Abstract
Fifteen years ago it was discovered that intramyocellular triglyceride (imcTG) content in skeletal muscle is abnormally high in lipid oversupply models and, later, in obesity, type 2 diabetes (T2D) and other metabolically diseased conditions. The imcTG abnormality was also found to be significantly correlated with muscle insulin resistance (MIR). As skeletal muscle is the main site for insulin-mediated glucose utilization, the research on this topic has been active since. However, to date the pathways responsible for the imcTG excess and the mechanisms underlying the imcTG-MIR correlation have not been identified. The current view is focused on a backward theory that fatty acid oxidation by muscle is impaired causing imcTG to accumulate. Therefore, an enlarged imcTG pool is merely a marker of MIR and thus is considered a non-player in the development and intervention of MIR. However, it is more likely that imcTG is a source of MIR. On one hand, an enlarged and fast turning over imcTG pool interferes with insulin signaling by producing excess amounts of signaling molecules that activate PKC pathways. On the other hand, it may promote mitochondrial β-oxidation that suppresses glucose metabolism via substrate competition. Therefore, it is hypothesized that imcTG is a source and contributor to MIR.
INTRODUCTION
Intramyocellular triglycerides (imcTG) is an indispensable energy source for skeletal muscle and thus plays pivotal roles in substrate metabolism not only for the tissue but also the whole body given its massive representation (40% of body wt in humans). More than 15 years ago, Storlien and associates discovered that imcTG is elevated by high fat feeding and muscle insulin sensitivity is inversely correlated with imcTG content (r=−0.86–0.93) 1. This metabolic abnormality has been repeatedly reported for other models as well such as obesity type 2 diabetes, hypertension and etc 2,3,4,5,6,7. As skeletal muscle is the main site for insulin-mediated glucose uptake and thus a key determinant of whole body insulin sensitivity for glucose metabolism, the implication of the imcTG-MIR correlation to health and diseases cannot be overestimated. Therefore, there is no wonder that the investigations on this issue have been very active. This is exemplified by the establishment and utilization of proton NMR spectroscopy technology for non-invasive measurement of imcTG content 8,9,10,11 that started in the early 1990s 12. The research primarily involved the mechanisms underlying the imcTG-MIR relation, such as the role of glucose-fatty acid cycling (Randle cycle) and signal transduction in MIR. It was shown that long chain acyl CoA (LCACoA), diacylglycerol (DAG) and ceramides in myocytes cause MIR via interfering with insulin signaling 13,14,15. In contrast, there have been much less research efforts to study the role of imcTG kinetics, such as imcTG turnover, in MIR.
THE HYPOTHESIS: imcTG as a contributor to MIR
It is hypothesized that enlarged imcTG pool in metabolic diseases is not only a marker of, but a significant and substantive contributor to, MIR. imcTG exerts the effects by releasing large amounts of fatty acids which interfere with insulin signaling via signal transduction pathways. The increased intracellular fatty acid availability also suppresses glucose metabolism by promoting their oxidation by skeletal muscle.
EVALUATION AND DISCUSSION
imcTG turnover
In general, turnover kinetics of imcTG has been scarcely investigated, letting alone for MIR or any metabolic conditions specifically. Nonetheless, limited reports indicated that in resting healthy humans, imcTG pool turns over slowly with a fractional rate of 0.0026/min (calculated from a rate of 29 h/pool based on monexponential e−kt) 16. In relatively young and healthy men and women exercising at 45% of VO2max, imcTG turned over at a rate of 0.0032±0.0007/min 17. As expected, imcTG turns over in rodents was much faster as we observed. In lean rats, imcTG fractional turnover rate (FTR) was determined to be 0.013±0.005/min, 0.016±0.005/min and 0.0072±0.003%/min for gastrocnemius, tibialis anterior and soleus muscle, respectively. By comparison, FTR of imcTG in high fat-fed obese rats is markedly accelerated in gastrocnemius (0.026±0.002/min, P=0.02) and tibialis anterior (0.030±0.002/min, P=0.01) 18. The findings are in contrast to the view that imcTG is a static lipid pool turning over slowly causing it to accumulate 19. Our observations pointed to the contrary that imcTG is a rather dynamic lipid pool with an enlarged pool size. The implication is that the combination of a large pool size and rapid turnover translates into marked increases by a factor of several folds in the absolute turnover and thus release of imcTG-fatty acids. The large amounts of fatty acids released from imcTG increase intracellular fatty acid availability in myocytes. Indeed, in addition to large imcTG pool, we constantly observed increased intramyocellular non-esterified fatty acids in obese rats compared to lean control (gastrocnemius by 70%, soleus by 89% and extensor digitorus longus by 106%, P<0.01). Under the action of acyl CoA synthase, long chain fatty acids are activated to acyl CoA. Long chain fatty acids participate in a number of metabolic pathways including mitochondrial β-oxidation and signal transduction pathways involving PKC, among others.
imcTG oxidation
As imcTG is a local energy source for muscle, an immediate fate for long chain acyl CoA released from imcTG is oxidation in mitochondria to produce ATP. Peroxisomal β-oxidation is minor as it is mainly for shortening very long fatty acids for further oxidation by mitochondria 20,21. Increased fatty acid availability can accelerate mitochondrial β-oxidation as high substrate concentration enhances enzymatic reactions (mass action). The close vicinity between imcTG droplets and mitochondria in myocytes 22 facilitates this process and thus enhances mitochondrial β-oxidation. Increased fatty acid oxidation is known to interfere with glucose metabolism via the mechanism of substrate competition (Randle cycle, 23), a widely accepted theory with a large body of literature supporting 24,25,26,27 although it appear not applicable to some conditions such as exercises 28. Our preliminary results confirmed this that imcTG-palmitate oxidation is significantly higher in high fat-fed obese rats than in lean control (unpublished data). This is consistent with reports
imcTG-fatty acid signaling
Fatty acids must be activated (thioacylated) to acyl CoA before any further steps of metabolism. As intracellular fatty acid flux and availability increase as a result of rapid imcTG turnover, the thioacylation reaction can be enhanced by mass action alone. This is consistent with the higher concentration of long chain acyl CoA in skeletal muscle of obese rats (above). As well known, long chain acyl CoA and diacylglycerol activate PKC (especially θ and ε isoforms) which in turn phosphorylates serine/threonine residues of insulin receptor (IR) and insulin receptor substrate-1 (IRS-1) 13,29. Phosphorylation of these residues inhibits activation of these proteins causing impairment of insulin signaling and thus insulin-mediated glucose uptake (i.e. MIR).
Meanwhile, imcTG hydrolysis during turnover also releases diacylglycerol such as 1,2-diacylglycerol 30 (triacylglycerol → diacylglycerol + fatty acid), a classical second messenger that acts on the same signaling pathway in a similar manner 19. Thus, one diacylglycerol and one acyl CoA molecule are produced from the hydrolysis of each imcTG molecule. Both can activate the PKC system to inhibit insulin signaling. In the high fat-fed obese rat model, the content of diacylglycerol in gastrocnemius, soleus and extensor digitorus longus is 52% (P<0.01), 37% (P<0.05) and 88% (P<0.01) higher than that in lean littermate control. And diacylglycerol content is significantly correlated with imcTG pool size and imcTG turnover rate (r=0.68, P<0.05). The results strongly suggest a precursor-product relationship between imcTG and diacylglycerol. This has been confirmed by using 14C-glycerol as a tracer to track the transition from imcTG to diacylglycerol in rat skeletal muscles using pulse-chase technique (unpublished data).
Elevated long chain acyl CoA and diacylglycerol in conditions of increased lipid supply and insulin resistance have been extensively reported 14, 31, 13, 32, 15. By comparison, the roles of increased imcTG pool size and accelerated imcTG turnover have not been directly studied and this area of research has not been given much attention. Therefore, the extent of imcTG contributing to the production of these signaling molecules is unknown. For example, what is the relative contribution compared to that from plasma fatty acids. The information is directly relevant to a potential causal role of imcTG in MIR. If a significant part of intramyocellular long chain acyl CoA or/and diacylglycerol is derived from imcTG, then it can be concluded that imcTG substantively contributes to MIR. In other words, imcTG is a contributor to, and not merely a marker of, MIR. This can be confirmed or verified experimentally by using 14C/13C-glycerol to trace the production of diacylglycerol from imcTG and 14C/13C-fatty acid isotopes to trace the release of acyl CoA by using pulse-chase technique (to prelabel the precursor pool first and then following the label flow to the product pools).
Fatty acids are also a precursor to ceramides, yet another member of the signaling molecule family with similar functions. Thus, increased fatty acid availability stimulates ceramide synthesis (e.g. palmitoyl ceramide). This further worsens the impairment of insulin signaling.
Taken together, long chain acyl CoA, diacylglycerol and ceramides can all be produced from imcTG. When imcTG pool is enlarged, the fluxes are increased. Rapid imcTG turnover amplifies this mass effect by dramatically increasing the rate of efflux of fatty acids and diacylglycerol from imcTG and thus local fatty acid trafficking and availability. As a result, excess amounts of these signaling molecules are produced and they can jointly activate PKC system. MIR results (Fig 1).
Figure 1.
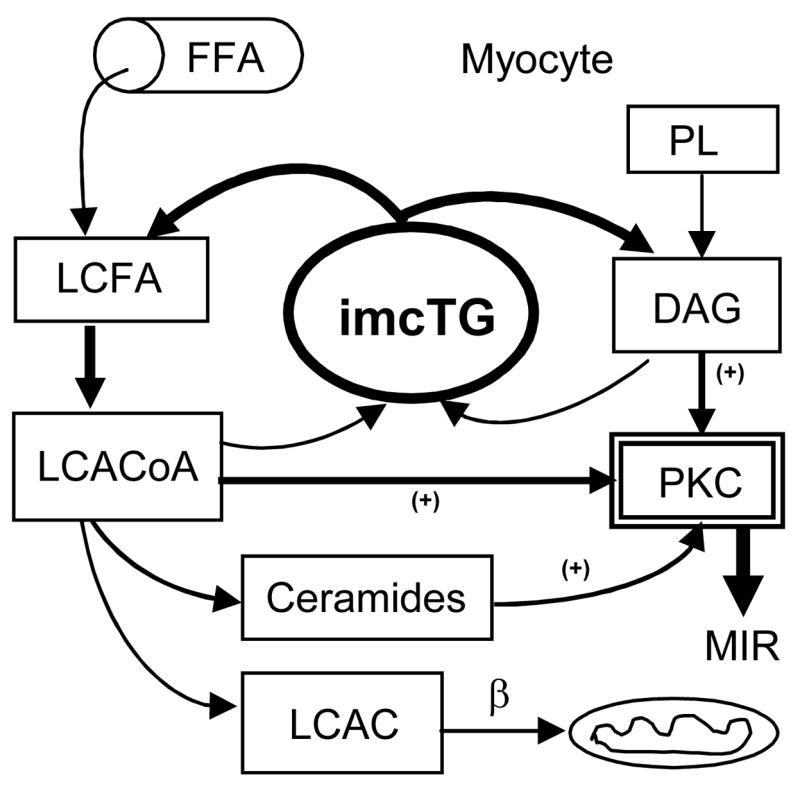
Hydrolysis of imcTG produces DAG and LCACoA which is also precursor to ceramides. All three can activate PKC and thus inhibit insulin signaling. A large, rapid turning over imcTG pool increases their production and thus MIR results. This continues even at increased lipid oxidation given the large imcTG-fatty acid flux and reduced mito β-oxidation worsens it. FFA, (plasma) free fatty acids; LCFA, long chain fatty acids; LCACoA, long chain acyl CoA; LCAC, long chain acylcarnitines; PL, phospholipids; DAG, diacylglycerol; PKC, protein kinase C; β, mitochondrial β-oxidation.
This mechanism can be further intensified with reduced fatty acid oxidation when more fatty acids become available for signaling. On the other hand, even increased fatty acid oxidation may not necessarily divert fatty acids away from PKC pathways to a degree that reverses the mechanism because the large fatty acid efflux from imcTG may suffice to maintain the needs for both pathways simultaneously. In such case, glucose utilization by muscle suffers dual suppressions, one being impaired insulin-mediated uptake as a result of impaired insulin signaling and the other being reduced glucose uptake via substrate competition due to increased fatty acid oxidation. This may well be the case as the evidence is overwhelming that muscle lipid oxidation in obesity and type 2 diabetes is elevated 24, 33, 34, 25, 35, 36, 37, 38, 39, 40. Our recent data showed the similar observation that mitochondrial β-oxidation in skeletal muscle of high fat-fed obese rats is greater than that in lean control (unpublished data). Since the obesity model has also accelerated imcTG turnover 18 and elevated diacylglycerol content (above), it appears that these indices of lipid hyper-metabolism co-exist in skeletal muscle of obesity and this supports the hypothesis.
On the other hand, impaired lipid oxidation by skeletal muscle has also been observed 41, 19. It is possible that certain metabolic conditions may modify the relationship among these parameters. For example, over weight and non-extreme obesity have higher but extreme obesity has lower lipid oxidation in muscle 37. The lack of reliable methodologies for measuring muscle lipid oxidation in vivo is another factor. For example, often the difference between total lipid oxidation (indirect calorimetry) and plasma fatty acid oxidation (fatty acid tracer) is used to represent lipid oxidation by muscle while it also includes oxidation by other tissues. Therefore, the issue of muscle lipid oxidation requires further investigation with more reliable techniques. Nonetheless, there seem no doubts that intramyocellular signaling molecules of PKC system are high in obesity and other metabolic conditions. As impairment of insulin signaling may be more powerful in suppressing glucose metabolism, this factor alone is powerful enough to cause MIR.
SUMMARY
Ultimate proof of increases in mitochondrial β-oxidation and in the fluxes of imcTG-fatty acids to PKC signaling pathways co-existing with MIR will question the validity of the marker theory. This will reverse the previous backward mechanism to a forward one where imcTG is a contributor to MIR rather than a marker. Such a reversal is fundamental to the understanding and intervention of MIR. For example, it will become a target for intervention of MIR, rather than a surrogate.
Acknowledgments
The work is supported by NIH research project R01 grant DK 60013 and ADA Research Award 7-05-RA-48. The author thanks Dr. Michael D. Jensen for providing access to research resources. The technical services provided by Lianzhen Zhou (Ph.Lic.) are appreciated used to obtain the data as mentioned herein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Storlien LH, Jenkins AB, Chrisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of Dietary Fat Composition on Development of Insulin Resistance in Rat. Relationship to Muscle Triglyceride and Omega-3 Fatty Acids in Muscle Phospholipid. Diabetes. 1991;40:280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 2.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal Muscle Triglyceride Levels Are Inversely Related to Insulin Action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 3.Kelley DE, Goodpaster BH. Skeletal Muscle Triglyceride. An Aspect of Regional Adiposity and Insulin Resistance. Diabetes Care. 2001;24:933–941. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 4.Guo ZK, Zhou L. Evidence for Increased Insulin-Resistant Lipolysis in Skeletal Muscle of High-Fat-Fed Rats. Metabolism. 2004;53:794–798. doi: 10.1016/j.metabol.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular Triglyceride Content Is a Determinant of in Vivo Insulin Resistance in Humans: a 1H-13C Nuclear Magnetic Resonance Spectroscopy Assessment in Offspring of Type 2 Diabetic Parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 6.Koyama K, Chen G, Lee Y, Unger RH. Tissue Triglycerides, Insulin Resistance, and Insulin Production: Implications for Hyperinsulinemia of Obesity. Am J Physiol. 1997;273:E708–E713. doi: 10.1152/ajpendo.1997.273.4.E708. [DOI] [PubMed] [Google Scholar]
- 7.Russull JC, Shillabeer G, Bar-Tana J, Lau DCW, Richardson M, Wenzel LM, Graham SE, Dolphin PJ. Development of Insulin Resistance in the JCR:LA-Cp Rat:Role of Triacylglycerol and Effects of Medica 16. Diabetes. 1998;47:770–778. doi: 10.2337/diabetes.47.5.770. [DOI] [PubMed] [Google Scholar]
- 8.Szczepaniak L, Babcock EE, Schick F, Bobbins RL, Garg A, Burns D, McGarry JD, Stein D. Measurement of Intracellular Triglyceride Stores by 1H Spectroscopy: Validation in Vivo. Am J Physiol. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 9.Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen CD, Haring HU. Association of Increased Intramyocellular Lipid Content With Insulin Resistance in Lean Nondiabetic Offersprings of Type 2 Diabetic Subjects. Diabetes. 1999;48:1113–1119. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 10.Boesch C, Slotboom J, Hoppeler H, Kreis R. In Vivo Determination of Intra-Myocellular Lipids in Human Muscle by Means of Localized 1H-MR-Spectroscopy. Magn Reson Med. 1997;37:484–493. doi: 10.1002/mrm.1910370403. [DOI] [PubMed] [Google Scholar]
- 11.Sinha RDSPKLVESMYSMRDSGCS. Assessment of Skeletal Muscle Triglyceride Content by (1)H Nuclear Magnetic Resonance Spectroscopy in Lean. Obese Adolescents: Relationships to Insulin Sensitivity, Total Body Fat, and Central Adiposity. Diabetes. 2002;51:1022–1027. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- 12.Schick F, Eismann B, Jung WI, Bongers H, Bunse M, Lutz O. Comparison of Localized Proton NMR Signals of Skeletal Muscle and Fat Tissue in Vivo: Two Lipid Compartments in Muscle Tissue. Magn Reson Med. 1993;29:158–167. doi: 10.1002/mrm.1910290203. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz-Peiffer C. Signalling Aspects of Insulin Resistance in Skeletal Muscle: Mechanisms Induced by Lipid Oversupply. Cell Signal. 2000;12:583–594. doi: 10.1016/s0898-6568(00)00110-8. [DOI] [PubMed] [Google Scholar]
- 14.Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. Peroxisome Proliferator-Activated Receptor (PPAR)-Alpha Activation Lowers Muscle Lipids and Improves Insulin Sensitivity in High Fat-Fed Rats: Comparison With PPAR-Gamma Activation. Diabetes. 2001;50:411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]
- 15.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by Which Fatty Acids Inhibit Insulin Activation of Insulin Receptor Substrate-1 (IRS-1)-Associated Phosphatidylinositol 3-Kinase Activity in Muscle. JBC. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 16.Sacchetti M, Saltin B, Olsen DB, van Hall G. High Triacylglycerol Turnover Rate in Human Skeletal Muscle. J Physiol. 2004;561:883–891. doi: 10.1113/jphysiol.2004.075135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo ZK, Burguera B, Jensen MD. Kinetics of Intramuscular Triglyceride Fatty Acids in Exercising Humans. J Appl Physiol. 2000;89:2057–2064. doi: 10.1152/jappl.2000.89.5.2057. [DOI] [PubMed] [Google Scholar]
- 18.Guo ZK, Zhou L. Muscle Type-Dependent Responses to Insulin in Intramyocellular Triglyceride Turnover in Obese Rats. Obes Res. 2005;13:2081–2087. doi: 10.1038/oby.2005.258. [DOI] [PubMed] [Google Scholar]
- 19.Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal Muscle Lipid Deposition and Insulin Resistance: Effect of Dietary Fatty Acids and Exercise. Am J Clin Nutr. 2007;85:662–677. doi: 10.1093/ajcn/85.3.662. [DOI] [PubMed] [Google Scholar]
- 20.Lee WN, Lim S, Bassilian S, Bergner EA, Edmond J. Fatty Acid Cycling in Human Hepatoma Cells and the Effects of Troglitazone. J Biol Chem. 1998;273:20929–20934. doi: 10.1074/jbc.273.33.20929. [DOI] [PubMed] [Google Scholar]
- 21.Pande SV. Carnitine-Acylcarnitine Translocase Deficiency. Am J Med Sci. 1999;318:22–27. doi: 10.1097/00000441-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Ogata T, Yamasaki Y. Scanning Electron-Microscopic Studies on the Three-Dimensional Structure of Mitochondria in the Mammalian Red, White and Intermediate Muscle Fibers. Cell Tissue Res. 1985;241:251–256. doi: 10.1007/BF00217168. [DOI] [PubMed] [Google Scholar]
- 23.Randle PJ, Garland PB, Hales CN. The Glucose-Fatty Acid Cycle. Its Role in Insulin Sensitivity and the Metabolic Disturbances of Diabetes Mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 24.Elks ML. Fat Oxidation and Diabetes of Obesity: the Randle Hypothesis Revisited. Med Hypotheses. 1990;33:257–260. doi: 10.1016/0306-9877(90)90138-5. [DOI] [PubMed] [Google Scholar]
- 25.Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP. A Potential Link Between Muscle Peroxisome Proliferator-Activated Receptor-Alpha Signaling and Obesity-Related Diabetes. Cell Metab. 2005;1:133–144. doi: 10.1016/j.cmet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Groop LC, Bonadonna RC, Shank M, Petrides AS, DeFronzo RA. Role of Free Fatty Acids and Insulin in Determining Free Fatty Acid and Lipid Oxidation in Man. J Clin Invest. 1991;87:83–89. doi: 10.1172/JCI115005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zorzano A, Balon TW, Brady LJ, Rivera P, Garetto LP, Young JC, Goodman MN, Ruderman NB. Effects of Starvation and Exercise on Concentrations of Citrate, Hexose Phosphates and Glycogen in Skeletal Muscle and Heart. Evidence for Selective Operation of the Glucose-Fatty Acid Cycle. Biochem J. 1985;232:585–591. doi: 10.1042/bj2320585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeukendrup AE. Regulation of Fat Metabolism in Skeletal Muscle. Ann NY Acad Sci. 2002;967:217–235. doi: 10.1111/j.1749-6632.2002.tb04278.x. [DOI] [PubMed] [Google Scholar]
- 29.Shulman GI. Cellular Mechanisms of Insulin Resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lykidis A, Mougios V, Arzoglou P. Kinetics of the Two-Step Hydrolysis of Triacylglycerol by Pancreatic Lipases. Eur J Biochem. 1995;230:892–898. doi: 10.1111/j.1432-1033.1995.tb20633.x. [DOI] [PubMed] [Google Scholar]
- 31.Peluso G, Petillo O, Margarucci S, Mingrone G, Greco AV, Indiveri C, Palmieri F, Melone MA, Reda E, Calvani M. Decreased Mitochondrial Carnitine Translocase in Skeletal Muscles Impairs Utilization of Fatty Acids in Insulin-Resistant Patients. Front Biosci. 2002;7:a109–a116. doi: 10.2741/A745. [DOI] [PubMed] [Google Scholar]
- 32.Shulman GI. Unraveling the Cellular Mechanism of Insulin Resistance in Humans: New Insights From Magnetic Resonance Spectroscopy. Physiology (Bethesda ) 2004;19:183–190. doi: 10.1152/physiol.00007.2004. [DOI] [PubMed] [Google Scholar]
- 33.Labayen I, Diez N, Parra D, Gonzalez A, Martinez JA. Basal and Postprandial Substrate Oxidation Rates in Obese Women Receiving Two Test Meals With Different Protein Content. Clin Nutr. 2004;23:571–578. doi: 10.1016/j.clnu.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Torgan CE, Brozinick JT, Jr, Kastello GM, Ivy JL. Muscle Morphological and Biochemical Adaptations to Training in Obese Zucker Rats. Physiol J Appl . 1989;67:1807–1813. doi: 10.1152/jappl.1989.67.5.1807. [DOI] [PubMed] [Google Scholar]
- 35.Franch J, Knudsen J, Ellis BA, Pedersen PK, Cooney GJ, Jensen J. Acyl-CoA Binding Protein Expression Is Fiber Type- Specific and Elevated in Muscles From the Obese Insulin-Resistant Zucker Rat. Diabetes. 2002;51:449–454. doi: 10.2337/diabetes.51.2.449. [DOI] [PubMed] [Google Scholar]
- 36.Goodpaster BH, Wolfe RR, Kelley DE. Effects of Obesity on Substrate Utilization During Exercise. Obes Res. 2002;10:575–584. doi: 10.1038/oby.2002.78. [DOI] [PubMed] [Google Scholar]
- 37.Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, MacDonald KG, Cline GW, Shulman GI, Dohm GL, Houmard JA. Skeletal Muscle Lipid Metabolism With Obesity. Am J Physiol Endocrinol Metab. 2003;284:E741–E747. doi: 10.1152/ajpendo.00514.2002. [DOI] [PubMed] [Google Scholar]
- 38.Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M. Insulin Downregulates Pyruvate Dehydrogenase Kinase (PDK) MRNA: Potential Mechanism Contributing to Increased Lipid Oxidation in Insulin-Resistant Subjects. Mol Genet Metab. 1998;65:181–186. doi: 10.1006/mgme.1998.2748. [DOI] [PubMed] [Google Scholar]
- 39.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired Cardiac Efficiency and Increased Fatty Acid Oxidation in Insulin-Resistant Ob/Ob Mouse Hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 40.Wang MY, Unger RH. Role of PP2C in Cardiac Lipid Accumulation in Obese Rodents and Its Prevention by Troglitazone. Am J Physiol Endocrinol Metab. 2005;288:E216–E221. doi: 10.1152/ajpendo.00004.2004. [DOI] [PubMed] [Google Scholar]
- 41.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal Muscle Fatty Acid Metabolism in Association With Insulin Resistance, Obesity, and Weight Loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]


