Figure 2D). AKT phosphorylation was strongly downregulated in the presence of MDK. As a control, we also pretreated the cells with TIMP-2 before VEGF-A stimulation and observed the same effect. Basic fibroblast growth factor.induced AKT activation was used as a specificity control and was not inhibited by MDK, indicating that MDK specifically downregulates VEGF-A signaling. These data support the notion that MDK negatively modulates VEGF-A-induced signaling pathways.
Figure 2.
MDK inhibits VEGF-A-mediated VEGFR-2, AKT, and mitogen-activated protein kinase phosphorylation in HMVECs. Serumstarved HMVECs, plated on collagen I-coated plates, were either stimulated with VEGF-A (20 ng/ml) and MDK (10µg/ml) or pretreated with MDK and TIMP-2 (100 ng/ml) before VEGF-A stimulation for the indicated time points. Cell lysates were subjected to immunoprecipitation using antibodies against VEGFR-2 (VEGFR-2; A), p85 (p85; B, left panel), or ErbB2 (ErbB2; B, right panel). Tyrosine phosphorylation was analyzed by Western blot analysis (WB) with monoclonal anti-phosphotyrosine antibody (α-PY); phosphorylation of ERK1/2 and AKT 1 were analyzed by Western blot analysis (WB) of whole-cell lysates (WCL) with phospho-specific anti.ERK1/2 antibody (PT/Y-ERK1/2; C, top panel), or anti.phospho-AKT antibody (S-473; D, top panel), respectively. Protein levels were checked by reblotting with antibodies against VEGFR-2 (A, lower panel) or β-actin (C and D, lower panel). Arrows indicate the detected proteins. Note that ErbB2 phosphorylation status serves as a specificity control for MDK and VEGF-A stimulation.
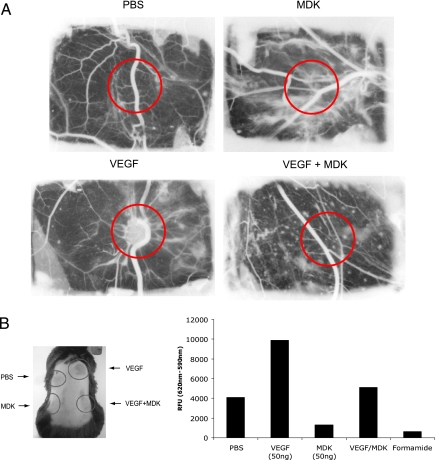
To further substantiate our findings, we investigated the effects of MDK on VEGF-induced angiogenesis in vivo. We first focused on primary network formation in the CAM assay [16]. We used fertilized special pathogen-free eggs and challenged them either with PBS, MDK or VEGF-A alone or in combination. This experiment was performed double-blinded, and after 48 hours, the angiogenic response was visually scored (Figure 3A).
Figure 3.
MDK inhibits VEGF-A-induced neovascularization and vascular permeability in vivo. (A) The CAM assay was performed either with PBS, VEGF-A, MDK, or both growth factors (see Materials and Methods). This study was performed double-blinded, and effects on angiogenesis were scored visually (the area where the stimuli were added and scored is marked with a red circle). The experiment was performed three times independently with 10 eggs per group. A representative micrograph is shown. (B) The Miles assay was performed three times independently, consisting of four treatment groups (n = 3 per group) and quantified as described in Materials and Methods. The left panel shows a representative animal (circles indicate where cytokines have been injected intradermally); the right panel shows the results of the quantification.
Again, PBS- and MDK-treated eggs displayed no angiogenic response (Figure 3A, top left and right panels). Consistent with published data, VEGF-A promoted neovascularization, leading to the formation of a primary network, whereas MDK- and VEGF-A-treated eggs failed to form a capillary network (Figure 3A, lower left and right panels). This result demonstrates for the first time an antiangiogenic effect of MDK on VEGF-A-induced signaling events in an in vivo setting.
This observation directly contradicts an older report, which states that MDK can induce angiogenesis in a rabbit cornea model [14]. To address this issue, we also tested MDK in the rat cornea assay, but did not observe a statistically significant stimulation, confirming that MDK alone does not act as a proangiogenic factor in this context (see Figure W1). These differences might be explained by the reagents used in the studies. Whereas we were able to use a highly purified recombinant MDK, earlier studies were limited to at best partially purified conditioned media from MDK-transfected mammalian cell lines, raising the possibility that the observed effects were not directly mediated by MDK.
Because it is well known that VEGF-A induces strong vascular permeability, we then examined if MDK could also abrogate VEGF-A-induced vascular permeability in vivo. To test MDK's antiangiogenic properties in combination with VEGF-A, we performed the Miles assay [17] (Figure 3B). We injected either PBS, VEGF-A, and MDK alone or a combination of VEGF-A and MDK. Compared to PBS, MDK treatment alone led to a decrease in vascular permeability, whereas VEGF-A alone induced permeability as expected (Figure 3B). Injecting MDK and VEGF-A together failed to induce vascular leakage, corroborating our previous observations that MDK acts as an antagonist in VEGF-A signaling in ECs.
Most previously published studies focused on the role of MDK on the growth of epithelial cell lines in vitro and in vivo, and it has been suggested, primarily based on circumstantial evidence, that MDK might play a proangiogenic role [13]. To the best of our knowledge, highly purified recombinant MDK has not been directly studied in combination with VEGF-A in HMVEC or in any in vivo angiogenic model systems. Our data clearly indicate that MDK can antagonize VEGF-A-induced signaling events both in vitro and in vivo.
Some discrepancies between this study and previously published observations linking MDK to angiogenesis can be explained by the different cell types that were studied. It was previously concluded that MDK is an attractive target for therapeutic intervention in various cancers, and this conclusion was based primarily on its effect on cancer cell lines of epithelial origin and its expression in human tumors. Our findings demonstrate that the role of MDK in tumorigenesis is more complex than previously appreciated. In light of the seemingly dual role of this secreted factor, promoting growth and survival of epithelial cells on one hand and interfering with the function of the proangiogenic factor VEGF-A on ECs on the other, a prediction of the potential therapeutic benefit of an anti-MDK strategy is very difficult and likely to be highly context-dependent.
Supplementary Material
Acknowledgments
We thank Juan Estrada and Stephen Kaufman for their help with the rat corneal angiogenesis assays.
Footnotes
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 3.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol. 2003;13:159–167. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 6.Muramatsu H, Zou P, Suzuki H, Oda Y, Chen GY, Sakaguchi N, Sakuma S, Maeda N, Noda M, Takada Y, et al. α4β1- and α6β1-integrins are functional receptors for midkine, a heparin-binding growth factor. J Cell Sci. 2004;117:5405–5415. doi: 10.1242/jcs.01423. [DOI] [PubMed] [Google Scholar]
- 7.Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127–143. doi: 10.1016/S0304-3835(03)00450-6. [DOI] [PubMed] [Google Scholar]
- 8.Herradon G, Ezquerra L, Nguyen T, Silos-Santiago I, Deuel TF. Midkine regulates pleiotrophin organ-specific gene expression: evidence for transcriptional regulation and functional redundancy within the pleiotrophin/midkine developmental gene family. Biochem Biophys Res Commun. 2005;333:714–721. doi: 10.1016/j.bbrc.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 9.Ikematsu S, Yano A, Aridome K, Kikuchi M, Kumai H, Nagano H, Okamoto K, Oda M, Sakuma S, Aikou T, et al. Serum midkine levels are increased in patients with various types of carcinomas. Br J Cancer. 2000;83:701–706. doi: 10.1054/bjoc.2000.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada H, Nabeya Y, Tagawa M, Okazumi S, Matsubara H, Kadomatsu K, Muramatsu T, Ikematsu S, Sakuma S, Ochiai T. Preoperative serum midkine concentration is a prognostic marker for esophageal squamous cell carcinoma. Cancer Sci. 2003;94:628–632. doi: 10.1111/j.1349-7006.2003.tb01494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maehara H, Kaname T, Yanagi K, Hanzawa H, Owan I, Kinjou T, Kadomatsu K, Ikematsu S, Iwamasa T, Kanaya F, et al. Midkine as a novel target for antibody therapy in osteosarcoma. Biochem Biophys Res Commun. 2007;358:757–762. doi: 10.1016/j.bbrc.2007.04.183. [DOI] [PubMed] [Google Scholar]
- 12.Ruan M, Ji T, Wu Z, Zhou J, Zhang C. Evaluation of expression of midkine in oral squamous cell carcinoma and its correlation with tumour angiogenesis. Int J Oral Maxillofac Surg. 2007;36:159–164. doi: 10.1016/j.ijom.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Muramaki M, Miyake H, Hara I, Kamidono S. Introduction of midkine gene into human bladder cancer cells enhances their malignant phenotype but increases their sensitivity to antiangiogenic therapy. Clin Cancer Res. 2003;9:5152–5160. [PubMed] [Google Scholar]
- 14.Choudhuri R, Zhang HT, Donnini S, Ziche M, Bicknell R. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997;57:1814–1819. [PubMed] [Google Scholar]
- 15.van der Horst EH, Weber I, Ullrich A. Tyrosine phosphorylation of PYK2 mediates heregulin-induced glioma invasion: novel heregulin/HER3-stimulated signaling pathway in glioma. Int J Cancer. 2005;113:689–698. doi: 10.1002/ijc.20643. [DOI] [PubMed] [Google Scholar]
- 16.Storgard C, Mikolon D, Stupack DG. Angiogenesis assays in the chick CAM. Methods Mol Biol. 2005;294:123–136. doi: 10.1385/1-59259-860-9:123. [DOI] [PubMed] [Google Scholar]
- 17.Satchi-Fainaro R, Mamluk R, Wang L, Short SM, Nagy JA, Feng D, Dvorak AM, Dvorak HF, Puder M, Mukhopadhyay D, et al. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer Cell. 2005;7:251–261. doi: 10.1016/j.ccr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Coxon A, Bolon B, Estrada J, Kaufman S, Scully S, Rattan A, Duryea D, Hu YL, Rex K, Pacheco E, et al. Inhibition of interleukin-1 but not tumor necrosis factor suppresses neovascularization in rat models of corneal angiogenesis and adjuvant arthritis. Arthritis Rheum. 2002;46:2604–2612. doi: 10.1002/art.10546. [DOI] [PubMed] [Google Scholar]
- 19.Leong KG, Hu X, Li L, Noseda M, Larrivee B, Hull C, Hood L, Wong F, Karsan A. Activated Notch4 inhibits angiogenesis: role of β1-integrin activation. Mol Cell Biol. 2002;22:2830–2841. doi: 10.1128/MCB.22.8.2830-2841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem (Tokyo) 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9(Supp:1), 2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- 22.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMPindependent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.