Abstract
Females of many socially monogamous species accept or even actively seek copulations outside the social pair bond. As females cannot increase the number of offspring with promiscuous behaviour, the question arises why they engage in extra-pair mating. We used microsatellite data to determine paternity, heterozygosity and genetic relatedness in the reed bunting (Emberiza schoeniclus), a species with high levels of extra-pair paternity (EPP). We found that extra-pair young (EPY) were more heterozygous than within-pair young (WPY). The high heterozygosity of the EPY resulted from a low genetic similarity between females and their extra-pair mates. EPY were heavier and larger when compared with their maternal half-siblings shortly before they left the nest. Recapture data indicated a higher fledgling survival of EPY compared with WPY. Our data suggest that reed bunting females increase the viability of their offspring and thus fitness through extra-pair mating with genetically dissimilar males.
Keywords: reed bunting, promiscuity, extra-pair paternity, heterozygosity, genetic relatedness, genetic compatibility
1. Introduction
Promiscuity, whereby males and females mate with other than their social partner, is common in socially monogamous bird species (Griffith et al. 2002). Males may initiate extra-pair copulations (Westneat & Stewart 2003) but females have pre- and post-copulatory control over fertilization (Andersson 1994; Birkhead & Pizzari 2002). While males can increase the number of offspring via extra-pair fertilizations, it is less obvious how females could gain fitness due to promiscuous behaviour. It has been proposed that females increase offspring heterozygosity and, thus, the viability of their offspring through extra-pair fertilizations (Brown 1997). Evidence has been found in several species (Foerster et al. 2003; Masters et al. 2003; Bishop et al. 2007). However, other studies do not support these findings (Schmoll et al. 2005; Smith et al. 2005). A high heterozygosity reduces the risk that recessive deleterious alleles are expressed and prevents other negative effects of inbreeding, which would lead to reduced fitness. However, females may not only engage in extra-pair copulations to avoid inbreeding but also benefit from mating with more dissimilar mates (Amos et al. 2001). In that case, high heterozygosity in the offspring will be advantageous owing to potentially useful gene products. A higher heterozygosity at the major histocompatibility complex (MHC) does, for example, lead to an increased resistance against diseases (McClelland et al. 2003). In bluethroats (Luscinia s. svecica), Johnsen et al. (2000) found that extra-pair young (EPY) have a higher immunocompetence when compared with within-pair young (WPY). Females can maximize the heterozygosity of their offspring if they mate to males that are either highly heterozygous or genetically different from themselves (Masters et al. 2003).
Females that engage in extra-pair copulations may envisage different costs such as the loss of the social mate's investment in the offspring (Dixon et al. 1994), enhanced risk of sexually transmitted diseases, injury and predation. Therefore, females should engage in extra-pair mating only if it leads to substantial benefits. In a meta-analysis, Arnqvist & Kirkpatrick (2005) suggest that infidelity may not be adaptive for females because costs caused by depressed paternal investment outweigh indirect genetic benefits, but see Griffith (2007).
In this study we show that EPY were more heterozygous, heavier and larger when compared with WPY in a population of reed buntings (Emberiza schoeniclus) in Switzerland. Additionally we found that extra-pair fledglings survive at a higher rate when compared with within-pair fledglings. The observed extra-pair fertilizations resulted from matings between genetically dissimilar mates. Our findings suggest that reed bunting females increase the viability of their offspring through extra-pair mating.
2. Material and methods
(a) Field methods
The study was conducted on a reed bunting population in the Grande Cariçaie at the southeast shore of the lake of Neuchâtel in Switzerland for 6 years (during 2000–2002 and 2004–2006). Adult birds were caught with mist nets either at the nest site or (for some males) with additional help of playback within the territory. For individual identification, the birds were ringed with three coloured plastic rings and a numbered aluminium ring from the Swiss Ornithological Institute. From all birds we took 5–50μl blood from the brachial or leg vein for paternity analyses. Blood samples were collected in a 70 μl capillary tube and put on ice in the field and stored in a freezer at −18°C on the same day. Nestlings were bled from day 2 onwards and measured and ringed between days 6 and 11 after hatching (day 0=hatching day). Weight was measured with a Pesola balance (to the nearest 0.1 g) and tarsus length with a calliper (to the nearest 0.1 mm). Dead nestlings and eggs that failed to hatch were collected. Nests were monitored daily to determine hatch dates. However, some nests were found after hatching. We confirmed the social parents of a brood while they were feeding the young either by direct observation or with a colour camera installed near the nest.
Some of the birds that had been ringed as nestlings were recaptured later in the season from July to October. In the analysis, we used only data from birds that were recaptured at least 30 days after they had been ringed at the nest, thus, they were independent from their parents at the time of recapture. Birds were recaptured by our own research group at the study site at the end of the breeding season or by different other research groups during the autumn migration. All birds were recaptured along the southeast shore of the lake of Neuchâtel (0–25 km) except two of which one was caught at La Touvière (Geneva; 73 km) and the other at Romans-sur-Isère (France; 250 km).
(b) Molecular methods
DNA was extracted from blood and tissue samples using a Promega protocol during 2000–2002 and Peqgold blood DNA isolation kit (Peqlab) during 2004–2006.
For paternity analyses, we used fluorescently labelled primers for six different variable microsatellite loci: Escμ1; Escμ3; Escμ4; Escμ6 (Hanotte et al. 1994); Pdoμ5 (Griffith et al. 1999); and Ppi2 (Martinez et al. 1999). They were amplified by PCR and products were run on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems Instrument). The alleles were determined using DNA fragment analysis software (Genescan v. 3.1). The microsatellite loci did not deviate from Hardy–Weinberg equilibrium except Escμ3 indicating null alleles at this locus. The combined exclusion probability of the six microsatellite was higher than 0.995 for the first parent and 0.999 for the second parent using the program Cervus (v. 3.0; Kalinowski et al. 2007).
To sex the young we used the primers P2 (reverse) and P8 (forward) that anneal to conserved regions and amplify across introns that differ in length between the CHD-W and the CHD-Z gene (Griffiths et al. 1998).
(c) Statistical analyses
We used a general linear mixed model to test the effect of paternity on tarsus length and weight of nestlings from broods with mixed paternity. Sex was included as a fixed factor in the model because males are heavier and larger than females. The age of the brood was included as a linear covariate because broods were measured between days 6 and 8 after hatching. Brood identity was included as a random factor in the model to take into account the seasonal differences (e.g. food abundance) and the quality of the social parents.
Standardized individual heterozygosity (SIH) was calculated as the proportion of heterozygous loci divided by the mean heterozygosity of the typed loci in the population (Coltman et al. 1999). Genetic similarity between individuals was calculated using the program ML-Relate that takes into account the presence of null alleles (Kalinowski et al. 2006). A permutation test was conducted to test whether females mate randomly to their social mate with regard to the genetic relatedness. The observed number of social pairs was randomly formed 1000 times using all adult birds present at the site. The observed mean relatedness of the social pairs was then compared to the distribution of the means that had been generated under the assumption of random mating. To test whether the extra-pair fertilizations resulted from matings between genetically dissimilar mates, we compared the genetic relatedness between the female and the actual extra-pair sire or sires to the mean genetic relatedness between the female and the four nearest residents that did not sire EPY in the focal female's nest. Since almost all EPY were sired by males that have their territories in the neighbourhood of the female's nest (S. Suter 2007, unpublished data), this procedure allows a comparison between actual and potential extra-pair mates (Masters et al. 2003).
If there was more than one extra-pair father within the same brood, then the mean of all extra-pair sires was used in the analysis. Pairs where social partners changed between years were treated as independent. Statistical analysis was performed using JMP v. 5.0.1, R v. 2.4.1 and Excel. Non-parametric tests were used in case of non-normal distribution of the data. All tests are two tailed with a significance level of p<0.05.
3. Results
(a) Frequency and distribution of extra-pair paternity
We genotyped 915 offspring, of which 65 were fledged young, 786 nestlings and 64 embryos from eggs that did not hatch. The genetic father was determined for 835 (91%) of the young and the social father for 801 (88%). Overall there were 486 (61%) WPY and 315 (39%) EPY. The proportion of EPY in different years varied between 33 and 46% and the proportion of broods that contained EPY varied between 54 and 72%. No significant differences between years were found (G=8.23 and 4.09, d.f.=5, p=0.14 and 0.54, respectively). We could determine the sex of 792 out of the 801 young where the social father was known. Overall, EPY were not more likely to be males: there were 47% (224/481) WPY males and 51% EPY males (159/311; Fisher's exact test, p=0.22).
The EPY were not equally distributed among broods; there were 36 entire extra-pair broods, 98 mixed broods and 79 broods containing only WPY. In each year, the distribution of EPY among broods differed from what is expected under a binomial distribution (G-tests, all p<0.01), except in the year 2001 (G=4.92, d.f.=5, p=0.42). No egg dumping was detected as the social mother of a brood always corresponded to the genetic mother. A summary of the microsatellite loci used for paternity analysis can be found in table 3 of electronic supplementary material.
(b) Paternity and offspring heterozygosity
SIH of the 915 offspring varied between 0.28 and 1.18 (mean SIH±s.d., 1.00±0.17). EPY were more heterozygous when compared with WPY (mean SIH±s.e., EPY n=315, 1.02±0.01; WPY n=486, 0.98±0.01, Wilcoxon rank–sum test, Z=3.55, p=0.0004).
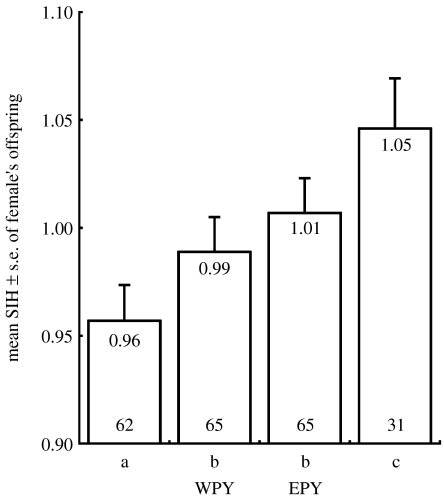
However, if we look only at mixed broods, the difference between maternal half-siblings is not significant (Wilcoxon signed-rank test, Z=0.55, n=57, p=0.58). The high overall SIH of EPY resulted from high SIH of the young that came from entire extra-pair broods and the low overall SIH of WPY resulted from low SIH of the young that came from entire within-pair broods (figure 1). In a pairwise comparison, the mean SIH of extra-pair offspring showed a tendency to be higher compared with that of within-pair offspring a female produced (Wilcoxon signed-rank test, Z=1.67, n=67, p=0.09). A paired test of the mean SIH between offspring of entire within- (mean SIH ± s.e.: 0.99±0.03) and entire extra-pair broods (1.05±0.03) of the same female revealed a significant difference (Wilcoxon signed-rank test, Z=2.03, n=13, p=0.04). Allele frequencies of different microsatellite loci are presented in table 4 of the electronic supplementary material.
Figure 1.
Mean standardized individual heterozygosity (SIH) of female's offspring over different brood categories and paternities (mean values at the top, number of females at the bottom of the bars). Different brood categories are: a, entire within-pair broods; b, mixed broods; c, entire extra-pair broods. Mixed broods are separated by paternity: within-pair young (WPY) and extra-pair young (EPY). Offspring of a female may occur in more than one brood category, in total 117 different mothers are included.
(c) Paternity and offspring size parameters
Paternity had a significant effect on weight and tarsus length, controlling for sex, age and brood identity in a general linear mixed model (table 1). EPY were on average 0.5 g (3.3%) heavier when compared with WPY, and male nestlings were on average 0.8 g (5.8%) heavier compared with female nestlings. The effect of paternity on tarsus length was greater than the effect of sex. The tarsi of EPY were on average 0.24 mm (1.3%) longer than that of WPY, and the tarsi of male nestlings were on average 0.15 mm (0.8%) longer than that of female nestlings. Only mixed broods where the size parameters had been measured between days 6 and 8 after hatching were included in the analyses.
Table 1.
General linear mixed model to test the effect of paternity on weight and tarsus length in nestlings from 83 broods with mixed paternity. (Extra-pair young (n=163) were heavier and they had longer tarsi compared with within-pair young (n=165). The different factors in the model are: paternity; sex; and age. Brood identity had a significant effect and was included as a random factor in the model. Interactions between factors were not significant.)
| parameter | effect | estimate | F | p |
|---|---|---|---|---|
| weight (g) | paternity | 0.226 | 7.1 | 0.010 |
| sex | 0.392 | 20.2 | 0.001 | |
| age | 1.100 | 23.3 | 0.001 | |
| tarsus length (mm) | paternity | 0.121 | 5.7 | 0.020 |
| sex | 0.076 | 2.2 | 0.150 | |
| age | 0.901 | 34.3 | 0.001 |
(d) Paternity, offspring survival and recapture data
Overall there was no difference in hatching success between WPY (450/486) and EPY (299/315; Fisher's exact test p=0.24) or in fledging success WPY (385/486), EPY (264/315; Fisher's exact test p=0.12). A pairwise comparison in mixed broods revealed no differences in mortality from egg to fledging between maternal half-siblings (Wilcoxon signed-rank test, Z=0.54, n=18, p=0.58).
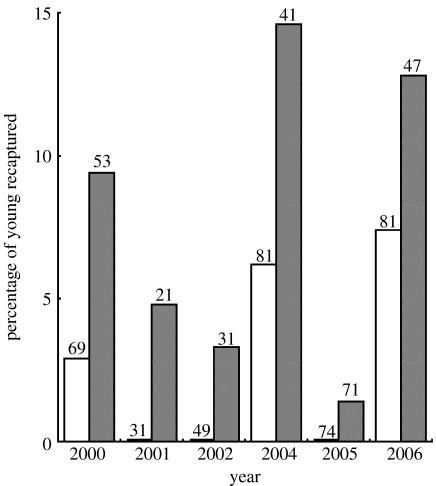
However, a larger percentage of EPY compared with WPY was recaptured later in the season (figure 2). This pattern is consistent over the 6 years. A pairwise comparison in mixed broods revealed a significantly higher recapture rate for EPY compared with WPY (Wilcoxon signed-rank test, Z=2.31, n=13, p=0.02). Also a pairwise comparison between within- and extra-pair offspring of the same female shows that more EPY were recaptured (Wilcoxon signed-rank test, Z=2.03, n=22, p=0.05).
Figure 2.
The percentage of fledglings recaptured in different years. In all years a larger percentage of EPY (grey bars) compared with WPY (white bars) was recaptured. Pooled data over all years show that more EPY were recaptured (WPY 3.4% (13/385), EPY 7.6% (20/264); Fisher's exact test p=0.02). The numbers above bars are the number of ringed nestlings.
Birds that fledged early in the season were not more likely to be recaptured (logistic regression: Χ12=0.92, p=0.34). EPY did not hatch earlier in the season compared with WPY (mean relative hatching date±s.e., WPY n=450, 22.25±0.86; EPY n=299, 21.74±1.05, Wilcoxon rank–sum test, Z=−0.09, p=0.93). The relative hatching date of a brood is the difference in days between the hatching date of the focused brood and the hatching date of the first brood that hatched in a given year. No difference in recapture rate was recorded between male and female fledglings (recaptured by sex: males 5.03% (16/318), females 5.14% (17/331); Fisher's exact test p=1).
(e) Adult heterozygosity and extra-pair paternity
All tests within single year led to the same conclusions. Therefore, only results of pooled data are shown here. Males that sired EPY were not more heterozygous compared with males that did not (mean SIH±s.e. of males that sired: no EPY n=67, 1.01±0.02, EPY n=58, 1.02±0.02; Wilcoxon rank–sum test, Z=0.29, p=0.78). A paired test revealed no difference in heterozygosity between social males and their cuckolders (Wilcoxon signed-rank test, Z=−0.02, n=96, p=0.98) nor were extra-pair males that gained entire paternity in a brood more heterozygous compared with the corresponding social males (Wilcoxon signed-rank test, Z=0.25, n=31, p=0.80). There was no difference in heterozygosity between females that produced EPY and females that produced only WPY (mean SIH±s.e. of females with: no EPY n=35, 1.02±0.03, EPY n=81, 1.02±0.02; Wilcoxon rank–sum test, Z=−0.40, p=0.69).
(f) Relatedness and extra-pair paternity
A higher genetic relatedness between parents led to a lower heterozygosity in the offspring (rs=−0.20, n=258, p=0.002). Females did not choose genetically dissimilar males as breeding partners as the mean relatedness of females to their social mates was not different from that of randomly drawn pairs (table 2a). The relatedness of a female to her social mate did not predict the likelihood of having extra-pair offspring within a breeding season. For this analysis, we pooled the data from all years and included each pair only once (binomial logistic regression: Χ12=1.52, p=0.22, n=145). Successful extra-pair mates were genetically more dissimilar to the females than nearby potential unsuccessful extra-pair mates (table 2b). A test with pooled data over all years where each female was only included once revealed a significant difference (Wilcoxon signed-rank test, Z=3.84, n=73, p=0.0003).
Table 2.
Mean genetic relatedness±s.e. (n) between females and males. ((a) Comparison between female relatedness to all theoretically possible mates and female relatedness to their social mates (p values from permutation tests). (b) Comparison between female relatedness to potential extra-pair (ep) mates and female relatedness to actual extra-pair mates. (p values from Wilcoxon signed-rank test).)
| year | all males | social mates | p |
|---|---|---|---|
| (a) | |||
| 2000 | 0.061±0.018 | 0.052±0.016 (29) | 0.70 |
| 2001 | 0.055±0.021 | 0.062±0.019 (16) | 0.52 |
| 2002 | 0.051±0.020 | 0.083±0.028 (20) | 0.12 |
| 2004 | 0.058±0.019 | 0.065±0.026 (27) | 0.66 |
| 2005 | 0.059±0.015 | 0.048±0.011 (36) | 0.54 |
| 2006 | 0.058±0.015 | 0.049±0.011 (39) | 0.54 |
| year | potential ep-mates | actual ep-mates | p |
|---|---|---|---|
| (b) | |||
| 2000 | 0.074±0.008 | 0.031±0.014 (16) | 0.02 |
| 2001 | 0.044±0.014 | 0.025±0.020 (10) | 0.04 |
| 2002 | 0.036±0.013 | 0.031±0.017 (11) | 0.11 |
| 2004 | 0.069±0.018 | 0.041±0.014 (16) | 0.12 |
| 2005 | 0.051±0.007 | 0.037±0.015 (27) | 0.01 |
| 2006 | 0.076±0.011 | 0.063±0.020 (24) | 0.09 |
In a pairwise comparison, extra-pair mates showed a tendency to be less related to females than the social mates (Wilcoxon signed-rank test, Z=1.77, n=83, p=0.07). The relatedness between female and social mate was not related to the percentage of EPP in a brood (rs=0.07, n=145, p=0.39). To test whether the percentage of EPP a brood contains depends on the relatedness between female and the extra-pair mate, we used one randomly chosen brood per female where a single male sired all EPY. The percentage of EPP a brood contained did not depend on the genetic relatedness between female and extra-pair mate (rs=0.16, n=73, p=0.16). The difference of genetic relatedness between female to the social mate and female to the extra-pair mate did not correlate with the amount of EPP (rs=0.05, n=73, p=0.68).
4. Discussion
Female fitness gains of extra-pair fertilizations could be assessed from different survival rates between EPY and WPY. Yet, dispersal makes it hard to get any long-term data on the survival of young birds in order to investigate differences based on paternity, but see Hasselquist et al. (1996), Lubjuhn et al. (1999) and Schmoll et al. (2003). A higher survival of EPY from hatching to fledging has been found in blue tits (Parus caeruleus; Kempenaers et al. 1997; Charmantier & Perret 2004). However, differences in nestling mortality may be less important compared with that in fledgling mortality. The time when a young bird leaves the nest until it becomes independent from its parents is known to be a critical phase in the life of a bird, and high mortality during this period has been reported (Ringsby et al. 1998; Naef-Daenzer et al. 2001). In our study, we assume that a within-pair fledgling that survived was as likely to be recaptured as an extra-pair fledgling. From the higher recapture rate of extra-pair fledglings, we suggest that they are more viable and therefore survive better compared with within-pair fledglings. Alternatively it might be argued that different dispersal patterns between EPY and WPY explain the higher recapture rate of EPY. Different migration patterns between males and females exist in the reed bunting (Schmitz & Steiner 2006). However, in our study we recaptured similar percentages of young males and females and the sex ratio did not differ between EPY and WPY.
EPY were heavier and had longer tarsi compared with their maternal half-siblings shortly before they left the nest. Weight has been shown to be important for fledgling survival (Magrath 1991; Naef-Daenzer et al. 2001). Longer tarsi of EPY compared with WPY have also been found in a population of reed buntings in The Netherlands (Bouwman et al. 2007) as well as in savannah sparrows (Passerculus sandwichensis; Freeman-Gallant et al. 2006). The tarsus was not full grown when we took measurements from the nestlings. It is therefore not a measure of the final size but an indicator of the development stage of a young. Fast development and thus the ability to leave the nest early are important in ground breeding birds owing to the high predation risk at nest sites. From the differences in the size parameters between EPY and WPY, we suggest that EPY develop faster and are therefore more viable. However, the size differences between extra- and within-pair nestlings could be based on other than genetic factors. The first eggs laid in a clutch might be more likely to contain EPY and therefore hatch earlier (Krist et al. 2005), but see Westneat et al. (1995), Whittingham et al. (2003) and Barber & Robertson (2007). Another alternative explanation would be that non-genetic maternal effects like increased female investment in the nutrient or hormonal content of the eggs that contain EPY cause these size differences (Gil et al. 1999; Cunningham & Russell 2000).
Females did choose their social partners randomly with regard to genetic relatedness. The choice of a social partner might be based on resources like territory quality and potential paternal care (Andersson 1994). Furthermore, female's choice for a social partner is restricted because males can already be paired to other females. To become a secondary female is disadvantageous owing to the reduced paternal assistance at nest (Dixon et al. 1994). A better strategy would be to pair with an unpaired male to assure the help at the nest and then go for extra-pair copulations to correct for low genetic quality or low compatibility of the social partner. In our study the genetic relatedness between breeding partners did not predict the occurrence of EPP, but see Blomqvist et al. (2002) and Freeman-Gallant et al. (2006). For the females even the choice of an extra-pair mate can be restricted. The social male has an interest to assure his paternity and could achieve it through intense mate guarding and frequent copulations.
We do not know whether reed bunting females are able to estimate genetic relatedness or compatibility of a male. Our finding that successful extra-pair mates are less related to females than potential but unsuccessful extra-pair mates is based on the observed extra-pair fertilizations. As we do not have any observational data on extra-pair copulations, we do not know whether females engage selectively in extra-pair copulations with genetically dissimilar males. In humans, mice and fishes, females were able to estimate genetic compatibility through odours in studies investigating mating preferences for genes of the MHC (Wedekind et al. 1995; Penn & Potts 1998; Reusch et al. 2001). Whether odour is important for mate choice in birds has not been tested yet although it is known that birds have the necessary olfactory ability (Roper 1999; Bonadonna & Nevitt 2004; Mennerat et al. 2005). It has been shown that song plays a role in kin recognition in birds (Komdeur & Hatchwell 1999; Sharp et al. 2005), therefore song could be a potential trait on which females estimate the genetic relatedness of a male.
Cryptic female choice could play an important role in the fertilization process (Birkhead & Pizzari 2002). Females could simply eject sperm of less desirable mates (Davies et al. 1996; Pizzari & Birkhead 2000) or the selection of sperm could take place within the female's reproductive tract (Wilson et al. 1997). Artificial insemination experiments have to be done to find out more about possible sperm selection within the female's reproductive tract.
Studies on reed bunting populations in Norway and The Netherlands did not reveal differences in heterozygosity between EPY and WPY (Kleven & Lifjeld 2005; Bouwman et al. 2007). However, the authors were focusing on mixed broods. In our population, there was likewise no significant difference in heterozygosity between maternal half-siblings of mixed broods. The higher heterozygosity of EPY in our study is based on the high heterozygosity of EPY that came from entire extra-pair broods. This raises the question whether the percentage of EPP in a brood depends on the genetic relatedness between the female and the extra-pair mate. We could not establish such a correlation. It is possible that an existing relationship is hidden by confounding factors which influence female ability to engage in extra-pair copulations, such as weather conditions (Bouwman & Komdeur 2006) or paternity assurance behaviour (Marthinsen et al. 2005).
The two main explanations for indirect genetic benefits of female infidelity are the good genes hypothesis and the compatible genes hypothesis, reviewed by different authors (Jennions & Petrie 2000; Griffith et al. 2002; Mays & Hill 2004; Neff & Pitcher 2005). In the reed bunting, it has been shown that mainly old males gain extra-pair paternity (Bouwman et al. 2007). The fact that old males have shown their ability to survive might be an indicator of their good genes (Kokko & Lindstrom 1996; Brooks & Kemp 2001). Our findings that EPY are more heterozygous and that this is based on a low relatedness between females and their extra-pair mates support the genetic compatibility hypothesis. However, whether dissimilarity equals compatibility can be questioned (Mays & Hill 2004; Puurtinen et al. 2005). Mate choice based on dissimilarity maintains or even increases genetic diversity within populations contrary to mate choice based on good genes. Both good and compatible genes would lead to higher viability in the offspring. Therefore, female fitness gains of extra-pair fertilizations can be based on both mechanisms because they are not mutually exclusive (Colegrave et al. 2002; Neff & Pitcher 2005).
In conclusion, our findings show that females can increase the quality of their offspring and thus fitness through extra-pair mating. Our study provides evidence against the meta-analysis of Arnqvist & Kirkpatrick (2005). The positive indirect selection may outweigh the cost of extra-pair copulation behaviour and therefore infidelity could be adaptive for females in the reed bunting population we studied.
Acknowledgements
Access to nature reserves was granted by the cantons of Fribourg and Vaud. The birds were caught under licence of the Federal Office for the Environment and blood samples were taken under licence of the cantonal ethics committee on animal experiments.
We thank Joanna Bielanska, Lukas Escher, David Ermacora, Vanessa Heierli, Manuel Lingg, Stephanie Michler, Nadia Rieille, Sabine Röthlin-Spillman, Ludivine Strambini, Elham Tarbush and Karin Safi-Widmer for their help in the field and in the laboratory. We thank Tadeusz Kawecki and three anonymous referees for their comments on the manuscript. We also thank all the people who caught birds and the Swiss Ornithological Station for information about recaptured birds. Fieldwork was coordinated with Michel Antoniazza from the Groupe d'étude et de gestion de la Grande Cariçaie (GEG).
Supplementary Material
Summary of the microsatellite loci used for paternity analysis and allele frequencies of different microsatellite loci
References
- Amos W, Wilmer J.W, Fullard K, Burg T.M, Croxall J.P, Bloch D, Coulson T. The influence of parental relatedness on reproductive success. Proc. R. Soc. B. 2001;268:2021–2027. doi: 10.1098/rspb.2001.1751. doi:10.1098/rspb.2001.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Arnqvist G, Kirkpatrick M. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 2005;168:26–37. doi: 10.1086/429350. doi:10.1086/429350 [DOI] [PubMed] [Google Scholar]
- Barber C.A, Robertson R.J. Timing of copulations and the pattern of paternity in relation to laying order in tree swallows Tachycineta bicolor. J. Avian Biol. 2007;38:249–254. doi:10.1111/j.0908-8857.2007.04042.x [Google Scholar]
- Birkhead T.R, Pizzari T. Postcopulatory sexual selection. Nat. Rev. Genet. 2002;3:262–273. doi: 10.1038/nrg774. doi:10.1038/nrg774 [DOI] [PubMed] [Google Scholar]
- Bishop J.M, O'Ryan C, Jarvis J.U.M. Social common mole-rats enhance outbreeding via extra-pair mating. Biol. Lett. 2007;3:176–179. doi: 10.1098/rsbl.2006.0607. doi:10.1098/rsbl.2006.0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist D, et al. Genetic similarity between mates and extra-pair parentage in three species of shorebirds. Nature. 2002;419:613–615. doi: 10.1038/nature01104. doi:10.1038/nature01104 [DOI] [PubMed] [Google Scholar]
- Bonadonna F, Nevitt G.A. Partner-specific odor recognition in an Antarctic seabird. Science. 2004;306:835. doi: 10.1126/science.1103001. doi:10.1126/science.1103001 [DOI] [PubMed] [Google Scholar]
- Bouwman K.M, Komdeur J. Weather conditions affect levels of extra-pair paternity in the reed bunting Emberiza schoeniclus. J. Avian Biol. 2006;37:238–244. doi:10.1111/j.2006.0908-8857.03611.x [Google Scholar]
- Bouwman K.M, Van Dijk R.E, Wijmenga J.J, Komdeur J. Older male reed buntings are more successful at gaining extrapair fertilizations. Anim. Behav. 2007;73:15–27. doi:10.1016/j.anbehav.2006.01.031 [Google Scholar]
- Brooks R, Kemp D.J. Can older males deliver the good genes? Trends Ecol. Evol. 2001;16:308–313. doi: 10.1016/s0169-5347(01)02147-4. doi:10.1016/S0169-5347(01)02147-4 [DOI] [PubMed] [Google Scholar]
- Brown J.L. A theory of mate choice based on heterozygosity. Behav. Ecol. 1997;8:60–65. doi:10.1093/beheco/8.1.60 [Google Scholar]
- Charmantier A, Perret P. Manipulation of nest-box density affects extra-pair paternity in a population of blue tits (Parus caeruleus) Behav. Ecol. Sociobiol. 2004;56:360–365. doi:10.1007/s00265-004-0794-5 [Google Scholar]
- Colegrave N, Kotiaho J.S, Tomkins J.L. Mate choice or polyandry: reconciling genetic compatibility and good genes sexual selection. Evol. Ecol. Res. 2002;4:911–917. [Google Scholar]
- Coltman D.W, Pilkington J.G, Smith J.A, Pemberton J.M. Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution. 1999;53:1259–1267. doi: 10.1111/j.1558-5646.1999.tb04538.x. doi:10.2307/2640828 [DOI] [PubMed] [Google Scholar]
- Cunningham E.J.A, Russell A.F. Egg investment is influenced by male attractiveness in the mallard. Nature. 2000;404:74–77. doi: 10.1038/35003565. doi:10.1038/35003565 [DOI] [PubMed] [Google Scholar]
- Davies N.B, Hartley I.R, Hatchwell B.J, Langmore N.E. Female control of copulations to maximize male help: a comparison of polygynandrous alpine accentors, Prunella collaris, and dunnocks, P. modularis. Anim. Behav. 1996;51:27–47. doi:10.1006/anbe.1996.0003 [Google Scholar]
- Dixon A, Ross D, Omalley S.L.C, Burke T. Paternal investment inversely related to degree of extra-pair paternity in the reed bunting. Nature. 1994;371:698–700. doi:10.1038/371698a0 [Google Scholar]
- Foerster K, Delhey K, Johnsen A, Lifjeld J.T, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. doi:10.1038/nature01969 [DOI] [PubMed] [Google Scholar]
- Freeman-Gallant C.R, Wheelwright N.T, Meiklejohn K.E, Sollecito S.V. Genetic similarity, extrapair paternity, and offspring quality in savannah sparrows (Passerculus sandwichensis) Behav. Ecol. 2006;17:952–958. doi:10.1093/beheco/arl031 [Google Scholar]
- Gil D, Graves J, Hazon N, Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. doi:10.1126/science.286.5437.126 [DOI] [PubMed] [Google Scholar]
- Griffith S.C. The evolution of infidelity in socially monogamous passerines: neglected components of direct and indirect selection. Am. Nat. 2007;169:274–281. doi: 10.1086/510601. doi:10.1086/510601 [DOI] [PubMed] [Google Scholar]
- Griffith S.C, Owens I.P.F, Burke T. Female choice and annual reproductive success favour less-ornamented male house sparrows. Proc. R. Soc. B. 1999;266:765–770. doi:10.1098/rspb.1999.0703 [Google Scholar]
- Griffith S.C, Owens I.P.F, Thuman K.A. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. doi:10.1046/j.1365-294X.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Griffiths R, Double M.C, Orr K, Dawson R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. doi:10.1046/j.1365-294x.1998.00389.x [DOI] [PubMed] [Google Scholar]
- Hanotte O, Zanon C, Pugh A, Greig C, Dixon A, Burke T. Isolation and characterization of microsatellite loci in a passerine bird—the reed bunting Emberiza schoeniclus. Mol. Ecol. 1994;3:529–530. doi: 10.1111/j.1365-294x.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Bensch S, vonSchantz T. Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature. 1996;381:229–232. doi:10.1038/381229a0 [Google Scholar]
- Jennions M.D, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. doi:10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Johnsen A, Andersen V, Sunding C, Lifjeld J.T. Female bluethroats enhance offspring immunocompetence through extra-pair copulations. Nature. 2000;406:296–299. doi: 10.1038/35018556. doi:10.1038/35018556 [DOI] [PubMed] [Google Scholar]
- Kalinowski S.T, Wagner A.P, Taper M.L. ML-Relate: a computer program for maximum likelihood estimation of relatedness and relationship. Mol. Ecol. Notes. 2006;6:576–579. doi:10.1111/j.1471-8286.2006.01256.x [Google Scholar]
- Kalinowski S.T, Taper M.L, Marshall T.C. Revising how the computer program Cervus accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007;16:1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x. doi:10.1111/j.1365-294X.2007.03089.x [DOI] [PubMed] [Google Scholar]
- Kempenaers B, Verheyren G.R, Dhondt A.A. Extrapair paternity in the blue tit (Parus caeruleus): female choice, male characteristics, and offspring quality. Behav. Ecol. 1997;8:481–492. doi:10.1093/beheco/8.5.481 [Google Scholar]
- Kleven O, Lifjeld J.T. No evidence for increased offspring heterozygosity from extrapair mating in the reed bunting (Emberiza schoeniclus) Behav. Ecol. 2005;16:561–565. doi:10.1093/beheco/ari027 [Google Scholar]
- Kokko H, Lindstrom J. Evolution of female preference for old mates. Proc. R. Soc. B. 1996;263:1533–1538. doi:10.1098/rspb.1996.0224 [Google Scholar]
- Komdeur J, Hatchwell B.J. Kin recognition: function and mechanism in avian societies. Trends Ecol. Evol. 1999;14:237–241. doi: 10.1016/s0169-5347(98)01573-0. doi:10.1016/S0169-5347(98)01573-0 [DOI] [PubMed] [Google Scholar]
- Krist M, Nadvornik P, Uvirova L, Bures S. Paternity covaries with laying and hatching order in the collared flycatcher Ficedula albicollis. Behav. Ecol. Sociobiol. 2005;59:6–11. doi:10.1007/s00265-005-0002-2 [Google Scholar]
- Lubjuhn T, Strohbach S, Brun J, Gerken T, Epplen J.T. Extra-pair paternity in great tits (Parus major)—a long term study. Behaviour. 1999;136:1157–1172. doi:10.1163/156853999501810 [Google Scholar]
- Magrath R.D. Nestling weight and juvenile survival in the blackbird, Turdus merula. J. Anim. Ecol. 1991;60:335–351. doi:10.2307/5464 [Google Scholar]
- Marthinsen G, Kleven O, Brenna E, Lifjeld J.T. Part-time mate guarding affects paternity in male reed buntings (Emberiza schoeniclus) Ethology. 2005;111:397–409. doi:10.1111/j.1439-0310.2005.01079.x [Google Scholar]
- Martinez J.G, Soler J.J, Soler M, Moller A.P, Burke T. Comparative population structure and gene flow of a brood parasite, the great spotted cuckoo (Clamator glandarius), and its primary host, the magpie (Pica pica) Evolution. 1999;53:269–278. doi: 10.1111/j.1558-5646.1999.tb05352.x. doi:10.2307/2640939 [DOI] [PubMed] [Google Scholar]
- Masters B.S, Hicks B.G, Johnson L.S, Erb L.A. Genotype and extra-pair paternity in the house wren: a rare-male effect? Proc. R. Soc. B. 2003;270:1393–1397. doi: 10.1098/rspb.2003.2380. doi:10.1098/rspb.2003.2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays H.L, Hill G.E. Choosing mates: good genes versus genes that are a good fit. Trends Ecol. Evol. 2004;19:554–559. doi: 10.1016/j.tree.2004.07.018. doi:10.1016/j.tree.2004.07.018 [DOI] [PubMed] [Google Scholar]
- McClelland E.E, Penn D.J, Potts W.K. Major histocompatibility complex heterozygote superiority during coinfection. Infect. Immun. 2003;71:2079–2086. doi: 10.1128/IAI.71.4.2079-2086.2003. doi:10.1128/IAI.71.4.2079-2086.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerat A, Bonadonna F, Perret P, Lambrechts M.M. Olfactory conditioning experiments in a food-searching passerine bird in semi-natural conditions. Behav. Process. 2005;70:264–270. doi: 10.1016/j.beproc.2005.07.005. doi:10.1016/j.beproc.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Naef-Daenzer B, Widmer F, Nuber M. Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J. Anim. Ecol. 2001;70:730–738. doi:10.1046/j.0021-8790.2001.00533.x [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. doi:10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Penn D, Potts W.K. Untrained mice discriminate MHC-determined odors. Physiol. Behav. 1998;64:235–243. doi: 10.1016/s0031-9384(98)00052-3. doi:10.1016/S0031-9384(98)00052-3 [DOI] [PubMed] [Google Scholar]
- Pizzari T, Birkhead T.R. Female feral fowl eject sperm of subdominant males. Nature. 2000;405:787–789. doi: 10.1038/35015558. doi:10.1038/35015558 [DOI] [PubMed] [Google Scholar]
- Puurtinen M, Ketola T, Kotiaho J.S. Genetic compatibility and sexual selection. Trends Ecol. Evol. 2005;20:157–158. doi: 10.1016/j.tree.2005.02.005. doi:10.1016/j.tree.2005.02.005 [DOI] [PubMed] [Google Scholar]
- Reusch T.B.H, Haberli M.A, Aeschlimann P.B, Milinski M. Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature. 2001;414:300–302. doi: 10.1038/35104547. doi:10.1038/35104547 [DOI] [PubMed] [Google Scholar]
- Ringsby T.H, Saether B.E, Solberg E.J. Factors affecting juvenile survival in house sparrow Passer domesticus. J. Avian Biol. 1998;29:241–247. doi:10.2307/3677106 [Google Scholar]
- Roper T.J. Olfaction in birds. Adv. Study Behav. 1999;28:247–332. [Google Scholar]
- Schmitz P, Steiner F. Autumn migration of reed buntings Emberiza schoeniclus in Switzerland. Ring. Migr. 2006;23:33–38. [Google Scholar]
- Schmoll T, Dietrich V, Winkel W, Epplen J.T, Lubjuhn T. Long-term fitness consequences of female extra-pair matings in a socially monogamous passerine. Proc. R. Soc. B. 2003;270:259–264. doi: 10.1098/rspb.2002.2216. doi:10.1098/rspb.2002.2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T, Quellmalz A, Dietrich V, Winkel W, Epplen J.T, Lubjuhn T. Genetic similarity between pair mates is not related to extrapair paternity in the socially monogamous coal tit. Anim. Behav. 2005;69:1013–1022. doi:10.1016/j.anbehav.2004.08.010 [Google Scholar]
- Sharp S.P, McGowan A, Wood M.J, Hatchwell B.J. Learned kin recognition cues in a social bird. Nature. 2005;434:1127–1130. doi: 10.1038/nature03522. doi:10.1038/nature03522 [DOI] [PubMed] [Google Scholar]
- Smith S.B, Webster M.S, Holmes R.T. The heterozygosity theory of extra-pair mate choice in birds: a test and a cautionary note. J. Avian Biol. 2005;36:146–154. doi:10.1111/j.0908-8857.2005.03417.x [Google Scholar]
- Wedekind C, Seebeck T, Bettens F, Paepke A.J. MHC-dependent mate preferences in humans. Proc. R. Soc. B. 1995;260:245–249. doi: 10.1098/rspb.1995.0087. doi:10.1098/rspb.1995.0087 [DOI] [PubMed] [Google Scholar]
- Westneat D.F, Stewart I.R.K. Extra-pair paternity in birds: causes, correlates, and conflict. Annu. Rev. Ecol. Evol. Syst. 2003;34:365–396. doi:10.1146/annurev.ecolsys.34.011802.132439 [Google Scholar]
- Westneat D.F, Clark A.B, Rambo K.C. Within brood patterns of paternity and paternal behavior in red-winged blackbirds. Behav. Ecol. Sociobiol. 1995;37:349–356. doi:10.1007/s002650050201 [Google Scholar]
- Whittingham L.A, Dunn P.O, Clotfelter E.D. Parental allocation of food to nestling tree swallows: the influence of nestling behaviour, sex and paternity. Anim. Behav. 2003;65:1203–1210. doi:10.1006/anbe.2003.2178 [Google Scholar]
- Wilson N, Tubman S.C, Eady P.E, Robertson G.W. Female genotype affects male success in sperm competition. Proc. R. Soc. B. 1997;264:1491–1495. doi:10.1098/rspb.1997.0206 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the microsatellite loci used for paternity analysis and allele frequencies of different microsatellite loci