Abstract
Background
Ambient particulate matter (PM) is associated with cardiovascular morbidity and mortality. It has been proposed that PM induces a pro-thrombotic process, increasing the risk of cardiovascular events, with some support from epidemiological and laboratory-based models. Diesel exhaust is a major contributor to urban PM, and we conducted a controlled human exposure of diesel exhaust in healthy subjects.
Objective
To evaluate diesel exhaust exposure effects on fibrinolytic burden (D-dimer), platelet number, and endothelial injury (von Willebrand’s factor, VWF), inhibition of the fibrinolytic pathway (plasminogen activator inhibitor-1 [PAI-1]), and inflammation (C-reactive protein, CRP).
Materials and Methods
Randomized, crossover, double-blinded design, with 13 healthy participants exposed on three different days (≥ 2 weeks washout) to diesel exhaust at 0 (filtered air), 100μg PM2.5/m3 and 200μg PM2.5/m3. We assessed diesel exhaust-associated changes in D-dimer, VWF, PAI-1 and platelets at 3, 6 and 22 hours, and CRP at 22 hours, after exposure initiation.
Results
Significant changes did not occur in any primary endpoints. Among secondary endpoints, diesel exhaust (200μg PM2.5/m3) effect on PAI-1 levels at 22 hours was of borderline significance, with a 1.32-fold decrease after exposure to diesel exhaust (200μg PM2.5/m3), relative to filtered air (CI 1.00 to 1.54). Diurnal patterns in D-dimer and PAI-1 were observed.
Conclusions
In healthy individuals, exposure to 200μg PM2.5/m3 diesel exhaust did not affect primary pro-thrombotic endpoints. Thus, these data do not support a diesel exhaust-induced pro-thrombotic phenomenon. Replication of these studies should be carried out to ascertain whether or not they inform our mechanistic understanding of air pollution’s cardiovascular effects.
Keywords: diesel, coagulation, fibrinolysis, human, inhalation exposure, vehicle emissions
Ambient particulate matter (PM) is associated with cardiovascular morbidity and mortality [1]. Diesel exhaust (DE) is a major contributor to urban PM [2]. Proposed mechanisms for the association between PM and cardiovascular disease include endothelial dysfunction, alteration of autonomic control, systemic inflammation and increase in coagulability [3]. In this report, we evaluate coagulability and systemic inflammation in a controlled human experiment of DE exposure in healthy subjects.
The hypothesis of increased coagulability due to PM [4] is supported by epidemiologic evidence in both general and particularly susceptible populations [5, 6]. However, a DE-specific healthy animal model showed decreased plasminogen activator inhibitor-1 (PAI-1), implying increased thrombolysis, in response to DE [7]. Data from controlled human DE-exposure models are inconclusive, with one study of a susceptible population [8] unable to demonstrate increased pro-coagulative factors and another, using healthy subjects, suggesting impaired fibrinolysis associated with DE [9].
Cardiovascular death and several associated morbidities are associated with both direct and indirect markers of a “pro-coagulant” state, including those primarily reflecting inflammation (C-reactive protein, CRP) [10], fibrin burden (D-dimer) [11], endothelial injury (von Willebrand’s factor, VWF) [12], and inhibition of the fibrinolytic pathway (plasminogen activator inhibitor-1, PAI-1) [13]. Therefore, we used such markers as endpoints to investigate the hypothesis that DE induces a subclinical hyper-coagulable state, as reflected by increases in relevant peripheral blood markers.
Materials and Methods
Subject Recruitment and Inclusion Criteria
Subjects were eligible to participate in the study if they fulfilled the following inclusion criteria
age 18-49 years; non-smoking status at least six months prior to recruitment; no history of ongoing medical care for heart disease, hypertension, asthma, diabetes, hypercholesterolemia, or other chronic condition; body mass index below 30kg/m3; fasting blood sugar below 126mg/dL; lack of arrhythmia or ischemia on electrocardiogram; blood pressure below 130/85 mmHg; normal spirometry (MicroDL, Micro Medical Ltd, Kent, UK). Women of childbearing age underwent a urine pregnancy test at screening and before each exposure, and were instructed to practice effective contraception during the study. All subjects gave a written informed consent prior to the screening process. The consent form and study protocol were approved by the University of Washington Human Subjects Review Division; all subjects provided informed consent.
Experimental Design
A crossover design was used, in which study participants were exposed on three different days to each of three different concentrations, randomized and counter-balanced to order. Exposure was calibrated at 0 (filtered air, FA), 100μg/m3 (DE100) and 200μg/m3 (DE200) of particulate DE, based on the mass of particles less than 2.5 microns in diameter (PM2.5), and measured continuously using a tapered element oscillating microbalance (TEOM 1400A PM2.5, Rupprecht & Patashnick Co., Albany, NY). DE was derived from a 2002 model turbocharged direct-injection 5.9 liter Cummins B-series engine (6BT5.9G6, Cummins, Inc., Columbus, IN) operating at load. This system incorporates several features, including a fast primary dilution of 15:1 and a subsequent residence time of 2 seconds with secondary dilution assisted by turbulence in a 1000 cubic feet per minute tunnel, that result in a final breathing zone mimicking ambient exposures. Exposures were separated by at least 2 weeks. Subjects, staff interacting with subjects, and staff conducting outcome assessments, were blinded to exposure level.
Subjects fasted overnight and during the exposure. They ate a standardized meal, with the same food content and quantity, after each exposure. Exposure began consistently within 30 minutes of 9 a.m. on weekdays and lasted 2 hours, during which time subjects were resting.
D-dimer, VWF, PAI-1, and platelet counts were measured from a peripheral venous blood sample drawn from an indwelling IV catheter approximately 30 minutes prior to exposure (0h) and at 3 hours (3h), 6 hours (6h), and 22 hours (22h) after the start of each exposure. CRP was assessed similarly except that there was no assessment at 6h.
D-dimer antigen and VWF antigen (Diagnostica Stago, Parsippany, NJ) were measured by enzyme-linked immunosorbent assay and PAI-1 antigen level was measured by the Chromolyze assay (Trinity Biotech, St. Louis, MI). CRP was assessed by immunonephelometric assay (Dade Behring, Newark, DE). Platelet count was assessed by automated cell count.
Statistical Analysis
Our primary analyses evaluated DE-attributable changes (from baseline) in the following measures
CRP at 22h; D-dimer, VWF, PAI-1 and platelet count at 6h. Secondary analyses evaluated DE-attributable changes in D-dimer, VWF and PAI-1 at 3h and 22h.
To perform such analyses, raw outcome variable values were log-transformed in order to normalize their distributions. Subsequently, changes in each endpoint at the prescribed time intervals were assessed by paired t-tests, separately for the FA and DE200 exposures. To directly assess DE-attributable changes (“DE effect”), these exposure-specific changes were compared to each other by paired t-tests.
Although the primary analyses assessed the differences in effect between the exposure extremes (FA and DE200), three post-hoc sensitivity analyses were performed to assess the DE100 exposure. First, a repeated measures ANOVA test inclusive of the FA, DE100 and DE200 exposures was performed on the primary endpoints. Then, an analysis of threshold effect was performed by pooling the FA and DE100 exposures into a “low exposure” group and comparing that to the DE200 exposure for the primary endpoints noted above. Similarly, an analysis of “exposure” versus “no exposure” was performed by pooling the DE100 and DE200 exposures and comparing that to FA for the primary endpoints noted above. Both the threshold and “exposure” versus “no exposure” analyses were also performed for any secondary endpoints with significant results in their initial testing.
Other measures of analytic quality control were to repeat the primary analyses after removing values greater than 2 standard deviations from the mean and to assess an ANOVA model inclusive of period and carryover effect terms.
All analyses were performed with Stata version 8.0 (Stata Corp, College Station, TX). P-values less than 0.05 were considered significant.
Results
Seventeen healthy subjects entered the study; 13 of these were exposed to and provided data at both the FA and DE200 exposures. Dropouts were due to scheduling difficulties; no adverse effects were reported. The 13 subjects were young, with a median age of 24.8 years (range 20.7 - 42.6). 11 (84.6%) of the subjects were male. All subjects were never smokers. Levels of gaseous co-pollutants were low, with nitrogen dioxide ranging from 10-35 ppb and carbon monoxide ranging between 0.7-1.8 ppm.
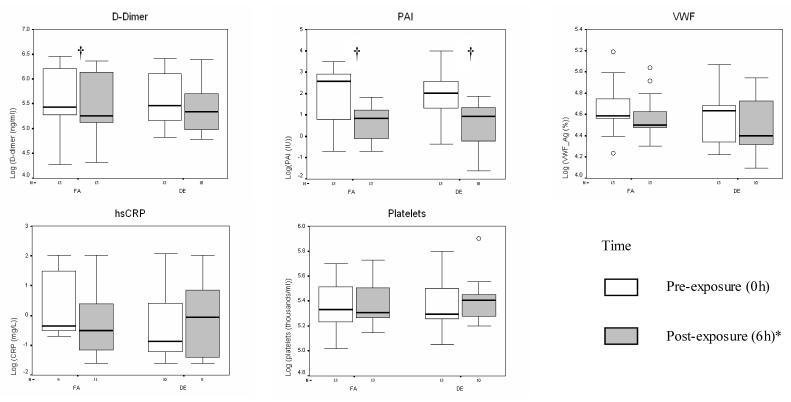
As shown in Figure 1 and Table 1, there were significant decreases in 6h D-dimer with FA exposure and significant decreases in 6h PAI-1 with both FA and DE200. However, in the “DE effects” analysis that accounts for diurnal variability, the 6h changes in PAI-1 associated with FA were not significantly different from the 6h changes associated with DE200. Similarly, changes in other primary analysis endpoints associated with FA were not significantly different from changes associated with DE200.
Figure 1.
Coagulation marker changes from 0 to 6 hours* post-exposure, by exposure level. Each panel depicts medians (bars) and interquartile ranges (whiskers) for each marker associated with given dose and time point (see legend); † 0 versus 6 hour* fold-change significant at p<0.05 (paired t-test); * Except hsCRP (22h); open circles represent data points outside of interquartile range; FA = filtered air; DE = 200μg/m3 (PM2.5) diesel exhaust.
Table 1.
Coagulation markers, fold-change in primary endpoints by exposure
| Change from Pre- to Post-Exposure, Fold* (95% CI) | |||
|---|---|---|---|
| FA | DE (200 μg/m3) | DE Effect¶ | |
| Ddimer (ng/ml) | -1.09 (-1.17, -1.02)† | -1.03 (-1.35, +1.23) | +1.10 (-1.19, +1.51) |
| PAI-1 (IU/ml) | -3.25 (-6.33, -1.68)† | -4.14 (-9.39, -1.76)† | -1.08 (-1.59, +2.02) |
| VWF (antigen %) | -1.07 (-1.18, +1.02) | -1.04 (-1.10, +1.01) | +1.03 (-1.12, +1.20) |
| Platelets (103/mL) | +1.01 (-1.04, +1.05) | 1.00 (-1.06, +1.05) | +1.01 (-1.06, +1.09) |
| CRP (mg/L) | -1.14 (-1.51, +1.04) | +1.01 (-1.29, +1.25) | +1.28 (-1.15, +1.91) |
Fold-change (using log-transformed data) between 0 and 6 hours, except for CRP (between 0 and 22 hours).
Positive difference indicates that change in given marker was relatively more positive over DE exposure interval, as compared to change over FA exposure interval.
Significant at 5% level.
Secondary analyses demonstrated non-significant differences in fold changes, with DE200 relative to FA, in 3h D-dimer (+1.01, 95% CI = -1.23, +1.32), PAI-1 (-1.03, -1.55, +2.06) and VWF (-1.01, 95% CI = -1.17, +1.17). 22h DE200-associated changes in D-dimer (+1.07, 95% CI = -1.07, +1.40) and VWF (+1.05, 95% CI = -1.08, +1.20) were also non-significant. However, there was an effect of borderline significance on 22h PAI-1 levels, with a 1.32-fold (95% CI = 1.00, +1.54; p=0.05) decrease with exposure to DE200 relative to FA. The PAI-1 data is detailed in Table 2.
Table 2.
Plasminogen activator inhibitor-1 (PAI-1)* at multiple time points
| Time from exposure initiation | FA | DE (200 μg/m3) |
|---|---|---|
| 0h | 11.7 (9.7) | 11.9 (14.3) |
| 3h | 5.2 (5.3) | 5.5 (5.5) |
| 6h | 2.6 (1.9) | 2.5 (1.9) |
| 22h | 12.1 (13.3) | 7.8 (8.8) |
mean for all subjects in IU/ml (SD); data log-transformed for subsequent analyses
There were no carryover or period effects. With removal of outliers, there was a non-significant sharpening of trends noted in primary analyses. There was no significant change in primary endpoints using repeated measures ANOVA regression including DE100 data.
Regarding the secondary analyses of threshold effect and of “exposure” versus “no exposure”, there were no statistically significant changes in either primary endpoints or 22h PAI-1 levels.
Discussion
Using a controlled human exposure to precisely-quantified DE, we were unable to demonstrate a pro-thrombotic effect of moderate-dose DE in healthy individuals. We observed significant circadian variation in D-dimer and PAI-1, as previously reported [14, 15]. After accounting for this variation, an independent effect of DE was not noted at 6 hours after exposure initiation. Although trends in most of the primary endpoints (Table 1) were in the direction expected under the hypothesis that DE causes a pro-thrombotic environment in blood, none of these trends reached statistical significance in our relatively small sample of healthy individuals.
Based on epidemiological evidence of traffic-related cardiac morbidity [16] and animal models of PM-related soluble components [17], we expected an early effect on pro-thrombotic markers, including PAI-1, which we did not find. One explanation for this is that early and relatively minor DE-associated effects on PAI-1 are obscured by significant diurnal variation in PAI-1, which is minimized at the 22h time point. However, our analysis should have controlled for diurnal variability, unless patterns were to differ considerably, within individuals, on different days. We did find a decrease of borderline significance in a secondary endpoint, 22h PAI-1 levels. Because PAI-1 is known to decrease the breakdown of fibrin clot by inhibiting the conversion of plasminogen to plasmin [18], this finding is surprising given the overall study hypothesis that DE induces a pro-thrombotic state in peripheral blood.
Though our attempt to broadly assess DE-induced pro-thrombotic markers was limited, our exposure model reflects ambient traffic-related exposures in some settings, and several of the negative findings may inform future study. We did not find a DE-associated increase in CRP, as anticipated by an epidemiological model [19]. One explanation for this is that PM-associated increases in CRP found in the epidemiological study were due to PM components other than those generated from DE. Alternatively, the middle-aged population in epidemiological studies, though free of known cardiovascular disease, may have had some subclinical disease making them relatively more prone to inflammation than our young population. We also did not find a DE-associated increase in platelet numbers, consistent with prior study in a hamster model of DE particle exposure [20]. However, we did not directly test platelet activation, a more sensitive measure for which there is evidence in this hamster model. Finally, though the lack of DE-associated change in VWF is consistent with prior study [21] and suggests a lack of endothelial activation [22] under our experimental conditions, the role of endothelial dysfunction needs to be further addressed given others’ findings of impaired vasomotor responses to both endothelium-dependent and -independent vasodilators at 6 hours [9].
The epidemiological research documenting PM-related cardiovascular disease is consistent, and its underlying mechanisms need to be better understood. Our data from a controlled human exposure model suggest a small DE-associated decrease in PAI-1 at 22 hours. However, our interpretation of this result is limited by having made comparisons in multiple endpoints. Had we performed a multiple testing correction, the finding of decreased 22h PAI-1 would no longer achieve even borderline statistical significance.
Nonetheless, with the exception of PAI-1, the trend in each of the primary endpoints was in the direction expected by our study hypothesis. One potential explanation for the lack of significant findings, in addition to those proposed above, relates to the effective dose administered by our protocol. Mills and colleagues [9] noted impairment of endogenous fibrinolysis (suppression of bradykinin-stimulated tissue plasminogen activator levels), but not significant changes in PAI-1, in association with DE exposure at 300μg/m3 and with moderate intermittent exercise in healthy individuals. However, using the same dose and a slightly decreased exercise level in a study of individuals with chronic obstructive lung disease, Blomberg and colleagues [8] did not find DE-associated changes in VWF, CRP, D-dimer or fibrinogen. In our study, we exposed subjects to lower concentrations of DE without exercise to more closely reflect ambient conditions of urban highway driving, which rarely exceed the levels of our study. Notably, the exposure system reported by both Mills and colleagues and Blomberg and colleagues generate NO2 in significantly higher concentrations (by approximately 2 orders of magnitude) than ours. While NO2 has been shown to cause airway inflammation [23], its effects on thrombotic parameters in blood is not adequately studied. Therefore, how any of these specific difference in protocol may explain the disparate findings in these studies is an important topic for future investigation.
It is also possible that the trends noted under the current protocol could be statistically significant in a larger population. Even changes of a relatively small absolute magnitude, they could be of considerable clinical significance to workers such as miners, who are already prone to several compromising work-related cardiopulmonary disorders and have exposure concentrations greater than those we generated [24], or to populations in developing world cities, where DE exposure levels are considerably higher than they are in the developed world. Further, these findings from healthy adults may not be generalizable to other populations; cautious DE-based experiments using at-risk groups with a greater susceptibility for cardiovascular events [25] or susceptibility to particulate matter [26] are needed. Furthermore, for some populations, even if an acute clinical effect cannot be demonstrated, a small increase in these endpoints might be important. For example, a slight absolute increase in CRP may be significant, particularly on a population level, given the potential for CRP-associated atherosclerosis progression [27], a chronic effect that may be influenced by subtle plasma-based inflammatory states. Our study was not designed to assess such chronic changes, but this warrants further investigation.
In conclusion, 200μg/m3 (PM2.5) DE led to borderline-significant (p=0.05) decrease in 22-hour post-exposure PAI-1 levels in healthy individuals, using a controlled human inhalational exposure model with randomized, double-blind, crossover design. The clinical significance of such an effect in a secondary endpoint is unclear. Primary and other secondary endpoints, designed to reflect diverse aspects of the thrombotic process, were not significantly altered by DE. Our ability to detect changes in such endpoints may have been limited by diurnal variability, for which we found evidence in D-dimer and PAI-1. Thus, while it appears that moderate-dose DE does not produce a strong coagulant effect in young healthy individuals, further study, including carefully accounting for diurnal factors, may be helpful in addressing the relevance of the noted late decrease in PAI-1.
Acknowledgements
We would like to thank Mary Aulet, Timothy Gould, Karen Jansen, Sara Jarvis, Jim Stewart, and the University of Washington’s General Clinical Research Center staff for invaluable assistance with the conduct of this study. Support for this study was provided by grants R830954 and R827355 from the Environmental Protection Agency, M01RR-00037 from the National Institutes of Health (University of Washington - General Clinical Research Center), and ES013195, ES011139, and P30ES07033 (University of Washington - Center for Ecogenetics and Environmental Health) from the National Institute for Environmental Health Sciences.
Abbreviations
- CRP
C-reactive protein
- DE
diesel exhaust
- DE100
100μg PM2.5/m3
- DE200
200μg PM2.5/m3
- FA
filtered air
- PAI-1
plasminogen activator inhibitor-1
- PM
particulate matter
- PM2.5
particulate matter of 2.5 microns or less
- TEOM
tapered element oscillating microbalance
- VWF
von Willebrand’s factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular Mortality and Long-Term Exposure to Particulate Air Pollution: Epidemiological Evidence of General Pathophysiological Pathways of Disease. Circulation. 2004;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 2.USEPA . National Air Pollutant Emission Trends. Office of Air Quality Planning and Research; 2000. 1990-1998. Available from: http://www.epa.gov/ttn/chief/trends/trends98/trends98.pdf, (Table 3-6, p 3-14) [Google Scholar]
- 3.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr., Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 4.Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995;345(8943):176–8. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 5.Peters A, Doring A, Wichmann HE, Koenig W. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet. 1997;349(9065):1582–7. doi: 10.1016/S0140-6736(97)01211-7. [DOI] [PubMed] [Google Scholar]
- 6.Ruckerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, Heinrich J, Marder V, Frampton M, Wichmann HE, Peters A. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173(4):432–41. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- 7.Furuyama A, Hirano S, Koike E, Kobayashi T. Induction of oxidative stress and inhibition of plasminogen activator inhibitor-1 production in endothelial cells following exposure to organic extracts of diesel exhaust particles and urban fine particles. Arch Toxicol. 2006;80(3):154–62. doi: 10.1007/s00204-005-0020-x. [DOI] [PubMed] [Google Scholar]
- 8.Blomberg A, Tornqvist H, Desmyter L, Deneys V, Hermans C. Exposure to diesel exhaust nanoparticles does not induce blood hypercoagulability in an at-risk population. J Thromb Haemost. 2005;3(9):2103–5. doi: 10.1111/j.1538-7836.2005.01559.x. [DOI] [PubMed] [Google Scholar]
- 9.Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112(25):3930–6. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 10.De Servi S, Mariani M, Mariani G, Mazzone A. C-reactive protein increase in unstable coronary disease cause or effect? J Am Coll Cardiol. 2005;46(8):1496–502. doi: 10.1016/j.jacc.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 11.Menown IB, Mathew TP, Gracey HM, Nesbitt GS, Murray P, Young IS, Adgey AA. Prediction of Recurrent Events by D-Dimer and Inflammatory Markers in Patients with Normal Cardiac Troponin I (PREDICT) Study. Am Heart J. 2003;145(6):986–92. doi: 10.1016/S0002-8703(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 12.Collet JP, Montalescot G, Vicaut E, Ankri A, Walylo F, Lesty C, Choussat R, Beygui F, Borentain M, Vignolles N, Thomas D. Acute release of plasminogen activator inhibitor-1 in ST-segment elevation myocardial infarction predicts mortality. Circulation. 2003;108(4):391–4. doi: 10.1161/01.CIR.0000083471.33820.3C. [DOI] [PubMed] [Google Scholar]
- 13.Sinkovic A, Pogacar V. Risk stratification in patients with unstable angina and/or non-ST-elevation myocardial infarction by Troponin T and plasminogen-activator-inhibitor-1 (PAI-1) Thromb Res. 2004;114(4):251–7. doi: 10.1016/j.thromres.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Angleton P, Chandler WL, Schmer G. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1) Circulation. 1989;79(1):101–6. doi: 10.1161/01.cir.79.1.101. [DOI] [PubMed] [Google Scholar]
- 15.Iversen PO, Groot PD, Hjeltnes N, Andersen TO, Mowinckel MC, Sandset PM. Impaired circadian variations of haemostatic and fibrinolytic parameters in tetraplegia. Br J Haematol. 2002;119(4):1011–6. doi: 10.1046/j.1365-2141.2002.03953.x. [DOI] [PubMed] [Google Scholar]
- 16.Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, Lowel H. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351(17):1721–30. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 17.Gilmour PS, Nyska A, Schladweiler MC, McGee JK, Wallenborn JG, Richards JH, Kodavanti UP. Cardiovascular and blood coagulative effects of pulmonary zinc exposure. Toxicol Appl Pharmacol. 2006;211(1):41–52. doi: 10.1016/j.taap.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood. 1987;69(2):381–7. [PubMed] [Google Scholar]
- 19.Peters A, Frohlich M, Doring A, Immervoll T, Wichmann HE, Hutchinson WL, Pepys MB, Koenig W. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J. 2001;22(14):1198–204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- 20.Nemmar A, Hoet PH, Dinsdale D, Vermylen J, Hoylaerts MF, Nemery B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation. 2003;107(8):1202–8. doi: 10.1161/01.cir.0000053568.13058.67. [DOI] [PubMed] [Google Scholar]
- 21.Nemmar A, Hoet PH, Vermylen J, Nemery B, Hoylaerts MF. Pharmacological stabilization of mast cells abrogates late thrombotic events induced by diesel exhaust particles in hamsters. Circulation. 2004;110(12):1670–7. doi: 10.1161/01.CIR.0000142053.13921.21. [DOI] [PubMed] [Google Scholar]
- 22.Lip GY, Foster W, Blann AD. Plasma von Willebrand factor levels and surrogates of atherosclerosis. J Thromb Haemost. 2005;3(4):659–61. doi: 10.1111/j.1538-7836.2005.01284.x. [DOI] [PubMed] [Google Scholar]
- 23.Solomon C, Christian DL, Chen LL, Welch BS, Kleinman MT, Dunham E, Erle DJ, Balmes JR. Effect of serial-day exposure to nitrogen dioxide on airway and blood leukocytes and lymphocyte subsets. Eur Respir J. 2000;15(5):922–8. doi: 10.1034/j.1399-3003.2000.15e19.x. [DOI] [PubMed] [Google Scholar]
- 24.Monforton C. Weight of the evidence or wait for the evidence? Protecting underground miners from diesel particulate matter. Am J Public Health. 2006;96(2):271–6. doi: 10.2105/AJPH.2005.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. Jama. 1998;279(18):1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 26.Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: are diabetics more susceptible? Epidemiology. 2002;13(5):588–92. doi: 10.1097/00001648-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Paffen E, DeMaat MP. C-reactive protein in atherosclerosis: A causal factor? Cardiovasc Res. 2006;71(1):30–9. doi: 10.1016/j.cardiores.2006.03.004. [DOI] [PubMed] [Google Scholar]