Abstract
Objective
To assess corticomotor excitability (CM) of the antagonist biceps brachii (BB) post-stroke in preparation for pronator contraction. In healthy subjects, we previously demonstrated that prior to pronator contraction CM excitability of the antagonist BB was suppressed.
Methods
Transcranial magnetic stimulation (TMS) was used to assess pre-contraction changes in motor evoked potential (MEP) amplitude of the BB, when BB was acting either as an antagonist or an agonist. TMS was applied 100−200 ms prior to rhythmic isometric BB or pronator contractions in chronic stroke survivors and age/gender matched healthy control subjects.
Results
Prior to pronator contraction, MEPs in BB were elicited in the stroke group but were absent in healthy controls indicating that CM excitability of the antagonist BB was increased post-stroke. The extent of the abnormal increase in excitability positively correlated with the extent of upper limb motor impairment.
Conclusions
Our results suggest that an alteration of cortical control mechanisms regulating motor excitability of the antagonist BB may contribute to the impairment of upper limb motor coordination post-stroke.
Significance
This study offers a unique approach to study the potential for a cortical origin of post-stroke motor discoordination.
Keywords: motor preparation, transcranial magnetic stimulation, abnormal flexor synergy, stroke
Introduction
Inhibition of antagonist muscle activity is essential for the execution of coordinated limb movements. Studies using transcranial magnetic stimulation (TMS) have provided experimental evidence of cortical inhibitory control of antagonists that is initiated during preparation for a motor action. Specifically, in intact humans, pre-contraction suppression has been shown to occur in wrist flexors, wrist extensors (Hoshiyama et al., 1997; Hoshiyama et al., 1996) and elbow flexors (Gerachshenko and Stinear, 2007) when these muscles were antagonists. Currently, there are no reports evaluating this inhibitory control mechanism in neurologically impaired populations. Therefore, a contribution of pre-contraction suppression of antagonists to the execution of coordinated limb movements is unclear.
Abnormal flexor muscle synergies are often present in the upper limb post-stroke. This impairment of motor coordination involves stereotypical movement patterns such as elbow flexion coupled with shoulder abduction. At the early stages of recovery, these movement patterns are the first movements many stroke survivors voluntarily produce (Brunnstrom, 1966; Brunnstrom, 1970; Twitchell, 1951). In the chronic stage of stroke, depending on the level of recovery, abnormal movement patterns may continue to dominate and accompany every movement that a stroke survivor attempts to perform. The strongest component of the upper limb abnormal flexor synergy is elbow flexion (Brunnstrom, 1970). This activity of elbow flexors interferes with the accomplishment of selective movements that normally are not dependent on contraction of elbow flexors. It has been suggested that alteration of control mechanisms regulating activity of antagonists may underlie this motor impairment (Lum et al., 2003).
We recently demonstrated that the excitability of corticospinal pathways projecting to the biceps brachii (BB) acting as an antagonist was suppressed in preparation for pronator contraction in neurologically intact individuals (Gerachshenko and Stinear, 2007). The present study was designed to examine whether pre-contraction corticomotor (CM) excitability of the antagonist BB was modified post-stroke. We hypothesized that CM excitability of the paretic BB antagonist prior to pronator contraction will be increased as a consequence of stroke. This hypothesis was based on clinical observations that abnormal contractions of elbow flexors post-stroke are associated with motor actions that do not require contractions of these muscles in healthy individuals. To this aim, TMS at intensities below resting threshold was employed to reveal abnormal CM excitability of the paretic BB antagonist preceding pronator contraction. We assessed CM excitability by comparing the amplitude of motor evoked potentials (MEPs) in the BB prior to pronator contraction with MEP amplitude prior to elbow flexor contraction separately in stroke survivors and healthy controls. Finally, we examined the extent of the abnormal increase in CM excitability of the paretic BB antagonist and the extent of upper limb motor impairment for a correlation.
Methods
Subjects
Sixteen stroke patients and eight control subjects participated in the present study. Stroke patients were recruited from the Sensory Motor Performance Program Stroke Registry and the Rehabilitation Institute of Chicago outpatient stroke group. Stroke patients' main inclusion criteria were: 1) first ever monohemispheric stroke documented by computerized tomography or magnetic resonance imaging (duration > 6 months); 2) ability to perform forearm pronation and elbow flexion against resistance; 3) no contraindications for the application of TMS. Eight stroke patients (two females and six males, 52−72 years old; mean age, 61.5 years; see Table 1) qualified for inclusion in the final analysis (see below for more details).
Table 1.
Demographic and clinical information for stroke subjects
| Patient | Gender | Age (years) | Hand preference | Years since onset | Affected hemisphere | Lesion location | F-M score | MAS score |
|---|---|---|---|---|---|---|---|---|
| I | M | 68 | R | 12.1 | L | MCA territory | 37 | 2 |
| II | M | 58 | R | 4.2 | R | BG, CR, IC & frontal lobe ICA infarct | 33 | 1+ |
| III | F | 55 | R | 19.6 | L | 31 | 1+ | |
| IV | M | 70 | R | 7.4 | L | MCA & ACA territories | 40 | 1 |
| V | F | 57 | R | 11.1 | R | Frontal & parietal lobes | 30 | 1+ |
| VI | M | 72 | R | 3.6 | R | ACA territory | 56 | 0 |
| VII | M | 60 | R | 6 | R | Frontal & parietal lobes | 27 | 1+ |
| VIII | M | 52 | L | 3.9 | L | CR & IC | 45 | 1+ |
M, male; F, female; R, right; L, left; MCA, middle cerebral artery; BG, basal ganglia; CR, corona radiata; IC, internal capsule; ICA, internal carotid artery; ACA, anterior cerebral artery; F-M, Fugl-Meyer; MAS, Modified Ashworth Scale.
The control group included neurologically intact individuals with no contraindications to TMS from the departmental staff and from outside the department. Control subjects were age and gender matched to the stroke patients whose data were included in the final analysis (two females and six males, 50−75 years old; mean age, 60.6 years).
All subjects gave their written informed consent to participate in the study. The experimental protocol was approved by the Northwestern University Institutional Review Board in accordance with the Declaration of Helsinki.
Clinical assessment
A licensed physical therapist blinded to the experimental data evaluated the motor function of the upper limb of stroke patients using the upper extremity portion of the Fugl-Meyer (FM) Motor Assessment Protocol (Fugl-Meyer et al., 1975). The FM protocol was chosen because it evaluates abnormal muscle synergy patterns and the ability of a patient to move outside of those patterns. High FM scores (maximum 66) indicate greater motor function and selectivity of movements. A score less than 20 indicates severe motor impairment.
Spasticity for the elbow was evaluated implementing the Modified Ashworth Scale (0−4: where 0 reflects normal muscle tone and 4 indicates severe spasticity) (Bohannon and Smith, 1987).
The investigators were blinded to the results of the clinical assessment until data analysis had been completed. Clinical assessment scores for the stroke patients included in the final analysis are provided in Table 1.
Technical considerations
TMS was employed in this study to assess pre-contraction CM excitability of the antagonist BB post-stroke. Pre-contraction suppression of antagonists has previously been examined using TMS intensities above resting motor threshold (Gerachshenko and Stinear, 2007; Hoshiyama et al., 1996). Suprathreshold TMS allows the direct comparison of target muscle MEP amplitude obtained at rest with MEP amplitude obtained in preparation for contraction. However, the application of this protocol in the present study is problematic for the following reasons. First, BB is a proximal upper limb muscle with typically high TMS-induced resting motor thresholds (RThs) and thus requires the application of high stimulation intensities (Gerachshenko and Stinear, 2007; Rothwell et al., 1991; Turton et al., 1996; Wassermann et al., 1992). Second, paretic muscle motor thresholds are increased following stroke (Talelli et al., 2006). Our preliminary experiments confirmed that the combination of those two factors results in a low probability of obtaining MEPs in a relaxed paretic BB. We were either unable to determine RTh, or RTh was so high that TMS just above RTh would have required a setting that exceeded maximum stimulator output. Therefore, pre-contraction changes in CM excitability of the antagonist BB were assessed in the present study using stimulus intensities below resting threshold. Subthreshold TMS has been previously used to examine changes in CM excitability during preparation for a motor action in a number of studies (Chen et al., 1998; MacKinnon and Rothwell, 2000; Pascual-Leone et al., 1992; Tomberg and Caramia, 1991).
Transcranial Magnetic Stimulation
For both groups, the excitability of corticospinal pathways projecting to BB was assessed with TMS delivered a) at rest, b) prior to an intended elbow flexor contraction (flexion task) and c) prior to a pronator contraction (pronation task). Magnetic stimuli were delivered over the optimal scalp site for the contralateral BB (the site which gave the largest MEP amplitude for a given stimulus intensity) via a figure-of-eight coil connected to a Magstim 200 unit (Magstim, Dyfed, Wales, UK). To maintain identical positioning of the stimulating coil throughout the experiment, the optimal site for BB stimulation was marked on a cotton cap, which was tightly but comfortably fixed on the subject's head. The coil was placed tangentially on the scalp with the handle rotated at 45 ° from the midline and pointing posterior to induce cortical current in the posterior to anterior direction. TMS intensity was adjusted to 120% of the active motor threshold (ATh). ATh was determined when subjects received visual feedback of their electromyographic (EMG) activity while maintaining a tonic isometric contraction of BB at 10% maximum voluntary contraction (MVC). ATh was defined as the stimulator output intensity that elicited MEPs that were discernible from the background EMG in four out of eight trials. For the two stroke patients in whom it was not possible to determine ATh at 10% MVC, the optimal site for BB stimulation was determined using strong tonic isometric contractions of BB above 10% MVC and TMS intensity was adjusted to the maximum intensity that the patient could tolerate (82% and 85% of maximum stimulator output respectively).
Electromyography
TMS-induced responses were recorded simultaneously from BB and pronator teres (PT) with surface EMG. Surface self-adhesive disposable electrodes (Bortec BioMed, Calgary AB, Canada) were applied to the skin over the muscle belly using a standard skin preparation procedure (removal of hair, light abrasion and cleansing with alcohol). The electrodes have a fixed distance of 2 cm between the centers of the gel pads. The location and orientation of each muscle belly was identified using palpation at the expected anatomical location. For this purpose, subjects were asked to perform actions that selectively activated the muscle of interest. Electrodes were placed longitudinally along the muscle belly midway between the musculotendinous junctions. To target PT and minimize cross talk onto PT from adjacent brachioradialis (BR) and flexor carpi radialis (FCR) muscles, identification of the PT belly location and orientation was achieved by palpation during rhythmic selective contractions of the BR, FCR and PT. EMG data were sampled at 2000 Hz, amplified (×500), band-pass-filtered (10−500 Hz) using an AMT-8 amplifier (Bortec Biomedical, Canada, Calgary, Alberta), and recorded using Spike 2 software via a Micro 1401 Mk II A/D board (Cambridge Electronic Design, Cambridge, England). Data were saved to disk for off-line analysis. The amplitude of EMG during MVC of the BB was measured by instructing the subject to perform a brief maximal elbow flexion against resistance three times with a rest of several seconds in between. Visual EMG feedback was displayed on a computer screen placed in front of the subject during the performance of all tasks.
Experimental protocol
The same experimental protocol was used for both groups. Subjects were seated in a comfortable chair with the forearm of interest (impaired arm for a stroke subject; self-reported dominant arm for a control subject) placed at approximately 90° elbow flexion into a custom-built arm restraint mounted on a table with adjustable height. The target forearm was supported in front of the subject parallel to the coronal plane with the fingers flexed. This arm posture resembles a “typical” flexed arm posture that many stroke survivors maintain when sitting. The arm restraint maintained the subject's forearm in a neutral position (thumb uppermost) and restricted movements at the wrist joint. In addition, Velcro straps were used to restrict movements that could occur during performance of the flexion task. During testing the other arm rested on the subject's lap and was kept relaxed.
Subjects were asked to perform a brief isometric contraction of the target muscle against the arm restraint in a rhythmic manner in time with an auditory metronome. They were instructed to keep the rhythm of a contraction as precise as possible and to keep their arm relaxed between the contractions. Subjects performed a sequence of either forearm pronator or elbow flexor contractions. Each sequence comprised several sets of trials during which TMS was delivered. The task order was randomized between subjects. Upon completion of all trials for one task, a subject was given a 10−15 minute break and then proceeded with the other task. The timing of TMS delivery was set with respect to the metronome signal (see Figure 1a). Therefore, to ensure maximum quality of rhythmic performance, the preferred frequency was determined for each subject. This was defined as the frequency at which the subject could comfortably and consistently time the onset of their agonist burst in the majority of trials either with or following the tone but not before the tone. Frequencies ranged from 0.7−1.0 Hz (mean, 0.8 Hz) and 0.6−0.8 Hz (mean, 0.7 Hz) for control and stroke groups respectively. Practice trials were performed for each muscle contraction task (pronation, flexion) prior to data collection. At the beginning of data collection, EMG was recorded with the subject at rest during which 5 TMS pulses at 120% ATh were delivered every 4 seconds to ensure that no MEPs were elicited in the relaxed BB. For each motor task, TMS was applied to record MEPs in a 200 ms window prior to the agonist contraction (see Figure 1b). This time interval was chosen because we have previously shown that significant changes in BB MEP amplitude occur mainly within this interval (Gerachshenko and Stinear, 2007). TMS was delivered every 4.8 to 6.8 seconds (depending on the preferred frequency of contractions) at randomly assigned time intervals with respect to the metronome signal in order to obtain MEPs between 200 and 100 ms prior to agonist contraction. During each set of trials, a randomly assigned number of MEPs (5 or 10) was collected and subjects were given breaks between sets (2−5 minutes). A minimum of 60 MEPs were recorded for each motor task.
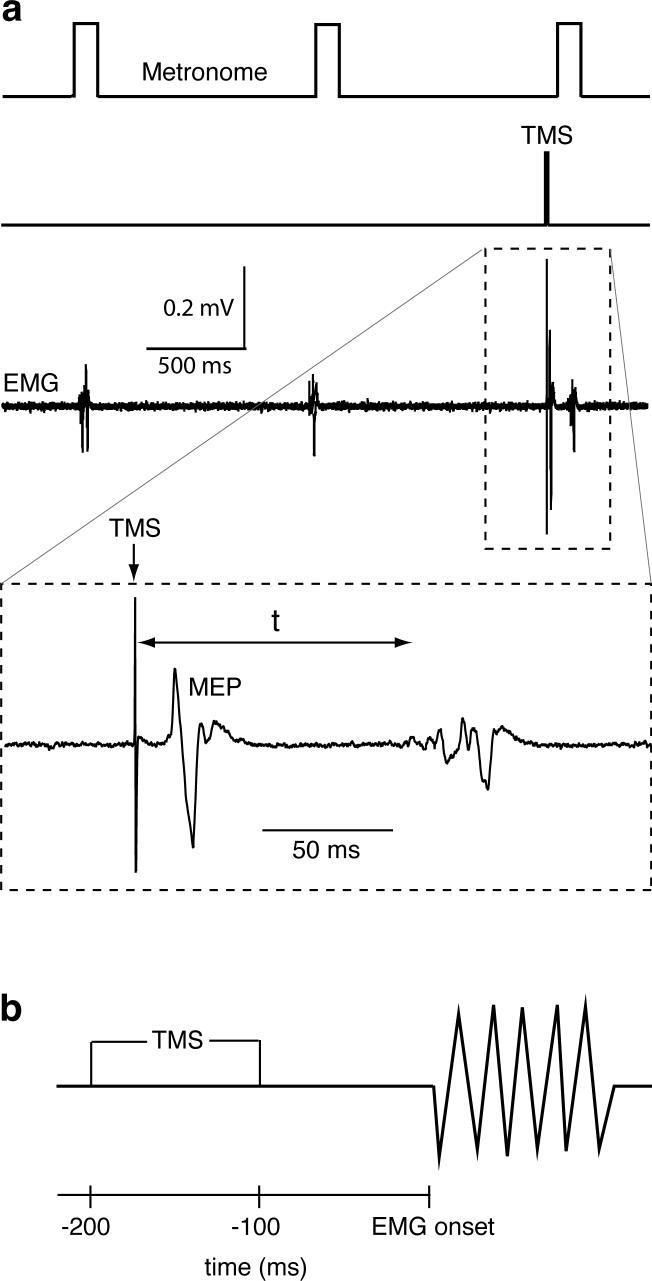
Fig. 1.
a) Schematic depicting the temporal relationship between three metronome signals (1st trace), a TMS trigger (2nd trace), and three short duration EMG bursts recorded from BB (3rd trace), as stroke patient IV performed a sequence of elbow flexor contractions. The 4th trace shows a TMS-induced MEP in BB prior to BB contraction on an expanded time scale. “t” indicates the time interval from TMS to the agonist burst onset. b) TMS was delivered in a pre-contraction window between 200 and 100 ms prior to agonist burst onset.
Data analysis
Analysis was performed using Signal software (Cambridge Electronic Design, Cambridge, UK). All frames were visually inspected and only the frames in which TMS was successfully delivered prior to the agonist EMG onset were accepted. In each frame the following parameters were measured for each muscle: MEP latency; the root mean square (RMS) amplitudes of a 30 ms pre-stimulus window of background EMG; the peak-to-peak MEP amplitude; and the time interval “t” from TMS delivery to the agonist EMG onset (see Figure 1a). An adequate estimate of the latter was achieved by visual inspection and cursor placement. For this experiment the resolution of “t” could be in the range of several milliseconds, and onsets of the low-amplitude short-duration bursts recorded in the rhythmic task were distinct. Data were further sorted to accept only the frames where t ≤ 200 ms. The average number of frames retained was 18 and 25 for stroke and healthy subjects respectively. Retained data were averaged for each task. Mean RMS amplitudes of the pre-stimulus background EMG were inspected for each muscle, and frames were excluded from further analysis to ensure that equivalent RMS values of the pre-stimulus background EMG were obtained for each task for a particular individual.
To evaluate the modulation of pre-contraction MEP amplitude of the antagonist BB for each subject, the mean BB MEP amplitude recorded prior to PT contraction (pronation task) was normalized to the mean value obtained prior to BB contraction (flexion task). The resulting fraction was called the “excitability ratio”.
Inclusion criteria for the final analysis
Of the sixteen stroke patients who participated in this study, data from eight patients (Table 1) qualified for inclusion in the final analysis. Exclusion criteria for the final analysis included: 1) insufficient data for between-task comparisons due to the inability of a patient to relax muscles between isometric contractions in the majority of trials; 2) pre-contraction BB MEP amplitudes were indiscernible from the background EMG in the flexion task, when TMS was applied at the maximum intensity that a patient could tolerate.
Statistical analysis
Between-group differences in mean RMS amplitude of background EMG, mean MEP amplitude, and mean time-to-agonist-onset were examined using “mixed” two-way ANOVAs where group and task were the factors and repeated measures were made on the factor task. Main effects were examined using Bonferroni corrected post hoc two-tailed t-tests including two sample tests for between-group comparisons and paired tests for within group comparisons. Two sample t-tests were used to determine if there was a between group difference in mean BB MEP latency prior to flexor activity, and a between group difference in excitability ratios. A regression analysis was conducted to examine for a correlation between the excitability ratio of the paretic BB antagonist and the Fugl-Meyer score. The coefficient of determination (R2) was used to assess the strength of the association between the excitability ratio and the FM score. The significance level was set at P < 0.05 for all but the post-hoc tests. Values are reported as mean ± s.e.m. (standard error of the mean) unless otherwise indicated.
Results
For control subjects, BB active motor thresholds (mean % ± one standard deviation) ranged from 33 to 64 % of the maximum stimulator output (53.3 ± 9.7). For six out of eight stroke subjects, in whom the ATh could be determined, BB AThs ranged from 50 to 71 % of the maximum stimulator output (60.2 ± 8.8).
RMS amplitude of pre-contraction background EMG
The mixed two-way ANOVA revealed main effects of group, F(1,14) = 8.53, P = 0.011, and task, F(1,14) = 6.26, P = 0.017. Post-hoc t-tests (with an adjusted P of 0.017) revealed that, prior to pronator activation, background EMG (μV ± s.e.m.) was greater for the stroke group (13.8 ± 2.0) than the healthy group (7.7 ± 0.3), P = 0.011, and prior to flexor activation, background EMG was also greater for the stroke group (14.1 ± 2.2) than the healthy group (7.3 ± 0.6), P = 0.011. For stroke group means, no difference was revealed between background EMG prior to pronator activation (13.8 ± 2.0) and flexor activation (14.1 ± 2.2), P = 0.091. Similarly, for healthy group means, no difference was revealed between background EMG prior to pronator activation (7.7 ± 0.3) and flexor activation (7.3 ± 0.6), P = 0.629.
Pre-contraction BB MEP amplitude
Figure 2 depicts typical MEPs induced by subthreshold TMS in the BB of a stroke and a healthy control subject at rest, prior to BB contraction (as an agonist) and prior to PT contraction (BB as an antagonist). For all subjects, MEPs were not elicited in the BB during muscle relaxation, and in healthy controls (Figure 2b), amplitudes were indiscernible from the background EMG amplitude in preparation for PT contraction (P = 0.179). The latter was determined using a paired t-test to compare peak-to-peak amplitude calculated from a window encompassing each subject's BB MEP window and peak-to-peak amplitude calculated from the same sized window prior to the stimulation artifact.
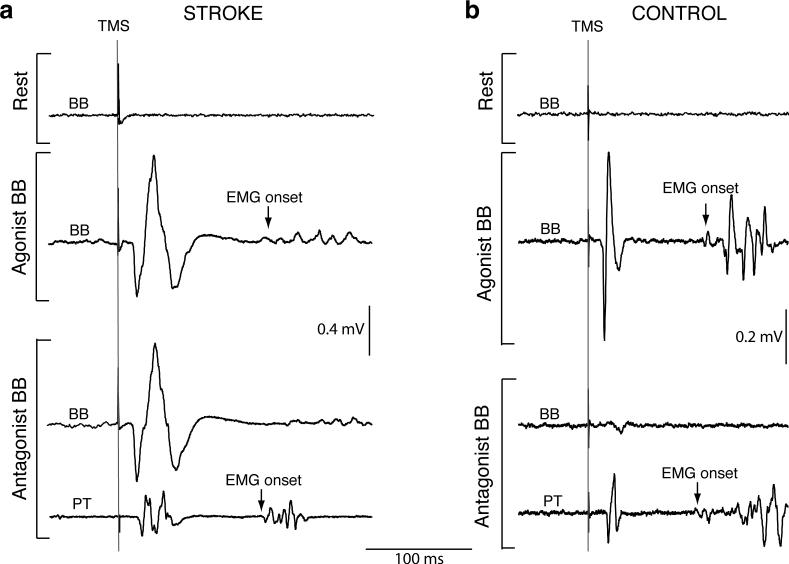
Fig. 2.
Single EMG traces from stroke patient II (a) and an age/gender matched control subject (b) recorded from BB at rest (top traces); prior to BB burst onset (middle traces); and prior to PT burst onset (bottom traces). The agonist's burst onset is indicated by an arrow. Vertical lines prior to each MEP represent TMS delivery. MEPs were not induced in the resting BB with subthreshold TMS. In the stroke patient (a), pre-contraction MEPs of similar amplitude were evident in BB acting as an agonist or as an antagonist. In the control subject (b), pre-contraction MEPs were elicited in the agonist BB but were absent in the antagonist BB. Two bottom traces are simultaneous recordings from BB and PT. Note, the control subject is one of two subjects in whom PT MEPs were elicited prior to pronator contraction.
The mixed two-way ANOVA just failed to reveal a main effect of group, F(1,14) = 4.3, P = 0.056, but did reveal a main effect of task F(1,14) = 17.7, P < 0.001. Post-hoc t-tests (with an adjusted P of 0.025) revealed that the stroke group MEP amplitude mean (mV ± s.e.m.) prior to flexor activation (0.48 ± 0.13) was greater than the mean prior to pronator activation (0.35 ± 0.14), P = 0.002, and the healthy group mean was greater prior to flexor activation (0.21 ± 0.07) than prior to pronator activation (0.03 ± 0.007), P = 0.018. The difference between the stroke group mean prior to pronator activation (0.35 ± 0.14) and the healthy group mean prior to pronator activation (0.03 ± 0.007), P = 0.041, did not reach the adjusted level of significance.
Time-to-agonist-onset
For stroke subjects, group mean time interval (ms ± s.e.m.) from TMS delivery to the agonist EMG onset was 123 ± 8 for the pronation task (range 96 − 165) and 129 ± 8 for the flexion task (range 102 − 162). For control subjects, TMS was delivered 109 ± 9 prior to PT contraction (range 84 − 147) and 115 ± 11 prior to BB contraction (range 83 − 175). The ANOVA did not reveal significant differences in mean time intervals between groups and tasks (all P > 0.1).
MEP latency
A between-group comparison of MEP latencies (ms ± s.e.m.) recorded from BB prior to flexor activity did not detect a difference between means (stroke, 13.2 ± 0.9; healthy, 12.8 ± 0.5), P = 0.60. However, stroke subjects' latencies ranged from 11 − 21 and healthy subjects' ranged from 13 − 16.
BB excitability ratio
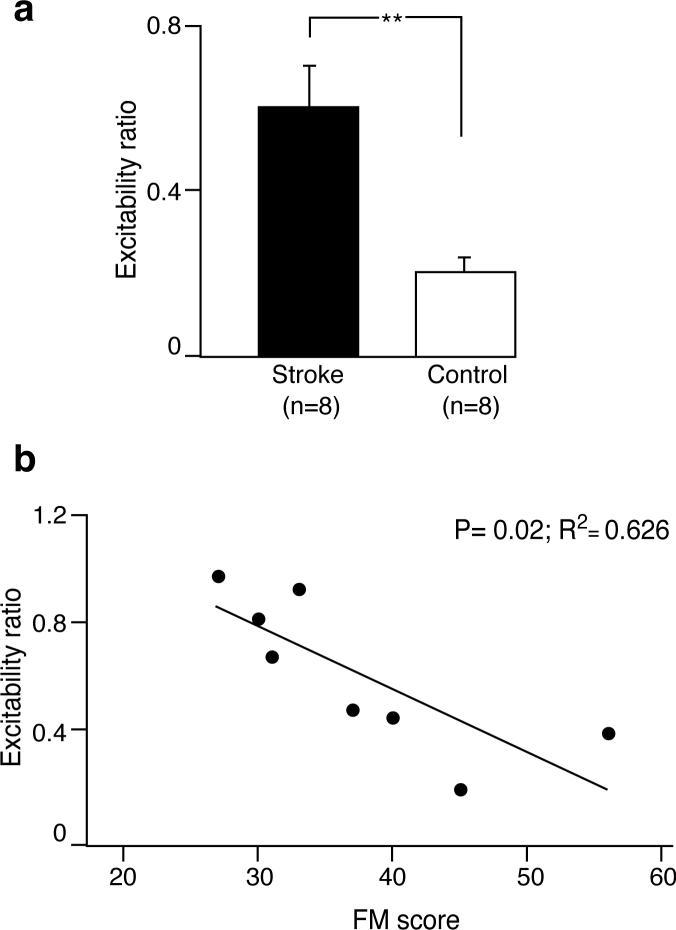
To help control for the variance in MEP amplitude for both group and task, we calculated group mean excitability ratios. MEP amplitudes prior to pronator activation were normalized to MEP amplitudes prior to flexor activation. The stroke group excitability ratio (0.61 ± 0.1) was greater than the healthy group ratio (0.21 ± 0.04), P = 0.002 (Figure 3a).
Fig. 3.
a) Mean excitability ratios (BB MEP amplitude prior to pronator contraction normalized to BB MEP amplitude prior to flexor contraction) for stroke and control groups. **, P = 0.002. b) Stroke patients' excitability ratios plotted as a function of their Fugl-Meyer (FM) scores. A line of best fit was drawn through data points. High excitability ratios were correlated with low FM scores.
Excitability ratio vs level of motor impairment
Figure 3b depicts the relationship between the excitability ratio and the Fugl-Meyer score. A linear regression analysis and coefficient of determination demonstrated that the excitability ratio was significantly and positively correlated with the extent of upper limb motor impairment post-stroke (R2 = 0.626, P = 0.019).
MEP amplitude changes in PT
Analysis of PT MEP amplitudes in stroke patients revealed that group mean MEP amplitudes (mV ± s.e.m.) were 0.39 ± 0.13 prior to PT contraction and 0.24 ± 0.06 prior to BB contraction. Analysis of PT MEP amplitudes in control subjects for both tasks revealed that individual mean values were ≤ 50 μV for all but two subjects as illustrated on Figure 2b. These low values precluded comparison of pre-contraction changes in PT MEP amplitude between the two groups.
Discussion
The main findings from this study were that the excitability of corticospinal pathways projecting to the BB antagonist as revealed by the excitability ratio was higher preceding paretic pronator contraction compared with healthy pronator contraction. The extent of this abnormal increase in CM excitability was correlated with the extent of upper limb motor impairment of stroke survivors. We have previously suggested that cortical inhibitory control of antagonists initiated during preparation for a motor action may play an important role for coordination of upper limb movements (Gerachshenko and Stinear, 2007). Here we provide evidence for the first time of a pre-contraction facilitation of the upper limb antagonist post-stroke suggesting that this abnormal excitability may contribute to the impairment of motor coordination.
This study is the continuation of our work investigating changes in CM excitability of antagonistic muscles in preparation for a motor action. Using suprathreshold TMS, we have previously shown in healthy subjects that CM excitability of the antagonist BB was significantly suppressed up to 200 ms prior to pronator contraction (Gerachshenko and Stinear, 2007). However, as discussed in Methods, application of the suprathreshold TMS protocol in a stroke population is difficult due to the high threshold of paretic proximal upper limb muscles at rest. Therefore, pre-contraction changes in CM excitability of the antagonist BB were assessed in the present study using subthreshold TMS intensities.
Pre-contraction modulation of the intact antagonist BB
A number of studies have previously demonstrated that stimulus intensities below resting motor threshold detected increases in CM excitability of an agonist during preparation for a motor action (Chen et al., 1998; MacKinnon and Rothwell, 2000; Pascual-Leone et al., 1992; Tomberg and Caramia, 1991). Indeed, in the present study, application of subthreshold TMS prior to intended muscle contractions in healthy subjects revealed that agonist BB CM excitability increased, because the stimulus intensities used were below rest threshold. In contrast, MEP amplitudes of the intact BB antagonist were indiscernible from background EMG in preparation for pronator contraction. This pre-contraction suppression of the intact BB antagonist was demonstrated in the present study with low excitability ratios.
Pre-contraction modulation of the antagonist BB post-stroke
In stroke survivors unwanted contraction of elbow flexors is associated with motor tasks that do not require contraction of these muscles in healthy individuals. In the present study, we used a pronator contraction task to reveal inappropriate activity in the paretic BB likely the result of an abnormal flexor synergy. The application of subthreshold TMS in the stroke group revealed levels of pre-contraction paretic BB antagonist and BB agonist CM excitability that were closer than the respective levels in the healthy group. High excitability ratios reflected this abnormal CM excitability of the antagonist BB post-stroke.
It is not unusual to find paretic limb MEP latencies to be longer than healthy subjects' latencies. In the present study mean MEP latencies did not differ statistically for the two groups despite stroke subjects' MEP latencies having a spread of 11 − 21 ms and healthy subjects having a spread of 13 − 16 ms. The longer conduction times in some stroke subjects may indicate partial conduction via alternate polysynaptic pathways, or slower temporal summation of descending volleys at the motoneuron level. However, an inspection of stroke subject data showed that one patient (I) with a long MEP latency (21 ms) had an excitability ratio (0.48) that was 5th largest, and two patients (III & VI) with latencies similar to healthy subjects' (14 & 13 ms) had high excitability ratios (0.93 & 0.82). There is no evidence in our present data that slower conduction times gave rise to higher excitability ratios.
Of prime concern in this type of study is whether the between-group differences in MEP amplitude merely result from differences in background motoneuron activity, even when absolute levels of background EMG are low as recorded in the present experiment. The effect on MEP amplitude of very small amounts of motoneuron activity is not clear, especially in stroke subjects. What was the magnitude of difference between stroke and healthy subjects' mean MEP amplitudes prior to pronator activation? For this key task, mean paretic MEP amplitude (0.35 mV ± 0.14) was ∼ 12 times larger than healthy subjects' (0.03 mV ± 0.007), even though this difference did not reach the adjusted level of significance. The lack of significance was due to paretic BB MEP amplitude variance being high compared with the mean (0.35 mV ± 0.14). Notwithstanding the lack of significance, the difference in means gave rise to the highly significant between-group difference in excitability ratios. It is possible that higher background EMG in paretic BB prior to pronator activation contributed to the ∼12-fold between-group difference in MEP amplitude, and therefore the between-group difference in excitability ratio. However, there are several lines of evidence from the present data that indicate the between-group difference in motoneuron activity alone was not responsible for the between-group difference in excitability ratio. First, if the between-group difference in excitability ratio was due to small differences in background motoneuron activity, background EMG in paretic BB prior to pronator activation would have been higher than prior to flexor activation, and been equivalent in healthy subjects' data. No between-task difference was detected for stroke group background EMG means (P = 0.09), and there was no difference between healthy group means (P = 0.63). Second, even if the excitability ratio was sensitive to statistically insignificant differences in between-task background EMG means, in order to explain the abnormal excitability ratio for the stroke group, background activity would need to have been higher prior to pronator activation than flexor activation, and it clearly was not. Prior to pronator activation the mean value was slightly smaller (13.8 μV) than prior to flexor activation (14.1 μV). Third, an inspection of data from two subjects in each group who had almost identical levels of background EMG for each task revealed differences in excitability ratios that were similar to the between group difference (stroke, 0.61; healthy, 0.21). Patients III and IV had ratios of 0.45 and 0.39 respectively with background EMG levels of 7.6 μV and 9.8 μV respectively, while the two healthy subjects had ratios of 0.18 and 0.11 and background EMG levels of 7.8 μV and 9.8 μV respectively. Fourth, a regression analysis of stroke subjects' excitability ratios and background EMG r.m.s. amplitudes prior to pronator activation demonstrated that there was no correlation (R2 = 0.167; P = 0.315). If the abnormal excitability ratio was merely the result of some stroke subjects being unable to maintain their background motoneuron activity quiet prior to pronation, subjects with higher excitability ratios should also have higher background levels of EMG, but this was not evident. Together these findings suggest that background motoneuron activity alone is unlikely to have modulated MEP amplitude in a direction that could explain our main findings.
Another consideration is whether EMG burst amplitude during the rhythmical task represented a larger proportion of MVC for stroke patients than healthy controls. MEP amplitudes recorded prior to burst onset may have been larger for stroke patients because they were applying a greater proportion of their maximum effort than control subjects, resulting in a general up-regulation of the lesioned motor cortex. To explore this, we calculated an EMG ratio (r.m.s. amplitude) of mean BB burst size to MVC during flexor activation for each subject. Stroke patients activated paretic BB (ratio ± s.e.m.) to 0.22 ± 0.16 of MVC, and healthy controls activated BB to 0.13 ± 0.08 of MVC, indicating that stroke patients applied greater effort relative to controls during the rhythmic task. However, a two sample t-test did not detect a difference between these means (P = 0.16), and a regression analysis of abnormal excitability ratio and EMG ratio for stroke patients indicated that there was no correlation between these two variables (R2 = 0.048; P = 0.60). For example, an inspection of individual subject's values revealed that patients III and VI with excitability ratios of 0.92 and 0.81 respectively had widely dissimilar EMG ratios of 0.08 and 0.35 respectively. If high excitability ratios were the result of patients employing greater effort, a high EMG ratio should have been consistently evident for patients with high excitability ratios, but this was not the case. This is an intriguing finding that suggests abnormal excitability ratios are independent of the amount of effort stroke patients employ in order to execute a rhythmical task with their paretic upper limb.
The disruption of pre-contraction suppression and motor coordination
In the present study, we aimed to recruit chronic stroke patients with different levels of upper limb motor impairment measured using the FM assessment. This assessment is considered to be “one of the most comprehensive quantitative measures of motor impairment following stroke” (Gladstone et al., 2002). Moreover, the FM scale is the most commonly used approach to evaluate abnormal muscle synergy patterns and the ability of a patient to move outside of those patterns. The FM scores of the stroke patients included in the final analysis ranged from 27 to 56. This wide range permitted us to examine relationships between the extent of abnormal excitability of the antagonist BB and the level of upper limb motor impairment. Stroke subjects with FM scores below 27 failed to qualify for this study because they were unable to pronate their paretic forearms in a rhythmic manner. The positive correlation between the extent of the abnormal increase in CM excitability of the paretic BB antagonist (defined by the excitability ratio) and the extent of upper limb motor impairment (defined by the Fugl-Meyer score) revealed that high excitability ratios were associated with low FM scores. This important finding suggests that the disruption of pre-contraction suppression of antagonists likely contributes to inappropriate elbow flexor activation post-stroke. Alternatively, abnormal pre-contraction CM excitability of the paretic BB antagonist may reflect an alteration in pre-contraction facilitatory mechanisms or in the balance between inhibitory and facilitatory control mechanisms. Our present findings are likely to be mediated by cortical mechanisms, because the TMS intensities used were below resting motor threshold, and excitability measures were taken 100 − 200 ms prior to muscle activation. However, our data do not rule out mediation at a subcortical or spinal level. A future experiment is being designed which will assess the pre-contraction motor excitability of a distal upper limb agonist-antagonist muscle pair. Electrical stimulation at the pyramidal decussation will be used to determine whether the effects are mediated at the spinal level.
This study did not aim to investigate the origin of abnormal flexor synergies post-stroke per se. The exact neural mechanisms underlying abnormal flexor synergies are unknown. Several potential mechanisms have been proposed to contribute to this motor impairment, including altered neuronal activity in the spinal cord, brain stem, or motor cortex (Dewald et al., 1995; Lum et al., 2003). It has been proposed that following stroke, the impaired descending commands from the lesioned cortex as well as modification of afferent input may cause an increase in excitability of flexion reflex spinal interneurons. Somatosensory impairments may have contributed to the abnormal excitability ratio but were not characterized in this study. Studies in patients with thalamic lesions suggest that impaired sensory pathways induce changes in motor system excitability (Liepert et al., 2005). Posterior cerebral artery infarctions are more likely to result in somatosensory impairments than the infarctions present in our group. A future investigation of the relationship between a range of somatosensory impairments and abnormal muscle synergies using the pre-contraction protocol reported in this study is warranted. The loss of descending input from the lesioned cortex may be partially compensated by increasing activity of intact descending motor routes originating in the brain stem, which are known to have the extensive intraspinal branching. However, the role of these efferents in humans is still unclear. Finally, the results of multiple studies in animals and humans strongly suggest that the injured brain is capable of structural and functional reorganization (Butefisch, 2004; Carmichael, 2003; Keyvani and Schallert, 2002). It has been demonstrated that rapid reorganization of motor cortex is mediated via activation of existing but silent synapses (Sanes and Donoghue, 2000) and modulation of synaptic strength in intracortical circuits (Hess and Donoghue, 1994; Hess and Donoghue, 1996; Rioult-Pedotti et al., 1998). In addition, lesion-induced structural reorganization including neurogenesis and synaptogenesis has been reported (Carmichael, 2003; Ming and Song, 2005; Zhang et al., 2005). Therefore, it was proposed that altered neuronal activity in the motor cortex may potentially underlie the manifestation of abnormal flexor synergies after stroke (Dewald et al., 1995; Lum et al., 2003).
Extensive interconnectivity between the motor cortical areas controlling various upper limb muscles requires a regulatory mechanism that could promote simultaneous activation of the muscles appropriate for a particular motor action and prevent unwanted co-activation of antagonists. Local modulation of the strength of intracortical connections between task-related corticospinal neurons has been proposed as a potential mechanism for mediating muscle synergies for a particular motor task (Schneider et al., 2002). Importantly, it was demonstrated that this modulation includes not only excitation but also a release from inhibition (i.e., disinhibition). It was shown that pharmacologically induced local removal of intracortical inhibition in cat motor cortex was capable of producing an artificial muscle synergy (Schneider et al., 2002). In addition, Devanne et al. demonstrated that co-activation of upper limb muscles during a pointing task was associated with a significant reduction of intracortical inhibition (Devanne et al., 2002). Therefore, these lines of evidence indicate that the functional coupling between task-related corticospinal neurons is associated with selective disinhibition of local inhibitory interneurons. On the other hand, the release from inhibition could potentially link functionally related and unrelated corticospinal neurons and thus recruit muscles that are inappropriate for a particular motor action. Therefore, disruption of the mechanisms involved in the functional selective coupling of the task-related motor cortical sites (e.g. abnormal release from inhibition) may underlie the emergence of abnormal muscle synergies during voluntary movements made by stroke survivors. Accordingly, the results of the current study suggest that disruption of pre-contraction suppression of antagonists post-stroke may underlie the abnormal co-activation of the antagonist BB. We have also noted with interest, that Lum et al. suggested that a loss or alteration of cortical control mechanisms regulating activity of antagonists due to stroke may result in synergistic activity (Lum et al., 2003). Finally, evidence of post-stroke motor cortical disinhibition has been provided in studies involving animals and humans (Cicinelli et al., 2003; Liepert et al., 2000; Talelli et al., 2006; Witte, 1998). Future studies are needed to elucidate the exact origin of this abnormal excitability.
Concluding remarks
This is the first report that demonstrates the feasibility of assessing CM excitability just prior to abnormal contraction of the paretic elbow flexor. Given that the pre-contraction window offers a relatively stable level of activity of spinal motoneurons and eliminates the contribution of afferent inputs from a contracting muscle, we suggest that this unique approach will be useful in the further assessment of the contribution of motor cortex to abnormal muscle synergies post-stroke.
Acknowledgments
We are grateful to Tobey DeMott, MS PT for assessment of stroke patients and to Mary Ellen Stoykov MS OTR/L, Carol Mottram, PT PhD and Michelle Prior MS for their help in recruitment of subjects. We are also grateful to subjects who volunteered for this study. Financial support was provided through a T32 grant from NIH (PI, WZR). Additional financial support was provided by the AHA (JWS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966;46:357–75. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. New York; Harper and Row: 1970. Movement therapy in hemiplegia: a neurophysiological approach. [Google Scholar]
- Butefisch CM. Plasticity in the human cerebral cortex: lessons from the normal brain and from stroke. Neuroscientist. 2004;10:163–73. doi: 10.1177/1073858403262152. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003;9:64–75. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol. 1998;44:317–25. doi: 10.1002/ana.410440306. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke. 2003;34:2653–8. doi: 10.1161/01.STR.0000092122.96722.72. [DOI] [PubMed] [Google Scholar]
- Devanne H, Cohen LG, Kouchtir-Devanne N, Capaday C. Integrated motor cortical control of task-related muscles during pointing in humans. J Neurophysiol. 2002;87:3006–17. doi: 10.1152/jn.2002.87.6.3006. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gerachshenko T, Stinear JW. Suppression of motor evoked potentials in biceps brachii preceding pronator contraction. Exp Brain Res. 2007 doi: 10.1007/s00221-007-1071-4. in press’. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–40. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol. 1994;71:2543–7. doi: 10.1152/jn.1994.71.6.2543. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term depression of horizontal connections in rat motor cortex. Eur J Neurosci. 1996;8:658–65. doi: 10.1111/j.1460-9568.1996.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R, Koyama S, Takeshima Y, Watanabe S, Shimojo M. Temporal changes of pyramidal tract activities after decision of movement: a study using transcranial magnetic stimulation of the motor cortex in humans. Electroencephalogr Clin Neurophysiol. 1997;105:255–61. doi: 10.1016/s0924-980x(97)00019-2. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M, Kitamura Y, Koyama S, Watanabe S, Shimojo M, Kakigi R. Reciprocal change of motor evoked potentials preceding voluntary movement in humans. Muscle Nerve. 1996;19:125–31. doi: 10.1002/(SICI)1097-4598(199602)19:2<125::AID-MUS1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Keyvani K, Schallert T. Plasticity-associated molecular and structural events in the injured brain. J Neuropathol Exp Neurol. 2002;61:831–40. doi: 10.1093/jnen/61.10.831. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000;111:671–6. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- Liepert J, Restemeyer C, Münchau A, Weiller C. Motor cortex excitability after thalamic infarction. Clin Neurophysiol. 2005;116:1621–1627. doi: 10.1016/j.clinph.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC. Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle Nerve. 2003;27:211–21. doi: 10.1002/mus.10305. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Rothwell JC. Time-varying changes in corticospinal excitability accompanying the triphasic EMG pattern in humans. J Physiol. 2000;528:633–45. doi: 10.1111/j.1469-7793.2000.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain. 1992;115(Pt 4):1045–59. doi: 10.1093/brain/115.4.1045. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–4. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Schneider C, Devanne H, Lavoie BA, Capaday C. Neural mechanisms involved in the functional linking of motor cortical points. Exp Brain Res. 2002;146:86–94. doi: 10.1007/s00221-002-1137-2. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: Neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006;117:1641–59. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Tomberg C, Caramia MD. Prime mover muscle in finger lift or finger flexion reaction times: identification with transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:319–22. doi: 10.1016/0168-5597(91)90019-t. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–28. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–80. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- Witte OW. Lesion-induced plasticity as a potential mechanism for recovery and rehabilitative training. Curr Opin Neurol. 1998;11:655–62. doi: 10.1097/00019052-199812000-00008. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–16. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]