Abstract
Although inbreeding can reduce individual fitness and contribute to population extinction, gene flow between inbred but unrelated populations may overcome these effects. Among extant Mexican wolves (Canis lupus baileyi), inbreeding had reduced genetic diversity and potentially lowered fitness, and as a result, three unrelated captive wolf lineages were merged beginning in 1995. We examined the effect of inbreeding and the merging of the founding lineages on three fitness traits in the captive population and on litter size in the reintroduced population. We found little evidence of inbreeding depression among captive wolves of the founding lineages, but large fitness increases, genetic rescue, for all traits examined among F1 offspring of the founding lineages. In addition, we observed strong inbreeding depression among wolves descended from F1 wolves. These results suggest a high load of deleterious alleles in the McBride lineage, the largest of the founding lineages. In the wild, reintroduced population, there were large fitness differences between McBride wolves and wolves with ancestry from two or more lineages, again indicating a genetic rescue. The low litter and pack sizes observed in the wild population are consistent with this genetic load, but it appears that there is still potential to establish vigorous wild populations.
Keywords: conservation genetics, genetic rescue, inbreeding, inbreeding depression, wolves
1. Introduction
Inbreeding reduces the fitness of wild (Keller & Waller 2002), captive (Ralls et al. 1988) and experimental populations (Lacy et al. 1996), and increases the risk of population extinction (Newman & Pilson 1997; Saccheri et al. 1998). Inbred populations may have fitness restored by immigration of unrelated individuals (Wang et al. 1999; Whitlock et al. 2000), a phenomenon termed ‘genetic rescue’ (Tallmon et al. 2004). Support for genetic rescue comes from experiments in which fitness was increased following translocation of outbred individuals into small, declining wild populations with low fitness (Westemeier et al. 1998; Madsen et al. 1999, 2004; Hogg et al. 2006). Populations with a history of small size may have a high fixed, or nearly fixed, load of deleterious alleles, and the detrimental effect of additional inbreeding may be limited (Hedrick 1994; Hedrick & Kalinowski 2000). Small populations isolated from one another, however, are expected to become fixed for deleterious alleles at different loci. In this case, crosses between inbred populations may produce offspring with increased fitness, resulting in genetic rescue. Whereas the effects of inbreeding in small populations may be a cause for concern among conservation managers, the prospect of fitness restoration and reduced extinction risk resulting from renewed gene flow may offer new conservation opportunities.
Mexican wolf (Canis lupus baileyi), an endangered subspecies of grey wolf, is the most genetically distinct subspecies in North America (Leonard et al. 2005). Human activities throughout its range reduced and isolated Mexican wolf populations such that by 1925 they were rare in the United States (Brown 1983), and by the 1950s their range and numbers in Mexico were greatly reduced (Leopold 1959). By 1980, fewer than 50 wild Mexican wolves were thought to remain in isolated groups spread across four Mexican states (McBride 1980). Surveys in Mexico since then have failed to detect Mexican wolves.
All Mexican wolves alive today originated from three captive lineages founded between 1961 and 1980 by a total of seven wolves (Hedrick et al. 1997). These lineages were managed independently until 1995 when the Aragón and Ghost Ranch lineages were merged into the McBride lineage (Hedrick et al. 1997). By this time, each lineage had accumulated substantial levels of inbreeding (see the electronic supplementary material, figure S1) and the heterozygosity at microsatellite markers was about one half of that observed in northern grey wolves (Wayne & Vila 2003).
Pairings between lineages began in 1995 with the first F1 pups (those resulting from pairings between lineages) being born in 1997 (figure S1). Since then, F1 wolves have been bred among themselves, backcrossed to McBride wolves, and bred with cross-lineage wolves (wolves with ancestry from two or more lineages other than F1 wolves). The initial goal was for the merged population to have 10% of its ancestry from each of the Aragón and Ghost Ranch lineages. Upon review of the fitness effects of the merger, ancestry from these lineages could be increased to a maximum of 25% each. At the end of 2005, the captive population numbered about 300 wolves, held in 48 facilities throughout the USA and Mexico. Releases of captive-bred Mexican wolves to re-establish a wild population began in Arizona in 1998 (Interagency Field Team 2005). Initial releases were from the McBride lineage and releases of cross-lineage wolves began in 2000. At the end of 2006, there were nine known packs of wild Mexican wolves in Arizona and New Mexico (Hedrick & Fredrickson in press).
Here, we used data collected over 44 years from the Mexican wolf captive breeding program and from the first 9 years (1998–2006) of the reintroduction program to look for evidence of inbreeding depression among Mexican wolves as well as genetic rescue from merging the three lineages. We addressed four questions: (i) did captive wolves from the McBride and Ghost Ranch founding lineages show inbreeding depression? Furthermore, did inbred descendants of crosses between lineages show inbreeding depression? (ii) Did crosses between captive wolves of different lineages produce wolves with increased fitness? (iii) Did Mexican wolves in the reintroduced population show inbreeding depression? (iv) Did cross-lineage wolves have greater fitness than McBride lineage wolves in the reintroduced population?
2. Material and methods
For the captive population, we investigated the effects of demographic and inbreeding variables on three fitness traits: the probability of live birth, litter size and pup survival to 180 days. We determined values of fitness traits and demographic covariates from the Mexican wolf studbook (Siminski 2005). Demographic covariates and inbreeding models considered in the analyses of each trait are listed in the electronic supplementary material, table S1.
For the reintroduced population, ‘litter size’ was the maximum number of pups observed with a pair from April through November. For two litters, the number of pups was determined from post-mortem examination of the mother. Only pairs that were free-ranging during the breeding season, and at least the month before, were included. Three females that conceived in the wild were captured and brought into captivity shortly before giving birth, and the numbers of pups in these litters were determined while in captivity. Inbreeding coefficients (f) for captive and wild wolves were estimated from pedigree information. Parentage of wild-born wolves was determined from genetic markers by the US Fish and Wildlife Service (USFWS). All statistical analyses were calculated using SAS v. 9.1.3.
(a) Estimating the effects of inbreeding
We used generalized estimating equations (GEE, Hardin & Hilbe 2003) to estimate the effects of inbreeding on the probability of live birth and litter size in the captive population, and on the litter size of wild pairs. GEE is an extension of generalized linear models for use when data are longitudinal or clustered (Hardin & Hilbe 2003). Logistic and identity link functions were used to model the probability of producing live pups and litter sizes, respectively. We used PROC GENMOD to calculate GEE regressions.
To estimate the effects of inbreeding on survival of captive wolves to 180 days, we used Cox's proportional hazards models with standard errors adjusted for non-independent failure times within litters using the method of Lee et al. (1992). We implemented Cox regressions using SAS PROC PHREG. For additional information on estimation, see the electronic supplementary material. To model the effects of inbreeding on fitness, we used a multi-model approach in an information–theoretic context (Burnham & Anderson 2002), in a two-step process (see the electronic supplementary material). We assessed the weight of evidence in support of the selected best model being the actual best model within the set using Akaike weights (w, Burnham & Anderson 2002), which range from 0 to 1, with greater values indicating greater support.
(b) Identifying fitness differences among Mexican wolves
We looked for evidence of genetic rescue by comparing values of fitness traits between F1 wolves (all resulting from crosses between McBride and Ghost Ranch or McBride and Aragón) and wolves from the McBride and Ghost Ranch lineages. Genetic rescue occurred if the F1 fitness was greater than that of inbred wolves from the founding lineages. For these comparisons, we used two groups of McBride pairings. ‘Contemporary McBride’ pairings occurred from 1999 to 2003, had the greatest inbreeding levels, and coincided with the cross-lineage pairings in this study (1999–2005). ‘Early McBride’ pairings included those from 1981 to 1993 or a minimally inbred subset. Similarly, Ghost Ranch litters were divided into three groups: litters born to the founding female; litters with intermediate levels of inbreeding; and maximally inbred litters. Similar information was not available for analysis of Aragón wolves.
3. Results
(a) Inbreeding effects on the captive founding lineages
Inbreeding appeared to have weak or no fitness effects on captive wolves from the founding lineages. For McBride wolves, there was some evidence that inbreeding in the sire and dam had small effects on the probability of producing live pups (N=180 pairings, 89 litters). The best model included only two dichotomous demographic variables, but support for this model was weak (w=0.22). The second and third best models included the level of inbreeding in the sire (w=0.19, electronic supplementary material, figure S2a) and the mean of inbreeding levels in the dam and sire (w=0.17), respectively, in addition to the two demographic variables from the best model. The odds ratios for the two models indicated that the odds of failing to produce live pups increased by factors of 1.76 and 2.11, respectively, for an increase in f by 0.1.
To investigate effects of inbreeding on the probability of live birth within Ghost Ranch and Aragón lineage wolves, we used 51 pairings between McBride and Ghost Ranch or McBride and Aragón wolves (MB×GR, MB×AR) and 52 contemporary pairings among McBride lineage wolves (31 total litters produced). The results provided some evidence that inbreeding in sires reduced the probability of live birth, but little evidence of inbreeding effects in the dams. The best model included inbreeding in the sire, but support was weak (w=0.39). This model suggested that the odds of failing to produce live pups were 1.51 and 5.68 times greater among Aragón and Ghost Ranch sires, respectively, than among McBride sires (electronic supplementary material, figure S2b).
Analyses of litter size within Ghost Ranch and McBride lineage wolves provided evidence for a small effect of inbreeding on Ghost Ranch litters, but no effect on McBride litters. For Ghost Ranch litters (N=39), the best model (w=0.80) and second best model (w=0.19) included inbreeding effects in the dam. The best model suggested litter size declined by 0.32 pups with an increase of 0.1 in the dam f. For McBride litters, the best model included only demographic variables and had strong support (w=0.91). Finally, there was no evidence of inbreeding effects on survival of Ghost Ranch pups and only slight evidence for inbreeding effects on McBride lineage pups (results not shown).
(b) Fitness effects of outbreeding among captive wolves
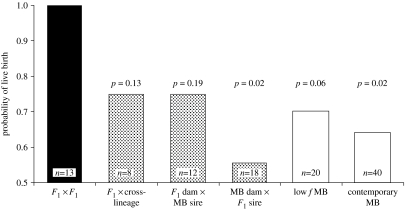
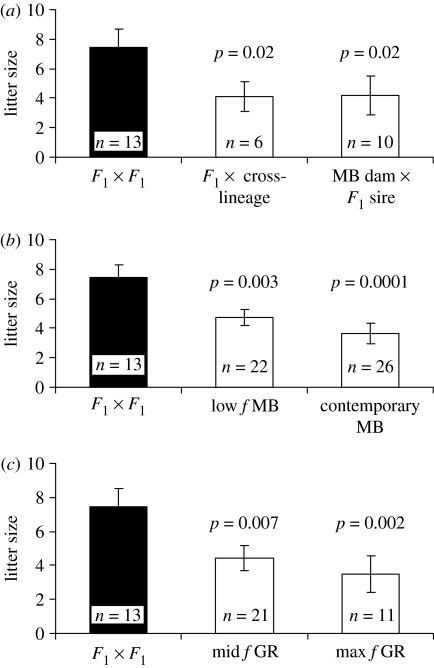
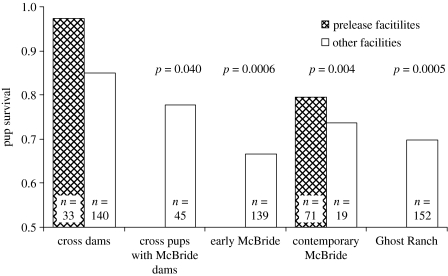
Although inbreeding appeared to have little or no effects on fitness in the founding lineages, F1 wolves showed large fitness increases. The proportion of live births for pairings between F1 wolves was 89% and 33% higher than that observed among contemporary McBride pairings in zoos and prerelease facilities, respectively (figure 1). Litters from F1×F1 pairings averaged more than twice the size of those from contemporary McBride wolves (7.5 versus 3.6, p=0.0001; figure 2b) and maximally inbred Ghost Ranch litters (7.5 versus 3.5, p=0.002; figure 2c). In addition, pups born to cross-lineage dams had 18% and 21% higher survival rates to 180 days than contemporary McBride lineage pups in zoos and prerelease facilities, respectively (p=0.004, figure 3).
Figure 1.
Probability of live birth among F1×F1 pairings (black bar) relative to other pairings by F1 wolves (stippled bars) and McBride (MB) lineage pairings (open bars). The p-values are for pairwise comparisons with F1×F1 pairings.
Figure 2.
Litter sizes of F1×F1 pairings (black bars) contrasted with (a) pairings between F1 and cross-lineage wolves (fixed effects, p=0.016), (b) early McBride (MB) lineage pairings with low inbreeding and contemporary McBride pairings (fixed effects, p=0.0002), and (c) Ghost Ranch (GR) pairings with intermediate and maximal inbreeding levels (fixed effects p=0.003). The p-values refer to pairwise contrasts with F1×F1 pairings. Error bars show standard errors.
Figure 3.
Survival of pups born to cross-lineage dams relative to cross-lineage pups born to McBride lineage dams, McBride lineage pairs and Ghost Ranch lineage pairs in prerelease facilities (hatched bars) and other facilities (open bars). The p-values are for comparisons with cross-lineage pups born to cross-lineage dams.
Fitness among F1 wolves was also higher than wolves early in the McBride lineage with low levels of inbreeding. Pairings between F1 wolves were more likely to produce live pups than pairings among McBride wolves with little inbreeding (1.0 versus 0.7, p=0.06; figure 1). The F1×F1 and McBride pairings in this comparison were closely matched in inbreeding levels (mean fmidparents=0.0 and 0.016, respectively). Litters from pairings between F1 wolves (mean f=0.0 dams, 0.057 pups), were also larger than those from early McBride lineage dams (7.5 versus 4.7, p=0.003; figure 2b) producing litters with low inbreeding (mean f=0.074 dams, 0.156 pups), and pups born to cross-lineage dams had greater survival than pups early in the McBride lineage (figure 3).
Overall, however, F1 and cross-lineage wolves showed a range of fitness levels for the three traits examined (figures 1–3). Pairings between McBride lineage dams and F1 sires were least likely to produce pups and produced nearly the smallest litters, and cross-lineage pups born to McBride dams had the lowest survival among cross-lineage wolves. These differences were significant when compared with F1×F1 pairs for birth probability (1.0 versus 0.56, one-tailed p=0.02) and litter size (7.4 versus 4.2 pups, one-tailed p=0.02). Cross-lineage pups born to McBride dams also had lower survival than pups born to cross-lineage dams (pups born in zoos 0.85 versus 0.78, one-tailed p=0.04). In fact, the performance of McBride dams in cross-lineage pairs showed no (birth probability) or only small improvement (litter size and pup survival) over contemporary McBride pairings (figures 1–3).
Surprisingly, pairings between F1 and cross-lineage wolves (F1×cross) produced the smallest litters (4.1 pups) among cross-lineage wolves (figure 2a), even though they had a relatively high probability of producing live pups (0.75, figure 1). By contrast, pairs with F1 dams and McBride sires averaged 5.7 pups per litter. This difference in litter size may have resulted from higher dam inbreeding in the former relative to the latter (mean fdam=0.059 versus 0.0); litter inbreeding was similar between the two pairing types (mean fpup=0.149 versus 0.142).
(c) Inbreeding effects among captive cross-lineage wolves
In contrast to wolves from the founding lineages, inbreeding had strong effects on the fitness of cross-lineage wolves. For the probability of producing live pups (N=54 pairings, 39 litters), the best two models (summed w=0.82) both indicated that inbreeding in the sire and dam reduced mating success. For parents with no inbreeding, the best model estimated the probability of live birth as 0.96, but for parents with mean inbreeding of 0.1 and 0.2, the probability of live birth dropped to 0.68 and 0.18, respectively (figure S2c). Odds ratios from this model indicated that the odds of failing to produce live pups increased 9.9 times with f=0.1 in the parents and 98.5 times with f=0.2.
There was also evidence of strong inbreeding depression in litter size among pairs including cross-lineage wolves (N=39 litters). The best model (w=0.99) indicated that inbreeding in the dam and pups affected litter size, and that litter size declined by 2.8 pups with an increase of 0.1 in f of the dam and pups.
For pups born to cross-lineage pairs, there was some evidence that inbreeding in the dam increased mortality, but there was no evidence that inbreeding in the pups affected their survival. For dams with f=0.1, the best model suggested that pup survival declined 12.6% and 3.8% for pups in zoos and prerelease facilities, respectively, relative to dams with no inbreeding, but this model had weak support (w=0.20).
(d) Inbreeding and genetic rescue among wild wolves
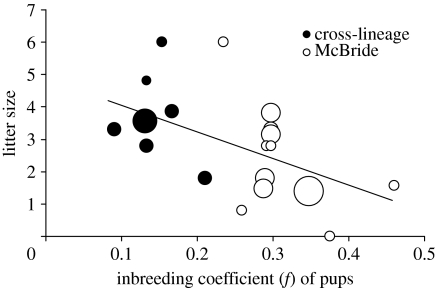
Inbreeding also had strong effects on observed litter sizes in the reintroduced population. The best model describing litter sizes (N=39 litters) among wild pairs included only inbreeding in the pups (figure 4). This model had strong support (w>0.99) and the regression coefficient (fpupβ=−8.23, 95% confidence interval (CI): −12.20, −4.26) was highly significant (p<0.0001). Two variables related to wolf monitoring efforts failed to substantially improve model fit relative to the constant only model (electronic supplementary material, table S1).
Figure 4.
Numbers of pups observed with wild wolf pairs and estimated regression function. For pairs with more than one litter, circles show mean litter sizes. Circle size reflects sample size (1–5), with a total sample size of 39 litters.
4. Discussion
In captive wolves of the McBride and Ghost Ranch lineages, inbreeding appeared to have weak or no effects on fitness. However, crosses between lineages produced wolves with greatly increased fitness, indicating genetic rescue. Subsequent inbreeding in cross-lineage wolves resulted in reduced fitness, revealing a high genetic load, the accumulation of deleterious or lethal alleles, not apparent in the analyses of the founding lineages. In the wild population, 52% more pups were observed among packs producing cross-lineage pups than those producing pure McBride lineage pups, underscoring the low fitness of wolves with only McBride ancestry and the restored fitness among cross-lineage wolves. Our study appears to be the first to explore the fitness effects of crosses between inbred but unrelated lineages in a wild vertebrate.
The range of inbreeding effects we observed was wide, affecting the fitness of sires, dams and pups. The apparently low mating success of inbred males probably resulted in part from reduced fertility. Semen samples from Mexican and generic grey wolves suggested that inbred Mexican wolves had reduced sperm quality (morphology and motility), and that some males may have been functionally infertile (Asa et al. in press). This is consistent with other studies that have found inbreeding or heterozygosity effects on ejaculate quality (Roldan et al. 1998; Gage et al. 2006). Our finding that inbreeding levels in the dam and pups affected litter size is consistent with observations from mice (Lacy et al. 1996) and foxes (Nordrum 1994) where maternal inbreeding was found to increase loss of ova or embryos before implantation, and pup inbreeding increased post-implantation mortality and mortality in the first 3 days after birth. In contrast to the strong negative effects of pup inbreeding on prenatal survival among cross-lineage wolves, there was no evidence of an effect of litter inbreeding on pup survival, perhaps due to the relatively benign conditions in captivity (Jimenez et al. 1994).
(a) Lethal equivalents
Among captive cross-lineage wolves, McBride ancestry accounted for all inbreeding in dams and sires and 94.4% of the total inbreeding in pups. McBride ancestry also accounted for all inbreeding in wild litters. Consequently, we calculated lethal equivalents (Morton et al. 1956) and litter-reducing equivalents (Liberg et al. 2005) using GEE for McBride ancestry only. For the probability of live birth, we estimated 5.64 (95% CI: 0.83–10.43) and 3.65 (95% CI: 0.19–7.12) lethal equivalents for captive dams and sires, respectively, in the McBride lineage, based on 54 pairings. In this context, a lethal equivalent is the cumulative effects of deleterious alleles sufficient to prevent a dam from producing live pups or a sire from successfully mating.
To estimate the genetic load associated with litter size among captive McBride lineage wolves, we used 23 litters produced from dams with f=0 and 10 litters produced by McBride dams paired with F1 sires (N=33 litters). The number of litter-reducing equivalents estimated from cross-lineage pups (6.89, 95% CI: 3.64–10.15) was nearly twice that estimated from McBride dams (3.52, 95% CI: 0.54–6.51). An analysis using only the 23 litters produced by F1 dams provided a similar estimate for pups (7.16, 95% CI: 4.37–9.94). This suggests that about two-thirds of the litter-reducing equivalents in the McBride lineage acted to reduce prenatal pup survival, and the remainder acted to reduce fertility in dams.
For the wild population, we estimated 5.19 (95% CI: 1.95–8.44) litter-reducing equivalents among pups. Because most breeding wolves and the litters produced had only McBride ancestry, estimates of litter-reducing equivalents may increase in the future as additional cross-lineage litters with inbreeding from their McBride ancestry are born into the wild population.
The numbers of lethal equivalents estimated for captive Mexican wolf pairs (dams+sires) and litter-reducing equivalents estimated for captive litters (dams+pups) were greater than the numbers of lethal equivalents estimated by Ralls et al. (1988) for juvenile survival in 35 out of 40 captive mammal populations. Litter-reducing equivalents among captive and wild pups only, were greater than that of 33 and 30, respectively, out of 40 populations examined, estimated by Ralls et al. (1988), but similar to those estimated for wild Scandinavian wolf pups (6.04, Liberg et al. 2005). Liberg et al. (2005) also noted that inbreeding in the dam and pups reduced winter litter sizes among Scandinavian wolves.
(b) Causes of inbreeding depression and heterosis in Mexican wolves
The Mexican wolf lineages may have been primed for strong heterosis by the combination of small effective sizes (Fredrickson 2007), isolation and rapid inbreeding in captivity resulting in divergence between lineages and the fixation of large numbers of moderately deleterious alleles within lineages (Wang et al. 1999; Whitlock et al. 2000). Before being merged, the Aragón and Ghost Ranch lineages were fixed at 45% of microsatellite loci surveyed, each lineage had substantial numbers of unique alleles, and levels of genetic differentiation between the three lineages were comparable to that seen between different populations of northern grey wolves (Hedrick et al. 1997). The presence of large heterotic effects among F1 wolves suggested that many loci in the founding lineages were differentially fixed or nearly fixed for deleterious alleles (Whitlock et al. 2000), and that the increased fitness among F1 offspring relative to contemporary inbred individuals resulted from the masking of deleterious alleles in heterozygotes (Wang et al. 1999). The weak inbreeding depression observed in the McBride and Ghost Ranch lineages coupled with the strong inbreeding depression within cross-lineage wolves is also consistent with a high fixed load within each of the founding lineages. Theory further predicts that the numbers of lethal equivalents will be elevated in the F1 offspring, providing the potential for strong inbreeding depression with renewed inbreeding (Wang et al. 1999).
In addition to the fitness increases among F1 wolves relative to inbred wolves from the McBride and Ghost Ranch lineages, F1 wolves showed greater fitness than earlier outbred Mexican wolves. Litter sizes among F1×F1 pairings (mean=7.45 pups) exceeded those from the McBride and Ghost Ranch founding females (mean=3.40 and 6.71, respectively), pairings between outbred offspring of the McBride founding female (5.12 pups), and a sample of unborn wild litters from Mexico in the 1970s (mean=6.75 pups, electronic supplementary material, table S2). In addition, survival of pups born to cross-lineage dams greatly exceeded survival of pups born early in the McBride and Ghost Ranch lineages (figure 3), but the greater survival of contemporary McBride versus early McBride pups suggested that some of the observed survival increases may have resulted from improvements in animal husbandry over time. Although we have focused on genetic rescue in Mexican wolves resulting from the masking of deleterious alleles, it is hoped that the merging of the three founding lineages also restored neutral genetic variation and retained adaptive variation, thereby allowing selection to act on the full range of genetic variation in future generations, a phenomenon termed ‘genetic restoration’ (Hedrick 2005).
(c) Reintroduction prospects for Mexican wolves
Thus far, the wild population has produced smaller pack and litter sizes than other grey wolf populations in North America, despite abundant prey in the reintroduction area (Interagency Field Team 2005). Our results suggest that this may result largely from the high fixed genetic load in McBride lineage wolves. By the end of 2006, relatively few cross-lineage wolves had been introduced, and one half of the alpha wolves had only McBride ancestry. The heterotic effects we observed suggested that cross-lineage wolves have the potential to increase the population growth rate and initiate a high effective migration rate of Ghost Ranch and Aragón ancestry into the wild population (Ingvarsson & Whitlock 2000; Saccheri & Brakefield 2002; Vila et al. 2003). This, however, has not occurred largely due to high rates of human-caused mortality and removals for management reasons (USFWS 2005). Therefore, it currently appears that there is the biological potential in Mexican wolves to establish vigorous wild populations if conflicts with humans can be resolved.
Acknowledgments
We appreciate data and cooperation from the USFWS, SEMARNAT, the Mexican Wolf Species Survival Plan, the Subcomite Tecnico Consultivo Nacional Para la Recuperacion del Lobo Mexicano, and their member institutions. P.W.H. thanks the Ullman Professorship for their partial support during this research.
Supplementary Material
Additional information on the analysis methods in the text and in Table S1. Table S2 and the figures present additional information regarding the results and the history of inbreeding in Mexican wolves
References
- Asa, C., Miller, P., Agnew, M., Rivera, A. R., Lindsey, S. L., Callahan, M. & Bauman, K. In press. Relationship of inbreeding to sperm quality and reproductive success in Mexican Gray wolves (Canis lupus baileyi). Anim. Conserv.
- Brown D.E. University of Arizona Press; Tucson, AZ: 1983. The wolf in the southwest: the making of an endangered species. [Google Scholar]
- Burnham K.P, Anderson D.R. 2nd edn. Springer Science; New York, NY: 2002. Model selection and multimodel inference, a practical information–theoretic approach. [Google Scholar]
- Fredrickson, R. F. 2007 Effects of inbreeding and outbreeding in Mexican and red wolves. PhD dissertation, Arizona State University, Tempe.
- Gage M.J.G, Surridge A.K, Tomkins J.L, Green E, Wiskin L, Bell D.J, Hewitt G.M. Reduced heterozygosity depresses sperm quality in wild rabbits Oryctolagus cuniculus. Curr. Biol. 2006;16:612–617. doi: 10.1016/j.cub.2006.02.059. doi:10.1016/j.cub.2006.02.059 [DOI] [PubMed] [Google Scholar]
- Hardin J.W, Hilbe J.M. Chapman & Hall/CRC; New York, NY: 2003. Generalized estimating equations. [Google Scholar]
- Hedrick P.W. Purging inbreeding depression and the probability of extinction: full-sib mating. Heredity. 1994;73:363–372. doi: 10.1038/hdy.1994.183. [DOI] [PubMed] [Google Scholar]
- Hedrick P.W. ‘Genetic restoration:’ a more comprehensive perspective than ‘genetic rescue’. Trends Ecol. Evol. 2005;20:109. doi: 10.1016/j.tree.2005.01.006. doi:10.1016/j.tree.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Hedrick, P. W. & Fredrickson, R. J. In press. Captive breeding and the reintroduction of Mexican and red wolves. Mol. Ecol. [DOI] [PubMed]
- Hedrick P.W, Kalinowski S.T. Inbreeding depression in conservation biology. Ann. Rev. Ecol. Syst. 2000;31:139–162. doi:10.1146/annurev.ecolsys.31.1.139 [Google Scholar]
- Hedrick P.W, Miller P.S, Geffen E, Wayne R. Genetic evaluation of the three captive Mexican wolf lineages. Zool. Biol. 1997;16:47–69. doi:10.1002/(SICI)1098-2361(1997)16:1<47::AID-ZOO7>3.0.CO;2-B [Google Scholar]
- Hogg J.T, Forbes S.H, Steele B.M, Luikart G. Genetic rescue of an insular population of large mammals. Proc. R. Soc. B. 2006;273:1491–1499. doi: 10.1098/rspb.2006.3477. doi:10.1098/rspb.2006.3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvarsson P.K, Whitlock M.C. Heterosis increases the effective migration rate. Proc. R. Soc. B. 2000;267:1321–1326. doi: 10.1098/rspb.2000.1145. doi:10.1098/rspb.2000.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interagency Field Team 2005 Mexican wolf Blue Range reintroduction project 5 year review: technical component. See http://www.azgfd.gov/w_c/es/wolf_reintroduction.shtml
- Jimenez J.A, Hughes K.A, Alaks G, Graham L, Lacy R.C. An experimental study of inbreeding depression in a natural habitat. Science. 1994;266:271–273. doi: 10.1126/science.7939661. doi:10.1126/science.7939661 [DOI] [PubMed] [Google Scholar]
- Keller L.F, Waller D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–241. doi:10.1016/S0169-5347(02)02489-8 [Google Scholar]
- Lacy R.C, Alaks G, Walsh A. Hierarchical analysis of inbreeding depression in Peromyscus polionotus. Evolution. 1996;50:2187–2200. doi: 10.1111/j.1558-5646.1996.tb03609.x. doi:10.2307/2410690 [DOI] [PubMed] [Google Scholar]
- Lee E.W, Wei L.J, Amato D.A. Cox-type regression analyses for large numbers of small groups of correlated failure time observations. In: Klein J.P, Goel P.K, editors. Survival analysis: state of the art. Kluwer Academic Publishers; Amsterdam, The Netherlands: 1992. pp. 237–247. [Google Scholar]
- Leonard J.A, Vilà C, Wayn R.K. Legacy lost: genetic variability and population size of extirpated US grey wolves (Canis lupus) Molec. Ecol. 2005;14:9–17. doi: 10.1111/j.1365-294X.2004.02389.x. doi:10.1111/j.1365-294X.2004.02389.x [DOI] [PubMed] [Google Scholar]
- Leopold A.S. University of California Press; Los Angeles, CA: 1959. Wildlife of Mexico. [Google Scholar]
- Liberg O, Andren H, Pedersen H, Sand H, Sejberg D, Wabakken P, Akesson M, Bensch S. Severe inbreeding depression in a wild wolf (Canis lupus) population. Biol. Lett. 2005;1:17–20. doi: 10.1098/rsbl.2004.0266. doi:10.1098/rsbl.2004.0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen T, Shine R, Olsson M, Wittzell H. Restoration of an inbred adder population. Nature. 1999;402:34–35. doi:10.1038/46941 [Google Scholar]
- Madsen T, Ujvari B, Olsson M. Novel genes continue to enhance population growth in adders (Vipera berus) Biol. Conserv. 2004;120:145–147. doi:10.1016/j.biocon.2004.01.022 [Google Scholar]
- McBride, R. T. 1980 The Mexican wolf (Canis lupus baileyi): a historical review and observations on its status and distribution. Endangered species report no. 8, pp. 38, U.S. Fish and Wildlife Service, Albuquerque, New Mexico.
- Morton N.E, Crow J.F, Muller H.J. An estimate of the mutational damage in man from data on consanguineous marriages. Proc. Natl Acad. Sci. USA. 1956;42:855–863. doi: 10.1073/pnas.42.11.855. doi:10.1073/pnas.42.11.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D, Pilson D. Increased probability of extinction due to decreased genetic effective population size: experimental populations of Clarkia pulchella. Evolution. 1997;51:354–362. doi: 10.1111/j.1558-5646.1997.tb02422.x. doi:10.2307/2411107 [DOI] [PubMed] [Google Scholar]
- Nordrum N.M.V. Effect of inbreeding on reproductive performance in Blue fox (Alopex lagopus) vixens. Acta Agric. Scand. A: Anim. Sci. 1994;44:214–221. [Google Scholar]
- Ralls K, Ballou J.D, Templeton A. Estimates of lethal equivalents and the cost of inbreeding in mammals. Conserv. Biol. 1988;2:185–193. doi:10.1111/j.1523-1739.1988.tb00169.x [Google Scholar]
- Roldan E.R.S, Cassinello J, Abaigar T, Gomendio M. Inbreeding, fluctuating asymmetry, and ejaculate quality in an endangered ungulate. Proc. R. Soc. B. 1998;265:243–248. doi: 10.1098/rspb.1998.0288. doi:10.1098/rspb.1998.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccheri I.J, Brakefield P.M. Rapid spread of immigrant genomes into inbred populations. Proc. R. Soc. B. 2002;269:1073–1078. doi: 10.1098/rspb.2002.1963. doi:10.1098/rspb.2002.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccheri I.J, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I. Inbreeding and extinction in a butterfly metapopulation. Nature. 1998;392:491–494. doi:10.1038/33136 [Google Scholar]
- Siminski D.P. The Living Desert; Palm Desert, CA: 2005. Mexican wolf, Canis lupus baileyi, international studbook. [Google Scholar]
- Tallmon D.A, Luikart G, Waples R. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 2004;19:489–496. doi: 10.1016/j.tree.2004.07.003. doi:10.1016/j.tree.2004.07.003 [DOI] [PubMed] [Google Scholar]
- United States Fish and Wildlife Service (USFWS) 2005 Mexican wolf recovery program: progress report no. 8. See http://www.fws.gov/ifw2es/mexicanwolf/documents.shtml
- Vila C, et al. Rescue of a severely bottlenecked wolf (Canis lupus) population by a single immigrant. Proc. R. Soc. B. 2003;270:91–97. doi: 10.1098/rspb.2002.2184. doi:10.1098/rspb.2002.2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hill W.G, Charlesworth D, Charlesworth B. Dynamics of inbreeding depression due to deleterious mutations in small populations: mutation parameters and inbreeding rate. Genet. Res. 1999;74:165–178. doi: 10.1017/s0016672399003900. doi:10.1017/S0016672399003900 [DOI] [PubMed] [Google Scholar]
- Wayne R.K, Vila C. Molecular genetic studies of wolves. In: Mech L.D, Boitani L, editors. Wolves behavior, ecology and conservation. University of Chicago Press; Chicago, IL: 2003. pp. 218–238. [Google Scholar]
- Westemeier R.L, Brawn L.D, Simpson S.A, Esker T.L, Jansen R.W, Walk J.W, Kershner E.L, Bouzat J.L, Paige K.N. Tracking the long-term decline and recovery of an isolated population. Science. 1998;282:1695–1698. doi: 10.1126/science.282.5394.1695. doi:10.1126/science.282.5394.1695 [DOI] [PubMed] [Google Scholar]
- Whitlock M.C, Ingvarsson P.K, Hatfield T. Local drift load and the heterosis of interconnected populations. Heredity. 2000;8:452–457. doi: 10.1046/j.1365-2540.2000.00693.x. doi:10.1046/j.1365-2540.2000.00693.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information on the analysis methods in the text and in Table S1. Table S2 and the figures present additional information regarding the results and the history of inbreeding in Mexican wolves