Abstract
Infection with the parasitic nematode Nippostrongylus brasiliensis induces a potent Th2 response; however, little is known about early stages of the innate response that may contribute to protective immunity. To examine early events in this response, chemokine expression in the draining lymph node was examined after N. brasiliensis inoculation. Pronounced increases of several chemokines, including CCL2, were observed. Compared with wild-type mice, elevations in a Gr-1bright population in the draining lymph node was significantly decreased in CCL2−/− mice after N. brasiliensis inoculation. Further flow cytometric and immunofluorescent analysis showed that in wild-type mice, Gr-1+ cells transiently entered and exited the draining lymph node shortly after N. brasiliensis inoculation. The Gr-1bright population was comprised of neutrophils expressing TGF-β and TNF-α. Following Gr-1+ cell depletion, N. brasiliensis infection resulted in transient, but significantly increased levels of IFN-γ, increased serum IgG2a, reduced Th2 cytokines and serum IgE, greatly increased mortality, and delayed worm expulsion. Furthermore, bacteria were readily detected in vital organs. Infection of Gr-1+ cell-depleted mice with N. brasiliensis larvae that were pretreated with antibiotics prevented bacterial dissemination, Th1 inflammatory responses, and decreases in host survival. This study indicates that parasitic nematodes can be an important vector of potentially harmful bacteria, which is typically controlled by CCL2-dependent neutrophils that ensure the optimal development of Th2 immune responses and parasite resistance.
Nippostrongylus brasiliensis inoculation of mice is a widely used model for studying intestinal nematode infections that begin with parenteral invasion routes. Following s.c. N. brasiliensis inoculation, a highly polarized Th2-type cytokine response develops, characterized by high levels of IL-4, IL-5, and IL-13, and low to undetectable IFN-γ. This Th2-type response is host protective and can lead to rapid worm expulsion following primary immunization (1). The initial events that contribute to the development of this in vivo Th2 immune response are not well understood, nor are the innate mechanisms that contribute to Th2 responses in general.
N. brasiliensis infective third-stage larvae (L3) first penetrate the host skin, then enter the circulatory system, and migrate to the lung at ~24~48 h postinfection. Larvae migrate from the lungs to the small intestine, where they mature into egg-producing adults (2). In addition to the polarized Th2 immune response in the local lymphoid tissue of the gut, N. brasiliensis also induces a strongly polarized Th2 response in the lymph node draining the infection site (3), and in the lung (4). We have previously shown that intracutaneous inoculation of N. brasiliensis L3 in the ear induces a potent Th2 response in the local cervical lymph node (CLN),4 and that larvae migrate normally to the lung and intestine, followed by a host-protective response resulting in rapid expulsion. Using this model, we demonstrated that N. brasiliensis infection could also function as a Th2 adjuvant to a nonparasite protein Ag (3, 5).
Dendritic cells at the site of infection can encounter and transfer Ags to the local lymph node, where an adaptive immune response follows (6). Several recent studies have suggested that at early stages of the immune response, other components of the innate response besides dendritic cells may promote Th cell polarization (7–9). A common characteristic of many of these different cell populations, including granulocytes (10), neutrophils (11), and subsets of dendritic cells (12, 13) or monocytes (14), is their expression of the cell surface marker Ly-6G (Gr-1) with neutrophils expressing the highest levels.
Neutrophils are classically viewed as phagocytes that rapidly accumulate at the site of infection, and play a pivotal role in host protection by killing invading bacteria. They also have important roles in regulation of inflammatory responses, in wound healing and tissue repair mechanisms (15). Recent studies suggest nonphagocytic roles of neutrophils in host immunity, including transfer of pathogen to local lymphoid tissues, Ag presentation, and T cell recruitment (16). The intracellular parasite Toxoplasma gondii has been shown to trigger neutrophil synthesis of CC chemokines that were strongly chemotactic for immature dendritic cells (17). Parasite-triggered neutrophils are also known to produce TNF-α and induce dendritic cell CD40 and CD86 up-regulation (17). In another report, neutrophils were found to produce IL-4 early in BALB/c mice infected with Leishmania major, and were responsible for priming counterprotective Th2 cells (18). Innate cell populations activated following invasion of N. brasiliensis larvae may help the development of the protective Th2 adaptive response; however, the initial stages of this response remain largely uncharacterized.
In this investigation, we used quantitative fluorogenic real-time RT-PCR to assess elevations in gene expression of the Th2-type immune response in the draining lymph nodes early after N. brasiliensis inoculation. Our studies identified increased levels of CCL2 gene expression shortly after inoculation of wild-type (WT) mice, and an absence of Gr-1bright populations in the draining lymph node of N. brasiliensis-inoculated CCL2−/− mice. The Gr-1bright cells were identified as neutrophils that transiently entered the lymph node within 18 h after inoculation. Gr-1+ cell depletion of N. brasiliensis-infected mice induced a pronounced early increase in IFN-γ expression, systemic bacterial infection, decreased host survival and Th2 cell differentiation, and delayed worm expulsion. Furthermore, treatment of infective L3 with an antibiotic mixture before inoculation eliminated associated bacteria and restored the protective Th2 response in anti-Gr-1 Ab-treated N. brasiliensis-inoculated WT mice. Our current study reveals a role for neutrophils in limiting inflammation and mortality from nematode parasite-associated bacterial infection and in the generation of optimal antihelminth Th2-type immune responses.
Materials and Methods
Mice
Female 6- to 10-wk-old BALB/c mice, C57BL/6, and CCL2−/− C57BL/6 mice were purchased from The Jackson Laboratory. All the mice were maintained in a specific pathogen-free, virus Ab-free facility during the experiments. Five mice were used per treatment group, if not otherwise indicated. The experiments in this study were conducted according to the principles set forth in the “Guide for the Care and Use of Laboratory Animals,” Institute of Animal Resources, National Research Council, Department of Health, Education, and Welfare (National Institutes of Health).
Parasite infection and Gr-1 depletion
Parasite eggs shed with the feces of N. brasiliensis-infected BALB/c mice were cultured in a mixture of charcoal and sphagnum moss stored in plastic petri dishes. The charcoal and moss culture was autoclaved before use to ensure the elimination of nonparasite-associated bacteria. Eggs hatch and develop from L1 through third stage (L3) infective larvae within 1 wk (19). Mice were inoculated intracutaneously in the ear with a 10 μl suspension of 500 L3 collected from cultures using a modified Baermann apparatus (19). The L3 were washed by centrifugation and replacement of sterile PBS three times before injection. Intracutaneous inoculation of L3 has been shown to produce the same level of infectivity as s.c. inoculation (3) (our unpublished observations). Parasite egg and adult worm numbers were evaluated in infected mice, as described previously (3). In several experiments, Gr-1bright cells were depleted in vivo by i.p. administration of anti-Gr-1 mAb (clone RB6-8C5) or isotype-matched control mAb (BD Biosciences) 1 day before larvae inoculation.
Antibiotic treatment of infective N. brasiliensis larvae
N. brasiliensis L3 were incubated with 400 U of penicillin, 400 μg/ml streptomycin plus 400 μg/ml neomycin (Invitrogen Life Technologies) or sterile PBS for 2 h at room temperature, after which the larvae were washed three times with sterile PBS. Antibiotic-treated and untreated N. brasiliensis L3 were placed on blood agar plates (Fisher Scientific) and cultured at room temperature for 24 h. In some experiments, the antibiotic-treated larvae were used to inoculate BALB/c mice in the ear.
Quantitative measurement of serum Igs
Blood samples were collected 10 days after N. brasiliensis inoculation and processed to obtain serum. Total serum IgE, IgG1, and IgG2a levels were detected by ELISA (3).
Gene expression by real-time PCR
Total RNA was extracted from draining lymph nodes using RNAzol B (AMS Biotechnology), and total RNA was then reverse transcribed to cDNA using Superscript II (Invitrogen Life Technologies), as previously described (20). Real-time PCR Taqman (Applied Biosystems) kits were used for assessing IL-4, IFN-γ, IL-4R, IL-10, IL-13, TNF-α, and 18S ribosome levels. Primers were designed for CCL2, TGF-β, and hypoxanthine phosphoribosyltransferase (HPRT), which were used to quantitate differences in gene expression. All data were normalized to 18S ribosomal or HPRT values. Primer sequences for SYBR Green assays were performed using the following primer pairs: CCL2-GCTG GAGCATCCACGTGTT (forward) and ATCTTGCTGGTGAATGAGTAGCA (reverse), and HPRT-TTGCTCGAGATGTCATGAAGGA (forward) and AGCAGGTCAGCAAAGAACTTATAGC (reverse), TGF-β (21). The Applied Biosystems 7700 sequence detector was used for amplification of target mRNA, and quantification of differences between treatment groups was calculated according to the manufacturer’s instructions.
Cell sorting and cytokine gene expression by RT-PCR
For sorting of Gr-1+ cells, CLN cells were labeled with PE-conjugated anti-Gr-1 Ab (BD Pharmingen). After washing, the cells were applied to FACSVantage high-speed sorter, and Gr-1bright and Gr-1dull cells were sorted and collected following standard protocol. For isolation of CD11c+ cells, CLN cells were labeled with anti-CD11c microbeads and passed through LS+ columns (Miltenyi Biotec). Purity of sorted cell populations was checked by FACS analysis. For RT-PCR, total RNA was extracted from purified cell populations with the RNA Isolation Kit (Stratagene), specially developed for isolating small RNA quantities. Total RNA was then reverse transcribed, as previously described. Real-time PCR kits (Applied Biosystems), specific for individual cytokines or rRNA, were used to quantitate differences in gene expression, and all data were normalized to constitutive rRNA values. Sorted cells were morphologically characterized on cytospin glass slides (Shandon Cytospin 4; Thermo Electron) after Wright-Giemsa staining (CAMCO).
Flow cytometry
Lymph node cells were harvested, and 1 × 106 cells were blocked with Fc Block (BD Pharmingen) and then incubated with anti-Gr-1-FITC, anti-CD11c-PE, and CD11b-PerCP-Cy5.5; anti-Gr-1-FITC, anti-MHC II-PE, and anti-B220-FITC; anti-Gr-1-PE, F4/80-FITC, and DX5-allophycocyanin; or anti-Gr-1-FITC and anti-CCR3-Alexa Fluor 647 (all from BD Pharmingen) for 30 min on ice. After washes, cells were fixed with 1% paraformaldehyde (Fisher Scientific) and data were collected by FACSCalibur (BD Pharmingen). Expression of cell surface markers was analyzed on gated Gr-1bright cells or Gr-1dull cells using WinList 5.0 software (Verity Software House).
Immunofluorescent staining and digital microscopy
Draining CLN were harvested from sacrificed mice and frozen in liquid nitrogen. Cryostat-cut tissue sections (8 μm) were fixed in acetone and stained, as described previously (3, 22), with the following reagents: FITC-conjugated anti-Gr-1 and Alex 647-conjugated anti-B220 (BD Pharmingen). Sections were mounted in Fluormount G (Southern Biotechnology Associates) and viewed with a fluorescence microscope (Leica DM6000B). Images were acquired on a digital camera and were processed with Image-Pro Plus software (Media Cybernetics).
Results
N. brasiliensis inoculation leads to rapid recruitment of Gr-1+ cells within the draining lymph node, which is dependent on CCL2 signaling
Previous studies have shown that a highly polarized Th2 response develops in the draining CLN by day 7 after N. brasiliensis intracutaneous inoculation in the ear (3), with elevated IL-4 gene expression in the draining lymph node detected as early as day 3 after inoculation (5). Gene expression changes that precede increased IL-4 production in the draining lymph node were detected using quantitative fluorogenic real-time RT-PCR. Lymph nodes from N. brasiliensis-infected mice (5/treatment group) were individually collected for RNA analysis at days 1, 2, 3, and 6 after inoculation. As shown in Fig. 1A, elevations in CCL2 (MCP-1) consistently peaked in the draining CLN at day 1 after N. brasiliensis inoculation, and quickly dropped back to baseline. CCL2 is the ligand for CCR2 that is expressed on populations of granulocytes (23), dendritic cells (12), and also Th1 and Th2 cells (24, 25). It has also been shown to be responsible for recruitment of rat neutrophils in the absence of alveolar macrophages after endotoxin-induced injury (12). Recent studies have also suggested that CCL2 may be required for the development of an effective Th2 response in peripheral tissues, although its actual function is unclear (25–28). Further analysis of lymph node samples collected early after parasite inoculation with Affymetrix oligonucleotide microarrays confirmed up-regulation of this chemokine (our unpublished observations).
FIGURE 1.
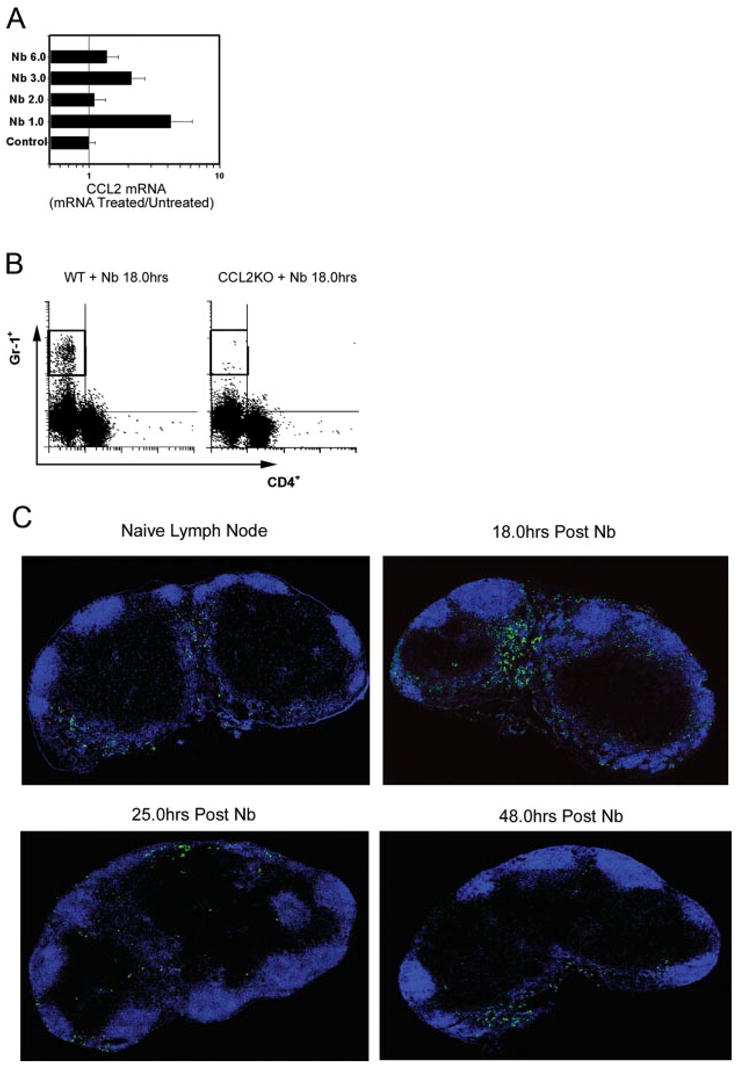
CCL2-dependent Gr-1bright cell infiltration of the draining lymph node shortly after N. brasiliensis inoculation. A, BALB/c mice (5/treatment group) were intracutaneously inoculated with 500 N. brasiliensis L3. On days 1, 2, 3, or 6 after inoculation, draining CLN were removed, RNA purified, and analyzed for CCL2 mRNA using real-time quantitative fluorogenic RT-PCR. Fold changes are expressed relative to the untreated BALB/c control. B, CCL2KO and WT control mice (5/treatment group) were inoculated with N. brasiliensis L3 at the ear. Draining CLN were collected 18 h later. CLN cell suspensions were prepared and stained for anti-Gr-1-PE and anti-CD4-FITC. C, BALB/c WT mice were inoculated intracutaneously in one ear with N. brasiliensis L3. Draining CLN were removed at 18, 24, or 48 h after inoculation, and also from untreated control mice. CLN frozen sections were fluorescently stained with anti-B220-Alexa Fluor 647 (B cells; blue) and anti-Gr-1-FITC (green). Individual × 400 digital images were tiled together to form a single high resolution composite image of the draining lymph node. These experiments were repeated twice with similar results.
Recent studies have shown that the host-protective Th2 response to the intestinal nematode parasite, Trichuris muris, is substantially inhibited in CCL2−/− mice (25). Our finding that N. brasiliensis triggered increased levels of a chemokine in the lymph node known to recruit immune cells suggested that a nonresident immune cell population may migrate to the draining lymph node at the initiation of an antihelminth response. Identification of such cell population(s) may provide useful information concerning the innate response that influences the development of a protective Th2-type immune response induced by N. brasiliensis infection. A common characteristic of many of these different cell populations, including granulocytes (10), neutrophils (11), and subsets of dendritic cells (12, 13), is their expression of the cell surface marker Gr-1. We thus examined whether a Gr-1+ cell population was recruited to the lymph node at the time of elevated CCL2 expression. In these studies, CCL2 knockout (KO) mice or BL/6 WT controls were inoculated with N. brasiliensis and, after 18 h when maximal CCL2 expression is observed, draining CLN were removed for analysis. As shown in Fig. 1B, in marked contrast to WT controls, CCL2KO BL/6 mice inoculated with N. brasiliensis showed a marked reduction in the recruitment of the Gr-1bright cell subset to the draining CLN. In contrast, the frequency of Gr-1dull cells was not significantly affected. This result suggests that CCR2-directed Gr-1bright cells infiltrate the draining lymph node after N. brasiliensis infection.
We next examined the dynamics of Gr-1+ cell infiltration into the draining lymph node at early stages of the Th2-type in vivo immune response. To perform these studies, we collected the draining lymph nodes from N. brasiliensis-inoculated WT mice or untreated WT controls, and stained lymph node sections with fluorescence-conjugated anti-Gr-1 and anti-B220 Abs. Individual images were taken using a fluorescent microscope and tiled together to form a single composite image of the entire draining lymph node (Fig. 1C). There are very few Gr-1+ cells in whole lymph node sections from untreated mice. In contrast, by 4 h after inoculation, Gr-1+ cells were readily detectable in peripheral regions of the lymph node (data not shown). By 18 h after inoculation, Gr-1+ cells had accumulated in large numbers in the subcapsular space, sinuses, and interfollicular areas (Fig. 1C). The number of Gr-1+ cells in the draining lymph node at 24 h was much lower than at 18 h after inoculation, and by 48 h the level of Gr-1+ cells in the lymph node was comparable to that of untreated controls (Fig. 1C). These results were highly reproducible in multiple independent experiments. We also examined Gr-1+ cell migration into other peripheral lymph nodes following inoculation with N. brasiliensis. Gr-1+ cells migrated into lymph nodes draining the site of inoculation, but not peripheral lymph nodes distant from the site of infection (data not shown).
Lymph node-infiltrating Gr-1bright cells are neutrophils that express TNF-α and TGF-β
We used flow cytometric analysis to determine the cell lineage of the Gr-1+ cell populations that infiltrated the draining lymph node after N. brasiliensis infection. At 18 h after N. brasiliensis inoculation, single-cell suspensions were prepared from the draining CLN of WT mice and stained with fluorochrome-conjugated Abs against an array of cell lineage markers. Forward and side scatter analysis of Gr-1bright cells revealed a characteristic granulocyte phenotype (data not shown). As shown in Fig. 2A, the majority of cells staining Gr-1bright also showed bright staining for CD11b, which is a β2 integrin component that is highly expressed by neutrophil and macrophage populations. Further analysis of gated Gr-1brightCD11bbright cells revealed a largely homogeneous population that was primarily negative for CCR3 (eosinophils), DX5 (basophils), CD11c (dendritic cells), and F4/80 (macrophages). Gr-1bright cells were electronically sorted by a high-speed FACSVantage cell sorter. Sorted cells were cytospun on glass slides and stained with Wright/Giemsa. The stained Gr-1bright cells showed a polymorphonuclear phenotype with clear cytoplasm (Fig. 2B). Taken together, the phenotype analysis of Gr-1bright cells indicated a homogenous population of neutrophils (29).
FIGURE 2.
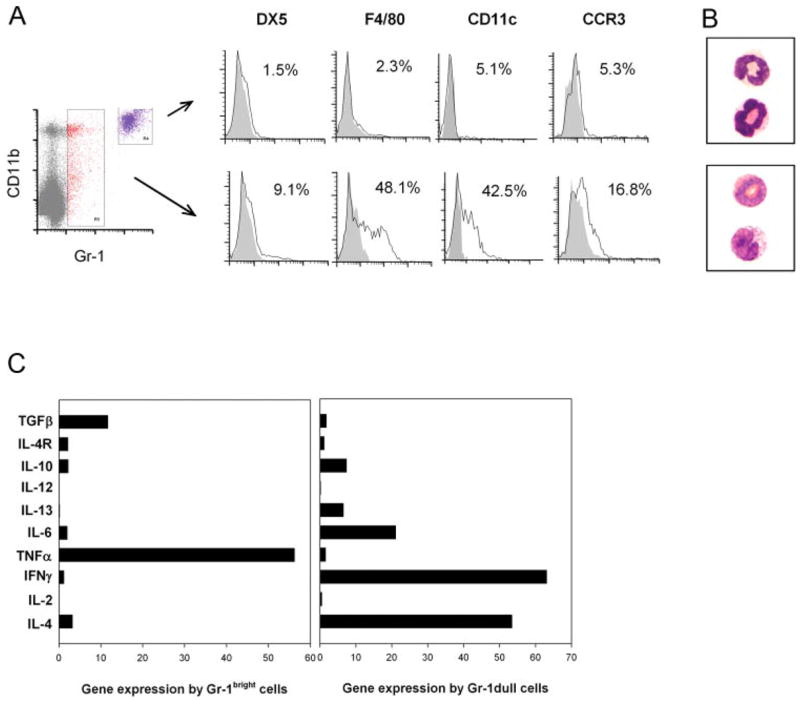
LN-infiltrating Gr-1bright cells are TNF-α- and TGF-μ-expressing neutrophils. A, BALB/c mice were inoculated with N. brasiliensis L3, and 18 h later CLN cells were harvested and stained with anti-Gr-1-PE, F480-FITC, anti-CD11b-PerCP-CY5.5, anti-CD11c-allophycocyanin, anti-CCR3-Alexa Fluor 647, or anti-DX5-allophycocyanin. B, In separate experiments, BALB/c mice (30/treatment group) were inoculated with N. brasiliensis L3, and 18 h later draining CLN were removed. Pooled cell suspensions were stained with anti-Gr-1 Ab. Gr-1bright and dull cells were isolated using high speed cell sorting (FACS). Sorted cells were centrifuged on slides using a Cytospin and then stained with DiffQuick to analyze morphology. C, RNA was purified from sorted cell populations, and real-time quantitative fluorogenic RT-PCR was used to assess cytokines and IL-4R gene expression. Sorted CD11c+ cells from untreated BALB/c mice were used as controls, and data are expressed as treated Gr-1+ cells/untreated CD11c+ cells. These experiments were repeated twice with similar results.
CD11b+Gr-1dull cells were moderately increased in the draining lymph node at 18 h after parasite inoculation, and were more heterogeneous with 16.8% CCR3+ cells, 42.5% CD11c+, and 48.1% F4/80+ cells. Consistent with this cell surface phenotype, electronic cell sorting and Wright/Giemsa staining of Gr-1dull cells revealed two major populations of dendritic cells/macrophages and eosinophils (Fig. 2A). Taken together, these data indicate that the Gr-1dull cells are composed of a heterogenous population, including eosinophils, dendritic cells, and macrophages. Migration of Gr-1dull cells to the draining lymph node seems independent of CCL2 expression, because a similar number of Gr-1dull was detected in parasite-infected CCL2KO mice (Fig. 1B).
To further characterize the LN-infiltrating Gr-1+ cells, RNA was isolated from the sorted Gr-1bright and Gr-1dull cells at 18 h after N. brasiliensis inoculation and reverse transcribed into cDNA. Cytokine gene expression by these two cell populations was assessed by quantitative fluorogenic real-time RT-PCR. Because the Gr-1+ cell number in untreated LN is too low for cell sorting, CD11c+ dendritic cells were purified from LN of untreated animals. Cytokine gene expression levels by sorted Gr-1+ populations were compared with the levels of naive CD11c dendritic cells. As shown in Fig. 2C, Gr-1bright cells express no or minimal levels of effector cytokines, including IL-4, IFN-γ, IL-12, IL-13, and IL-6. The only two cytokines that were up-regulated in Gr-1bright cells after N. brasiliensis infection were TNF-α and TGF-β. Expression of TNF-α by lymph node-infiltrating neutrophils was reported previously (29). In direct contrast, the Gr-1dull cells revealed no or very low levels of TNF-α and TGF-β expression; high levels of IL-4, IFN-γ, and IL-6; and lesser increases in IL-13 and IL-10 (Fig. 2C). Our findings now show for the first time that neutrophils infiltrate the draining lymph node transiently after helminth parasite inoculation.
Neutrophils prevent rapid immune cell activation and IFN-γ elevations following N. brasiliensis inoculation
To further assess the possible function of Gr-1 neutrophils in the Th2-type immune response induced by N. brasiliensis infection, we used an anti-Gr-1 Ab to deplete neutrophils before N. brasiliensis inoculation. Because neutrophil infiltration of draining lymph nodes peaked at 18 h postinfection, we selected this time point to collect the draining lymph nodes and further assessed cell activation and cytokine expression. Anti-Gr-1 Ab treatment effectively depleted the Gr-1bright population, as demonstrated by FACS analysis of lymph node cells, but left the Gr-1dull population unaffected (data not shown). To evaluate the cell activation status, lymph node cells were stained with anti-B220 and anti-CD11c Abs to identify B cells and dendritic cells, or stained with anti-CD4 or anti-CD8 for CD4+ or CD8+ T cells. Cell surface expression of B7.2, MHC II, or CD40 was analyzed on gated B cells or dendritic cells. Inoculation with N. brasiliensis induced increased expression of B7.2 on both B cells and dendritic cells in mice depleted of Gr-1bright cells with anti-Gr-1 Ab compared with N. brasiliensis-inoculated mice given control isotype Ab. The expression of MHC II molecules was slightly increased on lymph node B cells after anti-Gr-1 plus N. brasiliensis treatment; however, this treatment significantly increased MHC II and CD40 expression on lymph node dendritic cells (Fig. 3A). There were no significant changes of CD40 expression on B cells with or without anti-Gr-1 Ab treatment (data not shown). The general increase in expression of activation molecules on accessory cells after Gr-1 cell depletion correlated with increased activation of both CD4+ and CD8+ T lymphocytes. As shown in Fig. 3A, expression of the early activation marker CD69 increased markedly on CD4+ and CD8+ T cells after N. brasiliensis inoculation of anti-Gr-1 Ab-treated mice compared with isotype Ig-treated mice. These results indicate that the presence of Gr-1 cells in the N. brasiliensis-infected hosts is required to prevent a general activation of accessory cells and lymphocytes.
FIGURE 3.
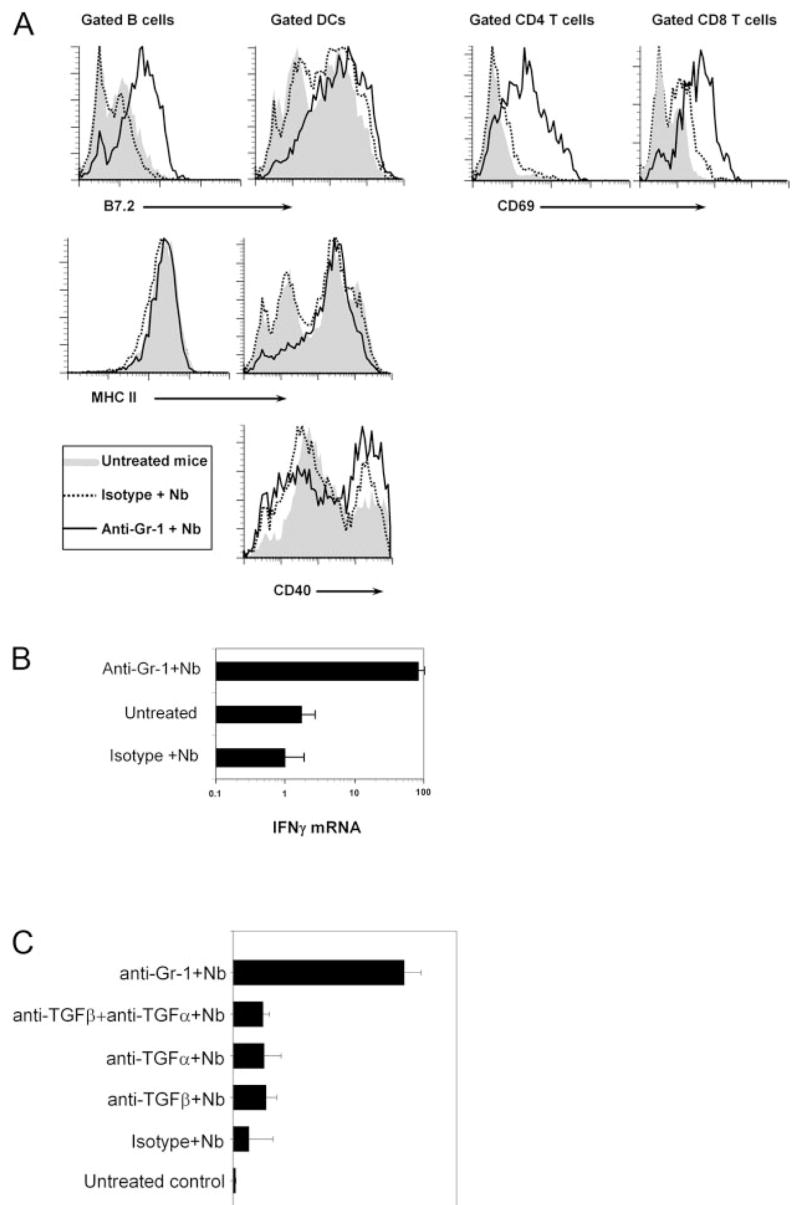
In the absence of Gr-1bright neutrophils, N. brasiliensis infection resulted in the rapid activation of immune cells and significantly increased IFN-γ expression. BALB/c mice were treated with anti-Gr-1 Ab to deplete Gr-1bright neutrophils, or given control Ig iso-type. One day later, the mice were inoculated in the ear with N. brasiliensis L3. The draining CLN were collected 18 h later. A, Single-cell suspensions were stained with anti-B220, anti-CD11c, and anti-CD4 or anti-CD8 plus anti-CD69, anti-B7.2, anti-MHC II, or anti-CD40 Abs and analyzed by FACS. Expression of cell surface costimulatory molecules was assessed on gated B220+ B cells and CD11c+ dendritic cells. Expression of CD69 was examined on gated CD4 T cells and CD8 T cells. This experiment was repeated twice with similar results. B, In a separate experiment, the collected CLN were further processed for RNA isolation and analyzed for IFN-γ gene expression using real-time quantitative fluorogenic RT-PCR. All data are expressed as relative to that of untreated controls, and the mean and SE are shown for each treatment group (5 mice/group). This experiment was repeated three times with similar results. C, BALB/c mice were administrated either with anti-Gr-1, anti-TGF-β, anti-TNF-α, or anti-TGF-β plus anti-TNF-α Abs, and 1 day later inoculated in the ear with N. brasiliensis L3. Gene expression of IFN-γ was analyzed by real-time quantitative fluorogenic RT-PCR. This experiment was repeated twice with similar results.
The increased activation of immune cells in the draining lymph nodes after N. brasiliensis infection in Gr-1-depleted mice may be associated with altered effector functions. Based on this, we were interested in examining the levels of effector cytokines at this early time point after N. brasiliensis inoculation. As shown in Fig. 3B, 18 h after N. brasiliensis infection, IFN-γ mRNA levels did not change in isotype-treated mice compared with untreated controls. However, N. brasiliensis infection induced increased IFN-γ expression after neutrophil depletion. Gene expression of IL-4 remained low in all groups at this time point (data not shown).
Neutrophils have been shown to express proinflammatory cytokines, including TNF-α, that can modify the Ag-specific T cell response (29, 30). TGF-β is one of the effector cytokines of T regulatory cells and was shown to suppress both Th1 and Th2 adaptive responses (31). Because the major cytokines produced by Gr-1bright neutrophils in this system were TGF-β and TNF-α, we next examined whether these cytokines inhibited IFN-γ expression after N. brasiliensis infection. To address this question, neutralizing Abs were used to block TGF-β and TNF-α bioactivity in vivo in mice inoculated with N. brasiliensis larvae, and IFN-γ expression in the draining lymph node was assessed 18 h later (Fig. 3C). Blocking TGF-β or TNF-α activity alone or together failed to increase IFN-γ expression in the draining lymph node similar to that observed in anti-Gr-1 Ab-treated and N. brasiliensis-infected mice. In addition, the cell surface expression of activation markers was not increased (data not shown). Thus, the increased immune cell activation and IFN-γ expression induced by N. brasiliensis after anti-Gr-1 Ab treatment were not due to the absence of TGF-β or TNF-α produced by neutrophils.
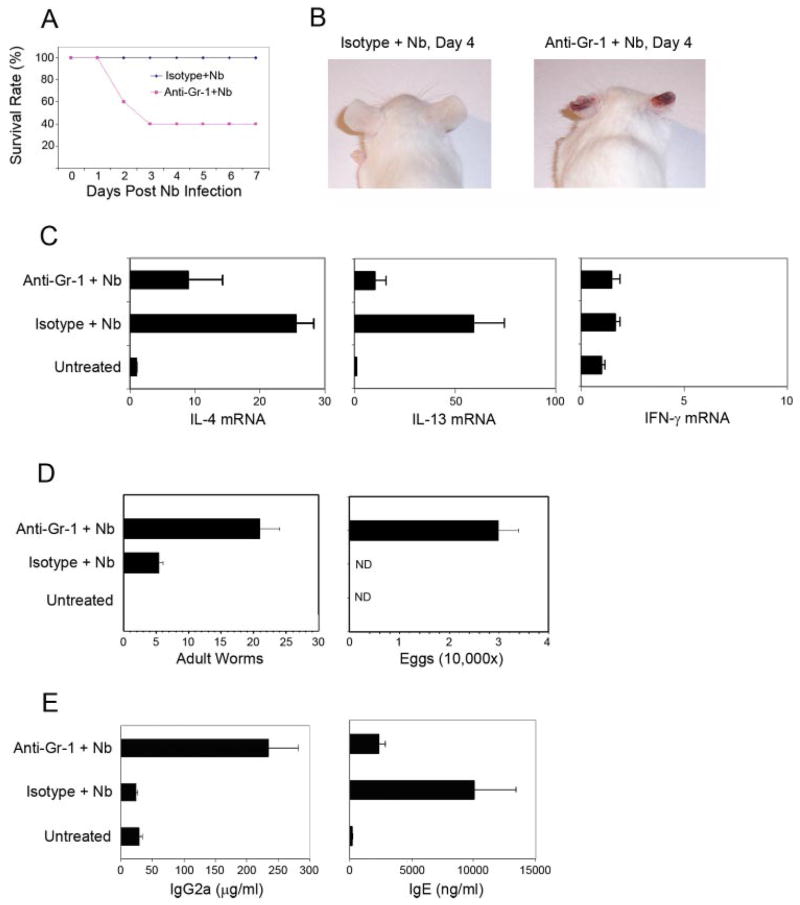
In the absence of neutrophils, N. brasiliensis infection results in significant mortality and impaired antihelminth Th2-type responses
The Th2-type immune response against N. brasiliensis infection is typically protective. Adult worms are expelled from the gut <2 wk after inoculation. After depletion of Gr-1bright neutrophils, N. brasiliensis inoculation resulted in a rapid and dramatic increase in IFN-γ expression. We hypothesized that this elevated IFN-γ expression during the early time point after N. brasiliensis infection could affect the development of the antihelminth Th2 immune response. Furthermore, because this Th2 response is critical for host protection, the absence of neutrophils might affect worm expulsion as well. To verify these possibilities, we monitored neutrophil-depleted mice after N. brasiliensis infection. By 48 h postinfection, a significant number of neutrophil-depleted mice died, and the mortality rate reached 60~80% by 72 h postinoculation (Fig. 4A). Several other neutrophil depletion experiments were performed in which a lower dose of larvae (300 instead of 500 L3) was administered and similar mortality rates were observed (data not shown). The surviving mice gradually recovered and were healthy at the end of these experiments (day 10 postinfection). In direct contrast, all isotype control Ab-treated N. brasiliensis-inoculated mice were healthy and survived. A slight inflammatory response was observed in the infection site (ear) of the isotype Ab-treated mice that resolved after day 3 postinfection (Fig. 4B); however, the inflammatory response at the infection site was much more severe in anti-Gr-1 Ab-treated mice. Areas of ear necrosis were observed in all of the Gr-1+ cell-depleted mice 3 days post-N. brasiliensis inoculation (Fig. 4B). The mice that were found dead in anti-Gr-1 Ab-treated groups were taken for necropsy (Research Animal Diagnostic Laboratory). A prominent Gram-negative rod bacterium colonized many tissues, including the lungs, liver, spleen, and CLN. Areas of cellular necrosis accompanied bacterial colonization in the spleen, liver, and CLN.
FIGURE 4.
Presence of Gr-1bright neutrophils promotes survival, wound healing, and host-protective Th2-type responses. BALB/c mice (5–10 mice/group) were injected with depleting anti-Gr-1 or isotype control Abs. One day later, these mice were inoculated in the ear with N. brasiliensis L3. Treated mice were monitored closely on a daily basis. A, Approximately 60–80% of Gr-1 cell-depleted mice were killed by N. brasiliensis infection on ~day 2 or 3. This experiment was repeated five times with similar results. B, A delayed-type hypersensitivity response developed at the infection site (ear) starting from day 2 postinfection in Gr-1 cell-depleted mice. This experiment was repeated five times with highly reproducible results. C, On day 7 postinfection, mesenteric lymph nodes were collected from surviving mice. RNA was purified and quantitative fluorogenic real-time RT-PCR was used to assess cytokine gene expression. This experiment was repeated three times with similar results. D, In a separate experiment, BALB/c mice were similarly treated and, 10 days post-N. brasiliensis inoculation, adult worms and egg production were determined. E, Total serum IgG2a and IgE levels were determined by ELISA. The mean and SE derived from five individual mice are shown for each treatment group. Graphs are representative of three individual experiments with similar results.
Mesenteric lymph nodes were collected from surviving mice at day 7 after N. brasiliensis inoculation, and processed to assess cytokine expression levels. As shown in Fig. 4C, significantly deceased levels of IL-4 expression were detected in N. brasiliensis-inoculated neutrophil-depleted mice compared with isotype Ig-treated mice; IL-13 expression levels were similarly down-regulated. Although Th2 cytokine expression was decreased after Gr-1 depletion, the immune response as detected on day 7 did not skew in a Th1 direction because IFN-γ expression was low (Fig. 4C, right panel). Similarly, draining CLN IL-4 expression on day 7 after N. brasiliensis infection was significantly decreased in mice administered anti-Gr-1 Ab with no elevation in IFN-γ expression (data not shown).
Consistent with the decreased Th2 response, worm burden and egg counts on day 10 were markedly increased in anti-Gr-1 Ab-treated mice (Fig. 4D). The Th2 response after N. brasiliensis infection is associated with significantly increased serum IgE levels. This IL-4-dependent serum IgE was decreased after anti-Gr-1 Ab treatment (Fig. 4E). Significantly increased levels of IFN-γ -dependent serum IgG2a were detected in N. brasiliensis-inoculated Gr-1-depleted mice compared with controls, providing confirmation of physiologically significant increased levels of IFN-γ. These data indicate that Gr-1bright neutrophils play critical roles in host protection against N. brasiliensis infection.
The selective inhibition of neutrophil recruitment to the draining lymph node of N. brasiliensis-inoculated CCL2KO mice provided the opportunity to confirm findings obtained with anti-Gr-1 Ab treatment. Draining CLN were removed from CCL2KO or control WT mice at 18 h after N. brasiliensis inoculation, and analyzed for Th1/Th2 cytokine mRNA. As shown in Fig. 5A, IFN-γ was markedly up-regulated in N. brasiliensis-inoculated CCL2-deficient BL/6 mice, but not in inoculated WT BL/6 mice by 18.0 h postinoculation. In contrast, IL-4 mRNA was not elevated in either CCL2-deficient or WT mice at this early time after N. brasiliensis inoculation. In addition, significantly increased levels of IFN-γ -dependent serum IgG2a were detected in N. brasiliensis-inoculated CCL2-deficient mice compared with inoculated WT controls (Fig. 5B). Furthermore, egg burden markedly increased in CCL2-deficient mice (Fig. 5B). These results are consistent with our previous data, obtained with anti-Gr-1 Ab treatment, suggesting that neutrophils are required for host protection and optimal Th2-type responses after N. brasiliensis infection.
FIGURE 5.
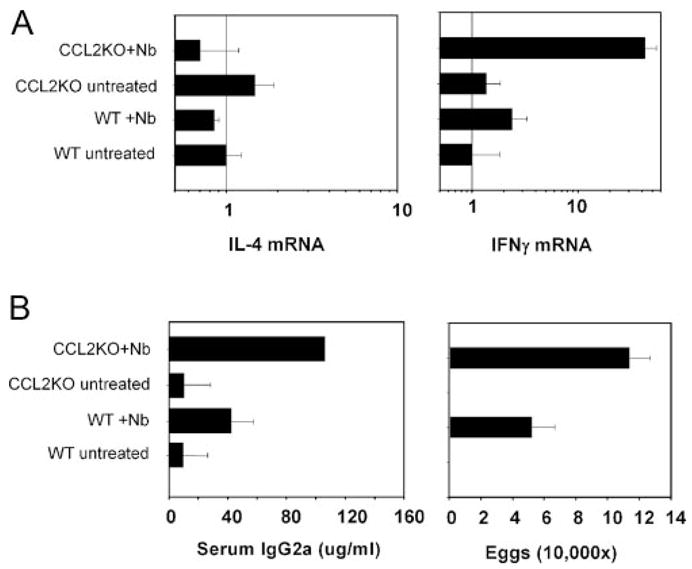
N. brasiliensis-infected CCL2KO mice showed increased Th1 response and suppressed host protectionA, CLN IL-4 and IFN-γ gene expression at 18 h after N. brasiliensis L3 inoculation was determined by real-time quantitative fluorogenic RT-PCR. B, In a separate experiment, tissue samples were collected 10 days post-N. brasiliensis infection, and serum IgG2a levels and egg production were assessed. This experiment was performed three times.
Control of larvae-associated bacteria by neutrophils is critical for host defense and optimal Th2 response development
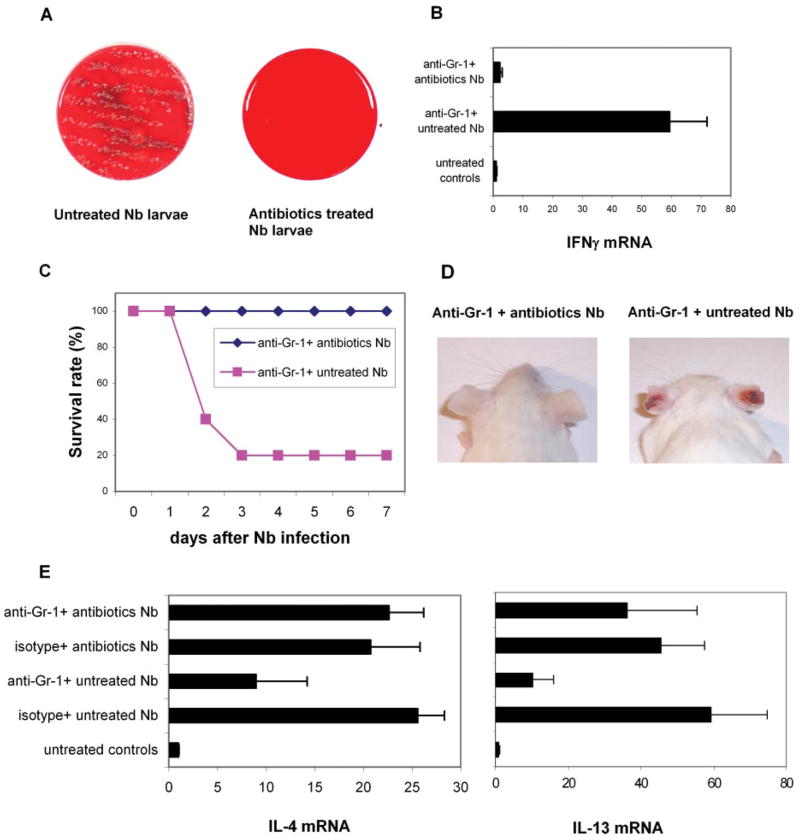
The findings of necrosis at the infection site and of systemic bacterial spreading in neutrophil-depleted N. brasiliensis-inoculated mice prompted us to investigate the influence of bacterial infection on the host response. It should be noted that we used standard N. brasiliensis propagation techniques, with charcoal and moss autoclaved before use. The larvae-associated bacteria most likely originate from feces in which the eggs developed. Bacteria are most likely ingested by free-living L3 and are also probably associated with their integument. Infective N. brasiliensis L3, along with any associated bacteria, penetrate (or are injected through) the host skin, enter the blood circulation, and 24 – 48 h later migrate to the lung. After neutrophil depletion, larvae-associated bacteria may spread quickly in the host. To test this hypothesis, we first examined whether bacteria are associated with N. brasiliensis infective larvae. Infective N. brasiliensis L3 were prepared following standard protocols. Five hundred larvae were washed with sterile PBS and then used to inoculate blood agar plates. After overnight culture at 37°C, large numbers of bacterial colonies were recorded (Fig. 6A, left panel). Another 500 N. brasiliensis L3 were treated with a mixture of antibiotics (penicillin plus streptomycin plus neomycin) for 2 h at room temperature. After several washes with sterile PBS, these larvae were inoculated onto another blood agar plate. Few, if any, bacteria colonies were observed on the agar plate after overnight culture in this condition (Fig. 6A, right panel).
FIGURE 6.
Infection with antibiotic-treated L3 blocked the effects of neutrophil depletion following N. brasiliensis inoculation. N. brasiliensis L3 were incubated with antibiotics (400 U of penicillin, 400 μg/ml streptomycin, and 400 μg/ml neomycin) or PBS for 2 h, and then washed with PBS three times. A, Treated and untreated N. brasiliensis L3 were placed on blood agar plate and cultured at 37°C for 24 h. This experiment was repeated three times with similar results. B, BALB/c mice (5/treatment group) were administered anti-Gr-1 Ab i.p., and 1 day later inoculated with antibiotic-treated or untreated N. brasiliensis L3. IFN-γ gene expression was assessed by real-time quantitative fluorogenic RT-PCR at 18 h after inoculation. C, In separate experiments, neutrophil-depleted mice were inoculated with antibiotic-treated or untreated N. brasiliensis L3, and the survival rate after N. brasiliensis infection was recorded. D, Delayed-type hypersensitivity developed at the infection site (right ear) of untreated, but not antibiotic-treated N. brasiliensis-inoculated neutrophil-depleted mice. E, On day 7 after infection, mesenteric lymph nodes were collected from surviving mice, and IL-4 and IL-13 mRNA levels were assessed by real-time quantitative fluorogenic RT-PCR. The mean and SE derived from five individual mice are shown for each treatment group. These experiments were repeated twice with similar results.
Because in vitro treatment with antibiotics could essentially eliminate the larvae-associated bacteria, we next examined the host response to antibiotic-treated N. brasiliensis L3 following administration of anti-Gr-1 Ab. Eighteen hours after ear inoculation, the draining CLN was removed and assessed for cytokine gene expression. Levels of IL-4 mRNA were unchanged in all groups at this early time point (data not shown). We have previously shown that inoculation of neutrophil-depleted mice with untreated N. brasiliensis L3 significantly increased immune cell activation and IFN-γ expression as detected at 18 h (Fig. 3, A and B). In direct contrast, after antibiotic mixture treatment, N. brasiliensis L3 failed to induce expression of immune cell surface activation markers (data not shown) or any elevation of IFN-γ expression in the lymph node (Fig. 6B).
Anti-Gr-1 Ab-treated mice inoculated with untreated N. brasiliensis L3 exhibit a markedly increased mortality rate (see Fig. 4A). We next tested whether the larvae-associated bacteria similarly contributed to the death of anti-Gr-1 Ab-treated mice inoculated with N. brasiliensis. L3 treated with or without antibiotics were inoculated in the ear of BALB/c WT mice 1 day after anti-Gr-1 Ab administration. Although 60~80% of anti-Gr-1 Ab-treated mice died after infection with untreated L3, none of the anti-Gr-1 Ab-treated mice that were infected with antibiotics-treated L3 showed any sign of illness, and all of them survived (Fig. 6C). In addition, there was no tissue necrosis at the infection site (ear) of the mice inoculated with antibiotics-treated larvae (Fig. 6D). It should be noted that Gr-1+ cells still migrated into the lymph node with a similar composition and frequency following injection with antibiotic-treated L3 (data not shown).
Because antibiotic-treated N. brasiliensis larvae did not induce a rapid IFN-γ response in the anti-Gr-1 Ab-treated host, we next examined whether antibiotic-treated larvae could dampen the protective Th2 response. Inoculation with antibiotic-treated L3 induced a potent IL-4 and IL-13 response in the draining lymph nodes following anti-Gr-1 Ab treatment, similar to that observed in isotype-treated mice inoculated with L3 (mesenteric lymph node, Fig. 6E; CLN, data not shown). In contrast, IFN-γ gene expression was not elevated on day 7 after N. brasiliensis inoculation. Furthermore, the potent Th2 response that developed in the presence of sterilized larvae was protective with all worms expelled around 2 wk and no eggs present in the feces after inoculation (data not shown). Thus, host protection was sustained following neutrophil depletion in mice infected with antibiotic-treated N. brasiliensis L3. These results suggest that the primary function of neutrophils during N. brasiliensis infection is to clear larvae-associated bacteria and provide an environment conducive to the development of a Th2-like immune response.
Discussion
N. brasiliensis induces one of the most potent and highly polarized Th2-type responses known. However, few studies have examined characteristics of the innate immune response that might influence the adaptive host-protective T cell response. In this study, we found that, shortly after N. brasiliensis inoculation, CCL2-dependent neutrophils transiently entered the lymph node, and that depletion of this population led to increased IFN-γ expression, mortality, decreased Th2 responses, and delayed worm expulsion. Treatment of infective larvae with antibiotics before inoculation abrogated the requirement of neutrophils, suggesting that neutrophils play a significant role in clearing bacteria associated with parasitic nematode infections, which would otherwise suppress the development of a host-protective Th2-type response.
By examining time points directly after inoculation with N. brasiliensis, we were able to identify multiple innate associated immune components, which were up-regulated before the development of IL-4-producing Th2 cells. Of particular interest, we noted the rapid accumulation in the draining lymph node of Gr-1bright neutrophils. Neutrophils comprise about two-thirds of peripheral blood leukocytes and are among the first cells that migrate to sites of inflammation, where they perform effector functions, including phagocytosis and the secretion of cytotoxic compounds. Early studies suggested that neutrophils are transcriptionally inactive due to their terminal differentiation; however, more recent studies have indicated that they are active components of the innate immune response (16). In addition, it has been suggested that neutrophils might carry pathogens/Ags to the lymphoid organs (29, 32, 33). This does not appear to be the case in N. brasiliensis-infected animals, because inoculation with larvae plus fluorescence-labeled protein Ag indicated that most of the lymph node-infiltrating neutrophils were not associated with the inoculated Ag (data not shown), although the distribution pattern of neutrophils in lymph nodes after N. brasiliensis infection is similar to previously reported data (29, 32, 33).
Neutrophils are also involved in host immunity to parasite infection. They are one of the first cell populations recruited to the site of T. gondii infection and are able to release proinflammatory cytokines and chemokines (including TNF-α and CCL2) (34, 35), which in turn contribute to recruitment, maturation, and activation of dendritic cells that can then drive the generation of the Th1-type immune response (17, 36). Although high levels of TGF--β and TNF-α were observed in lymph node-infiltrating neutrophils early after N. brasiliensis inoculation, our findings suggest that these cytokines did not have a significant impact on the development of the potent Th2-type response induced by N. brasiliensis inoculation.
Previous studies using various neutrophil depletion techniques have observed severe immunomodulatory consequences after exposure to fungal, protozoan, or nematode pathogens. Using CXCR2−/− mice, infection with T. gondii results in increased parasite burden and diminished Th1-associated immune factors (37), whereas the immune response to infection with the fungal agent Histoplasma capsulatum is significantly impaired in the absence of Gr-1+ neutrophils (38). Granulocytes directly affect Strongyloides stercoralis parasitic L3 housed in diffusion chambers in vivo (39), and neutrophil depletion before infection resulted in increased numbers of migrating larvae and egg production by day 5 after primary inoculation of Strongyloides ratti (40). Our findings indicate that during the immune response to N. brasiliensis, neutrophils control parasite-associated bacteria, which would otherwise impair the host-protective Th2-type immune response. When interpreting data showing regulatory function(s) for specific Gr-1+ cell populations, it is thus important to consider whether bacterial depletion by neutrophils may be an important modulating component of the immune response.
Our findings further showed that bacteria associated with N. brasiliensis initiate a potentially lethal bacterial infection following neutrophil depletion, which is most likely not observed in the Strongyloides model because parasites in this system are treated with antibiotics (40). Our data showed that antibiotic-treated or untreated larvae similarly induce Th2 immunity in mice with an intact neutrophil population. We interpret this as indicating that neutrophils play an essential role in clearing helminth-associated bacteria, which otherwise would induce a Th1-type response in the absence of neutrophils, thereby impairing the development of an effective Th2-type response. The importance of neutrophils in eliminating N. brasiliensis-associated bacteria was demonstrated by significantly increased mortality after anti-Gr-1 Ab treatment. This finding may be important during natural Strongyloides, or other similar parasite infections in immunocompromised patients.
As discussed in Results, the N. brasiliensis larvae-associated bacteria most likely originate from feces in which the eggs develop. Intestinal nematode larvae naturally develop under similar conditions, and it is thus likely that bacteria are also associated with parasitic nematodes during natural infection. Our experiments with the DO11.10 adoptive transfer system have been established with the intracutaneous ear injection inoculation model, partially because it induces a localized readily detectable response in the draining ear lymph node, while exhibiting a similar infection route and time course to s.c. inoculation. The process of skin penetration during natural infection may result in loss of bacteria to some degree. Further studies using different protocols, including studies in human parasitic nematode infections, are required to verify the significance of our observations.
Recent studies have shown that the host-protective Th2-type response to the intestinal nematode parasite, Trichuris muris, is substantially inhibited in CCL2−/− mice (25). We observed that the migration of neutrophils to the draining lymph nodes following inoculation with N. brasiliensis is CCL2 dependent. This is consistent with a previous study in which neutralization of CCL2 blocked airway accumulation of neutrophils (41). Although neutrophils failed to migrate to the draining lymph node in CCL2KO mice, they were capable of eradicating the bacteria, because the infected CCL2KO mice did not show increased mortality or ear necrosis (data not shown). It may be that, whereas neutrophils lack the ability to migrate to the lymph node in response to CCR2 signaling, the overall neutrophil population is still intact within the mouse and capable of controlling systemic bacterial infection, as opposed to anti-Gr-1 treatment, which systemically depletes the mice of neutrophils. It is important to note, however, that the inability of neutrophils to enter the lymph node early after infection did lead to significantly increased levels of IFN-γ production. Thus, CCL2-dependent rapid migration of neutrophils in response to infectious agents proves to be an important factor suppressing an alternative Th1-type response.
It should be noted that in the absence of neutrophils, N. brasiliensis inoculation resulted in greatly enhanced mortality despite marked and rapid elevations in IFN-γ production, suggesting that in the absence of neutrophils, the adaptive Th1-type response alone is not sufficient to effectively control bacterial infection. The early increased expression of IFN-γ in neutrophil-depleted mice also affected the N. brasiliensis-induced Th2 immune response. On day 7, IL-4/IL-13-expressing cells developed, but at a lower level, suggesting that early IFN-γ expression suppresses the optimal development of Th2-type immunity, leading to increased egg number and delayed worm expulsion. Our findings are supported by a previous study using exogenously administered IL-12 and anti-IFN-γ Ab, which suggested that Th1 cytokines can inhibit host protection against N. brasiliensis infection (42). The presence of neutrophils ensures an environment for optimal Th2 development by eliminating parasite-associated bacteria that otherwise induce a Th1-type response. Similarly, recent studies suggest that Gr-1+CD11b+ cells, which most likely include neutrophils, suppress Th1 responses while inducing Th2 polarization (43).
Our findings also suggest that, even following the early and pronounced Th1-type response resulting from neutrophil depletion, an active N. brasiliensis-dependent process ultimately drives a polarized, albeit attenuated, Th2-type response. This is not consistent with the default hypothesis, which postulates that a Th2-type response develops only in the absence of Th1-driving molecules (44), but does suggest that a bacteria-driven Th1-type response can suppress the development of an optimal Th2-type response if neutrophils are not present. In anti-Gr-1 Ab-treated mice, N. brasiliensis-associated bacteria induced rapid and dramatically increased expression of costimulatory molecules on dendritic cells and B cells. In contrast, when L3-associated bacteria were controlled by the presence of neutrophils, the activation level of dendritic cells was reduced (Fig. 4A). These data support the hypothesis that the development of Th2-type immune responses does not require optimal dendritic cell maturation (45). Endosymbiotic Wolbachia bacteria in filarid worms stimulate Th1-like proinflammatory responses (46). However, infection with live parasitic worms containing Wolbachia still induced a Th2-type dominant response (47, 48). Our results are similar, but also show the importance of innate immune components during N. brasiliensis infection that eliminate pathogenic bacteria exclusive of the pronounced adaptive Th1-type response. Our findings suggest that neutrophil recruitment to the lymph node prevents a bacterial-induced Th1-type response that would interfere with a host-protective Th2 response to worm infection.
In summary, the current study suggests a previously unknown essential role for neutrophils at early stages of nematode parasite infections. Our findings suggest that clearance of larvae-associated bacteria by neutrophils is critical for wound healing, host protection, and optimal development of the adaptive Th2-type response leading to worm expulsion. In addition to increased mortality, failure to control bacterial spreading significantly affects the developing Th2 immune response and delays worm expulsion. This may be of particular significance in immune compromised individuals infected by helminths or in the development of vaccines that may require stimulation of both Th1-type and Th2-type components of the response to protect against parenteral helminth infection.
Footnotes
This work was supported by National Institutes of Health Grants AI031678 and AI066188.
Abbreviations used in this paper: CLN, cervical lymph node; HPRT, hypoxanthine phosphoribosyltransferase; KO, knockout; WT, wild type.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Gause WC, Urban JF, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 2.Ogilvie BM, Hockley DJ. Effects of immunity of Nippostrongylus brasiliensis adult worms: reversible and irreversible changes in infectivity, reproduction, and morphology. J Parasitol. 1968;54:1073–1084. [PubMed] [Google Scholar]
- 3.Liu Z, Liu Q, Pesce J, Whitmire J, Ekkens MJ, Foster A, VanNoy J, Sharpe AH, Urban JF, Jr, Gause WC. Nippostrongylus brasiliensis can induce B7-independent antigen-specific development of IL-4-producing T cells from naive CD4 T cells in vivo. J Immunol. 2002;169:6959– 6968. doi: 10.4049/jimmunol.169.12.6959. [DOI] [PubMed] [Google Scholar]
- 4.Trujillo-Vargas CM, Mayer KD, Bickert T, Palmetshofer A, Grunewald S, Ramirez-Pineda JR, Polte T, Hansen G, Wohlleben G, Erb KJ. Vaccinations with T-helper type 1 directing adjuvants have different suppressive effects on the development of allergen-induced T-helper type 2 responses. Clin Exp Allergy. 2005;35:1003–1013. doi: 10.1111/j.1365-2222.2005.02287.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Liu Q, Hamed H, Anthony RM, Foster A, Finkelman FD, Urban JF, Jr, Gause WC. IL-2 and autocrine IL-4 drive the in vivo development of antigen-specific Th2 T cells elicited by nematode parasites. J Immunol. 2005;174:2242–2249. doi: 10.4049/jimmunol.174.4.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 7.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med. 2004;200:857– 870. doi: 10.1084/jem.20040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 11.Anderson KL, Smith KA, Pio F, Torbett BE, Maki RA. Neutrophils deficient in PU.1 do not terminally differentiate or become functionally competent. Blood. 1998;92:1576–1585. [PubMed] [Google Scholar]
- 12.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 13.Nakano H, Yanagita M, Gunn MD. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71– 82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 15.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 16.Appelberg R. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 2006;15:87–92. doi: 10.1016/j.tim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052– 6058. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- 18.Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P, Milon G, Louis JA. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol. 2000;165:2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 19.Camberis M, Le Gros G, Urban J., Jr . Current Protocols in Immunology. Vol. 19.12. John Wiley & Sons; Hoboken, NJ: 2003. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus; pp. 1–27. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Liu Q, Pesce J, Anthony RM, Lamb E, Whitmire J, Hamed H, Morimoto M, Urban JF, Jr, Gause WC. Requirements for the development of IL-4-producing T cells during intestinal nematode infections: what it takes to make a Th2 cell in vivo. Immunol Rev. 2004;201:57–74. doi: 10.1111/j.0105-2896.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 21.Pattyn F, Robbrecht P, De Paepe A, Speleman F, Vandesompele J. RTPrimerDB: the real-time PCR primer and probe database, major update 2006. Nucleic Acids Res. 2006;34:D684–D688. doi: 10.1093/nar/gkj155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekkens MJ, Liu Z, Liu Q, Whitmire J, Xiao S, Foster A, Pesce J, VanNoy J, Sharpe AH, Urban JF, Gause WC. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J Immunol. 2003;170:384–393. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 23.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601– 608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.deSchoolmeester ML, Little MC, Rollins BJ, Else KJ. Absence of CC chemokine ligand 2 results in an altered Th1/Th2 cytokine balance and failure to expel Trichuris muris infection. J Immunol. 2003;170:4693– 4700. doi: 10.4049/jimmunol.170.9.4693. [DOI] [PubMed] [Google Scholar]
- 26.Alam R, York J, Boyars M, Stafford S, Grant JA, Lee J, Forsythe P, Sim T, Ida N. Increased MCP-1, RANTES, and MIP-1α in bronchoalveolar lavage fluid of allergic asthmatic patients. Am J Respir Crit Care Med. 1996;153:1398–1404. doi: 10.1164/ajrccm.153.4.8616572. [DOI] [PubMed] [Google Scholar]
- 27.Sousa AR, Lane SJ, Nakhosteen JA, Yoshimura T, Lee TH, Poston RN. Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am J Respir Cell Mol Biol. 1994;10:142–147. doi: 10.1165/ajrcmb.10.2.8110469. [DOI] [PubMed] [Google Scholar]
- 28.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407– 411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 29.Maletto BA, Ropolo AS, Alignani DO, Liscovsky MV, Ranocchia RP, Moron VG, Pistoresi-Palencia MC. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood. 2006;108:3094–3102. doi: 10.1182/blood-2006-04-016659. [DOI] [PubMed] [Google Scholar]
- 30.Ashtekar AR, Saha B. Poly’s plea: membership to the club of APCs. Trends Immunol. 2003;24:485– 490. doi: 10.1016/s1471-4906(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 31.Wahl SM, Wen J, Moutsopoulos N. TGF--β: a mobile purveyor of immune privilege. Immunol Rev. 2006;213:213–227. doi: 10.1111/j.1600-065X.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 32.Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- 33.Bonneau M, Epardaud M, Payot F, Niborski V, Thoulouze MI, Bernex F, Charley B, Riffault S, Guilloteau LA, Schwartz-Cornil I. Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node. J Leukocyte Biol. 2006;79:268–276. doi: 10.1189/jlb.0605288. [DOI] [PubMed] [Google Scholar]
- 34.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J Immunol. 1996;157:798– 805. [PubMed] [Google Scholar]
- 35.Del Rio L, Butcher BA, Bennouna S, Hieny S, Sher A, Denkers EY. Toxoplasma gondii triggers myeloid differentiation factor 88-dependent IL-12 and chemokine ligand 2 (monocyte chemoattractant protein 1) responses using distinct parasite molecules and host receptors. J Immunol. 2004;172:6954– 6960. doi: 10.4049/jimmunol.172.11.6954. [DOI] [PubMed] [Google Scholar]
- 36.Denkers EY, Butcher BA, Del Rio L, Bennouna S. Neutrophils, dendritic cells and Toxoplasma. Int J Parasitol. 2004;34:411– 421. doi: 10.1016/j.ijpara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Del Rio L, Bennouna S, Salinas J, Denkers EY. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Tox-oplasma gondii infection. J Immunol. 2001;167:6503– 6509. doi: 10.4049/jimmunol.167.11.6503. [DOI] [PubMed] [Google Scholar]
- 38.Sa-Nunes A, Medeiros AI, Sorgi CA, Soares EG, Maffei CM, Silva CL, Faccioli LH. Gr-1+ cells play an essential role in an experimental model of disseminated histoplasmosis. Microbes Infect. 2007;9:1393–1401. doi: 10.1016/j.micinf.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Rotman HL, Yutanawiboonchai W, Brigandi RA, Leon O, Gleich GJ, Nolan TJ, Schad GA, Abraham D. Strongyloides stercoralis: eosinophil-dependent immune-mediated killing of third stage larvae in BALB/cByJ mice. Exp Parasitol. 1996;82:267–278. doi: 10.1006/expr.1996.0034. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe K, Noda K, Hamano S, Koga M, Kishihara K, Nomoto K, Tada I. The crucial role of granulocytes in the early host defense against Strongyloides ratti infection in mice. Parasitol Res. 2000;86:188–193. doi: 10.1007/s004360050030. [DOI] [PubMed] [Google Scholar]
- 41.Beck-Schimmer B, Schwendener R, Pasch T, Reyes L, Booy C, Schimmer RC. Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respir Res. 2005;6:61. doi: 10.1186/1465-9921-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finkelman FD, Madden KB, Cheever AW, Katona IM, Morris SC, Gately MK, Hubbard BR, Gause WC, Urban JF. Effects of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994;179:1563–1572. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al Quran SZ, et al. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jankovic D, Liu Z, Gause WC. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 2001;22:450– 457. doi: 10.1016/s1471-4906(01)01975-5. [DOI] [PubMed] [Google Scholar]
- 45.Pearce EJ, Kane CM, Sun J. Regulation of dendritic cell function by pathogen-derived molecules plays a key role in dictating the outcome of the adaptive immune response. Chem Immunol Allergy. 2006;90:82–90. doi: 10.1159/000088882. [DOI] [PubMed] [Google Scholar]
- 46.Taylor MJ, Cross HF, Bilo K. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J Exp Med. 2000;191:1429–1436. doi: 10.1084/jem.191.8.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loke P, MacDonald AS, Robb A, Maizels RM, Allen JE. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur J Immunol. 2000;30:2669–2678. doi: 10.1002/1521-4141(200009)30:9<2669::AID-IMMU2669>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]