Abstract
Ataxia-telangiectasia (AT) is a human disease caused by mutations in the ATM gene. The neural phenotype of AT includes progressive cerebellar neurodegeneration, which results in ataxia and eventual motor dysfunction. Surprisingly, mice in which the Atm gene has been inactivated lack distinct behavioral ataxia or pronounced cerebellar degeneration, the hallmarks of the human disease. To determine whether lack of the Atm protein can nonetheless lead to structural abnormalities in the brain, we compared brains from male Atm-deficient mice with male, age-matched controls. Atm-deficient mice exhibited severe degeneration of tyrosine hydroxylase-positive, dopaminergic nigro-striatal neurons, and their terminals in the striatum. This cell loss was accompanied by a large reduction in immunoreactivity for the dopamine transporter in the striatum. A reduction in dopaminergic neurons also was evident in the ventral tegmental area. This effect was selective in that the noradrenergic nucleus locus coeruleus was normal in these mice. Behaviorally, Atm-deficient mice expressed locomotor abnormalities manifested as stride-length asymmetry, which could be corrected by peripheral application of the dopaminergic precursor l-dopa. In addition, these mice were hypersensitive to the dopamine releasing drug d-amphetamine. These results indicate that ATM deficiency can severely affect dopaminergic neurons in the central nervous system and suggest possible strategies for treating this aspect of the disease.
Mutations in the ATM gene recently have been shown to underlie the human disease ataxia-telangiectasia (AT) (1, 2). AT is a pleiotropic disease (3) whose neurological symptoms include progressive degeneration of neural tissue, resulting in atrophy of the cerebellum and other brain regions, development of cerebellar ataxia, and eventual general neuromotor dysfunction. The ATM protein is a member of the phosphatidylinositol-3 (PI3) kinase-related protein kinase superfamily and has been proposed to be a key player in cellular processes that ensure genome integrity (3, 4). Mice lacking Atm and which recapitulate most of the symptoms of the disease have been produced by several research groups (5–7). Interestingly, ataxia seems to be mild in Atm-deficient mice in comparison to AT patients (5), although electron-microscopic studies have revealed degeneration of neurons in the cerebellar cortex of these mice (8). Here we report that Atm-deficient mice express a marked reduction in the dopaminergic nigro-striatal pathway, a defect that may contribute to the motor deficits seen in AT patients.
MATERIALS AND METHODS
Molecular Biology.
Atm-deficient mice, produced by targeted disruption of the Atm gene, have been previously described (6). Routine genotyping was performed on tail biopsy DNA, either by DNA blot analysis (6) or by PCR. These studies used male Atm-deficient and control, wild-type mice that were matched for age and genetic background.
Immunohistochemistry.
Adult (4–5 months old) male mice were anesthetized and perfused with 2.5% paraformaldehyde, and the brain was removed and stored for 5–10 days in 1% paraformaldehyde. For counting tyrosine hydroxylase (TH)-stained somata, serial 15-μm-thick coronal sections were cut from the anterior hypothalamic area down to the brainstem on a freezing microtome and subjected to immunohistochemical analysis using the avidin-biotin peroxidase technique (ABC, Vectastain, as described in ref. 14). For staining of TH-positive terminals or the dopamine transporter in the striatum, serial 40-μm-thick sections (from the rostral part of the above brains) were cut with a vibrotome. Monocolonal mouse anti-TH antibodies diluted 1:2,000 (Incstar, Stillwater, MN), or rat antidopamine transporter diluted 1:5,000 (Chemicon) were used. To minimize possible errors in intensity of immunostaining, sections of pairs of Atm-deficient and control, wild-type brains were processed in parallel. Cells were drawn on low-power images of the brain sections and were counted by an independent observer. Although the borders of areas A8, A9, and A10 are somewhat arbitrary, nerve cell counts were obtained in each area separately based on the distribution atlas of TH cells by Hokfelt et al. (15). The Abercrombie correction was applied (16) for the accurate counting of TH-positive somata in the region of the substantia nigra (SN). Brain sections at the level of the basal ganglia were imaged, and the staining intensity was analyzed by using the NIH image software package. Statistical analysis was performed by using Student’s unpaired t tests.
Behavior.
Mice (male, 3 months old) were placed individually in a 1-m2 open field surrounded by a wall, and their locomotor behavior in the open field was monitored for 4 min and scored, as described elsewhere (17). Mice then were injected i.p. with 5 mg/kg (one experiment) or 10 mg/kg (one experiment) of d-amphetamine sulfate and returned to the open field 15 min later for further observations. Walking patterns were analyzed by using the inked foot pad test: hind legs were dyed with ink, and the mouse was allowed to walk on filter paper. Footprints were used to estimate the stride length, and the difference between stride length of the two hind legs was measured to estimate stride-length asymmetry. The same mice then were injected with l-dopa (50 mg/kg, i.p.), and foot prints were measured 10–30 min later.
RESULTS AND DISCUSSION
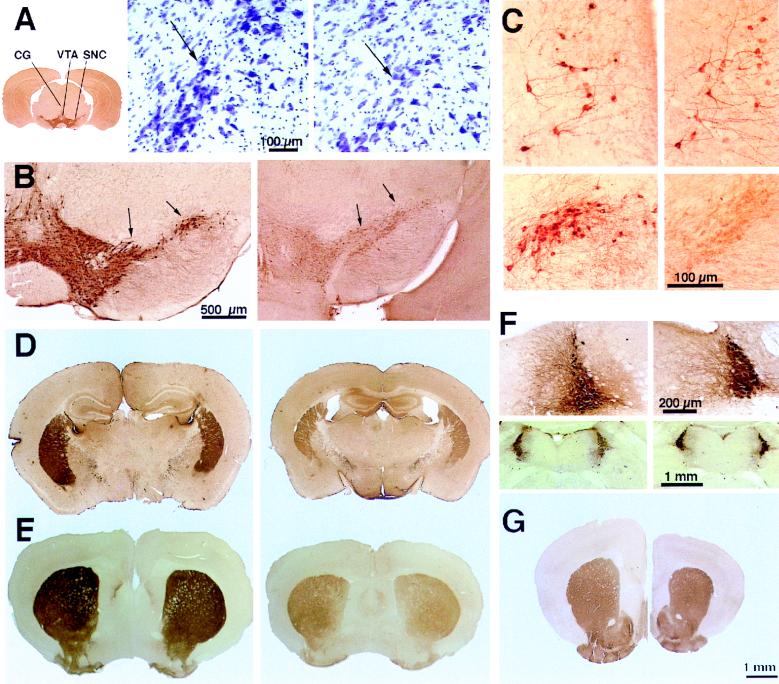
Brains of Atm-deficient 4-month-old mice are about 20% smaller than those of control littermates (Fig. 1 D, E, and G), in agreement with the smaller overall body dimensions of these mice (6). Reduction in brain size is not restricted to the cerebellum, as the cerebral cortex is similarly reduced in size. Thin brain sections of Atm-deficient or wild-type control mice were immunostained for TH, the enzyme that is responsible for synthesis of catecholamines. TH primarily is found in somata of the dopaminergic nuclei SN pars compacta (SNc, area A9 of Hokfelt et al., ref. 15), the mesolimbic area, and the noradrenergic nucleus locus coeruleus. A marked decrease of up to 75% in the number of TH-positive neurons was found in the SNc (Fig. 1B and Table 1). A similar reduction in cell density also was seen in cresyl violet-stained sections taken from the same region, indicating that the reduction in density of TH-positive cells represents a genuine reduction in cell number and not merely a loss of enzyme expression in affected neurons (Fig. 1A). A significant 66% reduction in TH staining also was seen in the dopaminergic cell group of the ventral tegmental area (A10 of Hokfelt et al., ref. 14) in four Atm-deficient mice compared with controls (Table 1). Significantly, a reduction in TH staining was not seen in the noradrenergic TH-positive nucleus locus coeruleus (Fig. 1F), which is the main source of noradrenergic innervation of the entire forebrain. Also, no reduction was seen in TH staining in scattered cells in the central gray matter (Fig. 1C), attesting to the specificity of the observed change.
Figure 1.
Selective depletion of TH immunoreactivity in the pars compacta of the SN of Atm-deficient mice and a corresponding loss of TH-positive terminals in the striatum. (A) (Left) Low-power (×2.5) view of a coronal section through the lower diencephalon illustrating the region of the SN, ventral tegmental area (VTA), and the central gray (CG) where TH-positive cells were found. (Right) Cresyl violet-staining of the region of the SN, illustrating cell loss in the Atm-deficient brain (Left, control, Right, Atm-deficient, arrows point to SN pars compacta cells). (B) TH immunostaining of the region of the SN demonstrating a marked reduction in cell and fiber staining in the region of pars compacta (arrows). (C) High-power (×120) images of cells in the central gray (Upper) and SN (Lower) in control (Left) and Atm-deficient (Right) mice. Note the normal appearance of cells in the central gray and the lack of cell staining in the SN. (D) Low-power (×7) images of the striatum, stained for TH in normal (Left) and Atm-deficient (Right) mice. Note the difference in size of the two age-matched brains. (E) Low-power (×7) view of coronal sections through the striatum, stained for the dopamine transporter in control (Left) and Atm-deficient (Right) mice. A clear reduction in staining for the DAT is seen in the Atm-deficient mouse. (F) Staining of the nucleus locus coeruleus for TH at high power (×35) (Upper) and low power (×9) (Lower), illustrating that the noradrenergic nucleus is not affected in the Atm-deficient mouse. (G) The striatum of control (Left) and Atm-deficient (Right) mice, stained for acetylcholine esterase, demonstrating a lack of difference, despite the smaller size of the Atm-deficient brain.
Table 1.
Number of TH-positive cells in the brainstem in control and Atm-deficient mice
| Area | Control, n = 3 | Atm-deficient, n = 4 |
|---|---|---|
| A9 | 5,964 ± 611 | 1,550 ± 289** |
| A91 | 402 ± 69 | 170 ± 58 |
| A10 | 6,670 ± 584 | 2,205 ± 601** |
| A8 | 1,484 ± 23 | 428 ± 280* |
*P < 0.05; **P < 0.01.
The efferents of the SN pars compacta reach the striatum in the nigro-striatal bundle, where they densely innervate the primary neurons of the caudate/putamen nuclei. Quantitative analysis of the TH-positive fiber density in the striatum revealed a significant 49% reduction in staining intensity in Atm-deficient mice (144 ± 10.7 arbitrary OD units in control vs. 76 ± 7.2 in Atm-deficient brains, n = 4 pairs of mice, P < 0.01, Fig. 1D). No significant reduction in TH-positive staining was found in the cerebral cortex, (control 49.9 ± 6.5, Atm-deficient 40.4 ± 6.9 arbitrary OD units), indicating the specificity of the observed defect in the striatum. Reduction in TH immunostaining could not be attributed to indirect effects in the caudate nucleus (e.g., reduction in brain tissue volume), as other cytochemical markers that heavily stain the caudate nucleus did not show significant reduction in staining intensity in Atm-deficient mice. For example, histochemical staining for acetylcholine-esterase was similar in the two groups of mice [control 127 ± 10, Atm-deficient 129 ± 10.8, arbitrary OD units, (Fig. 1G)], indicating that the deficit in the striatum was selective to the dopaminergic innervation.
Reduction in TH immunostaining in the striatum can reflect either a reduction in the number of dopaminergic fibers in the striatum, as implied from the reduction in dopaminergic cell bodies or down-regulation of TH in existing dopaminergic fibers. To examine these alternatives more directly we stained the striatum for a selective marker of dopaminergic terminals, the dopamine transporter (DAT, Fig. 1E). In four Atm-deficient mice striatum, there was a significant (t = 5.56, P < 0.01) reduction in immunostaining for DAT in the ventrolateral aspect of the striatum to 64% of control. Interestingly, in the medial dorsal striatum the reduction in DAT was even larger, to 46% of control, and was highly significant (t = 12.5, P < 0.0001, Fig. 1E).
D1 and D2 dopamine receptors mediate dopaminergic functions in the striatum. By using an antibody selective for the D2 receptor, we estimated the density of D2 receptors known to reside both on nigrostriatal dopaminergic terminals and postsynaptic striatal neurons. We expected to find a reduction in D2 receptor density in the Atm-deficient mice. Instead, a small and statistically insignificant increase of 16–19% in the density of D2 receptors was found in the striatum (data not shown). This increase may reflect a process of denervation supersensitivity seen in striatal neurons under conditions of removed dopaminergic afferents (9, 10). Similarly, a small and statistically nonsignificant increase also was seen in D2 receptor density in the overlying neocortex.
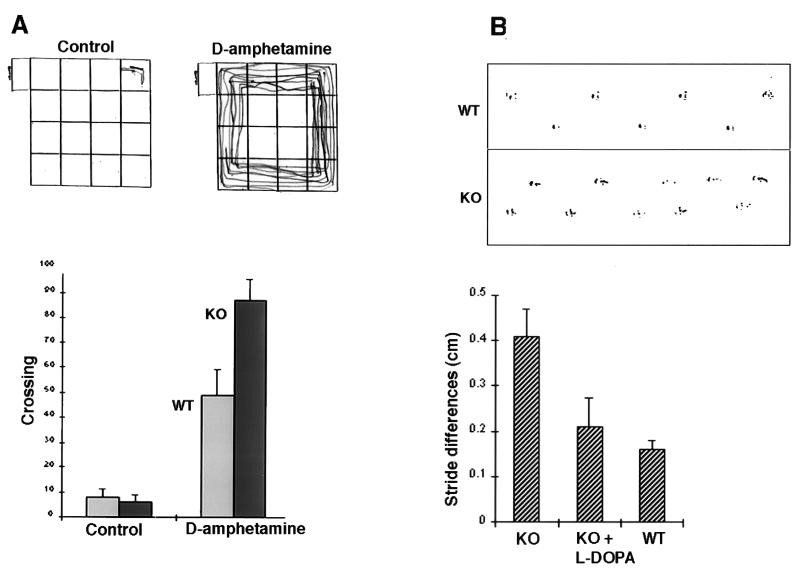
To assess the influence of reduction in nigrostriatal neurons on behavior, Atm-deficient and sex- and age-matched wild-type control mice were examined in an open-field test. Both groups of mice exhibited similar locomotor activity in the open field (Fig. 2A). Significantly, after an injection of the dopaminergic drug amphetamine, locomotion of the Atm-deficient mice increased by 78% above that of amphetamine-treated wild-type mice (n = 6 mice in each group, P < 0.01; the experiment was repeated with different groups of mice and scored by two independent observers). This finding indicates that Atm-deficient mice are supersensitive to amphetamine treatment, as previously reported for rodents suffering from nigrostriatal lesions (10).
Figure 2.
Behavioral phenotype of Atm-deficient mice. (A) The Atm−/− mouse is hyper-reactive to d-amphetamine. (Upper) Illustration of the locomotion of a KO mouse placed in the open field for 3 min under control conditions (Left) and after an injection of d-amphetamine (Right). (Lower) Group data for the locomotor activity of the mice before and after injection of d-amphetamine. The numbers of crossings of lines in the open field were counted for each mouse. Atm-deficient mice reacted to the drug much more than control mice. (B) Locomotor activity is impaired in Atm-deficient mice. (Upper) Illustration of footprints of control (wild type, WT) and Atm-deficient (KO) mice. (Lower) Asymmetry of stride length, measured as the difference between adjacent right and left strides, averaged across four Atm and five control mice. After an i.p. injection of l-dopa, the stride length of the same four Atm mice was as before (58.2-mm mean stride length before, 60.4-mm mean stride length after the drug) but it became more symmetric and not different from that of control mice. The mean stride length of control mice was 68.0 mm, and the asymmetry was small and not significant.
If impaired nigrostriatal function is involved in the behavioral phenotype of the Atm-deficient mouse, a beneficial effect might be expected from facilitation of dopaminergic neurotransmission. Indeed, the dopamine precursor l-dopa has been shown to enhance dopaminergic transmission and consequently is used as a standard treatment in Parkinson’s disease (12). As previously reported (5), Atm-deficient mice exhibit abnormal walking patterns, expressed as an asymmetry in stride length (ref. 5; Fig. 2B). Acute treatment with l-dopa corrected this stride-length asymmetry without affecting mean stride length (Fig. 2B).
Dopamine-containing nigrostriatal neurons undergo severe degeneration in Parkinson’s disease, an effect that is likely to be associated with their sensitivity to oxidative stress (11, 12). It has been suggested that Atm plays a role in cellular defense mechanisms against oxidative stress (13); lack of functional Atm could damage these mechanisms and lead to degeneration of dopaminergic neurons. Indeed, a nigrostriatal dopaminergic abnormality in a AT patient was reported (18), although this structure is not considered to be a major participant in AT disease (19). The fact that Atm-deficient mice do not express the more severe locomotor dysfunction and ataxia seen in humans suffering from AT is not surprising, because rodents depleted of dopamine by other means are almost symptom-less (9) and certainly do not share the major symptoms of the equivalent human disease. Nonetheless, careful examination of locomotor behavior of the mice reveals a deficit in rhythmic activity expressed as a stride-length asymmetry, which can be ameliorated by l-dopa, likely to restore synchronous activity in the nigrostriatal system and its related behaviors. Taken together, these results indicate that Atm deficiency has clear detrimental effects on dopaminergic neurons and that dopaminergic dysfunction may be a major, albeit probably not the only, constituent of the AT phenotype. Atm-deficient mice are therefore a useful model for the study of mechanisms of dopamine cell degeneration in AT and for developing pharmacological strategies for countering the effects of such degeneration.
Acknowledgments
We thank Ms. Keren Ross for the cell counts, Dr. Rafi Malach for the histology facility, Dr. Philip Leder (Harvard Medical School) for Atm-deficient mice, and Dr. Sara Fuchs (The Weizmann Institute) for the D2 antibody. This work was supported in part by a grant from the AT Children’s Project.
ABBREVIATIONS
- AT
ataxia telangiectasia
- TH
tyrosine hydroxylase
- SN
substantia nigra
References
- 1. Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle D A, Smith S, Uziel T, Sfez S, et al. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 2.Savitsky K, Sfez S, Tagle D A, Ziv Y, Sartiel A, Collins F S, Shiloh Y, Rotman G. Hum Mol Genet. 1995;4:2025–2032. doi: 10.1093/hmg/4.11.2025. [DOI] [PubMed] [Google Scholar]
- 3.Lavin M F, Shiloh Y. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 4.Hoekstra M F. Curr Opin Genet Dev. 1997;7:170–175. doi: 10.1016/s0959-437x(97)80125-6. [DOI] [PubMed] [Google Scholar]
- 5.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley J N, Ried T, Tagle D, Wynshaw-B A. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 6.Elson A, Wang Y, Daugherty C J, Morton C C, Zhou F, Campos-Torres J, Leder P. Proc Natl Acad Sci USA. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Ashley T, Brainerd E E, Bronson R T, Meyn M S, Baltimore D. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 8.Kuljis R O, Xu Y, Aguila M C, Baltimore D. Proc Natl Acad Sci USA. 1997;94:12688–12693. doi: 10.1073/pnas.94.23.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokry J. Physiol Res. 1995;44:143–150. [PubMed] [Google Scholar]
- 10.Takahashi N, Miner L L, Sora I, Ujike H, Revay R S, Kostic V, Jackson Lewis V, Przedborski S, Uhl G R. Proc Natl Acad Sci USA. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerlach M, Riederer P, Youdim M B. Adv Neurol. 1996;69:177–194. [PubMed] [Google Scholar]
- 12.Youdim M B, Riederer P. Sci Am. 1997;276:52–59. doi: 10.1038/scientificamerican0197-52. [DOI] [PubMed] [Google Scholar]
- 13.Rotman G, Shiloh Y. BioEssays. 1997;19:911–917. doi: 10.1002/bies.950191011. [DOI] [PubMed] [Google Scholar]
- 14.Eilam R, Pinkas-Kramarski R, Ratzkin B J, Segal M, Yarden Y. Proc Natl Acad Sci USA. 1998;95:1888–1893. doi: 10.1073/pnas.95.4.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hokfelt T, Martensson R, Bjorklund A, Kleinau S, Goldstein M. In: Handbook of Chemical Neuroanatomy. Bjorklund A, Hokfelt T, editors. Vol. 2. Amsterdam: Elsevier; 1984. pp. 277–379. [Google Scholar]
- 16.Abercrombie M. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 17.Gahtan E, Auerbach J M, Groner Y, Segal M. Eur J Neurosci. 1998;10:538–544. doi: 10.1046/j.1460-9568.1998.00058.x. [DOI] [PubMed] [Google Scholar]
- 18.Koepp M, Schelosky L, Cordes I, Cordes M, Poewe W. Movement Disorders. 1994;9:455–459. doi: 10.1002/mds.870090414. [DOI] [PubMed] [Google Scholar]
- 19.Sedgwick R P, Boder E. In: Handbook of Clinical Neurology. Vinken P, Bruyn G, Klawans H, editors. New York: Elsevier; 1991. pp. 347–423. [Google Scholar]