Abstract
The present studies examined whether the retrieval of an old ‘reactivated’ memory could be brought under the control of new contextual cues. In Experiment 1 rats trained in one context were exposed to different contextual cues either immediately, 60 min, or 120 min after a cued reactivation of the training memory. When tested in the shifted context, subjects exposed shortly after reactivation treated the shifted context as the original context. This transfer diminished with longer post-reactivation delays. Experiment 2 replicated the basic finding and demonstrated that the transfer of the old retrieval cues was specific to the contextual cues present during exposure. These findings are consistent with previous research (i.e., Briggs, Fitz, & Riccio, in press) showing the transfer of retrieval cues for a new memory, and demonstrating a similarity (in this case) between newly acquired and old reactivated memories.
Keywords: Reactivation, memory, retrieval, transfer, context, old memory, cues, rat
Memory retrieval cues, like conditional stimuli in learning, are generally assumed to acquire their value by virtue of being present at the time of the target episode. But temporal contiguity is not an essential condition in associative learning. For example, trace conditioning, in which the CS is not physically present at the time of UCS presentation, has been known since the early work of Pavlov (1927). What Pavlov referred to as a trace would more likely be called a “representation” in current parlance. However, only in recent years have investigators begun to examine other aspects of the associability of representations of stimuli. For example, using rats as subjects, Dwyer, Mackintosh, and Boakes (1998) demonstrated that two cues that were not present, but were simultaneously activated in memory, could become associated. More recently, Rovee-Collier and her colleagues (Cuevas, Rovee-Collier, & Learmonth, 2006) have shown similar results using infants as subjects.
With respect to memory processes, could contextual stimuli not present at the time of a learning episode become effective retrieval cues? Along the lines of the study by Rovee-Collier (Cuevas et al., 2006), but using rats rather than human infants, Briggs et al. (Briggs, Fitz, & Riccio, in press) investigated the possibility that neutral contextual stimuli might acquire value as retrieval cues if introduced shortly after a learning episode. In their study, rats exposed to a new context (B) immediately after fear conditioning showed markedly less impairment of performance than non-exposed controls when later tested in the new context. One plausible interpretation of this finding was that the active memory representation became associated with the novel context (B). Furthermore, the alleviation of the “context shift effect” was time dependent, in that that manipulation was less effective as the interval increased between training and exposure. This time-limited effect is important in two respects. First, it rules out mere familiarity with test context (B) as the basis for the improved performance. Second, the time dependent effect is consistent with evidence from other phenomena that active memory representations do not persist over long intervals. For example, both in studies of directed forgetting (Stonebraker & Rilling, 1981; Woodward, Bjork, & Jongeward, 1973; but see Roper & Zentall, 1993, for critique) and of retrograde amnesia (Duncan, 1949; McGaugh, 1966), the impact of the manipulation declines as the training to treatment interval increases.
In addition, some directly relevant work by Rovee-Collier and her colleagues has also shown time dependent effects of memory modification. In one example (Rossi-George & Rovee-Collier, 1999), retroactive interference was observed when infants were exposed to a novel context or a novel mobile shortly after learning to kick their leg to move a particular mobile in a distinct context. This interference effect also decreased as the timing of exposure increased, thus showing that new information could be incorporated while the training memory was still in an active state.
An exception to the time-dependent nature of retrograde amnesia can occur if an old memory is reactivated by a brief exposure to some aspect of the training episode just prior to the amnestic treatment. In that case the memory can again become susceptible to retrograde amnesia (Judge & Quartermain, 1982; Mactutus, Riccio, & Ferek, 1979; Misanin, Miller, & Lewis, 1968; Nader, Schafe, & Le Doux, 2000; Richardson, Riccio, & Mowrey, 1982; see also Millin, Moody, & Riccio, 2001). This effect has also been observed with human infants using a retroactive interference paradigm (Gulya, Rossi-George, & Rovee-Collier, 2002). These findings suggest that the activity level of the memory is more important than the age of the memory in terms of vulnerability to memory impairment. Given the evidence that a memory representation can be transferred to a new context shortly after conditioning (Briggs et al., in press; see also Boller & Rovee-Collier, 1992; Rossi-George & Rovee-Collier, 1999), the present experiments were designed to determine if similar effects would be obtained with an old reactivated memory. Thus, would exposure to the shifted context following reactivation of an old memory result in the transfer of retrieval cues to the new context? If so, would a temporal gradient be observed with respect to the interval between cuing and exposure treatment?
In an earlier study using rats, Gordon, McCracken, Dess-Beech, and Mowrer (1981) reported that the context shift effect was alleviated by exposing the rats to the training cues in the shifted contextual cues just prior to testing. Gordon et al. concluded that the shifted context “becomes attached in some way to the memory for prior training” (p. 208). However, their finding was puzzling because it is not clear how a cue exposure while in the shifted context could activate the training memory, given that performance was poor if rats were simply tested in that context. That is, how could memory be activated in the shifted context by cueing, but not by testing? To avoid this potential difficulty, we gave the reactivation exposure in the training context.
Thus, Experiment 1 investigated whether exposure to a new context immediately after a reactivation treatment in the training context would permit retrieval of the target information to be brought under the control of those new contextual cues. A second aim was to determine if such transfer declines with longer reactivation-to-exposure intervals.
Experiment 1
Experiment 1 was designed to investigate if retrieval cues for an old reactivated memory could be transferred to a new context by exposing subjects to that context immediately following a reminder cue, while the memory is presumably still active. To evaluate any transfer, our strategy exploited the tendency for performance to be impaired when the test context differs from that of training (Godden & Baddeley, 1975; Gordon et al., 1981; Smith, 1979; Zhou & Riccio, 1996). Thus, if retrieval cues become associated with the new context, the context shift effect should be alleviated.
Method
Subjects
The subjects were 70 Long-Evans rats, approximately 85 days of age. The animals were individually housed and were maintained on a 15/9 hr light/dark cycle. Food and water was available ad libitum throughout the course of the experiment.
Apparatus and contexts
Training and testing were conducted in two identical 43.18 × 17.78 × 17.78 cm black–white shuttle boxes with grid floors. Each shuttle box was divided into two compartments of equal size by a guillotine door. The exposure chamber was a 22 × 22 × 23.5 cm box made of clear Plexiglas walls and lid. The chamber was placed near the training/testing shuttle box in each context during exposure.
The two shuttle boxes were located in separate rooms that served as contexts. Context A was a 1.62 × 2.33 m room with white walls and scented with Airwick Wizard® air freshener with Country Berries® scented oil. White noise (76 dB) was presented at all times in this context. The room was illuminated by a 25-W red light bulb above the shuttle box. Context B was a brightly lit 1.83 × 2.74 m room with white walls. Posters were placed on each wall to provide visual cues. This room was illuminated by fluorescent houselights. The context was not artificially scented, and no white noise was present.
Procedure
Prior to the beginning of the experiment, all subjects were handled for two minutes on three consecutive days. Ten rats, randomly assigned to one of seven conditions, received fear conditioning in either Context A or Context B. Assignment to the contexts was counterbalanced such that within each group five rats were trained in Context A and five in Context B. For simplicity of exposition, we refer to context shifts (A to B) generically, regardless of the actual context used.
At training, the rat was brought into the context on the experimenter’s arm and remained there for 15 s to provide brief exposure to the context. The rat was then placed in the white compartment of the shuttle box facing away from the closed guillotine door. After 15 s, the guillotine door was raised and the latency to cross into the black compartment (all four paws) was recorded. The door was then lowered and two inescapable footshocks (1 s, 0.5 mA) were delivered 5 s and 10 s after the door was lowered. Five s after the last footshock, the animal was removed and returned to its home cage.
Twenty-four hours after training, three shift condition groups (Imm-Exp, 60-Exp, and 120-Exp) received a reminder session to reactivate the training memory before being exposed to the context that differed from training. The reminder consisted of a probe trial conducted in the original training context. The probe trial was similar to training, in that the animal was placed on the white side for 15 s before the guillotine door was raised, except that the doorway was only open for only 30 s and no shocks were delivered. The rationale for this form of cuing is that successful passive avoidance provides operational evidence that the memory was “reactivated” during the reminder. Conversely, failure to avoid during the probe trial permits the reasonable inference that memory was not activated, and was a criterion for excluding an animal. Three rats from group 120-Exp that crossed during the probe trial were removed and replaced.
Following memory reactivation, group Imm-Exp received an immediate exposure to the shifted context. Exposure consisted of bringing the rat into the shifted context and immediately placing the animal in the white compartment of the shuttle box in an attempt to maintain the activity of the reactivated memory. After 15 s of exposure to the white side, the rat was removed and placed in the exposure chamber for 4 min 45 s. Following the 5 min exposure treatment, the rat was returned to its home cage. Groups 60-Exp and 120-Exp received the exposure treatment in the shifted context 60 min and 120 min following reactivation, respectively. To control for the possibility of the reminder session producing new learning, a reactivation-only control group (React Only) received the reactivation session in the training context with no subsequent exposure to the other context. An exposure control group (Expose Only) was placed in the shifted context but without the prior probe (reactivation) trial to assess the effect of exposure per se on the context shift effect. These two control groups received their treatment 24 hours after training.
To evaluate the effect of the context shift on performance, two control groups (Same and Shift) that did not receive the reminder probe or the exposure treatment were tested 48 hours after training in either the same context as training (Same) or in the shifted context (Shift). The shift condition groups (Imm-Exp, 60-Exp, and 120-Exp) as well as the control groups (React Only and Expose Only) were tested 24 hours after the reminder/exposure (i.e., 48 hours after training) in the contexts that differed from training. All groups received a 10 min passive-avoidance test identical to training trials, except that no shocks were delivered and the guillotine door remained open. The rat was placed on the white side and allowed to choose between the white and black compartments. The cross-through latency and total time spent on the safe (white) side (TTS) were recorded as the dependent measures. As the patterns of results were generally similar on both measures, only TTS scores are reported.
Results and Discussion
Training
Rats in all seven groups exhibited short cross latencies at training with group means ranging from 7.5 s to 12.5 s. An analysis of variance (ANOVA) performed on training cross latencies revealed no differences between the seven groups (F (6, 63) = .89, p > .50).
Counterbalancing
There were no differences between training cross latencies, cross latencies at test, and TTS in either context. Accordingly, the contexts were collapsed within each group for all further analyses.
Testing
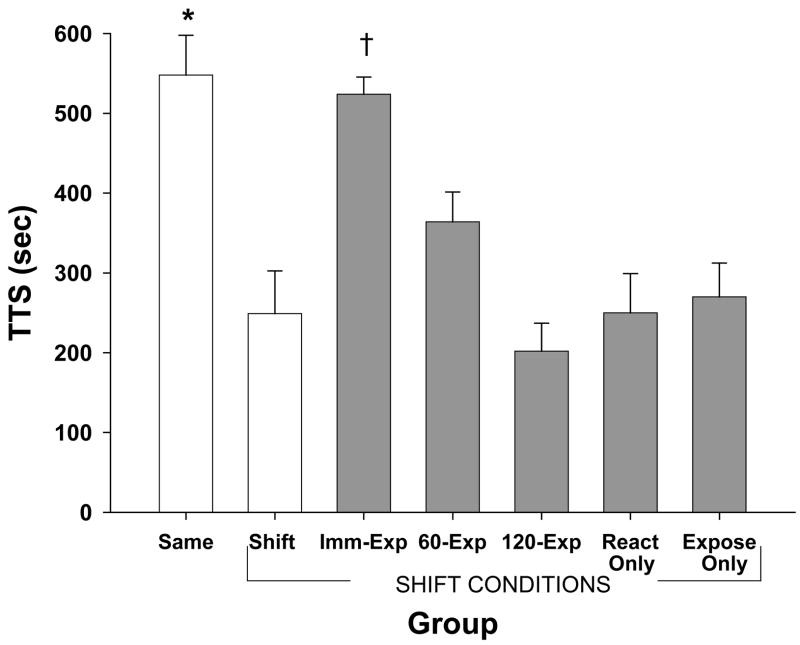
Figure 1 shows the mean TTS scores for all seven groups at test. An ANOVA revealed the groups differed significantly (F (6, 63) = 10.65, p < .001). Tukey’s honestly significantly difference (HSD) post-hoc tests were conducted to compare group differences.
Figure 1.
Mean total time spent on the safe (white) side in seconds for all groups in Experiment 1. Error bars represent standard errors of the means. Groups marked “shift conditions” were tested in the shifted context (B). Same and Shift groups represent a context shift effect. Asterisk represents a significant difference between control groups (open bars). Group Imm-Exp demonstrates exposure to the shifted context immediately after reactivation alleviates the shift effect. Groups 60-Exp and 120-Exp show this effect is time limited. Groups React Only and Expose Only demonstrate that neither the memory reactivation nor the exposure alone, respectively, attenuated the context shift effect. Dagger represents significant differences between group Imm-Exp and all experimental groups (shaded bars) except for group 60-Exp.
As can be seen in Figure 1, a context shift was obtained with the Same group showing significantly more fear (long TTS) than the Shift group (p < .001). Thus, being trained and tested in a different context impaired performance. The context shift was attenuated by the transfer of contextual cues to the shifted context, as the group that was immediately exposed to the shifted context after the memory was reactivated (Imm-Exp) displayed as much fear as the Same group (p > .50) and significantly more fear than the Shift group (p < .001).
The effectiveness of the shifted (exposed) context as a retrieval cue declined with longer reactivation-to-exposure intervals. The intermediate delay group (60-Exp) was not significantly different from the Same or Imm-Exp groups (ps > .05); however the group that was exposed to the shifted cues 120 min following reactivation (120-Exp) showed significantly less spatial avoidance than either the immediately exposed (Imm-Exp) group or the Same group (ps < .001). This finding indicates that mere exposure to the new context, irrespective of memory transfer, cannot account for the reduction of the context shift effect.
The reactivation control group (React Only) and the exposure only control group (Expose Only) demonstrated that neither the memory reactivation nor the exposure alone, respectively, attenuated the context shift effect shown by the immediate expose group (Imm-Exp). This was confirmed by post-hoc tests revealing groups React and Expose were significantly different than group Imm-Exp and Same (ps < .01).
In summary, Experiment 1 demonstrated that retrieval cues can become associated with a new context by exposing the animal to a different context immediately after reactivating an old memory. It also showed that with longer reactivation-to-exposure intervals the transfer of retrieval cues was less effective, an outcome similar to the time-dependency effects of retrograde amnesia. Thus, exposure to the context while the reactivated memory is active presumably allows the new retrieval cues to become encoded with the original training memory, but the encoding diminishes with post-reactivation delays.
The procedure used in this experiment is similar to a second-order conditioning paradigm (see Gewirtz & Davis, 2007; Rescorla, 1980; Rizley & Rescorla, 1972), where a second CS becomes associated with the conditioned response elicited by a first CS. Using a similar paradigm, Tronel, Milekic, & Alberini (2005) linked new information to an old reactivated memory. In their study rats were trained on a single trial inhibitory avoidance task in a particular context. During this initial training a light was presented as a visual CS. The avoidance memory was then reactivated 48 hr later in a second, different context where the light was presented during exposure to a second avoidance chamber. When tested two days later in the second context the rats that received the reactivation treatment combined with the light showed significantly more avoidance than control groups that did not receive the light presentation or that did not receive the reactivation treatment. These results demonstrated that a strong avoidance memory was formed for the second context, despite not receiving training in that particular context. Thus, the memory was transferred using a second-order conditioning paradigm.
The current investigation, although similar to a second-order conditioning paradigm, might better be described as involving second-order conditioning of an occasion setter. In our experiment the black compartment cues act as the first CS during training and reactivation. During the exposure treatment the context (occasion setter) appropriately took the place of the second CS. Thus, when the group was exposed to the new context immediately following memory reactivation an association was formed between fear induced by the original training cues and the new contextual cues. The new context could then serve as an occasion setter for fear to be elicited by the conditioning cues.
Experiment 2
Although Experiment 1 demonstrated that retrieval cues of an old reactivated memory could be transferred to a new context, it is not clear whether the exposure manipulation would have produced fear to any context, or if it was specific to the context in which the exposure took place. Previously, Briggs et al. (in press) found that for a newly acquired memory, exposure to an irrelevant context (C) did not improve performance in the shifted context. Whether this outcome would also obtain for a reactivated old memory is not clear, especially since some differences have been observed with respect to retrograde amnesia for new and old memory (for review, see Riccio, Millin, & Bogart, 2006). The aim of Experiment 2 was to determine if the retrieval cues are specific to those cues present at the time of the exposure. In order to make a relatively direct comparison with our previous study on transfer, the design involved exposure to a new context (C) followed by testing in the shifted context (B). As in the previous study, we attempted to avoid the potential contamination of generalization between contexts by making the third context (C) distinctly different from the test room (B).
Method
Subjects
Forty Long-Evans rats, approximately 85 days of age, were subjects. The animals were obtained and maintained as in Experiment 1.
Apparatus and contexts
The two shuttle boxes used were those described in Experiment 1. Contexts A and B were also the same as those used in Experiment 1. A third context (Context C) was added to this experiment. Context C was a 2.33 m × 1.62 m room with plain white walls. This context was illuminated by a single 15 W bulb located on the floor (approximately 1 m below the apparatus). The shuttle box was situated on top of a wooden table against one wall of the room. Context C was not artificially scented, and no white noise was present.
Procedure
Experiment 2 followed the procedures described in Experiment 1. Prior to training the rats were randomly divided into four groups of 10. As in Experiment 1, training conditions were counterbalanced with half of each group assigned to Context A and the other half to Context B.
Twenty-four hours after training, two shift condition groups (Expose-B and Expose-C) received a reactivation session (probe trial described in Experiment 1) in the training context to reactivate the training memory, followed immediately by exposure to a shifted context that differed from training. As a replication of the transfer of retrieval cues finding of Experiment 1, group Expose-B was exposed to Context B. To determine if the transfer of cues was context specific, group Expose-C was exposed to the third context (Context C). In each case, rats were placed briefly (15 s) in the white side of the shuttle box that was otherwise located in the test context, then placed in the exposure chamber for the remainder of the 5 min treatment. Context C was not counterbalanced but was always the different context for the Expose-C group. Two other groups (Same and Shift) did not receive the exposure treatment to the shifted context.
Forty-eight hours after training, all groups were tested as described in Experiment 1. The two groups that did not receive an exposure treatment were tested in either the same context as training (Same) or in the shifted context (Shift). Both groups in the shift conditions (Expose-B and Expose-C) were tested in context B.
Results and Discussion
Training
Rats in all four groups exhibited short cross latencies at training with group means ranging from 7.4 s to 8.7 s. An ANOVA performed on training cross latencies revealed no differences between the four groups (F (3, 36) = .28, p > .50).
Testing
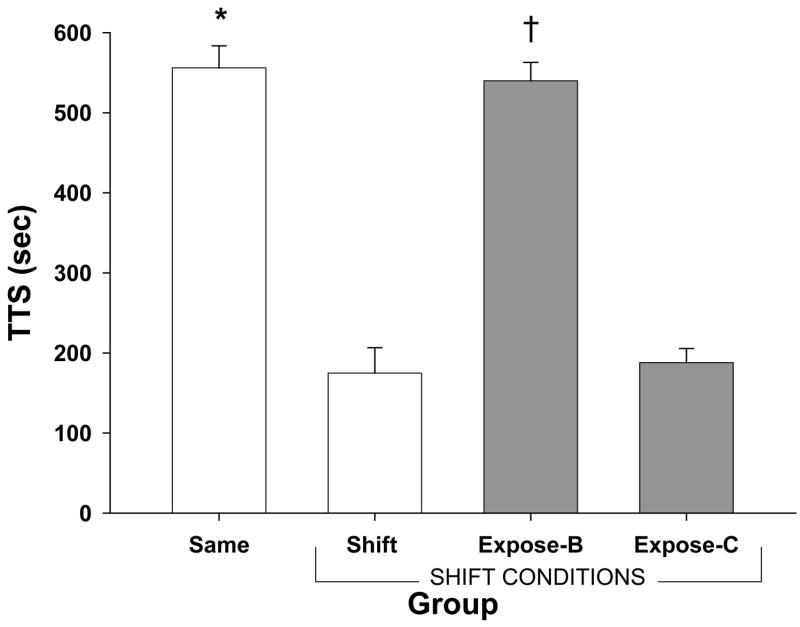
Figure 2 shows the mean TTS score for all four groups at test. An ANOVA revealed the groups differed significantly (F (3, 36) = 68.86, p < .001). Tukey’s HSD post-hoc tests were conducted to compare group differences.
Figure 2.
Mean total time spent on the safe (white) side in seconds for all groups in Experiment 2. Error bars represent standard errors of the means. Groups marked “shift conditions” were tested in the shifted context (B). Same and Shift groups represent a context shift effect. Asterisk represents a significant difference between control groups (open bars). Group Expose-B shows being exposed to the shifted context immediately after reactivation alleviated the context shift effect. Group Expose-C demonstrates this effect is context specific. Dagger represents significant differences between experimental groups (shaded bars).
As can be seen in Figure 2, a context shift was obtained with the Same group showing significantly more fear (long TTS) than the Shift group (p < .001). As in Experiment 1, being trained and tested in a different context impairs performance. The Expose-B group replicated the transfer of reactivated memory retrieval cues to a new context. Post-hoc tests confirmed this conclusion with group Expose-B showing as much fear as the Same group (p > .50). The group exposed to a third context (Expose-C) but tested in context B showed significantly less fear than groups Expose-B and Same (ps < .001), as well as a level of fear similar to the Shift group (p > .50). Thus, it appears that the transfer of retrieval for an old reactivated memory was specific to the context in which exposure occurred.
These data again indicate that by exposing rats to a context shortly after the reactivation of an old memory can incorporate the memory into those new cues, as reflected in the elimination or alleviation of the usual context shift decrement. As this interpretation suggests, the transfer of control over a reactivated memory is not achieved simply by exposure to any context, since placing rats in a context unrelated to testing produced no improvement in memory. Thus, transfer of retrieval cues for an old memory is relatively specific to the particular cues present at the time of re-encoding exposure.
General Discussion
The present experiments sought to determine if representations of an old reactivated memory could be transferred to a new context. The data showed that exposing rats to a new context shortly after memory reactivation alleviated the context shift effect. Moreover, the transfer of retrieval cues to a new context using an old reactivated memory is similar to that obtained for a new memory (Briggs et al., in press) in that the transfer of retrieval cues for an old memory was time dependent. That is, with longer reactivation-to-exposure intervals the exposure was less effective in alleviating the context shift effect. Thus, once the old memory was reactivated, the exposure to the shifted retrieval cues became associated with the original memory but only when the old memory was being re-processed.
The second experiment replicated the initial finding and demonstrated that the transfer of retrieval cues for an old reactivated memory was specific to those cues present at the time of exposure, as exposure to an irrelevant context following reactivation did not alleviate the context shift effect. Thus, the exposure manipulation alone was not sufficient to enhance retrieval in the novel context (B).
Although no data were presented here, we are in the process of determining if the transfer results in the retrieval cues being removed from the original context or if the fear memory continues to be associated with the training context. It is important to determine whether the memory is updated by allowing the fear memory to become associated with other retrieval cues while remaining with the original contextual cues, or whether the transfer results in the loss of effectiveness of the original context. Preliminary results suggest that the transfer has no effect on the original memory. It appears that this transfer of retrieval cues is not a case of erase-and-update, but rather fear remains for the original context and also becomes associated with the shifted context where exposure took place.
That retrieval cues for an old reactivated memory can be brought under the control of a new context establishes not only the importance of the activity level of the memory, but also the similarity between a newly formed memory and a stored memory that has been reactivated. These results are of particular interest because they provide further evidence that old reactivated memories share at least some characteristics similar to newly formed memories; a finding consistent with research comparing retrograde amnesia in old reactivated memories and new memories (Judge & Quartermain, 1982; Mactutus, Ferek, George, & Riccio, 1982; Mactutus et al., 1979; Misanin et al., 1968; Nader et al., 2000; Przybyslawski, Roullet, & Sara, 1999; for a review see Sara, 2000).
The present findings are in conflict with other work attempting to modify an old reactivated memory using an appetitive task with human infants as subjects (Boller & Rovee-Collier, 1994). Using procedures similar to those employed in the current study, Rovee-Collier and her colleague reported that a reactivated memory failed to transfer to a novel context despite the fact that a successful modification was observed using a newly acquired memory. The discrepancies between the findings may derive from the aversive nature of those used in animal studies. As described above, Tronel et al. (2005) have also demonstrated the modification of an old memory using aversively motivated learning (inhibitory avoidance) in rats. The fear memories encoded in the rats may be more salient and more significant for survival. Thus when reactivated there is greater importance of the fear memory becoming encoded with the contextual cues present. This transfer of old reactivated memories, as well as the transfer of newly acquired ones, could be examined with rats using an appetitive task.
These findings add to a growing interest in the modification of learning or memory through manipulations designed to act on representations of events, rather than the events themselves. Some years ago, Richardson et al. (1982) reported a study in which memory for an aversive event was counterconditioned by presenting a highly positive reward immediately after fear conditioning. As with retrograde amnesia, the effectiveness of counterconditioning was time dependent. More recently, research using infants (Cuevas et al., 2006; Galluccio, 2005; Galluccio & Rovee-Collier, 2005) or nonhuman animals (Dwyer et al., 1998) has shown that various manipulations can alter the target information (CS; reinforcer) when it is in an active (or activated) state.
At a theoretical level, the present findings demonstrate that cues not present during acquisition can come to serve as stimuli for that event. Furthermore, this transfer is not dependent on a newly formed association: activating the representation of the initial event allows the retrieval cues to become associated with a new environment. Thus, these transfer studies using old reactivated memories provide another mechanism by which neutral contexts can come to control retrieval of an episode.
Acknowledgments
Funding of this research was provided by NIMH Grant No. 37535 to David C. Riccio. The authors acknowledge the helpful assistance of Kelly Fitz in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boller K, Rovee-Collier C. Contextual coding and recoding of infants’ memories. Journal of Experimental Child Psychology. 1992;53:1–23. doi: 10.1016/s0022-0965(05)80002-5. [DOI] [PubMed] [Google Scholar]
- Boller K, Rovee-Collier C. Contextual updating of infants’ reactivated memories. Developmental Psychobiology. 1994;27:241–256. doi: 10.1002/dev.420270406. [DOI] [PubMed] [Google Scholar]
- Briggs JF, Fitz KI, Riccio DC. Transfer of Old Memory Retrieval Cues in rats. Psychonomic Bulletin & Review. doi: 10.3758/bf03194096. In press. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Rovee-Collier C, Learmonth AE. Infants form associations between memory representations of stimuli that are absent. Psychological Science. 2006;17:543–549. doi: 10.1111/j.1467-9280.2006.01741.x. [DOI] [PubMed] [Google Scholar]
- Duncan CP. The retroactive effect of electroshock on learning. Journal of Comparative and Physiological Psychology. 1949;42:32–44. doi: 10.1037/h0058173. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Mackintosh NJ, Boakes RA. Simultaneous activation of the representations of absent cues results in the formation of an excitatory association between them. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:163–171. [Google Scholar]
- Galluccio L. Updating reactivated memories in infancy: I. Passive- and active-exposure effects. Developmental Psychobiology. 2005;47:1–17. doi: 10.1002/dev.20073. [DOI] [PubMed] [Google Scholar]
- Galluccio L, Rovee-Collier C. Updating reactivated memories in infancy: II. Time passage and repetition effects. Developmental Psychobiology. 2005;47:18–30. doi: 10.1002/dev.20072. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Using Pavlovian higher-order conditioning paradigms to investigate the neural substrates of emotional learning and memory. Learning & Memory. 2007;7:257–266. doi: 10.1101/lm.35200. [DOI] [PubMed] [Google Scholar]
- Godden DR, Baddeley AD. Context-dependent memory in two natural environments: On land and underwater. British Journal of Psychology. 1975;66:325–331. [Google Scholar]
- Gordon WC, McCracken KM, Dess-Beech N, Mowrer RR. Mechanisms for the cueing phenomenon: The addition of the cueing context to the training memory. Learning and Motivation. 1981;12:196–211. [Google Scholar]
- Gulya M, Rossi-George A, Rovee-Collier C. Dissipation of retroactive interference in human infants. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:151–162. [PubMed] [Google Scholar]
- Judge ME, Quartermain D. Characteristics of retrograde amnesia following reactivation of memory in mice. Physiology & Behavior. 1982;28:585–590. doi: 10.1016/0031-9384(82)90034-8. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Ferek JM, George CA, Riccio DC. Hypothermia-induced amnesia for newly acquired and old reactivated memories: Commonalities and distinctions. Physiological Psychology. 1982;10:79–95. [Google Scholar]
- Mactutus CF, Riccio DC, Ferek JM. Retrograde amnesia for old (reactivated) memory: Some anomalous characteristics. Science. 1979;204:1319–1320. doi: 10.1126/science.572083. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Time dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- Millin PM, Moody EW, Riccio DC. Interpretations of retrograde amnesia: Old problems redux. Nature Reviews Neuroscience. 2001;2:68–70. doi: 10.1038/35049075. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: Role of β Adrenergic receptors. The Journal of Neuroscience. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian second-order conditioning: Studies in associative learning. Hillsdale, New Jersey: L. Erlbaum Associates; 1980. [Google Scholar]
- Riccio DC, Millin PM, Bogart AR. Reconsolidation: A brief history, a retrieval view, and some recent issues. Learning & Memory. 2006;13:536–544. doi: 10.1101/lm.290706. [DOI] [PubMed] [Google Scholar]
- Richardson R, Riccio DC, Mowrey H. Retrograde amnesia for previously acquired Pavlovian conditioning: UCS exposure as a reactivation treatment. Physiological Psychology. 1982;10:384–390. [Google Scholar]
- Rizley RC, Rescorla RA. Associations in second-order conditioning and sensory preconditioning. Journal of Comparative and Physiological Psychology. 1972;81:1–11. doi: 10.1037/h0033333. [DOI] [PubMed] [Google Scholar]
- Roper KL, Zentall TR. Directed forgetting in animals. Psychological Bulletin. 1993;113:513–532. doi: 10.1037/0033-2909.113.3.513. [DOI] [PubMed] [Google Scholar]
- Rossi-George A, Rovee-Collier C. Retroactive interference in 3-month-old infants. Developmental Psychobiology. 1999;35:167–177. doi: 10.1002/(sici)1098-2302(199911)35:3<167::aid-dev1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learning & Memory. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Smith SM. Remembering in and out of context. Journal of Experimental Psychology: Human Learning and Memory. 1979;5:460–471. [Google Scholar]
- Stonebraker TB, Rilling M. Control of delayed matching-to-sample performance using directed forgetting techniques. Animal Learning & Behavior. 1981:196–201. doi: 10.1901/jeab.1981.36-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Milekic MH, Alberini CM. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biology. 2005;3:1630–1638. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AE, Bjork RA, Jongeward RH. Recall and recognition as a function of primary rehearsal. Journal of Verbal Learning and Verbal Behavior. 1973;12:608–617. [Google Scholar]
- Zhou Y, Riccio DC. Manipulation of components of context: The context shift effect and forgetting of stimulus attributes. Learning and Motivation. 1996;27:400–407. doi: 10.1006/lmot.1996.0023. [DOI] [PubMed] [Google Scholar]