Summary
Background and purpose
Serotonin is a major regulator of structural brain plasticity, which may occur following cortical resection in humans. In this study we used positron emission tomography (PET) with alpha[11C]methyl-L-tryptophan (AMT) to evaluate serotonergic alterations in subcortical structures following cortical resection in children with intractable epilepsy.
Methods
AMT uptake in the thalamus and lentiform nucleus was evaluated postoperatively (1–89 months following resection) in 19 children (mean age: 8.7 years) with a previous cortical resection due to intractable epilepsy. Ten children with partial epilepsy but without resection and seven normal children served as controls.
Results
There was an increased AMT uptake in the lentiform nucleus ipsilateral to the resection as compared to the contralateral side (mean asymmetry: 4.2 ± 3.0%), and the asymmetries were significantly higher than those measured in the control groups (p ≤ 0.001). Post-resection asymmetry indices in the lentiform nucleus correlated inversely with postoperative time (r = −0.67; p = 0.002), but not with age (p = 0.29) or the extent of resection (p = 0.77). In contrast, thalamic AMT uptake asymmetries were not different among the three groups (p = 0.63).
Conclusions
Cortical resection results in a sustained increase of AMT uptake in the lentiform nucleus, suggesting increased serotonin synthesis. Serotonergic activation in the deafferented striatum may play a role in the functional reorganization of cortico-striatal projections in humans.
Keywords: Serotonin, Epilepsy, Cortical resection, Positron emission tomography, Alpha[11C]methyl-L-tryptophan, Striatum
Introduction
Children who have undergone large cortical resections for the alleviation of medically refractory epilepsy provide a unique opportunity for studying plasticity in the developing brain. Using positron emission tomography (PET) in children treated with cerebral hemispherectomy, we have previously reported a recovery of glucose utilization in the caudate nucleus following an initial period of post-operative hypometabolism (Chugani and Jacobs, 1994); this recovery is presumably related to reorganization of cortico-striatal projections following their disruption by the surgery. In a rat model of this phenomenon, we also showed increased expression of transforming growth factor alpha in the striatum following hemidecortication in P6, but not adult, animals (Kornblum et al., 1994). In humans, striatal plasticity following unilateral decortication may account for the observation that inclusion of the striatum in the resection results in more profound hemiplegia than hemidecortication alone (Krynauw, 1950). Therefore, a better understanding of the role of striatal plasticity in reorganization of the brain following cortical injury or resection is essential to maximizing functional recovery. Serotonin is one of the major regulators of structural brain plasticity (Banasr et al., 2001; Brezun and Daszuta, 2000), not only functioning as a neurotransmitter, but also as a protective compound following brain injury (Kline et al., 2001), and an enhancer of axonal growth factors (Hisaoka et al., 2007). In order to study post-resection serotonergic alterations in human striatal circuitry, we evaluated postoperative images of serotonin synthesis in the thalamus and striatum using positron emission tomography (PET) and the tracer alpha[11C]methyl-L-tryptophan (AMT) in children who had undergone epilepsy surgery.
Methods
Subjects
Nineteen children (12 boys, 7 girls, age: 2.1–17.5 years, mean age: 8.7 years) with intractable epilepsy and a failed cortical resection (resection group) were included in this study (Table 1). The resections were either left (12) or right (7) sided, and included regions of the frontal cortex in all 19 children, but also included portions of temporal and/or parietal cortices in 15 of the 19. To evaluate these children for further surgery, AMT PET scans were performed at varying times following the initial cortical resection (range = 1–89 months, 23 ± 24 months) (Juhász et al., 2003, 2004). Two pediatric control groups were used for comparison. The first consisted of 10 children (5 boys, 5 girls; age 1.5–15.2 years, mean age 5.8 years) with intractable epilepsy who were being evaluated for surgery (epilepsy controls). None had undergone previous cortical resections. In each of these children, the seizures emanated from the frontal lobes (5 left, 5 right) as indicated by EEG; however, interictal epileptiform activity also involved temporal and/or parietal cortex in 8 of the 10 children. The other control group consisted of seven normal children (without MRI scans, mean age 9.7 years) who were siblings of autistic children, and were described previously (normal controls) (Chugani et al., 1999). All protocols were approved by the Human Investigation Committee at Wayne State University, and a written informed consent of the parent or guardian was obtained.
Table 1.
Clinical data and alpha-[11C]methyl-tryptophan (AMT) uptake asymmetries in subcortical structures in the 19 patients with intractable epilepsy and a prior cortical resection
| Sex | Previous resection site
|
Age at AMT PET (years) | Time following resection (months) | Lentiform nucleus asymmetry index | Thalamic asymmetry index | Total volume of resection (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hemisphere | F | T | P | |||||||
| 1 | M | Left | × | × | 8.8 | 1 | 7.8 | 2.8 | 13 | |
| 2 | M | Right | × | × | × | 15.5 | 2 | 5.8 | 4.5 | 11 |
| 3 | M | Left | × | × | × | 7.8 | 7 | 4.7 | −0.4 | 5 |
| 4 | M | Left | × | × | × | 2.1 | 7 | 4.8 | −4.6 | 3 |
| 5 | F | Right | × | × | × | 16.1 | 7 | 11.8 | 3.1 | 8 |
| 6 | F | Left | × | × | 12.4 | 9 | 9.3 | −2.1 | 44 | |
| 7 | F | Left | × | 3.8 | 11 | 4.4 | −0.9 | 24 | ||
| 8 | M | Right | × | × | 4.1 | 11 | 4.6 | −3.7 | 6 | |
| 9 | M | Left | × | × | × | 4.3 | 11 | 3.7 | 4 | 7 |
| 10 | M | Right | × | × | × | 7.1 | 11 | 4.7 | −3.3 | 12 |
| 11 | M | Right | × | × | 9.0 | 17 | 3.1 | −1.4 | 9 | |
| 12 | M | Left | × | × | 4.1 | 20 | 4.6 | −4.2 | 4 | |
| 13 | M | Right | × | 17.5 | 21 | 2.6 | −1.2 | 2 | ||
| 14 | M | Left | × | 8.8 | 23 | 2.7 | 0.3 | 7 | ||
| 15 | M | Right | × | × | 4.2 | 23 | 1.7 | 2.8 | 18 | |
| 16 | F | Left | × | × | 11.0 | 42 | 3.6 | −7.3 | 2 | |
| 17 | F | Left | × | × | 10.3 | 61 | 2.1 | 2.4 | 2 | |
| 18 | F | Left | × | × | 8.0 | 70 | 2.3 | 0.9 | 6 | |
| 19 | F | Left | × | 11.0 | 89 | −1.2 | −0.5 | 12 | ||
| x̄ | 8.7 | 23 | 4.2 | −0.5 | 10 | |||||
| S.D. | 4.4 | 24 | 3.0 | 3.2 | 10 | |||||
Asymmetry index = (AI − AC)/[AC + AI)/2] × 100%, where AI and AC are the radioactivity concentrations (μCi/ml) ipsilateral and contralateral to the resection, respectively. Sex: M = male, F = female; resection sites: F = frontal, T = temporal, P = parietal. Volume of resection is given as a percentage of total volume of the intact hemispheric volume (contralateral to the resection).
MRI
MRI scans of the brain of all epilepsy patients were performed using a GE 1.5-T Signa 5.4 unit (GE Medical Systems, Milwaukee, WI). The MRI protocol included axial and coronal T1 and T2 weighted as well as fluid attenuation inversion recovery (FLAIR) and volumetric spoiled gradient echo (SPGR) (1.5 mm sections of the entire head using a 35/5/1 [TR/TE/NEX] pulse sequence, flip angle of 35°, matrix size of 256 × 256, and 240 mm field of view) sequences. MRI was used to delineate subcortical regions (thalamus, lentiform nucleus) in the epilepsy groups.
PET
The AMT PET studies were performed using a CTI/Siemens EXACT/HR whole-body positron tomograph (Knoxville, TN) located in the Children’s Hospital of Michigan, Detroit. This scanner has a 15 cm field of view and generates 47 image planes with a slice thickness of 3.125 mm. The reconstructed image in-plane resolution obtained is 7.5 ± 0.38 mm at FWHM and 7.0 ± 0.49 mm in the axial direction (reconstruction parameters: Hanning filter with 1.26 cycles/cm cutoff frequency). The procedure for the AMT PET scanning has been described previously (Chugani et al., 1998). Briefly, patients were fasted for 6 h prior to the AMT PET studies to ensure stable plasma tryptophan and large neutral amino acid levels during the study. A venous line was established for injection of AMT (0.1 mCi/kg) as a slow bolus over 2 min. A second venous line was established for collection of timed blood samples, and plasma tryptophan concentration was measured as previously described to confirm stable values during the study (Chugani et al., 1998). Children were sedated intravenously with either nembutal (5 mg/kg) or midazolam (0.2–0.4 mg/kg), if necessary. Prior studies performed in our laboratory on five adults each scanned twice (once without and once with sedation using midazolam) have shown no significant global difference in serotonin synthesis between the two testing conditions (Muzik et al., 1998). Twenty-five minutes after tracer injection, a dynamic emission scan of the brain (7 × 5 min) was acquired in three-dimensional mode. Measured attenuation and decay correction were applied to the AMT PET images. Summed images representing five frames of the dynamic scan (30–55 min after injection) were used for further analysis.
Postacquisition analysis
The AMT PET and MRI image volumes were co-registered in the resection and epilepsy controls groups using MPI-Tool (Pietrzyk et al., 1994). Regions of interest (ROIs) were defined on the co-registered MRI image volumes (epilepsy patients) or directly on the PET scans (normal pediatric controls, where MRI was not available) for the thalamus and lentiform nucleus (including the putamen and globus pallidus). Because of its close proximity and small volume, the caudate nucleus was not evaluated separately. MRI-defined ROIs were then copied to the co-registered AMT PET images (Figure 1), and a weighted average radioactivity concentration of AMT for each delineated structure was obtained. AMT uptake was characterized by using an asymmetry index (AI) for the defined structures on the side of the focus/resection and contralateral to it, respectively. AI (%) = (AI − AC)/[AC + AI)/2] × 100%, where AI and AC are the radioactivity concentrations (μCi/ml) ipsilateral and contralateral to the epileptic focus. Positive values represented higher AMT uptake ipsilateral to the epileptic focus. In the normal controls, positive values represented higher values in the right-sided structures. In order to analyze the effect of the extent of cortical on subcortical AMT uptake, ROIs were also drawn on the postoperative MRI volumes over the resected areas, and resection volumes were calculated in cm3. These values were then normalized to the volume of the contralateral (non-resected) hemisphere (including all supratentorial brain structures), and expressed as a percentage.
Figure 1.
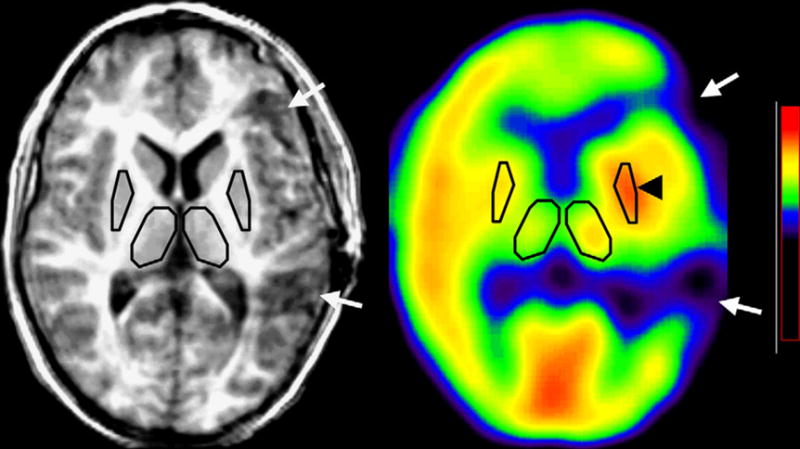
Increased AMT uptake (asymmetry index: 7.8%) in the ipsilateral lentiform nucleus (arrowhead) in a 8.8-year-old boy (patient 1), 1 month after a left fronto-temporal resection (see arrows on co-registered MRI and PET scans).
Statistical analysis
Statistical analyses were performed using the SPSS 11.5 software package (Chicago, IL). AMT uptake (characterized by AIs) of the analyzed structures in the three groups was compared with analysis of variance (ANOVA) followed by post hoc analysis with unpaired t-tests. Data are reported as mean ± standard deviation. In the resection group, subcortical AMT uptake AI values were correlated with the age of the patients (at the time of the postoperative PET scans), postoperative time, and the extent of the total resection using Pearson’s correlations. For variables showing significant correlation or a trend for significance (p < 0.1) in these correlations, a secondary regression analysis was performed using non-linear (quadratic and logarithmic) terms. p < 0.05 was considered to be significant.
Results
Postoperative AMT uptake was higher in the lentiform nucleus ipsilateral to the resection in all but one of the patients in the resection group (Table 1, p Figures 1 and 2). Mean AI values for the lentiform nucleus were significantly higher in the resection group (AI = 4.2 ± 3.0%; range: −1.2–11.8%) when compared to epilepsy patients without prior resections (AI = −0.1 ± 1.8%; < 0.001) and the normal pediatric controls (AI = 0.3 ± 1.3%; p = 0.001). The two control groups did not differ from each other (p = 0.23). In the resection group, asymmetry indices in the lentiform nucleus correlated inversely with postoperative time (r = −0.67; p = 0.002) (Figure 3), but not with age (r = 0.26, p = 0.29) or the extent of resection (r = 0.07, p = 0.77). The correlation between lentiform nucleus AIs and postoperative time could be best characterized by a logarithmic function (r = −0.69; p < 0.001).
Figure 2.
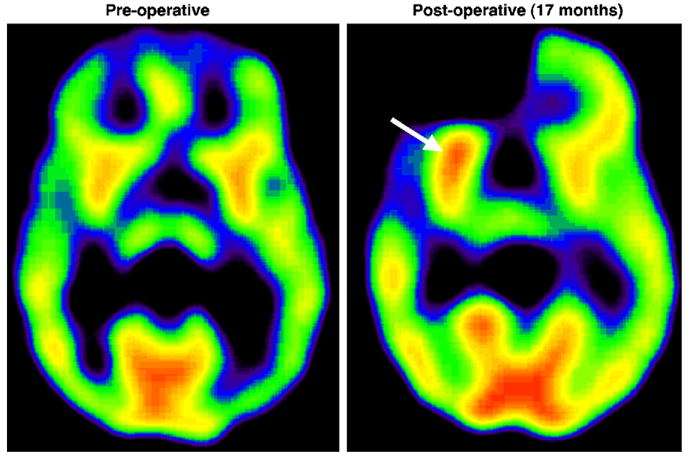
(A) PET scan showing a typical pattern of symmetric preoperative AMT uptake in a 9-year-old boy (patient 11). (B) A repeated AMT PET scan of the same patient 17 months after a right fronto-temporal resection demonstrated an increase in AMT uptake in the lentiform nucleus ipsilateral to the resection (arrow).
Figure 3.
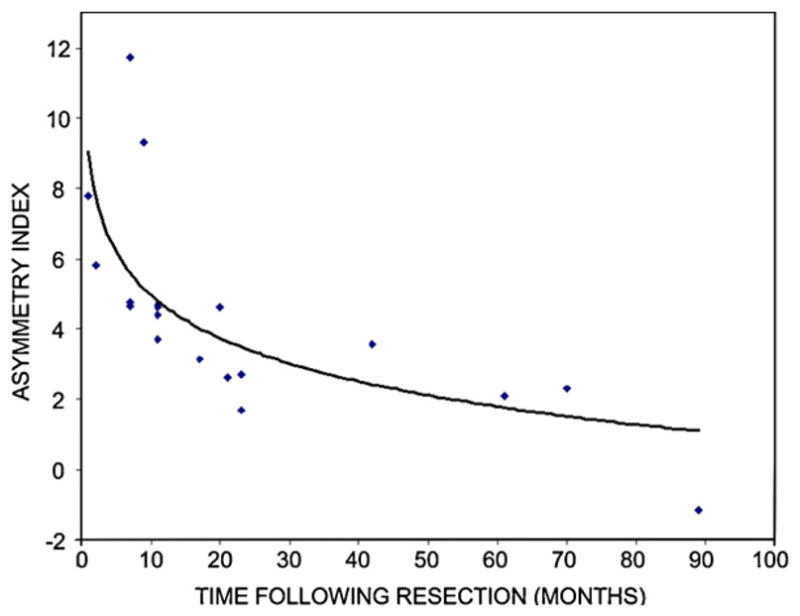
AMT uptake in the lentiform nucleus as a function of post-resection time. The asymmetry index was the highest (indicating increased uptake ipsilateral to the resection) in patients scanned early after surgery, and diminished with increasing postoperative time. The correlation could be well characterized by a logarithmic function (r = −0.69; p < 0.001).
AMT uptake in the thalamus was not different among the three groups (resection = −0.46 ± 0.32% vs. epilepsy controls = 0.71 ± 3.1% vs. normal controls = 0.14 ± 2.9%; ANOVA: p = 0.63). For the resection group, there was a negative correlation between the AI in the thalamus and the size of resection (r = −0.48, p = 0.038), but not with age (p = 0.29) or postoperative time (p = 0.85), indicating that larger resections were associated with lower ipsilateral thalamic AMT uptake.
Discussion
Our study provides evidence that cortical resection in humans results in a sustained increase of AMT uptake, indicating increased serotonin synthesis in the ipsilateral lentiform nucleus. These increases appear to slowly diminish with time. Although the present study was limited to pediatric patients, neither age, nor extent of the cortical resection significantly affected the measured increases of ipsilateral striatal AMT uptake. In contrast, thalamic AMT uptake ipsilateral to the resection was often decreased and related to the extent of resection but not post-resection time. The findings indicate differential effects of cortical resection on thalamic and striatal serotonin synthesis and implicate a role for increased striatal serotonin in the compensatory response to cortical resection.
AMT is a tryptophan analog that, when used with positron emission tomography, is a useful tool to measure serotonin synthesis in humans (Muzik et al., 1997; Diksic and Young, 2001). However, following ischemic brain injury or immune activation, induction of indolamine 2,3-dioxygenase can result in the conversion of tryptophan and AMT into kynurenine pathway metabolites (Saito et al., 1993). In the current study, we believe that AMT uptake reflects serotonin synthesis rather than activation of the kynurenine pathway for several reasons. First, widespread peri-resection increases in AMT uptake (sparing the striatum) have previously been reported 6 days post cortical resection but not at later time points (Juhász et al., 2004). This short-term increase in AMT uptake is presumably due to the inflammatory response to the subdural recording electrodes (Stephan et al., 2001) which were placed 3 days prior to resection and the subsequent incorporation of AMT into kynurenine pathway metabolites (Juhász et al., 2004). The increased AMT uptake seen in the lentiform nucleus of patients in the current study differs in that it persists for months to years after the resection. In addition, alterations in caudate nucleus metabolism following hemispherectomy have been imaged in children using serial 2-deoxy-2-[18F]fluoro-D-glucose (FDG) PET scanning. These studies revealed decreased glucose metabolism in the ipsilateral caudate 3–7 months posthemispherectomy, with recovery nearing pre-surgical levels in scans acquired 12–30 months posthemispherectomy and support a role for the caudate nucleus in compensatory changes following hemispherectomy (Chugani and Jacobs, 1994). There is a great deal of evidence that inflammatory processes can lead to increased FDG-PET uptake in animal studies (Ishimori et al., 2002) and in human disease (Bakheet and Powe, 1998; Brudin et al., 1994; Guhlmann et al., 1998; Stumpe et al., 2000). If striatal inflammation were responsible for increased AMT uptake in the current study, then we would expect a similar temporo-spatial pattern of FDG and AMT uptake in the ipsilateral striatum. However, this does not occur. Taken together, these findings suggest that the increased AMT uptake in the ipsilateral striatum more likely reflects serotonin synthesis than the formation of kynurenine pathway metabolites. Decreased AMT uptake seen in the ipsilateral thalamus of several patients may reflect diaschisis or neuronal loss following ipsilateral thalamic deafferentation (which may be more robust in patients with extensive cortical resection), resulting in decreased serotonin synthesis in this structure. Retrograde neuronal degeneration and thalamic atrophy following cortical resection (Matthews, 1973; Loopuijt et al., 1995; Kodama et al., 2003) may obscure an increase in thalamic serotonin synthesis in patients with large resections.
A major effect of cortical resection is the disruption of descending glutamatergic projections to the striatum (Hassler et al., 1982; Scatton et al., 1982; Young et al., 1981) and a subsequent imbalance between glutamatergic and monoaminergic afferents, which are important for the normal function of the basal ganglia (Carlsson and Carlsson, 1990). The effect of glutamatergic impairment on striatal serotonergic function is currently not well understood. Based on the findings that local administration of glutamate onto the caudate nucleus decreases serotonin release, presumably via GABAergic interneurons (Becquet et al., 1990), one might presume that the removal of glutamatergic neurons would increase serotonin release. Serotonergic innervation of the striatum is provided by the dorsal raphe nucleus, which also sends serotonergic projections to several other forebrain regions, including the frontal cortex (Vertes, 1991). Animal studies have demonstrated that the raphe nuclei also receive afferent (feedback) projections from widespread forebrain, including prefrontal, regions (Peyron et al., 1998). The ventral medial prefrontal cortex exerts a powerful inhibitory influence on serotonergic neurons in the dorsal raphe nucleus (Hajós et al., 1998; Varga et al., 2001). Thus, we speculate that a massive loss of prefrontal afferents to the raphe nuclei due to surgical resection may disinhibit raphe neurons thus leading to increased serotonin release in some of its efferent areas, including the ipsilateral striatum. It has been also shown that changes in striatal neurotransmitters following cortical resection are accompanied by massive axonal sprouting into the striatum, including axons originating from contralateral cortical regions (Carmichael and Chesselet, 2002; Cheng et al., 1997, 1998; Napieralski et al., 1996). This sprouting may be facilitated by increased striatal serotonin release during a finite period following the surgery. Patients scanned at later time points after surgery may be in various stages of axonal pruning and synaptic elimination (Cowan et al., 1984; Low and Cheng, 2005).
Extensive cortical resection during epilepsy surgery induces a profound reorganization to restore lost cortical (e.g. motor) functions, as evidenced by non-invasive studies using various electrophysiological and functional imaging modalities (Muller et al., 1998; Bernasconi et al., 2000; Holloway et al., 2000). Increased serotonin synthesis in the deafferented striatum may play a role in the functional reorganization of cortico-striatal projections and motor control, either by facilitating axonal sprouting from remote cortical areas, or by enhancing neurogenesis and guiding the migration of neuronal progenitor cells, or both. This latter mechanism has been demonstrated to be a potent self-repair mechanism that persists in the adult brain (Arvidsson et al., 2002; Parent et al., 2002). Serotonin plays a role in regulation of neurogenesis in the hippocampus and the subventricular zone, two regions where neuronal progenitor cells reside through adulthood in primates (Brezun and Daszuta, 1999, 2000; Gould, 1999). Therefore, manipulations of serotonergic mechanisms may be useful in future attempts to exogenously stimulate the brain’s inherent capacity for repair through axonal reorganization and neuronal replacement.
Acknowledgments
The authors thank the PET Center staff at Children’s Hospital of Michigan for their assistance in performing the studies. This work was partially supported by funding from grant NIH RO1 NS 45151 (to D.C. Chugani).
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bakheet SM, Powe J. Benign causes of 18-FDG uptake on whole body imaging. Semin Nucl Med. 1998;28:352–358. doi: 10.1016/s0001-2998(98)80038-x. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- Becquet D, Faudon M, Hery F. In vivo evidence for an inhibitory glutamatergic control of serotonin release in the cat caudate nucleus: involvement of GABA neurons. Brain Res. 1990;519:82–88. doi: 10.1016/0006-8993(90)90063-h. [DOI] [PubMed] [Google Scholar]
- Bernasconi A, Bernasconi N, Lassonde M, Toussaint PJ, Meyer E, Reutens DC, Gotman J, Andermann F, Villemure JG. Sensorimotor organization in patients who have undergone hemispherectomy: a study with (15)O-water PET and somatosensory evoked potentials. Neuroreport. 2000;11:3085–3090. doi: 10.1097/00001756-200009280-00010. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonin depletion in the adult rat produces differential changes in highly polysialylated form of neural cell adhesion molecule and tenascin-C immunoreactivity. J Neurosci Res. 1999;55:54–70. doi: 10.1002/(SICI)1097-4547(19990101)55:1<54::AID-JNR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus. 2000;10:37–46. doi: 10.1002/(SICI)1098-1063(2000)10:1<37::AID-HIPO4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Brudin LH, Valind SO, Rhodes CG, Pantin CF, Sweatman M, Jones T, Hughes JM. Fluorine-18 deoxyglucose uptake in sarcoidosis measured with positron emission tomography. Eur J Nucl Med. 1994;21:297–305. doi: 10.1007/BF00947964. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia-implications for schizophrenia and Parkinson’s disease. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HW, Rafols JA, Goshgarian HG, Anavi Y, Tong J, McNeill TH. Differential spine loss and regrowth of striatal neurons following multiple forms of deafferentation: a Golgi study. Exp Neurol. 1997;147:287–298. doi: 10.1006/exnr.1997.6618. [DOI] [PubMed] [Google Scholar]
- Cheng HW, Tong J, McNeill TH. Lesion-induced axon sprouting in the deafferented striatum of adult rat. Neurosci Lett. 1998;242:69–72. doi: 10.1016/s0304-3940(98)00050-0. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Jacobs B. Metabolic recovery in caudate nucleus of children following cerebral hemispherectomy. Ann Neurol. 1994;36:794–797. doi: 10.1002/ana.410360518. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Chakraborty P, Mangner T, Chugani HT. Human brain serotonin synthesis capacity measured in vivo with alpha-[C-11]methyl-L-tryptophan. Synapse. 1998;28:33–43. doi: 10.1002/(SICI)1098-2396(199801)28:1<33::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, Chugani HT. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Cowan WMFJ, O’Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225:1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Diksic M, Young SN. Study of the brain serotonergic system with labeled alpha-methyl-L-tryptophan. J Neurochem. 2001;78:1185–1200. doi: 10.1046/j.1471-4159.2001.00536.x. [DOI] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Guhlmann A, Brecht-Krauss D, Suger G, Glatting G, Kotzerke J, Kinzl L, Reske SN. Chronic osteomyelitis: detection with FDG PET and correlation with histopathologic findings. Radiology. 1998;206:749–754. doi: 10.1148/radiology.206.3.9494496. [DOI] [PubMed] [Google Scholar]
- Hajós M, Richards CD, Székely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Hassler R, Haug P, Nitsch C, Kim JS, Paik K. Effect of motor and premotor cortex ablation on concentrations of amino acids, monoamines, and acetylcholine and on the ultrastructure in rat striatum. A confirmation of glutamate as the specific cortico-striatal transmitter. J Neurochem. 1982;38:1087–1098. doi: 10.1111/j.1471-4159.1982.tb05352.x. [DOI] [PubMed] [Google Scholar]
- Hisaoka K, Takebayashi M, Tsuchioka M, Maeda N, Nakata Y, Yamawaki S. Antidepressants increase glial cell line-derived neurotrophic factor production through monoamine-independent activation of protein tyrosine kinase and extracellular signal-regulated kinase in glial cells. J Pharmacol Exp Ther. 2007;321:148–157. doi: 10.1124/jpet.106.116558. [DOI] [PubMed] [Google Scholar]
- Holloway V, Gadian DG, Vargha-Khadem F, Porter DA, Boyd SG, Connelly A. The reorganization of sensorimotor function in children after hemispherectomy. A functional MRI and somatosensory evoked potential study. Brain. 2000;123:2432–2444. doi: 10.1093/brain/123.12.2432. [DOI] [PubMed] [Google Scholar]
- Ishimori T, Saga T, Mamede M, Kobayashi H, Higashi T, Nakamoto Y, Sato N, Konishi J. Increased 18F-FDG uptake in a model of inflammation: concanavalin A-mediated lymphocyte activation. J Nucl Med. 2002;43:658–663. [PubMed] [Google Scholar]
- Juhász C, Chugani DC, Muzik O, Shah A, Asano E, Mangner T, Chakraborty PK, Sood S, Chugani HT. Alpha-methyl-L-tryptophan PET detects epileptogenic cortex in children with intractable epilepsy. Neurology. 2003;60:960–968. doi: 10.1212/01.wnl.0000049468.05050.f2. [DOI] [PubMed] [Google Scholar]
- Juhász C, Chugani DC, Padhye UN, Muzik O, Shah A, Asano E, Mangner TJ, Chakraborty PK, Sood S, Chugani HT. Evaluation with alpha-[11C]methyl-L-tryptophan positron emission tomography for reoperation after failed epilepsy surgery. Epilepsia. 2004;45:124–130. doi: 10.1111/j.0013-9580.2004.30303.x. [DOI] [PubMed] [Google Scholar]
- Kline AE, Yu J, Horvath E, Marion DW, Dixon CE. The selective 5-HT1A receptor agonist repinotan HCl attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience. 2001;106:547–555. doi: 10.1016/s0306-4522(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Kodama F, Ogawa T, Sugihara S, Kamba M, Kohaya N, Kondo S, Kinoshita T. Transneuronal degeneration in patients with temporal lobe epilepsy: evaluation by MR imaging. Eur Radiol. 2003;13:2180–2185. doi: 10.1007/s00330-003-1875-y. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Chugani HT, Tatsukawa K, Gall CM. Cerebral hemidecortication alters expression of transforming growth factor alpha mRNA in the neostriatum of developing rats. Brain Res Mol Brain Res. 1994;21:107–114. doi: 10.1016/0169-328x(94)90383-2. [DOI] [PubMed] [Google Scholar]
- Krynauw RA. Infantile hemiplegia treated by removing one cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1950;13:243–267. doi: 10.1136/jnnp.13.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loopuijt LD, Villablanca JR, Hovda DA. Morphological changes in the thalamus and neocortex of the cat brain after a restricted unilateral fetal neocortical lesion. Brain Res Dev Brain Res. 1995;85:259–272. doi: 10.1016/0165-3806(95)00003-v. [DOI] [PubMed] [Google Scholar]
- Low L, Cheng H. A little nip and tuck: axon refinement during development and axonal injury. Curr Opin Neurobiol. 2005;15:549–556. doi: 10.1016/j.conb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Matthews MA. Death of the central neuron: an electron microscopic study of thalamic retrograde degeneration following cortical ablation. J Neurocytol. 1973;2:265–288. doi: 10.1007/BF01104030. [DOI] [PubMed] [Google Scholar]
- Muller RA, Chugani HT, Muzik O, Mangner TJ. Brain organization of motor and language functions following hemispherectomy: a [(15)O]-water positron emission tomography study. J Child Neurol. 1998;13:16–22. doi: 10.1177/088307389801300103. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Chakraborty P, Mangner T, Chugani HT. Analysis of [C-11]alpha-methyl-tryptophan kinetics for the estimation of serotonin synthesis rate in vivo. J Cereb Blood Flow Metab. 1997;17:659–669. doi: 10.1097/00004647-199706000-00007. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Shen C, Chugani HT. Non-invasive imaging of serotonin synthesis rate using PET and a-methyltryptophan in autistic children. In: Carson RE, Daube-Witherspoon DE, Hesrcovitch P, editors. Quantitative Functional Brain Imaging with Positron Emission Tomography. Academic Press; San Diego: 1998. pp. 201–206. [Google Scholar]
- Napieralski JA, Butler AK, Chesselet MF. Anatomical and functional evidence for lesion-specific sprouting of corticos-triatal input in the adult rat. J Comp Neurol. 1996;373:484–497. doi: 10.1002/(SICI)1096-9861(19960930)373:4<484::AID-CNE2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pietrzyk U, Herholz K, Fink G, Jacobs A, Mielke R, Slansky I, Wurker M, Heiss WD. An interactive technique for three-dimensional image registration: validation for PET, SPECT, MRI and CT brain studies. J Nucl Med. 1994;35:2011–2018. [PubMed] [Google Scholar]
- Saito K, Nowak TS, Jr, Suyama K, Quearry BJ, Saito M, Crowley JS, Markey SP, Heyes MP. Kynurenine pathway enzymes in brain: responses to ischemic brain injury versus systemic immune activation. J Neurochem. 1993;61:2061–2070. doi: 10.1111/j.1471-4159.1993.tb07443.x. [DOI] [PubMed] [Google Scholar]
- Scatton B, Worms P, Lloyd KG, Bartholini G. Cortical modulation of striatal function. Brain Res. 1982;232:331–343. doi: 10.1016/0006-8993(82)90277-3. [DOI] [PubMed] [Google Scholar]
- Stephan CL, Kepes JJ, SantaCruz K, Wilkinson SB, Fegley B, Osorio I. Spectrum of clinical and histopathologic responses to intracranial electrodes: from multifocal aseptic meningitis to multifocal hypersensitivity-type meningovasculitis. Epilepsia. 2001;42:895–901. doi: 10.1046/j.1528-1157.2001.042007895.x. [DOI] [PubMed] [Google Scholar]
- Stumpe KD, Dazzi H, Schaffner A, von Schulthess GK. Infection imaging using whole-body FDG-PET. Eur J Nucl Med. 2000;27:822–832. doi: 10.1007/s002590000277. [DOI] [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, Sharp T, Hajós M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Young AB, Bromberg MB, Penney JB., Jr Decreased glutamate uptake in subcortical areas deafferented by sensorimotor cortical ablation in the cat. J Neurosci. 1981;1:241–249. doi: 10.1523/JNEUROSCI.01-03-00241.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


