Abstract
The anti-apoptosis protein, survivin, promotes cell survival and mitosis. Recent studies have demonstrated that survivin is expressed in normal gastric mucosa. Using an in vitro model, we examined whether survivin plays a role in the cytoprotection produced in gastric mucosa by mild irritant ethanol (ETOH) against subsequent exposure to concentrated ETOH. Pretreatment of rat gastric epithelial cells with 1% ETOH reduced cell death, in response to subsequent incubation with 5% ETOH, by 94% (P<0.005). This pretreatment also resulted in increased total and phosphorylated survivin protein levels by 180% (P<0.0001) and 540% (P<0.0002), respectively, which required the p34cdc2 cell cycle-dependent kinase. The cytoprotective effect was abrogated upon siRNA knockdown of survivin protein levels. Further, overexpression of exogenous survivin resulted in significant cytoprotection by 62% (P<0.02) in the absence of any pretreatment. We further examined the in vivo relevance of these findings. In fasted rats, administration of 20% ETOH, which we found to be 93% (P<0.0001) cytoprotective against 50% ETOH challenge, resulted in increased total and phosphorylated survivin protein levels by 234% (P<0.001) and 214% (P<0.02), respectively. Administration of 20% ETOH resulted in increased gastric p34cdc2 activity by 146% (P<0.01). Inhibition of p34cdc2 by the potent inhibitor, roscovitine, abolished the increased survivin levels in response to pre-administration of 20% ETOH and reduced the cytoprotection against 50% ETOH challenge by 59% (P<0.01). These results indicate that survivin is a key mediator of cytoprotection against ETOH-induced gastric injury, acting at the epithelial cell level, by a mechanism that is dependent, in part, on p34cdc2.
Keywords: • Adaptation, • Apoptosis, • Cell death, • Inhibitor, • Phosphorylation
Introduction
Adaptive cytoprotection is the term describing the phenomenon whereby necrotizing agents in low or ‘mild irritant’ concentration (e.g. 10−25% ETOH, 0.35N HCl), when applied to the gastric mucosa as a pre-treatment, prevent the necrosis produced by full-strength necrotizing agents such as absolute ETOH, 0.6N HCl, 0.2N NaOH and 80mM taurocholate (Robert et al., 1983; Smith et al., 1991; Kokoska et al., 1998a). The underlying molecular mechanisms that mediate adaptive cytoprotection at the cellular level remain incompletely defined. “Adaptive cytoprotection,” as originally defined, has been primarily postulated to be a consequence of increased endogenous prostaglandin synthesis. However, several studies indicate that other factors, which are either unrelated or are only indirectly related to prostaglandin function, likely play significant roles in the phenomenon of cytoprotection (Hawkey et al., 1988; Balaa, 1989; Schmidt et al., 1989; Mutoh et al., 1995; Ko and Cho, 1996; Ko and Cho, 1998; Kokoska et al., 1998b; Kokoska et al., 1998c; Rokutan et al., 1998). Other proposed mediators of cytoprotection include nitric oxide, vagal innervation, sensory nerves, blood flow, Ca2+ influx, heat shock proteins and a physical barrier resulting from mucosal surface exfoliation (Hawkey et al., 1988; Ko and Cho, 1998; Kokoska et al., 1998c, Rokutan et al., 1998). Consistent with the involvement of other factors, several studies using in vitro models have demonstrated that cytoprotection at the gastric epithelial cell level is either entirely prostaglandin-independent or only partly dependent on prostaglandins (Mutoh et al., 1995; Tsutsumi et al., 2003; Tanaka et al., 2004).
Survivin, a member of the Inhibitor of Apoptosis Protein (IAP) family, regulates both cell division and cell survival (Chiou et al., 2003a; Wheatley and McNeish, 2005; Lens et al., 2006). Survivin is expressed in most tissues during development and survivin gene deletion is embryonic lethal. Although survivin expression is absent in most non-cancerous adult differentiated tissues, some normal adult cell types do express survivin including thymocytes, CD34-expressing bone marrow stem cells, and basal colonic and gastric mucosal epithelial cells (Fukuda and Pelus, 2001; Gianani et al., 2001; Kobayashi et al., 2002; Chiou et al., 2003b). A role for survivin in maintaining the cellular integrity of differentiated tissues in which it is normally expressed has not been extensively explored. In light of the recent intensity with which survivin is being studied as a target for cancer therapy that should have little or no effect on the function of normal, non-cancerous tissue cells, we examined whether survivin might play a role in normal gastric mucosal integrity and specifically in gastric cytoprotection. We chose to first examine an in vitro model of gastric epithelial cell cytoprotection to avoid possible confounding contributions of blood flow/systemic factors and neuronal and immune responses; and, so that we could more easily manipulate survivin expression and function. We next examined the relevance of our in vitro findings using an in vivo rat model of gastric cytoprotection against ethanol-induced injury.
Materials and Methods
Alcohol-induced gastric Injury
All animal studies were approved by the Institutional Animal Care and Use Committee of the VA Long Beach Healthcare System. Male Sprague-Dawley rats (Charles River Labs, Wilmington, MA) weighing 225−250 g were used and were fasted for 18 hours prior to the studies. Thirty minutes prior to intragastric (i.g.) administration of 1.5 ml 50 % ethanol (v/v in water) or water (controls), rats received i.g. either vehicle (water) or 20% ethanol (v/v in water). In some studies, rats received 2.8 mg/kg roscovitine (LC Laboratories, Woburn, MA) or vehicle (dimethyl sulphoxide) control by intraperitoneal injection one hour prior to i.g. administration of the water or 20% ethanol pretreatment. The dosage of roscovitine was based on a previous study showing that this dose produced no apparent cytotoxic effects in rats (Pippin et al., 1997). For cytoprotection studies, groups of these rats were also administered 1.5 ml 50% ethanol or water 30 minutes following the mild irritant ethanol pretreatment. At 30 minutes and 6 hours after final ethanol or water administration, the rats were anesthetized, their stomachs were excised, and the animals were euthanized. The stomachs were then opened along the greater curvature, rinsed with 0.9% NaCl, and examined visually. The macroscopically visible hemorrhagic mucosal erosions were photographed in a standardized manner and evaluated by image analysis (Tarnawski et al., 1985). The area of hemorrhagic erosion was expressed as percentage of total glandular area (Tarnawski et al., 1985). Gastric tissue (1.5−2 mm in width) from non-hemorrhagic areas immediately adjacent to necrotic lesions was excised and flash-frozen in liquid nitrogen for subsequent molecular biology studies.
Thymocyte isolation
Rat primary thymocytes were isolated following an established method (Culvenor and Weidemann, 1976). Briefly, thymus glands were removed from euthanized rats and the thymocytes were teased out by passage through a 100 μm steel mesh. Fat cells, dead cells and cellular debris were removed by low speed centrifugation and thymocyte viability (>98%) was determined by trypan blue exclusion. The thymocytes were first washed several times in serum-free RPMI 1640 basal medium and then incubated in the same medium containing the indicated concentration(s) of ethanol or equal volume of water (control). Cells were lysed or medium was collected following the indicated incubation times.
Cell culture
RGM1, an epithelial cell line derived from normal rat gastric mucosa, was obtained from Riken Cell Bank (Tsukuba, Japan) and was used at passages 11−20. The cells were maintained in DMEM/F12 medium containing 20% FBS and 2 mM of L-glutamine at 37°C with 5% CO2 and 95% air in a humidified incubator. IEC-6, a non-transformed intestinal epithelial cell line, was maintained in DMEM medium supplemented with 4 mM L-glutamine, 10% FBS and 0.1 Unit/ml bovine insulin. Cells were plated in 100 mm dishes at a density of 4.0×106 cells/dish, or in 6-well plates at a density of 4.0×105 cells/well and incubated until ∼80% confluent. Cells were serum starved for 24 hours prior to experiments. Serum-free conditions were employed to avoid possible confounding effects of protective factors contained in the serum and because serum resulted in interference with the LDH assay used to assess cell damage (see below). The cells were then incubated in serum-free medium containing vehicle, 0.1μM PGE2, 0.1μM PGI2 (Cayman Chemical, Ann Arbor, Michigan) or 0.1μM PGE2 + 0.1μM PGI2 for 30 minutes prior to incubation in serum-free medium containing the indicated concentration of ethanol or equal volume of water (control). Cells were lysed or medium was collected following the indicated incubation times. For the inhibitor studies, cells were incubated in serum-free medium containing 10 μg/ml cycloheximide (Sigma Chemicals), 2 μg/ml actinomycin-D (Sigma Chemicals) or vehicle (controls) for 30 minutes; or, serum-free medium containing 2 μM roscovitine (LC Laboratories, Woburn, MA), 0.2 μM olomoucine II (Sigma Chemicals, St Louis, MO), 0.2 μM purvalanol A (Sigma Chemicals) or vehicle (controls) for 1 hour, prior to incubation in serum-free medium containing the indicated concentration(s) of ethanol or equal volume of water (control). For dose-response studies, cells were incubated in serum-free medium containing the indicated concentrations of roscovitine for 1 hour, prior to incubation in serum-free medium containing 1% ethanol. In some studies, cells were incubated in serum-free medium containing the indicated concentration(s) of sodium deoxycholate (Sigma Chemicals) or equal volume of water (control). Cells were lysed or medium was collected following the indicated incubation times.
Immunoblot analysis
Frozen specimens of gastric tissue were homogenized with a Polytron homogenizer in a lysis buffer containing 62.5 mM ethylenediaminetetraacetic acid; 50 mM Tris-HCL, pH 8.0; 0.4% deoxycholic acid; 1% Nonidet P-40; 0.5 μg/ml leupeptin; 0.5 μg/ml pepstatin; 0.5 μg/ml aprotinin; 0.2 mM phenylmethylsulfonyl fluoride; 0.05 mM aminoethyl benzene sulfonyl fluoride 0.1 mM sodium vanadate. Primary thymocytes, RGM1 and IEC-6 cells were lysed in the same lysis buffer following the indicated treatment(s). The tissue homogenates and cell lysates were then centrifuged (14,000 rpm for 10 minutes at 4°C). The protein content of the homogenates/lysates was determined by the bicinchoninic acid protein assay using a commercial kit (BCA Protein Assay Reagent, Pierce Chemical Company, Rockford, IL.) or by modified Bradford method using a commercial Bradford protein assay kit (Biorad, Hercules, CA). Total survivin protein, representing both unphosphorylated and phosphorylated survivin protein, phosphorylated survivin (p-survivin) protein, total p34cdc2 protein, representing both unphosphorylated and phosphorylated p34cdc2 protein, and phosphorylated p34cdc2 (p-Tyr15 p34cdc2) protein were determined from equal amounts of total tissue homogenate or cell lysate protein. The total survivin signal was detected with rabbit polyclonal anti-survivin antibody (Novus Biologicals, Littleton, CO). The phosphorylated survivin signal was detected with goat polyclonal anti-phosphorylated survivin at threonine-34 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Expression of the survivin HA-tagged fusion protein was detected with rabbit polyclonal anti-HA antibody (Sigma Chemical Co., St. Louis, MO). For determination of phosphorylated p34cdc2, the p-Tyr15 p34cdc2 signal was detected with mouse monoclonal anti-phosphorylated p34cdc2 at tyrosine-15 antibody (Stressgen, Ann Arbor, Michigan). The total p34cdc2 protein signal was detected with rabbit polyclonal anti-p34cdc2 antibody (Abcam, Cambridge, MA). All membranes were also stripped and reprobed with mouse monoclonal anti-β-actin antibody (Sigma Chemical Co., St. Louis, MO) to control for protein loading and membrane transfer. Signals were visualized by enhanced chemiluminescence (Amersham Life Science, Arlington Heights, IL). Quantification of the data was performed using a video image analysis system (Image-1/FL; Universal Imaging, Westchester, PA) after normalizing for the corresponding total protein and/or β-actin signal.
Determination of p34cdc2 kinase activity
The relative activities of p34cdc2 kinase were determined using an ELISA-based assay kit (MBL International, Woburn, MA). Briefly, RGM1 and IEC-6 cells or gastric tissues were homogenized in lysis buffer [50 mM Tris (pH 7.5), 0.5 M NaCl, 5 mM EDTA, 2 mM EGTA, 0.01% Brij35, 25 mM glycerophosphate, 50 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 1 mM sodium fluoride and 1 mM sodium orthovanadate]. Protein content of tissue homogenates and cell lysates was determined by modified Bradford method using a commercial Bradford protein assay kit (Biorad, Hercules, CA). Equal amounts of tissue homogenates or cell lysates (300 μg in 500 μl) were added to 20 μl HCK (p34cdc2 binding peptide-linked sepharose) gel suspension (MBL, Woburn, MA) and incubated overnight at 4 °C. The p34cdc2 precipitates were centrifuged and washed 3 times with lysis buffer and 2 times with 25 mM HEPES, pH 7.5, 10 mM MgCl2. The p34cdc2-kinase activity of the precipitates was determined by incubation in 50 μl of reaction buffer [25 mM HEPES, 10 mM MgCl2, 0.1 mM ATP, and 0.6 mM biotinylated p34cdc2-specific (MV) peptide substrate] for 10 min at 30 °C. Reactions were stopped by addition of 200 μl of 20% H3PO4, and the relative amounts of phosphorylated peptide substrate captured on a 96-well plate coated with specific antibody were determined by reaction with horse radish peroxidase-conjugated streptavidin using a plate reader at 492 nm.
Immunofluorescence staining and in vitro TUNEL assay
Formalin fixed, paraffin-embedded tissue sections were used for staining. For immunofluorescence staining, sections were incubated with rabbit polyclonal anti-survivin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for either 1 hr at room temperature or 16 hours at 4°C. The sections were then stained with fluorescein isothiocyanate (FITC)-conjugated goat-anti-rabbit secondary antibody (Sigma Chemical Co., St. Louis, MO) for one hour. Staining was visualized using a Nikon Optiphot epifluoresence microscope (Nikon, Inc.) with Omega FITC/Texas Red filter. Specificity of staining was confirmed by omitting the primary antibody and using appropriate blocking peptide. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end-labeling (TUNEL) assay was performed using an Apop Tag in situ detection kit (Serologicals Corporation, Norcross, GA) according to the manufacturer's instructions. For assessing apoptosis in vitro by TUNEL assay, RGM1 transfected or control cells grown on sterile microscope coverslips in 6 well culture dishes were serum-starved and treated with the indicated concentrations of ETOH in serum-free culture medium for the indicated time increment(s). The cells were then gently washed with PBS and fixed with 1% paraformaldehyde in PBS for 20 minutes at room temperature. Percent apoptosis was determined by counting the number of TUNEL-positive cells in 5 random fields and dividing by the total number of cells in the same field under 100X magnification. Images were captured using a camera attached to a Nikon Optiphot microscope (Nikon, Inc., Melville, NY).
Survivin suppression using siRNA
Double stranded short interfering RNA (siRNA) oligonucleotides were designed with a software program (www.qiagen.com) to be specific for rat survivin. The double stranded siRNA was composed of the following oligonucleotides: r(UGAGCCUGAUUUGGCCCAG)d(TT) and r(CUGGGCCAAAUCAGGCUCA)d(TT) (Qiagen-Xeragon, Germantown, MD). A fluorescein labeled double stranded siRNA having no known homology with mammalian genes (Qiagen-Xeragon) was used to control for nonspecific silencing effects and to assess efficiency of transfection. RGM1 cells were seeded in 6-well plates (or on sterile microscope coverslips in 6-well plates) at 105 cells per well 24 hours prior to transfection. The cells were transfected with 200nM double-stranded survivin siRNA (or equimolar control RNA) complexed with 3 μl of Oligofectamine transfection reagent (Invitrogen, Carlsbad, CA), according to manufacturers protocol, in 1 ml of Opti-MEM I reduced serum medium (Invitrogen). Control cells were also treated with the oligofectamine reagent in the absence of RNA to control for possible effects of the reagent. The growth medium was replaced 24 hours post transfection with serum-free medium and the cells were incubated an additional 24 hours. For determining the extent of suppressed survivin expression levels, cells were lysed on ice and survivin protein levels were assessed by immunoblot analysis as described above. For determination of resistance to ETOH-induced cell damage (see below) and apoptosis (TUNEL), serum-starved transfected cells were incubated in serum-free medium containing the indicated concentrations of ETOH for the indicated times.
Survivin overexpression studies
Total RNA from normal (non-injured) rat gastric tissue was reverse transcribed and used to amplify a cDNA fragment encoding the complete wild type rat survivin sequence with the following primers: 5'-GAATTCGATCATGGGTGCTACGGC-3' (sense) and 5'-GGATCCGCGTAAGGCAGCTGCTCAATG-3' (antisense). The added restriction sites for cloning (EcoRI and BamHI, respectively) are italicized and the corresponding specific rat survivin sequences are underlined. The resulting PCR product was directionally ligated into both the pcDNA3.1(−) (Invitrogen, Carlsbad, CA) mammalian expression vector, which does not encode a peptide tag sequence, and the phCMV3 mammalian expression vector (Genlantis, San Diego, CA) to create a C-terminal HA tag. The C-terminal HA tag construct was also used to create a threonine-117-to-alanine (T117A) survivin point mutation using a QuickChange II site-directed mutagenesis kit (Stratagene, San Diego, CA) according to the manufacture's instructions. Each construct was subsequently sequenced to ensure proper insertion with respect to the HA-tag sequence and/or plasmid promoter as well as fidelity of the RT-PCR reaction (Davis Sequencing, Inc., Davis CA). The pcDNA3 plasmid constructs harboring the threonine-34-to-alanine (T34A) HA tagged and threonine-34-to-glutamate (T34E) HA tagged survivin mutants were purchased from Health Research, Inc. (Buffalo, NY). RGM1 cells seeded at 5 × 105 in 60 mm culture dishes were transfected with 2 μg each of survivin pcDNA3.1 plasmid, survivin-HA phCMV3 plasmid, T117A mutant survivin-HA phCMV3 plasmid, T34A mutant survivin-HA pcDNA3 plasmid, T34E mutant survivin-HA pcDNA3 plasmid or 2 μg of the respective “empty” pcDNA3.1, phCMV3 and pcDNA3 (Invitrogen) plasmids (to obtain corresponding control transfectants) complexed with LipofectAMINE reagent (Invitrogen) according to manufacturer's instructions. Following the transfection, cells were allowed to recover for 2−3 days until confluent and stable transfectants were selected in growth medium containing 400 μg/ml Geneticin (Invitrogen). Clones were selected and survivin, HA-tagged survivin and HA-tagged mutant survivin protein expression levels were assessed by immunoblot analysis as described above. The clones expressing the highest level of survivin, HA-tagged survivin and HA-tagged mutant survivin protein by 3 independent determinations were used for subsequent studies. Although clonal cell populations were maintained in selection medium, non-selection culture medium was used for subsequent studies. For determination of resistance to ETOH-induced cell damage (see below) and apoptosis (TUNEL), serum-starved transfected cells were incubated in serum-free medium containing the indicated concentrations of ETOH for the indicated times.
Determination of cell damage
Culture medium, collected following cell incubation under the indicated conditions, was assayed for lactate dehydrogenase (LDH) release (an indicator of cellular damage) using a colorimetric assay kit (Genotech, St. Louis, MO) according to the manufacturer's instructions. Optical density (OD) was measured at 490 nm using a DU640B spectrophotometer (Beckman Coulter, Fullerton, CA). Percent cell damage was quantified as: % Cell Damage = (experimental OD490 − spontaneous OD490) ÷ maximum OD490 × 100 Experimental OD was determined from serum-free culture medium of cells cultured under the indicated condition (e.g. ETOH concentration and incubation time). Spontaneous OD was determined from serum-free culture medium of like cells (e.g. thymocytes, IEC-6, RGM1). Maximum OD was determined by sonication disruption of an equal number of like cells in an equal volume of serum-free culture medium (Chiou et al., 2005).
Determination of Caspase-3-like activity
RGM1 cellular extracts were assayed for caspase-3-like activity using a commercial colorimetric assay kit (Biomol, Plymouth Meeting, PA) based on a tetrapeptide conjugated to phenylnitroaniline (DEVD-pNA). Briefly, serum-starved RGM1 cells were exposed to the indicated concentration of ETOH or equal volume of water (control) in serum-free DMEM/F12. At the indicated time following ETOH exposure, the cells were lysed in lysis buffer (50 mM HEPES, pH 7.4, 0.1% CHAPS, 0.1% NP-40, 5 mM dithiothreitol, 0.1 mM EDTA) and the lysates were then centrifuged at 10,000 × g for 10 min at 4°C. The total protein contents of the supernatants were determined by modified Bradford method using a commercial Bradford protein assay kit (Biorad, Hercules, CA). The released chromophore was measured by determining the absorbance at 405 nm over a 60 minute period following addition of substrate. Specific activity was determined using a phenylnitroaniline (pNA) calibration standard and is expressed as pmol pNA/min/μg total protein after normalizing for protein content.
Statistical analysis
Comparisons of macroscopic gastric injury were performed using the Kruskal-Wilcoxon tests (Tarnawski et al., 1985). Results are expressed as mean ± standard deviation (SD). Student's 2-tailed t test was used to determine statistical significance between control and experimental groups. A P value of <0.05 was considered statistically significant. Comparisons of data between multiple groups were made with analysis of variance (ANOVA) followed by Bonferroni correction.
Results
Pretreatment of gastric mucosal epithelial cells with cytoprotective ethanol (ETOH) concentrations produces increased expression levels of total and phosphorylated survivin protein
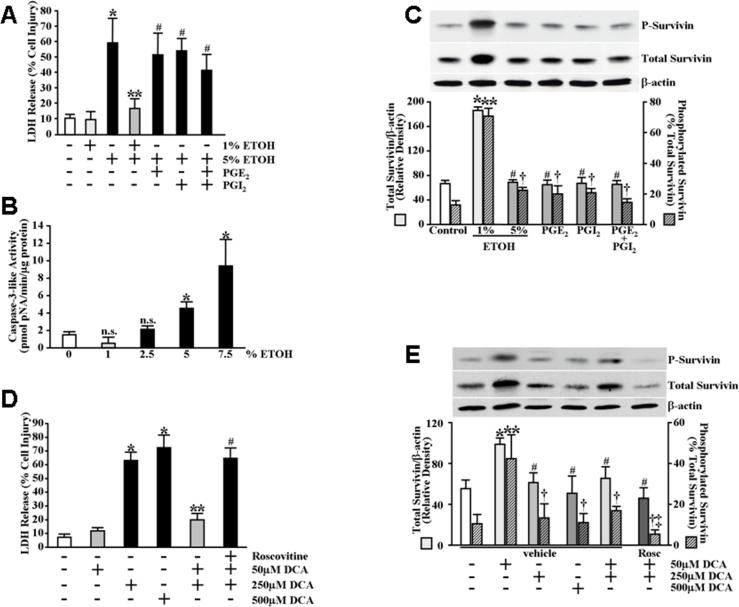
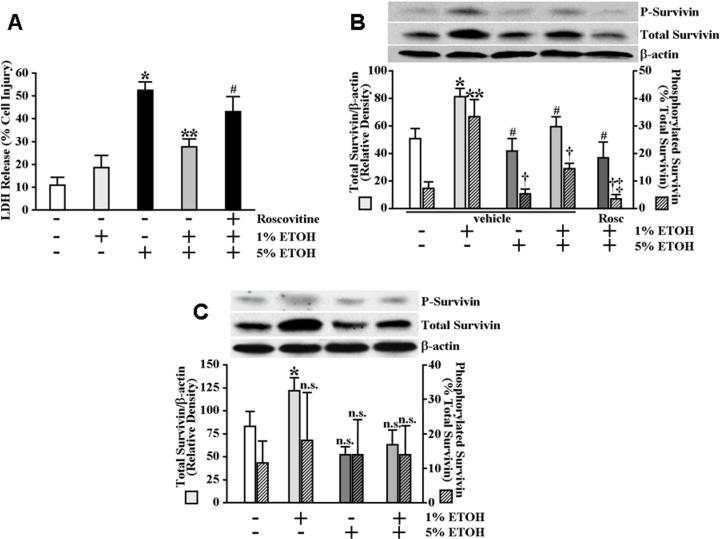
Although survivin expression has been demonstrated in normal (non-cancerous) human and rat gastric mucosa (Chiou et al., 2003b; Chiou et al., 2005), a role for survivin in maintaining gastric mucosal integrity, and more specifically as a mediator of cytoprotection, has not been explored. As a starting point to examine the possibility that survivin plays a role in gastric cytoprotection, we first utilized an in vitro gastric epithelial (RGM1) cell model. Using this model, we established that a 30 minute pretreatment of serum-starved gastric epithelial cells with a sub-cytotoxic concentration (1%) of ethanol (ETOH) produced significant cytoprotection against subsequent exposure to a cytotoxic concentration (5%) of ETOH (Figure 1A). In our model, neither pretreatment with prostaglandin E2 (PGE2), prostacyclin (PGI2) nor a combination of PGE2 and PGI2 affected significant cytoprotection against subsequent exposure to 5% ETOH (Figure 1A). Although our assay for cellular damage (LDH release) does not distinguish between necrosis and apoptosis, exposure of serum-starved RGM1 cells to ETOH concentrations ≥5% did result in significant caspase-3-like activity indicating that apoptosis was likely a significant component of the ETOH-induced cytotoxicity (Figure 1B). We next examined the effects of treating serum-starved RGM1 cells with various ETOH concentrations on survivin expression. As shown in Figure 1C, treatment of RGM1 cells for 30 minutes with 1% ETOH resulted in significantly increased expression levels of both total and phosphorylated survivin protein. Treatment of the cells for 30 minutes with 5% ETOH failed to significantly affect either total or phosphorylated survivin protein levels (Figure 1C). Similarly, treatment with PGE2, PGI2 or a combination of PGE2 and PGI2 also failed to significantly affect either total or phosphorylated survivin protein levels (Figure 1C). Interestingly, however, the role of survivin in cytoprotection was not limited to ETOH-induced cell damage as preconditioning with 50 μM deoxycholate, previously demonstrated to produce cytoprotection against subsequent exposure to 250 μM deoxycholate (Kokoska et al., 1998c) as well as the present study (Figure 1D), also resulted in significantly increased expression levels of both total and phosphorylated survivin protein (Figure 1E). Moreover, we found that pretreatment of serum-starved intestinal epithelial IEC-6 cells with 1% ETOH produced moderate but significant cytoprotection against subsequent exposure to 5% ETOH (Figure 2A) and that treatment of the IEC-6 cells with 1% ETOH also resulted in significantly increased expression levels of both total and phosphorylated survivin protein (Figure 2B). In contrast, although exposure of primary rat thymocytes to mild ETOH concentrations resulted in moderate, but significant, increases in total survivin expression levels (Figure 2C), we were unable to demonstrate an enhancement in cytoprotection against subsequent exposure to 5% ETOH (data not shown). Moreover, unlike the RGM1 and IEC-6 cells, ETOH exposure did not significantly affect survivin phosphorylation levels in the thymocytes (Figure 2C).
Figure 1.
ETOH- and deoxycholate-induced cytoprotection of gastric mucosal epithelial cells results in increased survivin expression levels. (A) Serum-starved RGM1 cells were pretreated for 30 minutes in serum-free medium containing 1% ETOH, 0.1μM PGE2, 0.1μM PGI2, 0.1μM PGE2 + 0.1μM PGI2 or an equal volume of water (control). The medium was then replaced with serum-free medium containing 5% ETOH or an equal volume of water (control) and the cells were incubated an additional 4 hours. Medium was collected and assayed for LDH release as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as % cell damage. *P<0.005 compared with control cells treated only with vehicle (equal volume of water). **P<0.005 compared with cells pretreated only with vehicle (equal volume of water) followed by treatment with 5% ETOH. #P<0.02 compared with vehicle-pretreated controls receiving 1% ETOH pretreatment followed by treatment with 5% ETOH. (B) Serum-starved RGM1 cells were incubated for 3 hours in serum-free medium containing the indicated concentrations of ETOH and caspase-3-like activity was determined as described under Materials and Methods. Experiments were performed in triplicate and specific activity is expressed as pmol pNA/min/μg total protein. *P<0.02 compared with control cells treated only with vehicle (equal volume of water). n.s. not significant compared with control cells treated only with vehicle (equal volume of water). (C) Serum-starved RGM1 cells were treated for 30 minutes in serum-free medium containing 1% ETOH, 5% ETOH, 0.1μM PGE2, 0.1μM PGI2, 0.1μM PGE2 + 0.1μM PGI2 or an equal volume of water (control). Phosphorylated survivin (P-survivin, upper panel) and total survivin (middle panel) expression and β-actin (lower panel) levels, as a control for protein loading and transfer, were determined by immunoblot analysis. Bottom graph shows relative quantification. Note the unit and scale differences for total survivin/β-actin (solid bars) and survivin phosphorylation, as a percentage of total survivin (striped bars). *P<0.0001 compared with total survivin expression levels of control cells treated only with vehicle (equal volume of water). **P<0.0002 compared with percent survivin phosphorylation of control cells treated only with vehicle (equal volume of water). #P<0.0001 compared with total survivin expression levels of cells treated with 1% ETOH. †P<0.002 compared with percent survivin phosphorylation of cells treated with 1% ETOH. (D) Serum-starved RGM1 cells were pretreated for 1 hour in serum-free medium containing 2 μM roscovitine or vehicle. The cells were then pretreated for an additional 20 minutes in serum-free medium containing 50 μM sodium deoxycholate (DCA) or an equal volume of water (control). The medium was then replaced with serum-free medium containing 250 μM DCA, 500 μM DCA or an equal volume of water (control) and the cells were incubated an additional 20 minutes. Medium was collected and assayed for LDH release as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as % cell damage. *P<0.01 compared with control cells treated only with vehicle (equal volume of water). **P<0.0002 compared with cells pretreated only with vehicle (equal volume of water) followed by treatment with 250 μM DCA. #P<0.0005 compared with vehicle-pretreated cells receiving 50 μM DCA pretreatment followed by treatment with 250 μM DCA. (E) Serum-starved RGM1 cells were treated as described in (D). Phosphorylated survivin (P-survivin, upper panel) and total survivin (middle panel) expression and β-actin (lower panel) levels, as a control for protein loading and transfer, were determined by immunoblot analysis. Bottom graph shows relative quantification. *P<0.002 compared with total survivin expression levels of control cells treated only with vehicle (equal volume of water). **P<0.05 compared with percent survivin phosphorylation of control cells treated only with vehicle (equal volume of water). #P<0.01 compared with total survivin expression levels of cells treated with 50 μM DCA. †P<0.05 compared with percent survivin phosphorylation of cells treated with 50 μM DCA. ‡P<0.005 compared with percent survivin phosphorylation of vehicle-pretreated cells receiving 50 μM DCA pretreatment followed by treatment with 250 μM DCA.
Figure 2.
Mild irritant ETOH exposure results in cytoprotection to intestinal epithelial cells as well as increased survivin expression levels in both intestinal epithelial cells and primary thymocytes. (A) Serum-starved IEC-6 cells were pretreated for 1 hour in serum-free medium containing 2 μM roscovitine or vehicle. The cells were then pretreated for an additional 30 minutes in serum-free medium containing 1% ETOH or an equal volume of water (control). The medium was then replaced with serum-free medium containing 5% ETOH or an equal volume of water (control) and the cells were incubated an additional 4 hours. Medium was collected and assayed for LDH release as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as % cell damage. *P<0.0002 compared with control cells treated only with vehicle (equal volume of water). **P<0.001 compared with cells pretreated only with vehicle (equal volume of water) followed by treatment with 5% ETOH. #P<0.02 compared with vehicle-pretreated cells receiving 1% ETOH pretreatment followed by treatment with 5% ETOH. (B) Serum-starved IEC-6 cells were treated as described in (A). Phosphorylated survivin (P-survivin, upper panel) and total survivin (middle panel) expression and β-actin (lower panel) levels, as a control for protein loading and transfer, were determined by immunoblot analysis. Bottom graph shows relative quantification. *P<0.005 compared with total survivin expression levels of control cells treated only with vehicle (equal volume of water). **P<0.005 compared with percent survivin phosphorylation of control cells treated only with vehicle (equal volume of water). #P<0.02 compared with total survivin expression levels of cells treated with 1% ETOH. †P<0.01 compared with percent survivin phosphorylation of cells treated with 1% ETOH. ‡P<0.002 compared with percent survivin phosphorylation of vehicle-pretreated cells receiving 1% ETOH pretreatment followed by treatment with 5% ETOH. (C) Primary rat thymocytes were isolated as described under Material and Methods. The cells were pretreated for 30 minutes in serum-free RPMI 1640 medium containing 1% ETOH or an equal volume of water (control). The medium was then replaced with serum-free medium containing 5% ETOH or an equal volume of water (control) and the cells were incubated an additional 4 hours. Phosphorylated survivin (P-survivin upper panel) and total survivin (middle panel) expression and β-actin (lower panel) levels, as a control for protein loading and transfer, were determined by immunoblot analysis. Bottom graph shows relative quantification. *P<0.05 compared with total survivin expression levels of control cells treated only with vehicle (equal volume of water). n.s. not significant.
Increased survivin protein expression levels and cytoprotection of gastric mucosal epithelial cells in response to cytoprotective ETOH exposure involves de novo protein synthesis but does not require de novo mRNA synthesis
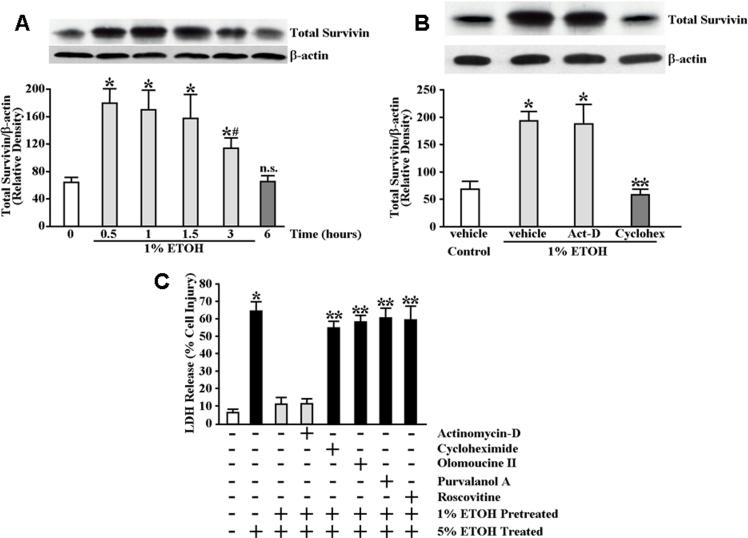
We next determined the time course over which total survivin expression levels remained significantly elevated following initial exposure to 1% ETOH. As shown in Figure 3A, the expression levels of total survivin remained significantly increased above control (baseline) levels for up to 3 hours following exposure to 1% ETOH. By 6 hours, however, survivin expression levels had returned to baseline (Figure 3A). We examined whether the increase in survivin protein expression levels in response to cytoprotective ETOH required either de novo mRNA synthesis or de novo protein synthesis. For this, serum-starved RGM1 cells were pretreated for 30 minutes in serum-free medium containing vehicle, actinomycin-D or cycloheximide prior to incubating the cells for 30 minutes in serum-free medium containing 1% ETOH. As shown in Figure 3B, pretreatment of the cells with cycloheximide abolished the increase in total survivin protein expression levels in response to 1% ETOH exposure whereas pretreatment with actinomycin-D was without significant affect suggesting the requirement for de novo protein synthesis but not de novo mRNA synthesis. We next determined the effects of blocking de novo mRNA and protein synthesis on cytoprotection. Serum-starved RGM1 cells were pretreated for 30 minutes in serum-free medium containing vehicle, actinomycin-D or cycloheximide followed by incubation for 30 minutes in serum-free medium containing 1% ETOH. The cells were then exposed for 4 hours to serum-free medium containing 5% ETOH. As shown in Figure 3C, pretreatment of the cells with cycloheximide significantly attenuated cytoprotection compared to vehicle-pretreated control cells whereas pretreatment with actinomycin-D had no significant affect on cytoprotection.
Figure 3.
Elevated survivin expression levels are sustained up to 3 hours following mild irritant ETOH exposure whereas inhibition of de novo protein synthesis abolishes cytoprotective ETOH-induced survivin expression and attenuates cytoprotection against ETOH challenge. (A) Serum-starved RGM1 cells were incubated in serum-free medium containing 1% ETOH or equal volume of water (control) for the indicated time interval before being lysed for determination of total survivin expression levels. Total (upper panel) survivin expression levels were determined by immunoblot analysis in which relative expression levels were normalized to β-actin signal as a control for protein loading and transfer (lower panel). Bottom graph shows relative quantification. *P<0.005 compared with total survivin expression levels of control cells treated only with vehicle (equal volume of water). #P<0.02 compared with total survivin expression levels of cells treated with 1% ETOH for 0.5−1.5 hours. n.s. not significant. (B) Serum-starved RGM1 cells were pretreated for 30 minutes in serum-free medium containing 2 μg/ml actinomycin-D, 10 μg/ml cycloheximide or vehicle (control) followed by treatment for 30 minutes in serum-free medium containing 1% ETOH or an equal volume of water (control). Total (upper panel) survivin expression levels were determined by immunoblot analysis in which relative expression levels were normalized to β-actin signal as a control for protein loading and transfer (lower panel). Bottom graph shows relative quantification. *P<0.0001 compared with total survivin expression levels of vehicle pretreated, vehicle (equal volume of water) treated control cells. **P<0.0001 compared with total survivin expression levels of vehicle pretreated cells subsequently treated with 1% ETOH. (C) Serum-starved RGM1 cells were pretreated for 30 minutes in serum-free medium containing 2 μg/ml actinomycin-D, 10 μg/ml cycloheximide or vehicle (control), or for 1 hour in serum-free medium containing 2 μM roscovitine, 0.2 μM olomoucine II, 0.2 μM purvalanol A or vehicle (control), followed by treatment for 30 minutes in serum-free medium containing 1% ETOH or an equal volume of water (control). The cells were then incubated for an additional 4 hours in serum-free medium containing 5% ETOH or an equal volume of water (control). Medium was collected and assayed for LDH release as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as % cell damage. *P<0.0001 compared with vehicle-pretreated controls receiving no ETOH pretreatment or treatment. **P<0.001 compared with vehicle-pretreated controls receiving 1% ETOH pretreatment followed by treatment with 5% ETOH.
Treatment of gastric mucosal epithelial cells with cytoprotective ETOH concentrations increases activity of the p34cdc2 cyclin-dependent kinase
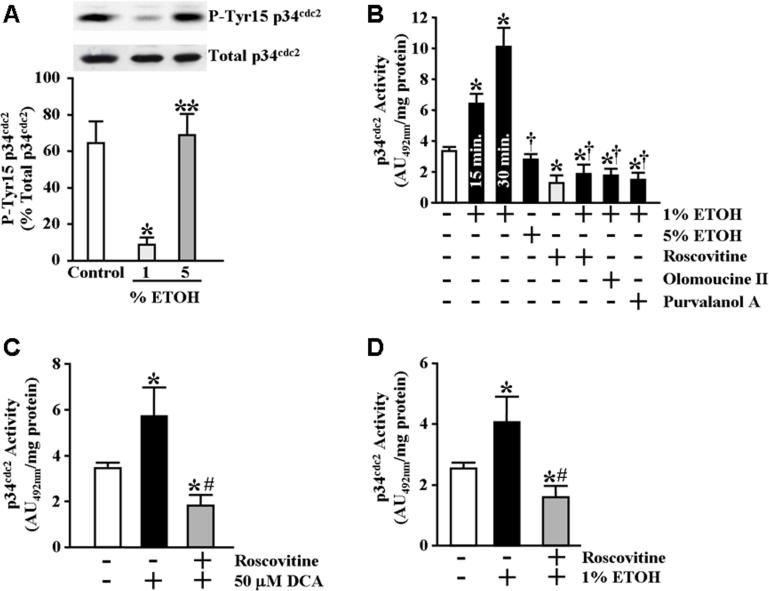
In light of the observed increase in phosphorylated survivin following exposure of the RGM1 cells to cytoprotective ETOH concentrations, we examined whether this treatment also affected the activity of p34cdc2, a kinase known to both phosphorylate and stabilize survivin (O'Connor et al., 2000a). Since the activity of p34cdc2 is negatively regulated by phosphorylation of amino acid residues Threonine-14 and Tyrosine-15 (Norbury et al., 1991; Atherton-Fesssler et al., 1993; Clemens et al., 2003), we first examined the effect of ETOH treatment on tyrosine-15 phosphorylation of p34cdc2 in the RGM1 cells. As shown in Figure 4A, treatment of serum-starved RGM1 cells for 30 minutes with 1% ETOH resulted in reduced p34cdc2 tyrosine-15 phosphorylation without significantly affecting total p34cdc2 protein expression levels. We next examined p34cdc2 activity directly from p34cdc2 precipitates of RGM1 cell lysates obtained following treatment. As shown in Figure 4B, treatment of serum-starved RGM1 cells for either 15 or 30 minutes with 1% ETOH resulted in a significant increase in p34cdc2 activity compared to vehicle-treated control cells. In addition, treatment of serum-starved RGM1 cells for 20 minutes with 25−50 μM DCA also resulted in a significant increase in p34cdc2 activity compared to vehicle-treated control cells (Figure 4C). Similarly, we found that treatment of IEC-6 cells for 30 minutes with 1% ETOH resulted in a significant increase in p34cdc2 activity (Figure 4D).
Figure 4.
Cytoprotective ETOH induces p34cdc2 activation. (A) Serum-starved RGM1 cells were incubated for 30 minutes in serum-free medium containing 1% ETOH, 5% ETOH or an equal volume of water (control). Phosphorylated on tyrosine-15 p34cdc2 (P-Tyr15 p34cdc2, upper panel) and total p34cdc2 (lower panel) expression levels were determined by immunoblot analysis as described in Materials and Methods. Bottom graph shows relative quantification of percent p34cdc2 phosphorylation (as a ratio of phosphorylated to total p34cdc2). *P<0.002 compared with control cells treated only with vehicle (equal volume of water). **P<0.005 compared with cells treated with 1% ETOH. (B) Relative p34cdc2 activity was determined as described under Materials and Methods. For cells treated with 1% ETOH, p34cdc2 activity was determined following incubation for both 15 and 30 minutes. For all other treatments, p34cdc2 activity was determined following incubation for 30 minutes. For inhibition determination, cells were pretreated for 1 hour in serum-free medium containing 2 μM roscovitine, 0.2 μM olomoucine II, 0.2 μM purvalanol A or vehicle (control), followed by treatment for 30 minutes in serum-free medium containing 1% ETOH or an equal volume of water (control). Experiments were performed in triplicate and results are expressed as absorbance units (AU492) per milligram total protein. *P<0.02 compared with control cells treated only with vehicle (equal volume of water). †P<0.0002 compared with cells treated for 30 minutes with 1% ETOH. (C) Serum-starved RGM1 cells were pretreated for 1 hour in serum-free medium containing 2 μM roscovitine or vehicle (control) followed by treatment for 20 minutes in serum-free medium containing 50 μM DCA or an equal volume of water (control). Relative p34cdc2 activity was determined as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as absorbance units (AU492) per milligram total protein. *P<0.05 compared with control cells treated only with vehicle (equal volume of water). #P<0.01 compared with vehicle pretreated cells treated with 50 μM DCA. (D) Serum-starved IEC-6 cells were pretreated for 1 hour in serum-free medium containing 2 μM roscovitine or vehicle (control) followed by treatment for 30 minutes in serum-free medium containing 1% ETOH or an equal volume of water (control). Relative p34cdc2 activity was determined as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as absorbance units (AU492) per milligram total protein. *P<0.03 compared with control cells treated only with vehicle (equal volume of water). #P<0.01 compared with vehicle pretreated cells treated with 1% ETOH.
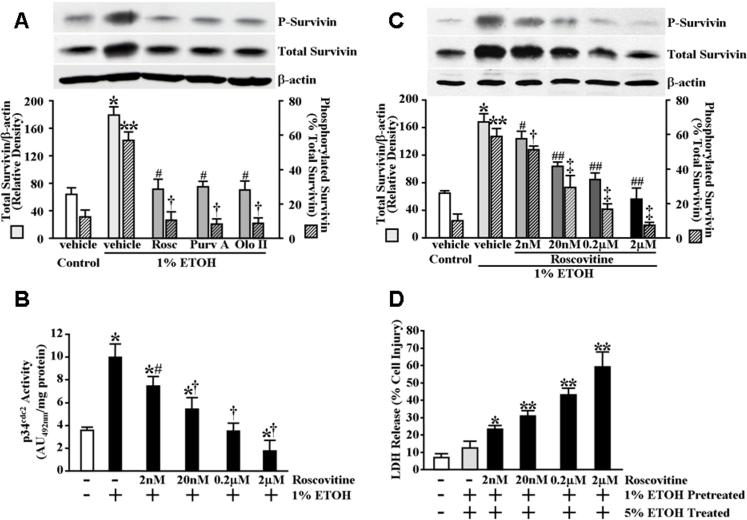
Inhibition of p34cdc2 abolishes the increased survivin protein expression levels produced in gastric mucosal epithelial cells by pretreatment with cytoprotective ETOH concentrations and significantly attenuates cytoprotection against ETOH-induced cytotoxicity
To determine whether p34cdc2 activity was necessary for the modulation of survivin phosphorylation and increased survivin protein expression levels obtained following exposure of serum-starved RGM1 cells to the cytoprotective 1% ETOH concentration, we examined the effect of inhibiting p34cdc2 with the potent cyclin-dependent kinase inhibitor, roscovitine (De Azevedo et al., 1997). As shown in Figure 4B, pretreatment of serum-starved RGM1 cells for 1 hour with 2 μM roscovitine completely prevented the increased p34cdc2 activity in response to cytoprotective ETOH exposure. Moreover, as shown in Figure 5A, pretreatment of the cells for 1 hour with roscovitine completely abolished the increase in phosphorylated and total survivin protein levels obtained in vehicle-pretreated control cells following exposure for 30 minutes to 1% ETOH. We next examined the effect of p34cdc2 inhibition on cytoprotection. As shown in Figure 3C, inhibition of p34cdc2 also abolished the cytoprotection produced in the vehicle-treated control cells by pre-exposure for 30 minutes to 1% ETOH against subsequent exposure to cytotoxic ETOH. Similar results were obtained using two other inhibitors of p34cdc2, purvalanol A and olomoucine II, which are more potent and specific than roscovitine (Gray et al., 1998; Krystof et al., 2002) (Figure 3C). Furthermore, the effect of p34cdc2 inhibition on ETOH-induced survivin expression and cytoprotection was found to be dose-dependent (Figures 5B, 5C and 5D) and inhibition of p34cdc2 completely abrogated the cytoprotection produced in IEC-6 cells by pretreatment with 1% ETOH (Figure 2A) as well as the increased expression levels of total and phosphorylated survivin (Figure 2B). Inhibition of p34cdc2 also significantly attenuated both the cytoprotection (Figure 1D) and increased survivin levels (Figure 1E) conferred by pre-exposure of RGM1 to 50 μM deoxycholate against subsequent exposure to 250 μM deoxycholate.
Figure 5.
Inhibition of p34cdc2 abolishes cytoprotective ETOH-induced survivin expression. (A) Serum-starved RGM1 cells were pretreated for 1 hour in serum-free medium containing 2 μM roscovitine, 0.2 μM olomoucine II, 0.2 μM purvalanol A or vehicle (control) followed by treatment for 30 minutes in serum-free medium containing 1% ETOH or an equal volume of water (control). Phosphorylated survivin (P-survivin, upper panel) and total survivin (middle panel) expression and β-actin (lower panel) levels, as a control for protein loading and transfer, were determined by immunoblot analysis. Bottom graph shows relative quantification. *P<0.0001 compared with total survivin expression levels of vehicle-pretreated control cells subsequently treated with vehicle (equal volume of water). **P<0.0002 compared with percent survivin phosphorylation of vehicle-pretreated control cells subsequently treated with vehicle (equal volume of water). #P<0.0002 compared with total survivin expression levels of vehicle-pretreated cells subsequently treated with 1% ETOH. †P<0.0005 compared with percent survivin phosphorylation of vehicle-pretreated cells subsequently treated with 1% ETOH. (B) Dose-response of roscovitine on p34cdc2 activity. Serum-starved RGM1 cells were pretreated for 1 hour in serum-free medium containing 2 nM roscovitine, 20 nM roscovitine, 0.2 μM roscovitine, 2 μM roscovitine or vehicle (control), followed by treatment for 30 minutes in serum-free medium containing 1% ETOH or an equal volume of water (control). Relative p34cdc2 activity was determined as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as absorbance units (AU492) per milligram total protein. *P<0.02 compared with control cells treated only with vehicle (equal volume of water). #P<0.04 compared with vehicle-pretreated cells subsequently treated with 1% ETOH. †P<0.05 compared with cells pretreated with 0.1 × the same concentration of roscovitine and subsequently treated with 1% ETOH. (C) Dose-response of roscovitine on phosphorylated and total survivin expression levels. Serum-starved RGM1 cells were treated as described in (B). Phosphorylated survivin (P-survivin, upper panel) and total survivin (middle panel) expression and β-actin (lower panel) levels, as a control for protein loading and transfer, were determined by immunoblot analysis. Bottom graph shows relative quantification. *P<0.0001 compared with total survivin expression levels of vehicle-pretreated control cells subsequently treated with vehicle (equal volume of water). **P<0.0002 compared with percent survivin phosphorylation of vehicle-pretreated control cells subsequently treated with vehicle (equal volume of water). #P<0.05 compared with total survivin expression levels of vehicle-pretreated cells subsequently treated with 1% ETOH. †P<0.05 compared with percent survivin phosphorylation of vehicle-pretreated cells subsequently treated with 1% ETOH. ##P<0.05 compared with total survivin expression levels of cells pretreated with 0.1 × the same concentration of roscovitine and subsequently treated with 1% ETOH. ‡P<0.05 compared with percent survivin phosphorylation of cells pretreated with 0.1 × the same concentration of roscovitine and subsequently treated with 1% ETOH. (D) Dose-response of roscovitine on cytoprotection. Serum-starved RGM1 cells were pretreated for 1 hour in serum-free medium containing 2 nM roscovitine, 20 nM roscovitine, 0.2 μM roscovitine, 2 μM roscovitine or vehicle (control), followed by pretreatment for 30 minutes in serum-free medium containing 1% ETOH or an equal volume of water (control). The cells were then incubated for an additional 4 hours in serum-free medium containing 5% ETOH or an equal volume of water (control). Medium was collected and assayed for LDH release as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as % cell damage. *P<0.01 compared with vehicle-pretreated controls receiving 1% ETOH pretreatment followed by treatment with 5% ETOH. **P<0.02 compared with cells pretreated with 0.1 × the same concentration of roscovitine prior to receiving 1% ETOH pretreatment followed by treatment with 5% ETOH.
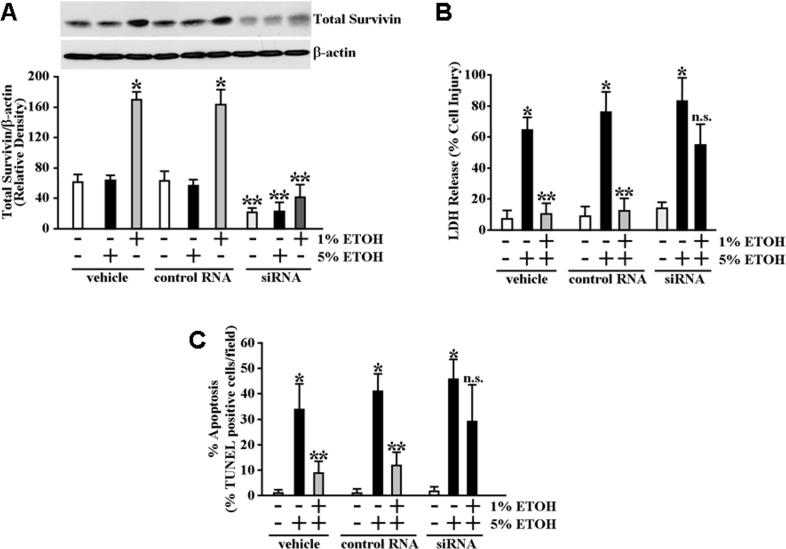
Knockdown of survivin protein expression in gastric mucosal epithelial cells significantly attenuates cytoprotection against ETOH-induced cytotoxicity and apoptosis
To examine the involvement of survivin in cytoprotection more directly, we used siRNA to specifically reduce survivin protein levels in the RGM1 cells. As shown in Figure 6A, siRNA treatment significantly reduced survivin protein levels within 48 hours by 65% (P<0.005) compared with cells treated with control RNA. Survivin-targeted siRNA treatment also significantly reduced survivin protein levels in response to 1% ETOH exposure by 75% (P<0.0002) compared with cells treated with control RNA (Figure 6A). We next examined the effect of the survivin siRNA treatment on cytoprotection. As shown in Figure 6B, pretreatment of the RGM1 cells with survivin siRNA 48 hours prior to exposing the cells for 30 minutes to 1% ETOH resulted in a statistical abolishment of cytoprotection such that the extent of cell death following subsequent exposure to 5% ETOH was comparable within significance to the control cells exposed to 5% ETOH without prior exposure to cytoprotective 1% ETOH. Pretreatment with the survivin siRNA also resulted in significantly greater apoptosis in response to subsequent cytotoxic ETOH exposure compared to control cells (Figure 6C).
Figure 6.
Knockdown of survivin protein levels attenuates ETOH-induced cytoprotection. (A) RGM1 cells were transiently transfected with survivin-specific siRNA, control RNA or treated only with transfection reagent (vehicle). Forty-eight hours following transfection the cells were treated for 30 minutes with 1% ETOH, 5% ETOH or an equal volume of water (control). Survivin protein expression levels were determined by immunoblot analysis. *P<0.0005 compared with survivin protein expression levels of vehicle treated or control RNA transfected cells subsequently treated with only with water (as a control for ETOH). **P<0.01 compared with survivin protein expression levels of vehicle treated or control RNA transfected cells subsequently treated with the corresponding (1% or 5%) concentration of ETOH or water (as a control for ETOH). (B) RGM1 cells transiently transfected with survivin-specific siRNA, control siRNA or treated only with transfection reagent (vehicle) were pretreated 48 hours later by incubation for 30 minutes in serum-free medium containing 1% ETOH or an equal volume of water (control). The medium was then replaced with serum-free medium containing 5% ETOH or an equal volume of water (control) and the cells were incubated an additional 4 hours. Medium was collected and assayed for LDH release as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as % cell damage. *P<0.002 compared with vehicle (transfection reagent)-treated, control RNA transfected and survivin-specific siRNA cells subsequently treated only with water (as a control for ETOH). **P<0.005 compared with vehicle-treated, control RNA transfected and survivin-specific siRNA cells subsequently pretreated with water followed by exposure to 5%ETOH for 4 hours. n.s. not significant. (C) Percentage of apoptotic cells following similar treatment conditions as in (B). RGM1 cells transiently transfected with survivin-specific siRNA, control siRNA or treated only with transfection reagent (vehicle) were pretreated 48 hours later by incubation for 30 minutes in serum-free medium containing 1% ETOH or an equal volume of water (control). The medium was then replaced with serum-free medium containing 5% ETOH or an equal volume of water (control) and the cells were incubated an additional 3 hours (the shortened incubation time was to reduce confounding effects of cellular necrosis). Apoptosis was determined and quantified by TUNEL staining as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as percentage TUNEL positive cells of total cell number in each field. *P<0.0001 compared with vehicle (transfection reagent)-treated, control RNA transfected and survivin-specific siRNA cells subsequently treated only with water (as a control for ETOH). **P<0.001 compared with vehicle-treated, control RNA transfected and survivin-specific siRNA cells subsequently pretreated with water followed by exposure to 5%ETOH for 3 hours. n.s. not significant.
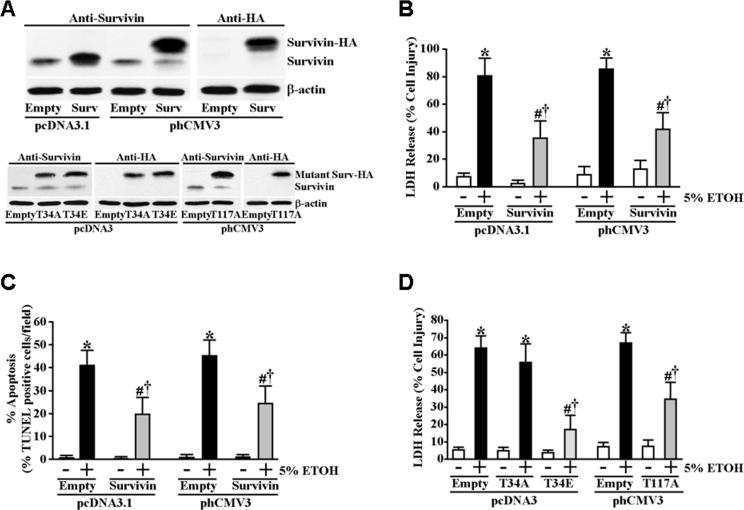
Overexpression of survivin increases the resistance of gastric mucosal epithelial cells to ETOH-induced cytotoxicity and apoptosis
We next examined whether increased survivin protein expression levels could be cytoprotective in the absence of pre-conditioning with prior cytoprotective ETOH exposure. To accomplish this, we transfected RGM1 cells with either an expression plasmid encoding the full-length wild-type survivin gene or an expression plasmid encoding the full-length wild-type survivin gene harboring a C-terminal HA tag sequence to verify transgene expression. For controls, we also transfected cells with the same plasmids that lacked a survivin transgene coding sequence. Stable transfectants were selected and screened for high survivin protein expression levels (Figure 7A). To determine whether forced overexpression of survivin could elicit cytoprotection, we exposed serum-starved survivin overexpressing and non-expressing control transfectants for 4 hours to 5% ETOH. As shown in Figure 7B, both the RGM1 transfectant overexpressing full-length untagged wild-type survivin and the transfectant overexpressing the full-length HA-tagged wild-type survivin were significantly more resistant to ETOH-induced cell death compared to the non-expressing control transfectants. Moreover, both the untagged and HA-tagged survivin overexpressing transfectants were significantly more resistant to ETOH-induced apoptosis (Figure 7C). To determine whether phosphorylation of residue threonine-34 was important for functionality of survivin in cytoprotection, we tested whether overexpression of mutant survivin having either threonine-34-to-alanine (T34A) or threonine-34-to-glutamate (T34E) substitutions could effectively confer cytoprotection in the absence of pretreatment with 1% ETOH. Stable transfectants expressing both mutant forms of survivin were selected and screened for high mutant survivin protein expression levels (Figure 7A). As shown in Figure 7D, overexpression of the T34A mutant survivin failed to confer significant cytoprotection compared to the non-expressing control transfectants in the absence of preconditioning whereas overexpression of the phosphorylation mimic T34E mutant survivin was significantly more potent in conferring cytoprotection compared to overexpression of wild-type survivin. These data indicate that phosphorylation at threonine-34 is likely important not only to the stabilization/accumulation of survivin in response to cytoprotective ETOH exposure, but also that phosphorylation at threonine-34 plays a functional role in survivin as a cytoprotective factor. Recently, phosphorylation of threonine-117 on survivin by aurora B kinase was shown to regulate cell cycle progression into anaphase (Wheatley et al., 2007). To determine whether phosphorylation of threonine-117 was also involved in the modulation of cytoprotection mediated by survivin, we overexpressed a mutant survivin having a threonine-117-to-alanine (T117A) substitution (Figure 7A) to test whether it was as effective as wild-type survivin at eliciting cytoprotection in the absence of 1% ETOH preconditioning. As shown in Figure 7D, overexpression of the T117A mutant survivin conferred statistically similar cytoprotection as that of overexpressed wild type survivin indicating that, unlike phosphorylation of threonine-34, phosphorylation at threonine-117 is apparently not integral to the function of survivin as a cytoprotective factor.
Figure 7.
Survivin overexpression is cytoprotective in the absence of ETOH preconditioning, which is dependent on threonine-34 but independent of threonine-117. (A) RGM1 cells were stably transfected with untagged (pcDNA3.1) or HA-tagged (phCMV3) full-length wild-type survivin expression plasmids. In addition, cells were stably transfected with HA-tagged T34A, T34E (both in pcDNA) and T117A (in phCMV3) mutant survivin expression plasmids. Wild-type and mutant survivin protein expression of clones was compared with control cells stably transfected with the corresponding “empty” plasmids. (B) The corresponding control, untagged and HA-tagged survivin transfectants were incubated in serum-free medium containing 5% ETOH or an equal volume of water (control) for 4 hours. Medium was collected and assayed for LDH release as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as % cell damage. *P<0.005 compared with “empty” or survivin expressing pcDNA3.1 and phCMV3 transfected cells treated only with water (as a control for ETOH). #P<0.02 compared with “empty” pcDNA3.1 and phCMV3 transfected cells incubated in serum-free medium containing 5% ETOH for 4 hours. †P<0.05 compared with “empty” or survivin expressing pcDNA3.1 and phCMV3 transfected cells treated only with water. (C) Percentage of apoptotic cells following similar treatment conditions as in (B). Control, untagged and HA-tagged survivin transfectants were incubated in serum-free medium containing 5% ETOH or an equal volume of water (control) for 3 hours. Apoptosis was determined and quantified by TUNEL staining as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as percentage TUNEL positive cells of total cell number in each field. *P<0.0001 compared with “empty” or survivin expressing pcDNA3.1 and phCMV3 transfected cells treated only with water (as a control for ETOH). #P<0.002 compared with “empty” pcDNA3.1 and phCMV3 transfected cells incubated in serum-free medium containing 5% ETOH for 3 hours. †P<0.001 compared with “empty” or survivin expressing pcDNA3.1 and phCMV3 transfected cells treated only with water. (D) The corresponding control and HA-tagged T34A, T34E and T117 mutant survivin transfectants were incubated in serum-free medium containing 5% ETOH or an equal volume of water (control) for 4 hours. Medium was collected and assayed for LDH release as described under Materials and Methods. Experiments were performed in triplicate and results are expressed as % cell damage. *P<0.002 compared with “empty” or mutant survivin expressing pcDNA3 and phCMV3 transfected cells treated only with water (as a control for ETOH). #P<0.02 compared with “empty” pcDNA3 and phCMV3 transfected cells incubated in serum-free medium containing 5% ETOH for 4 hours. †P<0.01 compared with “empty” or survivin expressing pcDNA3 and phCMV3 transfected cells treated only with water.
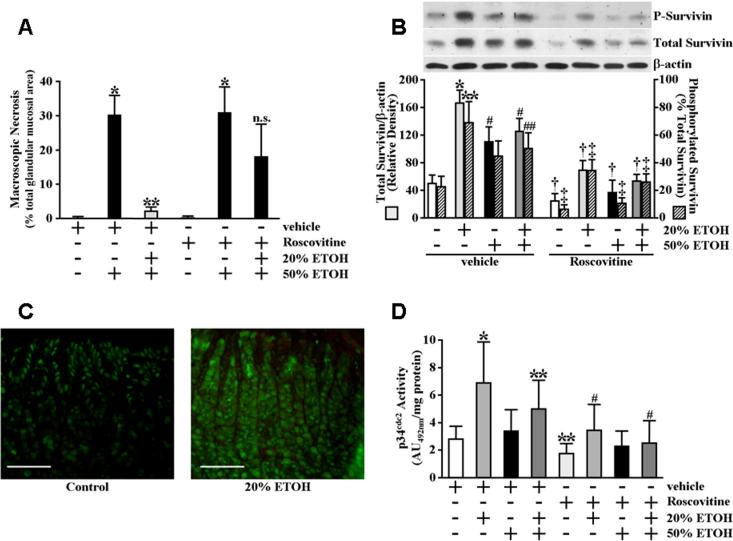
Expression levels of total and phosphorylated survivin protein are increased in the gastric mucosa of rats administered cytoprotective ETOH concentrations
Studies utilizing in vitro models indicate the possibility of differential mechanisms mediating cytoprotection at the gastric mucosal epithelial cell level from those mediating cytoprotection to the gastric mucosa in vivo (Smith et al., 1991; Kokoska et al., 1998b; Kokoska et al., 1998c; Tsutsumi et al., 2003; Tanaka et al., 2004). To examine whether gastric mucosal survivin expression levels are affected by cytoprotective conditioning, fasted rats were intragastrically administered 20% ETOH, a concentration we established produced greater than 93% (P<0.0001) gastric mucosal cytoprotection against subsequent challenge with 50% ETOH (Figure 8A). As shown in Figure 8B, both survivin phosphorylation and total survivin protein expression levels were significantly increased in the gastric mucosa of fasted rats 30 minutes following intragastric administration of 20% ETOH compared to water-administered control rats. Interestingly, whereas survivin expression in the gastric mucosa of water-administered control rats was mainly confined to the nuclei of surface epithelial cells, appreciable survivin expression was found in cells extending the entire mucosal depth of the rats administered cytoprotective ETOH (Figure 8C).
Figure 8.
Intragastric administration of cytoprotective ETOH increases gastric mucosal p34cdc2 activity whereas p34cdc2 inhibition both abolishes the increases in total and phosphorylated gastric survivin protein levels in response to cytoprotective ETOH administration and significantly attenuates gastric cytoprotection. (A) Gastric cytoprotection produced by mild irritant ETOH exposure. Rats were administered roscovitine (2.8 mg/kg) or vehicle by peritoneal injection. Fasted rats received 1 ml of 20% ETOH or water (control) by intragastric gavage 30 minutes prior to administration of 1.5 ml of 50% ETOH or water (control) by intragastric gavage. Stomachs were excised from euthanized rats at 6 hours and the extents of gastric injury were determined and quantified as described in Materials and Methods. Results are expressed as percentage of total glandular area. *P<0.0001 compared with vehicle-treated control rats gavaged with water only. **P<0.0001 compared with vehicle-treated rats administered water 30 minutes prior to administration of 50% ETOH. n.s. not significant. (B) Survivin phosphorylation and total survivin protein expression levels increase in the gastric mucosa in response to cytoprotective ETOH exposure. Rats were administered roscovitine or vehicle by peritoneal injection. Fasted rats received 1 ml of 20% ETOH, 50% ETOH or water (control) by intragastric gavage and gastric tissue was obtained 30 minutes later. One group of fasted rats received 1 ml of 20% ETOH followed 30 minutes later by intragastric gavage of 1.5 ml 50% ETOH. In this group, gastric tissue was obtained 6 hours later. Phosphorylated survivin (P-survivin, upper panel) and total survivin (middle panel) expression and β-actin (lower panel) levels, as a control for protein loading and transfer, were determined by immunoblot analysis. Bottom graph shows relative quantification. *P<0.001 compared with total gastric mucosal survivin expression levels of vehicle-administered rats receiving only water. **P<0.02 compared with percent survivin phosphorylation in the gastric mucosa of vehicle-administered rats receiving only water. #P<0.02 compared with total gastric mucosal survivin expression levels of vehicle-administered rats receiving only 20% ETOH. ##P<0.05 compared with percent survivin phosphorylation in the gastric mucosa of vehicle-administered rats receiving only 20% ETOH. †P<0.05 compared with total gastric mucosal survivin expression levels of vehicle-administered rats receiving the same administration of water, 20% ETOH, 50% ETOH or 20% ETOH followed by 50% ETOH. ‡P<0.05 compared with percent survivin phosphorylation in the gastric mucosa of vehicle-administered rats receiving the same administration of water, 20% ETOH, 50% ETOH or 20% ETOH followed by 50% ETOH. (C) Survivin expression and localization were examined by immunofluorescence staining of paraffin-embedded sections of gastric tissue obtained from fasted rats 30 minutes following administration of 1 ml of 20% ETOH or water (control) by intragastric gavage. Left panel shows representative gastric tissue section taken 30 minutes following administration of water control; right panel shows representative gastric tissue section taken 30 minutes following administration of 20% ETOH. (scale bars, 200 μm). Staining specificity was determined by exclusion of primary antibody in which no fluorescence signal was obtained (data not shown). (D) Cytoprotective ETOH increases gastric mucosal p34cdc2 activity. Rats were administered roscovitine or vehicle by peritoneal injection. Fasted rats received 1 ml of 20% ETOH, 50% ETOH or water (control) by intragastric gavage and gastric tissue was obtained 30 minutes later. One group of fasted rats received 1 ml of 20% ETOH followed 30 minutes later by intragastric gavage of 1.5 ml 50% ETOH. In this group, gastric tissue was obtained 6 hours later. Relative p34cdc2 activity was determined as described under Materials and Methods. Experiments were performed with 6 rats per group and results are expressed as absorbance units (AU492) per milligram total protein. *P<0.01 compared with the gastric mucosal p34cdc2 activity of vehicle-administered rats receiving only water. **P<0.05 compared with the gastric mucosal p34cdc2 activity of vehicle-administered rats receiving only water. #P<0.05 compared with the gastric mucosal p34cdc2 activity of vehicle-administered rats receiving the same administration of water, 20% ETOH, 50% ETOH or 20% ETOH followed by 50% ETOH.
Activity of p34cdc2 is increased in the gastric mucosa of rats administered cytoprotective ETOH concentrations
To further explore the in vivo relevance of our findings regarding a possible mechanism by which cytoprotective ETOH pre-exposure results in increased survivin phosphorylation and total survivin protein expression levels in gastric epithelial cells, we examined the effect of cytoprotective ETOH administration on gastric mucosal p34cdc2 activity. As shown in Figure 8D, p34cdc2 activity was significantly increased in the gastric mucosa of fasted rats 30 minutes following intragastric administration of 20% ETOH compared to water-administered control rats. In contrast, the gastric mucosal p34cdc2 activity was not significantly affected in fasted rats administered 50% ETOH (Figure 8D).
Inhibition of p34cdc2 abolishes the increased survivin protein expression levels produced in the gastric mucosa of rats administered cytoprotective ETOH concentrations and significantly attenuates cytoprotection against challenge with concentrated ETOH
We next examined whether p34cdc2 activity was involved in either the increased survivin protein expression levels in response to intragastric administration of 20% ETOH or gastric cytoprotection against subsequent challenge with 50% ETOH. For this, fasted rats were administered roscovitine or vehicle by intraperitoneal injection prior to intragastric instillation of 20% ETOH or water, as a control, by gavage. Gastric tissue was obtained 30 minutes later and used to determine phosphorylated and total survivin expression levels, as well as to verify p34cdc2 inhibition (Figure 8D). As shown in Figure 8B, inhibition of p34cdc2 abolished the increases in phosphorylated and total survivin expression levels obtained from the vehicle-pretreated controls administered cytoprotective ETOH. Moreover, in groups of rats subsequently challenged with 50% ETOH, pretreatment with roscovitine prior to cytoprotective ETOH conditioning significantly attenuated gastric cytoprotection compared to vehicle-pretreated control rats (Figure 8A). Roscovitine pretreatment did not further affect the extent of ETOH-induced damage in the absence of cytoprotective conditioning (Figure 8A).
Discussion
Survivin regulates mitogenesis and cell survival during development and reappears concomitant with tumorigenesis of most all human cancers (Chiou et al., 2003a; Li, 2005). However, survivin expression has been documented in non-cancerous organ/cell systems having a normally high turnover rate including the colon and gastric mucosa (Gianani et al., 2001; Chiou et al, 2003b). Gastric epithelial cells have an obligatorily high turnover rate as a result of their roles as first-line defenses against both physical (e.g. food intake) and infectious (e.g. bacterial) insult. It was recently documented that survivin is expressed in normal human and rat gastric mucosa and that nonsteroidal anti-inflammatory drugs (NSAIDs) reduce survivin expression by a mechanism that is prostaglandin-independent (Chiou et al, 2003b; Chiou et al, 2005).
Here, we demonstrate for first time that exposure to cytoprotective ETOH concentrations results in the accumulation of survivin both in gastric mucosal epithelial cells in vitro and gastric mucosa in vivo. Our data suggest that the increase in total survivin protein expression levels in response to cytoprotective ETOH exposure is a possible consequence of increased protein stability mediated via phosphorylation by the cyclin-dependent p34cdc2 kinase. Regulation of survivin stability via phosphorylation by p34cdc2 has been previously reported (O'Connor et al., 2002; Chang, et al., 2004). Modulation of p34cdc2 phosphorylation and activity by ETOH exposure of transfected hepatoblastoma HepG2 cells (Clemens et al., 2003) and human oral and colon carcinoma epithelial cells (Guo et al., 1997) has also been reported.
We are currently investigating possible mechanism(s) underlying the modulation of p34cdc2 activity in the gastric mucosal epithelial cells following exposure to ETOH. At least two mechanisms appear plausible. First, short exposure of mammary epithelial cells to ETOH for 5−15 minutes results in activation of several intracellular signaling factors including MAP (ERK) kinase and Akt (Ma et al., 2003). Both ERK and Akt positively regulate p34cdc2 activation (Roberts et al., 2002). A second possible mechanism is that activation of p34cdc2 in the gastric mucosal epithelial cells may be mediated by transient calcium (Ca2+) influx. Intracellular [Ca2+]i transients have been demonstrated to cause p34cdc2 activation by a cascade mechanism involving Ca2+ activation of calcium/calmodulin-dependent protein kinase (CaM kinase II) resulting in activation of tyrosine phosphatase p54cdc25-c, which in turn leads to subsequent activation of p34cdc2 (Patel et al., 1999; Suprynowicz et al., 2000). Since in vitro studies have shown that cytoprotection of gastric epithelial cells produced by mild irritant pre-exposure, including that produced by pre-exposure to low concentrations of ETOH and sodium deoxycholate, involves intracellular [Ca2+]i transients (Kokoska et al., 1998a; Kokoska et al., 1998c), it is possible in the present study that exposure of RGM1 cells to cytoprotective ETOH concentrations mediated p34cdc2 activation by affecting intracellular Ca2+ mobilization. Interestingly, we also found that exposure of gastric epithelial cells to a cytoprotective concentration of sodium deoxycholate resulted in both increased p34cdc2 activity and elevated survivin expression levels. The possibility that multiple mechanisms may act in concert to elicit the activation of p34cdc2, with the resultant phosphorylation and accumulation of survivin, is also being explored.
We found that mutation of threonine-34 to alanine abrogated the cytoprotective effect that was obtained by overexpression of wild-type survivin. Conversely, a greater extent of cytoprotection was obtained upon overexpression of the mutant survivin in which threonine-34 was substituted with glutamate. This is in agreement with phosphorylation of threonine-34 being important in the cell survival and/or anti-apoptosis function of survivin (O'Connor et al., 2000a; Marusawa et al., 2003). In contrast, the extent of cytoprotection obtained upon overexpression of a threonine-117 to alanine mutant survivin was comparable to the cytoprotection conferred by overexpression of wild-type survivin. Phosphorylation of threonine-117 has been shown to play a role in negatively regulating the function of survivin during mitosis (Wheatley et al., 2007). In the present study, treatment of gastric epithelial cells with cytoprotective ETOH was not found to cause re-entry into the cell cycle (data not shown). It is therefore possible that the regulatory role of threonine-117 phosphorylation is either specific to mitosis or that, as with its function in mitosis, the survival/anti-apoptosis function of survivin requires that threonine-117 be in the non-phosphorylated state.
While we are postulating that increased phosphorylation in response to cytoprotective ETOH exposure results in stabilization of survivin leading to its accumulation in gastric epithelial cells in vitro, we also found that continual survivin translation is required. Furthermore, although our studies utilizing actinomycin-D and cycloheximide indicate that cytoprotection in vitro requires de novo survivin protein synthesis but not de novo gene transcription, such may not be the case in vivo. We have preliminarily found that intragastric administration of 20% ETOH in vivo results in a significant increase in survivin mRNA levels within 30 minutes (unpublished results). In addition, although exposure of gastric epithelial cells in vitro to cytotoxic (5%) ETOH concentrations did not significantly modulate either total or phosphorylated survivin expression levels, administration of 50% ETOH in the absence of cytoprotective ETOH pre-administration resulted in significantly increased total survivin protein expression levels in the gastric mucosa immediately bordering necrotic lesions. Therefore, it is possible that increased survivin plays an important general role in regulating the extent of gastric injury in addition to its possible role in mediating cytoprotection against gastric injury.
Numerous reports have verified that prostaglandin synthesis/release is integral to maintaining gastric homeostasis in vivo (e.g. Wallace and Tigley, 1995; Peskar, 2001; Takeuchi et al., 2002; Brzozowski et al., 2005; Gyires, 2005), and that prostaglandins have a role in gastric mucosal cytoprotection. Nevertheless, important mechanisms underlying prostaglandin-independent components of gastric cytoprotection in vivo have likely been overlooked as a result of the complexity created by the interactions of several different cell types, in addition to the effects of gastric blood flow and neuronal and immune responses. It is also important to note in that because NSAIDs have been shown to inhibit gastric mucosal survivin expression in a manner independent of prostaglandin synthesis or function (Chiou et al., 2005), the reported findings that NSAIDs abolish gastric cytoprotection cannot necessarily be considered definitive evidence that prostaglandins are the sole mediators of gastric cytoprotection caused by mild irritant exposure.
On the other hand, it should be noted that while knockdown of survivin protein expression using siRNA was found in the present study to statistically abolish cytoprotection in vitro, forced overexpression of survivin did not completely mimic the extent of cytoprotection produced by mild irritant ETOH pre-exposure. These findings suggest that survivin plays a crucial role in gastric cytoprotection, at least with regards to gastric epithelial cells in vitro, but also that survivin alone is not sufficient to elicit the full cytoprotective response. Therefore, even in gastric epithelial cells in vitro, other adaptation responses are also required in order for complete cytoprotection to occur. In addition, although both our studies regarding knockdown of survivin and its forced overexpression support a role for survivin as a mediator of apoptosis resistance against cytotoxic ethanol exposure, we cannot rule out the possibility that increased endogenous survivin expression levels resulting from pre-exposure to mild irritant ethanol contribute to cytoprotection via a mechanism that is either partly or entirely apoptosis-independent.
While we have emphasized the likely role of p34cdc2 kinase as a primary mediator of survivin phosphorylation, we cannot rule out the possibility that other kinases contribute to the phosphorylation of gastric epithelial cell survivin − particularly in vivo. It is also possible that exposure to mild irritants affects gastric mucosal survivin ubiquitination and/or degradation independently of phosphorylation state. In addition, exposure to mild irritants may also act to enhance gastric mucosal survivin levels via activation of a transcriptional program in vivo.
In the present study, we have demonstrated for the first time that administration of mild irritant ETOH, at concentrations that produce cytoprotection against the gastric damaging effects of concentrated (i.e. ≥ 50%) ETOH, results in increased protein expression levels of gastric mucosal survivin. Our present study also suggests that the role of survivin in cytoprotection might not be limited to gastric mucosal epithelial cells as we also found significant correlation between total and phosphorylated survivin expression levels and cytoprotection produced in intestinal epithelial cells by mild irritant ETOH. However, there appears to be at least some cell-type specificity to the role of survivin in cytoprotection as we were unable to demonstrate a relationship between survivin expression and cytoprotection in primary thymocytes. Nevertheless, we believe that our findings extend beyond the demonstration of a novel factor involved in mediating gastric cytoprotection. Indeed, it is possible that survivin plays a much broader role in regulating the integrity and/or homeostasis of at least some normal adult differentiated tissues than is presently realized. In this regard, survivin has also been shown to be a factor in liver regeneration (Deguchi et al., 2002), angiogenesis (O'Connor et al., 2000b) and vascular injury and repair (Simosa et al., 2005; Conte and Altieri, 2006). In at least some normal adult organ tissues, survivin may be a crucial player in maintaining the balance between cellular proliferation and apoptosis. However, in some cancers survivin expression has been shown to be differentially regulated at the transcriptional level from normal tissue expression (Xu et al., 2004). Small molecule transcriptional inhibitors such as tetra-O-methyl nordihydroguaiaretic acid that have been shown to inhibit transcriptional expression of both survivin and p34cdc2 (Chang et al., 2004) may, therefore, represent a potential class of novel cancer therapeutics harboring relatively low toxicity. Nevertheless, our present demonstration that gastric epithelial cell cytoprotection involves both enhanced survivin expression and p34cdc2 activation supports a case for closer scrutiny of the possible important physiological roles of survivin, particularly when undertaking the development of therapeutics to universally target it.
Acknowledgments
This study was supported by the VA Biomedical Laboratory Research & Development Service and an award to M.K.J. from NIH (AA014946). The authors wish to thank Dr. Martin Jadus for assistance in the isolation of thymocytes and Dr. Andrzej Tarnawski for providing the IEC-6 cells.
Literature Cited
- Atherton-Fessler S, Parker LL, Geahlen RL, Piwnica-Worms H. Mechanisms of p34cdc2 regulation. Mol Cell Biol. 1993;13:1675–1685. doi: 10.1128/mcb.13.3.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaa MA. Release of protective products, different from prostaglandins, by rat stomachs exposed to mild irritant. Dig Dis Sci. 1989;34:429–435. doi: 10.1007/BF01536267. [DOI] [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Konturek SJ, Brzozowska I, Pawlik T. Role of prostaglandins in gastroprotection and gastric adaptation. J Physiol Pharmacol. 2005;56(Suppl 5):33–55. [PubMed] [Google Scholar]
- Chang CC, Heller JD, Kuo J, Huang RC. Tetra-O-methyl nordihydroguaiaretic acid induces growth arrest and cellular apoptosis by inhibiting Cdc2 and survivin expression. Proc Natl Acad Sci USA. 2004;101:13239–13244. doi: 10.1073/pnas.0405407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SK, Jones MK, Tarnawski AS. Survivin - an anti-apoptosis protein: its biological roles and implications for cancer and beyond. Med Sci Monit. 2003a;9:P125–P129. [PubMed] [Google Scholar]
- Chiou SK, Moon WS, Jones MK, Tarnawski AS. Survivin expression in the stomach: implications for mucosal integrity and protection. Biochem Biophys Res Commun. 2003b;305:374–379. doi: 10.1016/s0006-291x(03)00724-1. [DOI] [PubMed] [Google Scholar]
- Chiou SK, Tanigawa T, Akahoshi T, Abdelkari B, Jones MK, Tarnawski AS. Survivin: a novel target for indomethacin-induced gastric injury. Gastroenterology. 2005;128:63–73. doi: 10.1053/j.gastro.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Clemens DL, Calisto LE, Sorrell MF, Tuma DL. Ethanol metabolism results in a G2/M cell-cycle arrest in recombinant Hep G2 cells. Hepatology. 2003;38:385–393. doi: 10.1053/jhep.2003.50332. [DOI] [PubMed] [Google Scholar]
- Conte MS, Altieri DC. Survivin regulation of vascular injury. Trends Cardiovasc Med. 2006;16:114–117. doi: 10.1016/j.tcm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Culvenor JG, Weidemann MJ. Phytohaemagglutinin stimulation of rat thymus lymphocytes glycolysis. Biochim Biophys Acta. 1976;437:354–363. doi: 10.1016/0304-4165(76)90005-2. [DOI] [PubMed] [Google Scholar]
- De Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- Deguchi M, Shiraki K, Inoue H, Okano H, Ito T, Yamanaka T, Sugimoto K, Sakai T, Ohmori S, Murata K, Furusaka A, Hisatomi H, Nakano T. Expression of survivin during liver regeneration. Biochem Biophys Res Commun. 2002;297:59–64. doi: 10.1016/s0006-291x(02)02128-9. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Pelus LM. Regulation of the inhibitor-of-apoptosis family member survivin in normal cord blood and bone marrow CD34(+) cells by hematopoietic growth factors: implication of survivin expression in normal hematopoiesis. Blood. 2001;98:2091–2100. doi: 10.1182/blood.v98.7.2091. [DOI] [PubMed] [Google Scholar]
- Gianani R, Jarboe E, Orlicky D, Frost M, Bobak J, Lehner R, Shroyer KR. Expression of survivin in normal, hyperplastic, and neoplastic colonic mucosa. Hum Pathol. 2001;32:119–125. doi: 10.1053/hupa.2001.21897. [DOI] [PubMed] [Google Scholar]
- Gray NS, Wodicka L, Thunnissen AM, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, Leclerc S, Meijer L, Kim SH, Lockhart DJ, Schultz PG. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science. 1998;281:533–538. doi: 10.1126/science.281.5376.533. [DOI] [PubMed] [Google Scholar]
- Guo W, Baluda MA, Park NH. Ethanol upregulates the expression of p21 WAF1/CIP1 and prolongs G1 transition via a p53-independent pathway in human epithelial cells. Oncogene. 1997;15:1143–1149. doi: 10.1038/sj.onc.1201287. [DOI] [PubMed] [Google Scholar]
- Gyires K. Gastric mucosal protection: from prostaglandins to gene-therapy. Curr Med Chem. 2005;12:203–215. doi: 10.2174/0929867053363478. [DOI] [PubMed] [Google Scholar]
- Hawkey CJ, Kemp RT, Walt RP, Bhaskar NK, Davies J, Filipowicz B. Evidence that adaptive cytoprotection in rats is not mediated by prostaglandins. Gastroenterology. 1988;94:948–954. doi: 10.1016/0016-5085(88)90552-5. [DOI] [PubMed] [Google Scholar]
- Ko JK, Cho CH. Adaptive gastric mucosal cytoprotection in rats: different models of action by three mild irritants. Digestion. 1996;57:54–59. doi: 10.1159/000201313. [DOI] [PubMed] [Google Scholar]
- Ko JK, Cho CH. Histological study of mechanisms of adaptive cytoprotection on ethanol-induced mucosal damage in rat stomachs. Dig Dis Sci. 1998;43:1248–1257. doi: 10.1023/a:1018807808088. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Yukiue H, Sasaki H, Fukai I, Yokoyama T, Kiriyama M, Yamakawa Y, Maeda M, Fujii Y. Developmentally regulated expression of survivin in the human thymus. Hum Immunol. 2002;63:101–107. doi: 10.1016/s0198-8859(01)00369-x. [DOI] [PubMed] [Google Scholar]
- Kokoska ER, Smith GS, Deshpande Y, Rieckenberg CL, Miller TA. Adaptive cytoprotection induced by ethanol in human intestinal cells: role of prostaglandins and calcium homeostasis. Ann Surg. 1998a;228:123–130. doi: 10.1097/00000658-199807000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoska ER, Smith GS, Rieckenberg CL, Deshpande Y, Banan A, Miller TA. Adaptive cytoprotection against deoxycholate-induced injury in human gastric cells in vitro: is there a role for endogenous prostaglandins? Dig Dis Sci. 1998b;43:806–815. doi: 10.1023/a:1018826416864. [DOI] [PubMed] [Google Scholar]
- Kokoska ER, Smith GS, Wolff AB, Deshpande Y, Rieckenberg CL, Banan A, Miller TA. Role of calcium in adaptive cytoprotection and cell injury induced by deoxycholate in human gastric cells. Am J Physiol. 1998c;275:G322–G330. doi: 10.1152/ajpgi.1998.275.2.G322. [DOI] [PubMed] [Google Scholar]
- Krystof V, Lenobel R, Havlicek L, Kuzma M, Strnad M. Synthesis and biological activity of olomoucine II. Bioorg Med Chem Lett. 2002;12:3283–3286. doi: 10.1016/s0960-894x(02)00693-5. [DOI] [PubMed] [Google Scholar]
- Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Curr Opin Cell Biol. 2006;18:616–622. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Li F. Role of survivin and its splice variants in tumorigenesis. Br J Cancer. 2005;92:212–216. doi: 10.1038/sj.bjc.6602340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Lin H, Leonard SS, Shi X, Ye J, Luo J. Overexpression of ErbB2 enhances ethanol-stimulated intracellular signaling and invasion of human mammary epithelial and breast cancer cells in vitro. Oncogene. 2003;22:5281–5290. doi: 10.1038/sj.onc.1206675. [DOI] [PubMed] [Google Scholar]
- Marusawa H, Matsuzawa S, Welsh K, Zou H, Armstrong R, Tamm I, Reed JC. HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO J. 2003;22:2729–2740. doi: 10.1093/emboj/cdg263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh H, Ota S, Hiraishi H, Ivey KJ, Terano A, Sugimoto T. Adaptive cytoprotection in cultured rat gastric mucus-producing cells. Role of mucus and prostaglandin synthesis. Dig Dis Sci. 1995;40:872–878. doi: 10.1007/BF02064994. [DOI] [PubMed] [Google Scholar]
- Norbury C, Blow J, Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991;10:3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Altieri DC. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA. 2000a;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, Nath AK, Pober JS, Altieri DC. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol. 2000b;156:393–398. doi: 10.1016/S0002-9440(10)64742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DS, Wall NR, Porter AC, Altieri DC. A p34(cdc2) survival checkpoint in cancer. Cancer Cell. 2002;2:43–54. doi: 10.1016/s1535-6108(02)00084-3. [DOI] [PubMed] [Google Scholar]
- Patel R, Holt M, Philipova R, Moss S, Schulman H, Hidaka H, Whitaker M. Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells. J Biol Chem. 1999;274:7958–7968. doi: 10.1074/jbc.274.12.7958. [DOI] [PubMed] [Google Scholar]
- Peskar BM. Role of cyclooxygenase isoforms in gastric mucosal defence. J Physiol Paris. 2001;95:3–9. doi: 10.1016/s0928-4257(01)00003-1. [DOI] [PubMed] [Google Scholar]
- Pippin JW, Qu Q, Meijer L, Shankland SJ. Direct in vivo inhibition of the nuclear cell cycle cascade in experimental mesangial proliferative glomerulonephritis with Roscovitine, a novel cyclin-dependent kinase antagonist. J Clin Invest. 1997;100:2512–2520. doi: 10.1172/JCI119793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Nezamis JE, Lancaster C, Davis JP, Field SO, Hanchar AJ. Mild irritants prevent gastric necrosis through ”adaptive cytoprotection” mediated by prostaglandins. Am J Physiol. 1983;245:G113–G121. doi: 10.1152/ajpgi.1983.245.1.G113. [DOI] [PubMed] [Google Scholar]
- Roberts EC, Shapiro PS, Nahreini TS, Pages G, Pouyssegur J, Ahn NG. Distinct cell cycle timing requirements for extracellular signal-regulated kinase and phosphoinositide 3-kinase signaling pathways in somatic cell mitosis. Mol Cell Biol. 2002;22:226–7241. doi: 10.1128/MCB.22.20.7226-7241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokutan K, Hirakawa T, Teshima S, Nakano Y, Miyoshi M, Kawai T, Konda E, Morinaga H, Nikawa T, Kishi K. Implications of heat shock/stress proteins for medicine and disease. J Med Invest. 1998;44:137–147. [PubMed] [Google Scholar]
- Schmidt KL, Smith GS, Miller TA. Microscopic correlates of adaptive cytoprotection in an ethanol injury model. Histol Histopathol. 1989;4:105–115. [PubMed] [Google Scholar]
- Simosa HF, Wang G, Sui X, Peterson T, Narra V, Altieri DC, Conte MS. Survivin expression is up-regulated in vascular injury and identifies a distinct cellular phenotype. J Vasc Surg. 2005;41:682–690. doi: 10.1016/j.jvs.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Smith GS, Myers SI, Bartula LL, Miller TA. Adaptive cytoprotection against alcohol injury in the rat stomach is not due to increased prostanoid synthesis. Prostaglandins. 1991;41:207–223. doi: 10.1016/0090-6980(91)90041-d. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Groigno L, Whitaker M, Miller FJ, Sluder G, Sturrock J, Whalley T. Activation of protein kinase C alters p34(cdc2) phosphorylation state and kinase activity in early sea urchin embryos by abolishing intracellular Ca2+ transients. Biochem J. 2000;349:489–499. doi: 10.1042/0264-6021:3490489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Ogawa Y, Kagawa S, Ukawa H. Gastric ulcerogenic responses following barrier disruption in knockout mice lacking prostaglandin EP1 receptors. Aliment Pharmacol Ther. 2002;16(Suppl 2):74–82. doi: 10.1046/j.1365-2036.16.s2.21.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nishimoto K, Tomisato W, Tsutsumi S, Hoshino T, Tsuchiya T, Mizushima T. Adaptive cytoprotection induced by pretreatment with ethanol protects against gastric cell damage by NSAIDs. Dig Dis Sci. 2004;49:210–217. doi: 10.1023/b:ddas.0000017440.46863.56. [DOI] [PubMed] [Google Scholar]
- Tarnawski AS, Hollander D, Gergely H, Stachura J. Comparison of antacid, sucralfate, cimetidine, and ranitidine in protection of the gastric mucosa against ethanol injury. Am J Med. 1985;79(suppl 2C):19–23. doi: 10.1016/0002-9343(85)90567-4. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Mima S, Tomisato W, Hoshino T, Tsuchiya T, Mizushima T. Molecular mechanism of adaptive cytoprotection induced by ethanol in human gastric cells. Exp Biol Med (Maywood) 2003;228:1089–1095. doi: 10.1177/153537020322800917. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Tigley AW. Review article: new insights into prostaglandins and mucosal defence. Aliment Pharmacol Ther. 1995;9:227–235. doi: 10.1111/j.1365-2036.1995.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Wheatley SP, McNeish IA. Survivin: a protein with dual roles in mitosis and apoptosis. Int Rev Cytol. 2005;247:35–88. doi: 10.1016/S0074-7696(05)47002-3. [DOI] [PubMed] [Google Scholar]
- Wheatley SP, Barrett RM, Andrews PD, Medema RH, Morley SJ, Swedlow JR, Lens SMA. Phosphorylation by aurora-B negatively regulates survivin function during mitosis. Cell Cycle. 2007;6:1220–1230. doi: 10.4161/cc.6.10.4179. [DOI] [PubMed] [Google Scholar]
- Xu Y, Fang F, Ludewig G, Jones G, Jones D. A mutation found in the promoter region of the human survivin gene is correlated to overexpression of survivin in cancer cells. DNA Cell Biol. 2004;23:527–537. doi: 10.1089/dna.2004.23.527. [DOI] [PubMed] [Google Scholar]