Abstract
Living species of mammals, crocodiles and most species of birds exhibit parental care, but evidence of this behaviour is extremely rare in the fossil record. Here, we present a new specimen of varanopid ‘pelycosaur’ from the Middle Permian of South Africa. The specimen is an aggregation, consisting of five articulated individuals preserved in undisturbed, close, lifelike, dorsal-up, subparallel positions, indicating burial in ‘life position’. Two size classes are represented. One is 50% larger than the others, is well ossified, has fused neurocentral sutures and is distinguished by a coat of dermal ossifications that covers the neck and shoulder regions. We regard this individual to be an adult. The remaining four skeletons are considered to be juveniles as they are approximately the same size, are poorly ossified, have open neurocentral sutures and lack dermal ossifications. Aggregates of juvenile amniotes are usually siblings. Extant analogues of adult and juvenile groupings suggest that the adult is one of the parents, leading us to regard the aggregation as a family group. The Late Middle Permian age of the varanopid family predates the previously known oldest fossil evidence of parental care in terrestrial vertebrates by 140 Myr.
Keywords: parental care, ‘pelycosaur’, Varanopidae, South Africa, Permian
1. Introduction
Parental care of offspring has evolved numerous times in vertebrates. Among extant amniotes, it is present in all mammals and crocodilians, most species of birds and some squamates. However, evidence for parental care is extremely rare in fossil amniotes. The oldest known specimens of fossil amniotes that have been described as evincing parental care are assigned to dinosaurs of the Cretaceous Period. Meng et al. (2004) described a small assemblage of skeletons of the Early Cretaceous (approx. 120 Myr ago) ornithischian genus Psittacosaurus, consisting of a single adult preserved in association with 34 juvenile skeletons. All these individuals are preserved dorsal-up, in life poses, and there is no evidence of other taxa or isolated elements that might suggest that the animals had died elsewhere and were transported to their location. The heads of all the individuals are not covered by a limb or other elements of neighbouring individuals and those juveniles not adjacent to the adult are arranged in a subparallel fashion. Meng et al. (2004) propose several possible death scenarios including burial by volcanic debris, entrapment in a collapsed underground burrow and flooding of a nest. Varricchio et al. (2007) ascribed parental care to the Mid-Cretaceous (approx. 90 Myr ago) taxon Oryctodromeus cubicularis, on the basis of adult skeletal remains found with juvenile remains preserved in a burrow infilling. The relatively large size (i.e. larger than hatchling size) of both the Psittacosaurus and Oryctodromeus juveniles strongly suggested extended parental care in these dinosaurs (Meng et al. 2004; Varricchio et al. 2007).
There are numerous examples of monospecific assemblages of amniotes from pre-Cretaceous rocks. However, the skeletons in these assemblages are either of equivalent size or consist of a range of age classes. Given the absence of compelling evidence of two size classes (adult and juvenile), these monospecific assemblages probably represent social aggregations rather than a family group, i.e. evidence of parental care. One of the oldest examples is an Early Permian (approx. 280 Myr ago) slab that preserves six skeletons of the basal synapsid (‘pelycosaur’, or ‘primitive mammal-like reptile’ of previous parlance) Pantelosaurus saxonicus (von Huene 1925). Weigelt (1989) inferred that these skeletons had been water-transported based on the positions of the skeletons, the broad overlap of one of the skeletons by at least three of the others, the haphazard arrangement of the limb elements and the associated plant remains.
More compelling evidence of early sociality in the amniote fossil record comes from the Upper Permian rocks of the Beaufort Group, in the Karoo Basin of South Africa. Smith & Evans (1996) described a small aggregation of juvenile diapsid reptiles from the Tropidostoma assemblage zone (AZ). This aggregation is hypothesized to have been entombed within a burrow, although evidence for such a structure is lacking. Smith (1987) described a pair of individuals of the therapsid (‘advanced mammal-like reptile’) genus Diictodon from the same horizon. The therapsid specimen was found at the same locality as burrow infillings preserving single individuals, but direct evidence for entombment within a burrow for the paired skeletons was absent; Smith (1987) inferred that the pair died within the spiral tunnel of a burrow because the plane of the pair was inclined relative to the bedding plane. In none of the Tropidostoma AZ specimens is there evidence of parental care.
We present here a new specimen from the Karoo Basin of South Africa that preserves a small aggregation of five varanopid ‘pelycosaurs’. The specimen was initially identified as a skeleton of the therapsid genus Ictidosuchus, but recent preparation has exposed the anterior half of a large skeleton with unequivocal varanopid apomorphies and four smaller skeletons of the same morphology. Importantly, two size classes are present among the varanopids and, accordingly, the specimen can be regarded as the oldest evidence for parental care among terrestrial vertebrates.
2. Material and methods
The specimen (SAM-PK-K8305 in the Iziko South African Museum of Cape Town) consists of five well-preserved, articulated individuals in varying degrees of completeness. The specimen was prepared mechanically with an air scribe and then photographed using a Nikon Coolpix 5400 digital camera. In this paper, we provide a brief description of SAM-PK-K8305 and consider the taphonomy of the skeletons in order to infer the animals' behaviour. A detailed morphological description will be presented elsewhere.
3. Taxonomy
‘Pelycosaurs’ are a paraphyletic assemblage of basal synapsids that diversified during the Late Carboniferous and Early Permian in what is now North America (Reisz & Dilkes 2003). They were the dominant group of terrestrial tetrapods during the Early Permian, but were succeeded by the more mammal-like synapsids, the Therapsida, during the Middle Permian. The overwhelming majority of ‘pelycosaur’ specimens are known from North American and European rocks, and there are few unequivocal examples from the Southern Hemisphere. Varanopids are small, monitor-like carnivorous basal synapsids that were the only ‘pelycosaurs’ known to have coexisted with the more diverse and mammal-like therapsids in both the Northern and Southern Hemispheres during the late Middle Permian. Only three varanopid specimens have been described until now from South Africa.
The taxonomic history of South African varanopids is particularly thorny and requires a brief review in order to warrant a specific assignment for the specimens described here. Broom (1937) described Elliotsmithia longiceps on the basis of a partial skull. He recognized it as a ‘pelycosaur’ based on a comparison with the Early Permian ‘pelycosaur’ Mycterosaurus longiceps. Elliotsmithia was subsequently assigned by Romer & Price (1940) to their family ‘Varanopsidae’ (emended to Varanopidae by Reisz & Dilkes (2003), following Welles & Peachy (1953)). For decades, the holotype of E. longiceps was the only varanopid specimen known from Gondwana (but see Reisz 1986), until Modesto et al. (2001) described a skull of a Mycterosaurus-like ‘pelycosaur’ and referred it to E. longiceps. Reisz & Dilkes (2003) disputed the assignment of Modesto et al.'s (2001) specimen to E. longiceps, which they regarded as a species of varanodontine varanopid, and reinterpreted Modesto et al.'s (2001) specimen as a new type of mycterosaurine varanopid.
More recently, Reisz & Modesto (in press) restudied the small, enigmatic amniote Heleosaurus scholtzi, which was described by Broom (1907) as a diaptosaurian (=basal diapsid) and later redescribed by Carroll (1976) as a younginid diapsid. Reisz & Modesto (in press) concluded that H. scholtzi is in fact a varanopid synapsid. Heleosaurus scholtzi possesses cervical osteoderms, regarded as an autapomorphy of E. longiceps by Dilkes & Reisz (1996) and Reisz et al. (1998), but several aspects of its morphology (e.g. serrated dentition, femoral morphology) suggest strongly that it is a mycterosaurine varanopid. Although Reisz & Modesto (in press) reinterpreted H. scholtzi as a varanopid, they were unable to determine if it and E. longiceps were synonymous because the respective holotypes of each species largely preserve non-overlapping portions of the skeleton.
We assign SAM-PK-K8305 to H. scholtzi because the large individual has cervical dermal osteoderms, and the skeletal morphology of all the individuals is unequivocally mycterosaurine. However, we cannot determine if the surangular and the angular of any of the individuals bear ornamentation, which was regarded by Reisz & Modesto (in press) to be an autapomorphy of H. scholtzi. Although the holotype of E. longiceps also preserves cervical dermal osteoderms, there is disagreement on whether that taxon is a varanodontine or a mycterosaurine (Reisz et al. 1998; Modesto et al. 2001; Reisz & Dilkes 2003; Anderson & Reisz 2004; Maddin et al. 2006). We remain cognizant of the possibility that the two species are synonymous and, if so, H. scholtzi (Broom 1907) would have priority over E. longiceps (Broom 1937). Should the latter species be recognized as a junior synonym of the former, such an act would not require taxonomic reassignment of SAM-PK-K8305.
4. Geological setting
The specimen was collected in 1995 from the Tapinocephalus AZ, upper Abrahamskraal Formation, near Fraserburg in the Northern Cape Province, by Roger M. H. Smith. This biozone is thought to be Late Middle Permian in age (just over 260 Myr ago; Retallack et al. 2006). The specimen (figure 1) was preserved in situ in a fine-grained, greenish-grey mudstone level to the bedding plane. The sediment encasing the specimen is homogeneous and there are no structures indicating a burrow. The strata of the Tapinocephalus AZ were deposited in a predominantly fluvial floodplain environment and no volcanic or aeolian deposits are present in this geological unit (Rubidge 1995). The skeletons were probably complete when buried, but weathering has destroyed elements from all skeletons to varying degrees.
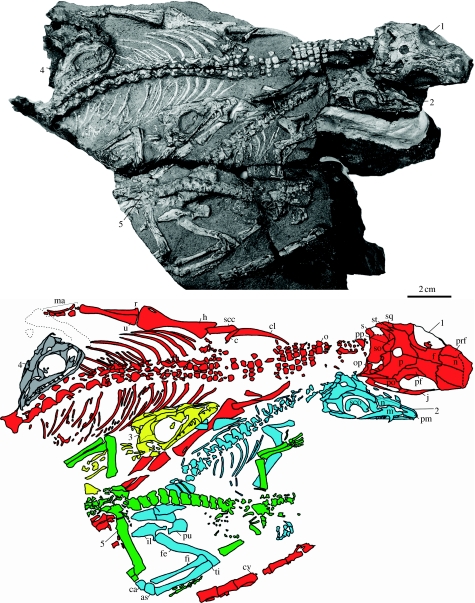
Figure 1.
The varanopid specimen SAM-PK-K8305. Numerals indicate individuals: leader lines point to the skulls of individuals 1–4, whereas that for individual 5 points to its hip region because the anterior half of its skeleton, including the skull, is missing. as, astragulus; c, cleithrum; ca, calcaneum; cl, clavicle; cv, caudal vertebra; f, frontal; fe, femur; fi, fibula; h, humerus; il, ilium; j, jugal; m, maxilla; ma, manus; n, nasal; o, osteoderm; op, opisthotic; p, parietal; pf, postfrontal; pm, premaxilla; po, postorbital; pp, postparietal; prf, prefrontal; pu, pubis; r, radius; s, stapes; scc, scapulocoracoid; sco, scleral ossicles; so, supraoccipital; sq, squamosal; st, supratemporal; ti, tibia; u, ulna.
5. Description
There is a suite of observations indicating that the varanopids were buried rapidly in ‘life position’ (Smith & Evans 1996). The undisturbed, lifelike articulation of the skeletons, consistent quality of the elements, and the presence of dermal osteoderms, which are perfectly arranged in transverse rows on the neck and shoulders of the largest specimen (individual 1) and would have been imbedded in its integument, indicate that the flesh was intact at burial. Furthermore, all the skeletons are preserved in a dorsal-up attitude and display a subparallel alignment, with the bodies oriented in the same direction. Although the head of individual 4 is oriented in a direction opposite to that of the others (figure 1), the curvature of its neck suggests that its body (not preserved posteriorly beyond the posterior end of the neck) was oriented in the same direction as the other skeletons.
Individual 1 consists of the skull (missing the anterior portion of the snout) with an articulated mandible and an articulated postcranial skeleton that includes the forelimbs with most of the presacral vertebrae, manual digits, ribs, gastralia and dermal osteoderms (figure 1). The pelvic girdle and hind limbs are missing from this individual. Although not connected to individual 1, there is an articulated series of distal caudal vertebrae of a size appropriate for this individual. The curvature of the presacral series of individual 1 relative to the orientation and position of this caudal series suggests that these two series were connected by a now missing series of vertebrae, implying that the tail of this individual was curled around individuals 2, 3 and 5 in life. Individual 1 is 50% larger than the other individuals, and judging from the anteroposterior length of the dorsal vertebrae, it is the same size as the holotype of Heleosaurus (Carroll 1976).
Individuals 2–5 are of equal size. Three of these individuals (2–4) consist of complete skulls (figure 2a–c), with mandibles in articulation, and articulated postcranial skeletons, consisting of vertebrae, ribs, pectoral girdles, forelimbs and hind limbs to varying degrees of preservation. In the skulls, the maxilla contacts both the nasal and the prefrontal, which is a mycterosaurine feature (Modesto et al. 2001; Reisz & Dilkes 2003). Individual 5 consists of an articulated portion of the vertebrae, a portion of the pectoral girdle, forelimbs, pelvic girdle and hind limbs. The skull is not preserved in this individual.
Figure 2.
Close up of the skulls of varanopid individuals 1–4. (a) Individuals 1 and 2, (b) individual 3 and (c) individual 4. Varanopid synapomorphies include a posterodorsally expanded external naris, a slender subtemporal bar and narrow quadratojugal, reduced occipital shelf of the squamosal, anteriorly extending parietal over the orbital region and a reduced tabular bone.
Individual 1 is distinguished from individuals 2–5 not only by its larger size but also by the presence of small, rounded dermal osteoderms covering the neck and shoulders. An examination of the exposed vertebrae of individual 1 reveals that the neurocentral sutures are fused in the cervicals and in the disconnected caudal series. The centra of most vertebrae of individuals 2–5 are not visible, but a posterior presacral vertebra of individual 2 indicates that the neurocentral suture is open. In individual 1, the elements of the appendicular skeleton are well ossified because they display minimal crushing. The limb bones of individuals 2–5, however, are crushed, indicating that their walls are thinner and less ossified than those of individual 1.
6. Discussion
The high degree of articulation of the varanopid skeletons, together with the preservation of delicate integumentary structures (dermal osteoderms), indicates that the carcasses were not subject to biological and physical processes of bone dispersal and/or destruction that most vertebrates experience after death. The specimen shows no evidence of scavenging or trampling, and the relative positions of the skeletons, together with the high degree of skeletal preservation, indicates that the aggregation was not formed as a result of a flood event or other post-mortem transport processes. If the varanopids died in an unprotected area during an overbank flood or other natural catastrophe, it is highly improbable that their bodies would have been transported and then come together, in such a neat, tight arrangement, oriented in the same direction and all on their ventral surfaces. Furthermore, the varanopid aggregate does not fit any of Weigelt's (1989) numerous examples of the positioning of vertebrate carcasses that have been transported by water. Weigelt (1989) described only a single case of a small, monospecific aggregation consisting of skeletons forming a tight, dorsal-up, subparallel formation (three individuals of the procolophonoid reptile Koiloskiosaurus coburgensis; Weigelt 1989, p. 154), which he regarded as very peculiar because he never saw such an aggregation of vertebrate carcasses in the modern cases with which he was familiar. Parenthetically, the arrangement of the three Koiloskiosaurus skeletons now makes sense if they died in a shelter, such as a burrow; it is noteworthy that burrowing abilities have been attributed recently to procolophonoid reptiles (Groenewald 1991; deBraga 2003).
Abdala et al. (2006) described an aggregate amniote fossil consisting of three skeletons from the Lower Triassic Katberg Formation of South Africa. They considered two possibilities in their interpretation of their specimen: (i) the skeletons were deposited together in an erosion gully or shrinkage crack during an overbank flood event or (ii) the aggregate formed as a result of behavioural activities of the amniotes and accumulated in a shelter of some kind. In the absence of sedimentological evidence for either scenario, Abdala et al. (2006) examined the evidence for the behavioural-mediated aggregation of the skeletons, and favoured an interpretation that the individuals died within a shelter. The evidence for a behavioural-mediated aggregation for varanopid specimen SAM-PK-K8305 is considerably stronger than that for the amniote aggregation described by Abdala et al. (2006). SAM-PK-K8305 is a monospecific aggregation consisting of carnivorous forms that are extremely rare components of the Middle Permian fauna of South Africa (Rubidge 1995; Modesto et al. 2001).
The size and osteological differences between individual 1 and individuals 2–5 allow us to identify two age classes in SAM-PK-K8305. Individual 1 is clearly an adult owing to the closed neurocentral sutures and the high degree of ossification. It is the same size as the holotype of Heleosaurus, which exhibits the same level of ossification (Carroll 1976). Individuals 2–5 are the smallest known varanopids from South Africa. The open neurocentral sutures and poor ossification of the elements indicates that they are juveniles. Furthermore, the identical size and close association of the individuals 2–5 implies that they are siblings, because aggregates of extant juvenile amniotes (‘sibling groups’ of Coombs 1982) are almost invariably derived from the same litter or clutch (Gardner et al. 2001). Thus, we interpret individual 1 as a parent and, using living reptilian analogues (Reynolds et al. 2001) infer that its parental responsibility was probably the protection of its offspring from predators.
The relatively large size of individuals 2–5 (approx. two-thirds the size of individual 1) indicates that parental care in Heleosaurus was extensive because it continued well beyond the post-natal stage. Similarly, Varricchio et al. (2007) inferred extensive parental care for Oryctodromeus in light of the large size (60–65% adult size) of the juvenile material, and Meng et al. (2004) inferred the same in Psittacosaurus. This level of parental care is completely unexpected in a Palaeozoic amniote, not only because it is characteristic of higher vertebrates (i.e. mammals and birds, as well as dinosaurs) but also because varanopids existed approximately 110 Myr prior to the last common ancestor of monotreme and therian mammals. Evidence of extended parental care in the vertebrate fossil record is extremely rare, and the varanopid specimen predates the previous earliest known evidence of extended parental care by the dinosaurs Psittacosaurus and Oryctodromeus by at least 140 Myr.
Extended parental care in a varanopid ‘pelycosaur’ is surprisingly given the utter absence of evidence for any form of parental care in the Palaeozoic and Mesozoic synapsids. Indeed, the recent description of a member of the mammalian crown group suggests that this behaviour must have appeared in synapsids by the Jurassic Period (Luo & Wible 2005). Karoo varanopids were among the last of their kind, coexisting with the more numerous and diverse therapsid synapsids. They may have been a Palaeozoic analogue of the Australian lizard Egernia stokesii, which forms long-lived family associations in which subadults of 3 years of age were found to be still living in the natal crevice with their parents (Gardner et al. 2001). Egernia subadults appear to benefit from parental vigilance, access to parental food and refuge resources, thermal advantages and perhaps even the ability to inherit the natal crevice from their parents (Gardner et al. 2001). The varanopid juveniles may have experienced the same advantages. It is evident that parental care must have provided an important survival advantage for varanopids in a Palaeozoic world dominated by their more mammal-like relatives, the therapsids.
Acknowledgments
We thank R. M. H. Smith for collecting the specimen and for its loan, and J. Nyaphuli for preparation. J. Botha-Brink is supported by a grant from the National Research Foundation of South Africa (GUN 2073037) and S.P.M. is supported by discovery grants (nos. 288126-04 and 288126-07) from the Natural Sciences and Engineering Research Council (NSERC) of Canada.
References
- Abdala F, Cisneros J.C, Smith R.M.H. Faunal aggregation in the Early Triassic Karoo Basin: earliest evidence of shelter-sharing behaviour among tetrapods? Palaios. 2006;21:507–512. doi:10.2110/palo.2005.P06-001R [Google Scholar]
- Anderson J.S, Reisz R.R. Pyozia mesenensis, a new small varanopid (Synapsida, Eupelycosauria) from Russia: “pelycosaur” diversity in the Middle Permian. J. Vert. Paleontol. 2004;24:173–179. doi:10.1671/1940-13 [Google Scholar]
- Broom R. On some new fossil reptiles from the Karroo Beds of Victoria West, South Africa. Trans. S. Afr. Philos. Soc. 1907;18:31–42. [Google Scholar]
- Broom R. A further contribution to our knowledge of the fossil reptiles of the Karroo. Proc. Zool. Soc. Lond. B. 1937;1937:299–318. [Google Scholar]
- Carroll R.L. Eosuchians and the origin of archosaurs. In: Churcher C.S, editor. Essays on palaeontology in honour of Loris Shano Russell. Miscellaneous Publications of the Royal Ontario Museum; Toronto, Canada: 1976. pp. 58–79. [Google Scholar]
- Coombs W.P., Jr Juvenile specimens of the ornithischian dinosaur Psittacosaurus. Palaeontology. 1982;25:89–107. [Google Scholar]
- deBraga M. The postcranial skeleton, phylogenetic position, and probable lifestyle of the Early Triassic reptile Procolophon trigoniceps. Can. J. Earth Sci. 2003;40:527–556. doi:10.1139/e02-082 [Google Scholar]
- Dilkes D.W, Reisz R.R. First record of a basal synapsid (‘mammal-like reptile’) in Gondwana. Proc. R. Soc. B. 1996;263:1165–1170. doi:10.1098/rspb.1996.0170 [Google Scholar]
- Gardner M.G, Bull C.M, Cooper S.J.B, Duffield G.A. Genetic evidence for a family structure in stable social aggregations of the Australian lizard Egernia stokesii. Mol. Ecol. 2001;10:175–183. doi: 10.1046/j.1365-294x.2001.01171.x. doi:10.1046/j.1365-294X.2001.01171.x [DOI] [PubMed] [Google Scholar]
- Groenewald G.H. Burrow casts from the Lystrosaurus–Procolophon assemblage zone. Koedoe. 1991;34:13–22. [Google Scholar]
- Luo Z.-X, Wible J.R. A Late Jurassic digging mammal and early mammalian diversification. Science. 2005;308:103–107. doi: 10.1126/science.1108875. doi:10.1126/science.1108875 [DOI] [PubMed] [Google Scholar]
- Maddin H.C, Evans D.C, Reisz R.R. An Early Permian varanodontine varanopid (Synapsida: Eupelycosauria) from the Richards Spur locality, Oklahoma. J. Vert. Paleontol. 2006;26:957–966. doi:10.1671/0272-4634(2006)26[957:AEPVVS]2.0.CO;2 [Google Scholar]
- Meng Q, Liu J, Varricchio D.J, Huang T, Gao C. Parental care in an ornithischian dinosaur. Nature. 2004;431:145–146. doi: 10.1038/431145a. doi:10.1038/431145a [DOI] [PubMed] [Google Scholar]
- Modesto S, Sidor C.A, Rubidge B.S, Welman J. A second varanopseid skull from the Upper Permian of South Africa: implications for Late Permian ‘pelycosaur’ evolution. Lethaia. 2001;34:249–259. doi:10.1080/002411601753292971 [Google Scholar]
- Reisz R.R. Handbuch der Paläoherpetologie. Gustav Fischer Verlag; Stuttgart, Germany: 1986. Pelycosauria. [Google Scholar]
- Reisz R.R, Dilkes D.W. Archaeovenator hamiltonensis, a new varanopid (Synapsid: Eupelycosauria) from the Upper Carboniferous of Kansas. Can. J. Earth Sci. 2003;40:667–678. doi:10.1139/e02-063 [Google Scholar]
- Reisz, R. R. & Modesto, S. P. In press. Heleosaurus scholtzi from the Permian of South Africa: a varanopid synapsid, not a diapsid reptile. J. Vert. Paleontol
- Reisz R.R, Dilkes D.W, Berman D.S. Anatomy and relationships of Elliotsmithia longiceps Broom, a small synapsid (Eupelycosauria: Varanopseidae) from the Late Permian of South Africa. J. Vert. Paleontol. 1998;18:602–611. [Google Scholar]
- Retallack G.J, Metzger C.A, Greaver T, Jahren A.H, Smith R.M.H, Sheldon N.D. Middle–Late Permian mass extinction on land. Geol. Soc. Am. Bull. 2006;118:1398–1411. doi:10.1130/B26011.1 [Google Scholar]
- Reynolds J.D, Goodwin N.B, Freckleton R.P. Evolutionary transitions in parental care and live bearing in vertebrates. Phil. Trans. R. Soc. B. 2001;357:269–281. doi: 10.1098/rstb.2001.0930. doi:10.1098/rstb.2001.0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer A.S, Price L.W. Review of the Pelycosauria. Geol. Soc. Am. Spec. Pap. 1940;28:1–538. [Google Scholar]
- Rubidge, B. S. (ed.) 1995 Biostratigraphy of the Beaufort Group (Karoo Supergroup) South African Committee for Stratigraphy, Biostratigraphic Series, vol. 1, pp. 1–46. Pretoria, Republic of South Africa: South African Committee for Stratigraphy.
- Smith R.M.H. Helical burrow casts of therapsid origin from the Beaufort Group (Permian) of South Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1987;60:155–169. doi:10.1016/0031-0182(87)90030-7 [Google Scholar]
- Smith R.M.H, Evans S.E. New material of Youngina: evidence of juvenile aggregation in Permian diapsid reptiles. Palaeontology. 1996;39:289–303. [Google Scholar]
- Varricchio D.J, Martin A.J, Katsura Y. First trace and body fossil of a burrowing, denning dinosaur. Proc. R. Soc. B. 2007;274:1361–1368. doi: 10.1098/rspb.2006.0443. doi:10.1098/rspb.2006.0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Huene F. Ein neuer Pelycosaurier aus der unteren Permformation Sachsens. Geol. Paläont. Abh., N.F. 1925;14:215–264. [Google Scholar]
- Weigelt J. University of Chicago Press; Chicago, IL: 1989. Recent vertebrate carcasses and their paleobiological implications. [English transl. of original 1927 publication.] [DOI] [PubMed] [Google Scholar]
- Welles S.P, Peachy F. Family endings. J. Paleontol. 1953;27:756–758. [Google Scholar]