Abstract
A number of postmortem studies have found decreased pH in brains of patients with schizophrenia. Insofar as lower pH has been associated with decreased mRNA expression in postmortem human brain, decreased pH in schizophrenia may represent an important potential confound in comparisons between patients and controls. We hypothesized that decreased pH may be related to increased concentration of lactic acid. However, in contrast to the previous notion that an increase in lactic acid represents evidence for primary metabolic abnormalities in schizophrenia, we hypothesized that this increase is secondary to prior antipsychotic treatment. We have tested this by first demonstrating that lactate levels in the cerebellum of patients with schizophrenia (n=35) are increased relative to control subjects (n=42) by 28%, p=0.001. Second, we have shown that there is an excellent correlation between lactate levels in the cerebellum and pH, and that this correlation is particularly strong in patients (r=− 0.78, p=3e-6). Third, we have shown in rats that chronic haloperidol (0.8 mg/kg/day) and clozapine (5 mg/kg/day) increase lactic acid concentration in the frontal cortex relative to vehicle (by 31% and 22% respectively, p<0.01). These data suggest that lactate increases in postmortem human brain of patients with schizophrenia are associated with decreased pH and that these changes are possibly related to antipsychotic treatment rather than a primary metabolic abnormality in the prefrontal cortex of patients with schizophrenia.
Introduction
Postmortem brain research is important in elucidating the pathophysiology of neurological and neuropsychiatric conditions. The interpretation of the results of postmortem studies must be evaluated carefully as the observed changes may be primary to the disease or may be the secondary effects of disease or medication or other confounding factors. For instance, a common feature of brain tissue from a number of cohorts of schizophrenic patients is a decreased brain pH relative to controls (Eastwood and Harrison 2005; Lipska et al 2006; Prabakaran et al 2004; Torrey et al 2005). The cause of the decreased pH is unclear, however. It may arise from differences in the manner of death between patients with schizophrenia and controls, premortem acidosis, medication-induced alterations, or it may reflect a primary feature of schizophrenia.
Recent studies have found altered expression of metabolic genes and altered levels of metabolites in the cerebrospinal fluid of live patients with schizophrenia (Holmes et al 2006, Huang et al 2007) and in the postmortem brain tissues of schizophrenia patients (Altar et al 2005; Prabakaran et al 2004; Prabakaran et al 2007). One of the postmortem studies reported altered transcription of genes in a large number of metabolic pathways and increased lactate levels in the prefrontal cortex (PFC) of patients with schizophrenia (Prabakaran et al 2004). The authors hypothesized that altered energy metabolism constitutes the “vulnerability” of the PFC and, when combined with a variety of genetic and/or epigenetic factors, results in the deficits that characterize schizophrenia. The authors argued that decreased pH and increased lactate levels are not postmortem artifacts but may underlie the pathophysiology of schizophrenia.
As antipsychotics induce numerous changes in metabolism both in vitro (Ferno et al 2005; Minet-Ringuet et al 2007; Vallejo-Illarramendi et al 2005) and in vivo (Bettinger et al 2000; Dwyer et al 2001; Newcomer 2004; Newcomer and Haupt 2006; Newcomer et al 2002), we hypothesized that increased lactate concentrations observed in postmortem brains of patients with schizophrenia are the result of treatment with antipsychotics and not a primary feature of the disease. Moreover, we hypothesized that increased lactic acid concentrations would be associated with decreased pH and perhaps decreased gene expression, and thus constitute an important confound in postmortem gene expression studies. To elucidate the significance of increased lactate concentrations in the postmortem brains of schizophrenic patients, we investigated whether increased lactate concentrations were found in the cerebellum, whether lactate levels correlated with cerebellar pH, and RNA integrity and expression of mRNA of housekeeping genes in the hippocampus and prefrontal cortex. Finally, we tested whether postmortem brain lactate levels were increased in the frontal cortex of rats chronically treated with haloperidol or clozapine.
Materials and Methods
Human Subjects
Human brain specimens were collected in the Section on Neuropathology of the Clinical Brain Disorders Branch at the National Institute of Mental Health (NIMH) through the Offices of the Chief Medical Examiner of the District of Columbia and of Northern Virginia, after autopsy, and through tissue donations via funeral homes. Informed consent to study brain tissue was obtained from the surviving next-of-kin for all cases, according to Protocol #90-M-0142 approved by the NIMH/National Institutes of Health Institutional Review Board. A telephone interview with the next-of-kin to gather basic demographic information and medical, substance use, and psychiatric history was conducted within one week of donation. Detailed information regarding diagnosis, antipsychotic medication history, neuropathology, toxicological analysis and other information is described elsewhere (Lipska et al 2006) and the subcohort used in this study is summarized in Table 1.
Table 1.
Summary of Cohort Demographics
| Subjects (n) | Schizophrenia (35) | Control (42) | t test p-value |
|---|---|---|---|
| Age (years) | 52.5 (18.3) | 45.8 (12.5) | 0.06 |
| Sex (M/F) | 21/14 | 28/14 | - |
| Race (AA/Cauc/Other) | 57.1%/ 42.9% | 61.9%/ 31.0% / 7.1% | - |
| pH | 6.38 (0.35) | 6.58 (0.33) | 0.02 |
| PMI (hours) | 35.6 (16.2) | 32.9 (16.2) | 0.44 |
| Manner of Death | Nat=71.4%;
Suic=17.1%; Acc=11.4% |
Nat=83.3%;
Hom=7.1%; Acc=9.5% |
- |
| Cigarette Smokers | 82.9% | 33.3% | - |
| Age Onset Illness (years) | 23.6 years | - | - |
| Lifetime Substance Abuse/Dep | 57.1% | - | - |
| Daily CPZ Equivalent Dose (mg) | 448 | - | - |
| Last CPZ Equivalent Dose (mg) | 524 | - | - |
| Lifetime CPZ Equivalent Dose (mg) | 3,938,291 | - | - |
Abbreviations: M – male, F – female, AA – African American, Cauc – Caucasian, PMI – postmortem interval, Nat – natural death, Suic – suicide, Acc – accident, Hom –homicide,
Drug Preparation
A stock solution of haloperidol (Sigma Chemicals, St Louis, MO) (20 mg/ml) was prepared by heating 200 mg of haloperidol in 10 ml 1% lactic acid until dissolved. To obtain a solution of 0.8 mg/ml haloperidol, the stock solution was diluted with distilled water and NaOH (1 N) was added to adjust the final solutions to a pH of 5.1. Clozapine (Sigma Chemicals) was prepared daily by dissolving 140 mg of clozapine in 0.6 ml of 1 N HCl with gentle heating, then diluting the solution with distilled water to 5 mg/ml and neutralized with 1N NaOH to a pH of 5.1. Vehicle consisted of 0.1% lactic acid.
Animals and Drug Administration
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) (n=15, weight 225–250 g) were housed two per cage with ad libitum access to food and water. All procedures were performed in accordance with the National Institutes of Health Guide for Use and Care of Laboratory Animals. After a 1-week habituation period, animals were administered haloperidol 0.8 mg/kg/day, n=5), clozapine (5 mg/kg/day, n=5) or vehicle (n=5) daily via intraperitoneal (i.p.) injections. This dose regimen was chosen to emulate the therapeutic doses given to patients (Kapur et al 2000). All animals were administered daily injections of drug or vehicle for 4 weeks. Rats were killed by decapitation, the brains were quickly removed, frontal cortex dissected and quickly frozen on dry ice.
Tissue Preparation and Lactate Measurements
Rat frontal cortex and human cerebellar tissue samples tissue samples (1 g tissue : 10 mL buffer) were homogenized in a protease inhibitor-Tris-glycerol extraction buffer (AEBSF 0.024%, aprotinin 0.005%, leupeptin 0.001%, pepstatin A 0.001%, glycerol 50%, Tris 0.6%) and centrifuged at 16,000 g for 20 minutes. Supernatant lactate measurements were obtained by a standard colorimetric method on a CMA 600 microdialysis analyser using a lactate reagent kit (CMA Microdialysis). This method has been widely used to measure the concentrations of lactate in blood and other biological material (Marbach & Weil 1967). The method is based on the enzymatic conversion of lactate to pyruvate by lactate oxidase and the formation of hydrogen peroxide. Hydrogen peroxide is in turn used in a reaction with 4-chlorophenol and 4-amino-antipyrine and yields the red-violet colored quinoneimine. The rate of formation is measured photometrically at 546 nm and is proportional to lactate concentration. The linear range is 0.02 – 12 mmol/L and the accuracy below 5% CV (coefficient of variation).
Measurement of pH
A portion of the cerebellum was used for pH measurement, as previously described (Romanczyk et al 2002). Briefly, tissue was pulverized over dry ice and 500 mg of tissue was weighed and placed in ice-cold 5 mL of distilled H2O. Samples were homogenized for 15 s using a 7-mm diameter generator probe with a processing range from 0.25 to 10 mLs (Omni International, Gainesville, VA, USA), attached to a hand-held tissue homogenizer (model OMNI TH, Omni International). The pH was measured on a model 370 PerpHeCT pH/ISE meter (ATI Orion Analytical Technology, Boston, MA, USA) equipped with a PerpHect Ross glass semimicro combination pH electrode (explicitly for tissue use) after a two-point calibration at pH 4.0 and pH 7.0. The pH was read for each sample and the electrode was rinsed twice with distilled H2O between samples.
Statistical Analysis
Two-tailed Student’s t-tests were used to examine if diagnostic groups (normal controls and schizophrenics) differed in variables such as brain pH, postmortem interval (PMI), and age. Multiple regression analysis was used for determining the contribution of multiple variables to pH. Pearson’s coefficients of correlation were calculated to examine if lactate levels were associated with age, pH, PMI, or any measure of antipsychotic treatment (i.e. daily, lifetime, or last chlorpromazine equivalents). Two-way ANCOVA was used to determine the effects of smoking and diagnosis on lactate and a one-way ANCOVA was used to determine whether lactate was different between groups of patients with positive and negative toxicology. A one-way ANOVA, followed by Fisher PLSD post hoc tests were used to test the effects of haloperidol and clozapine administration on lactate levels in the rat frontal cortex.
Results
Lactate Levels in Postmortem Human Brain
Patients with schizophrenia had significantly higher levels of lactate in the cerebellum compared to controls (by 28%, t= − 3.4, p = 0.001; Fig. 1). Furthermore, pH of the cerebellar tissue was significantly lower in patients as compared with controls (t = 2.7, p = 0.02; Table 1).
Figure 1.
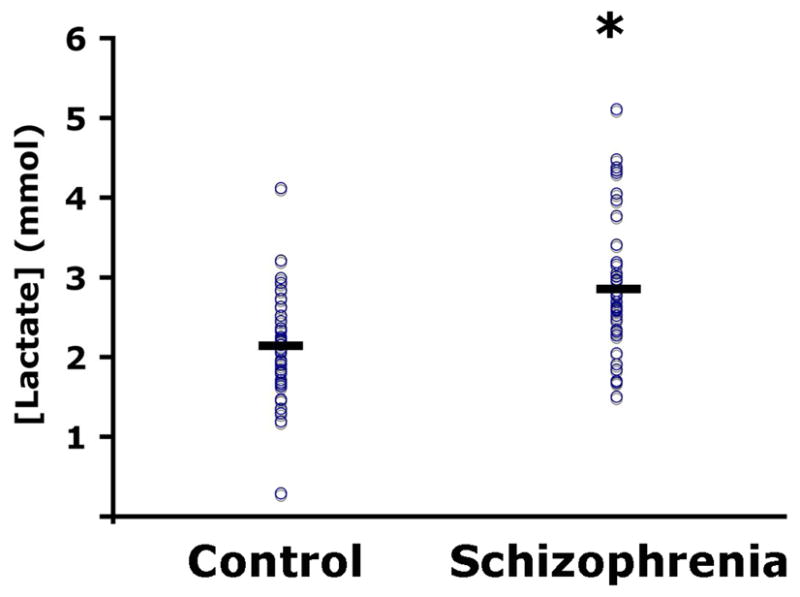
A scatter plot of lactate levels (mmol/L) in the cerebellum of control subjects (n = 42) and schizophrenic patients (n = 35). Lactate levels are significantly higher in patients with schizophrenia than control subjects (p = 0.001).
To assess the contribution of other variables to pH, we performed forward stepwise multiple regression analysis using lactate, age, sex, pH, post-mortem interval (PMI), smoking history (“yes” or “no”), agonal state (“short” or “extended”), manner of death (“natural” or “other”) and diagnosis as predicting variables in the whole cohort and then separately in subjects with schizophrenia (see Lipska et al 2006 for detailed definitions of these variables). The results showed that the only factor that contributed significantly to pH was the lactate level (adj R2 adjusted coefficients of determination = 0.36 and 0.56; β standardized regression coefficients = − 0.59 and − 0.69, p < 1e-7 and 1e-5, for the whole cohort and patients, respectively).
In the follow-up separate Pearson’s correlation analyses, cerebellar lactate levels correlated inversely with pH (r = − 0.61, p = 1e-5; Fig. 2A). This correlation was stronger in a group of patients with schizophrenia (r = − 0.71, p = 3e-6) than in normal controls (r = − 0.41, p < 0.001). There was a weak positive correlation of lactate levels with age at death (r = 0.32, p < 0.01; Fig. 2B). Correlations with age were similar in both diagnostic groups (r = 0.22 and 0.20, respectively). Lactate levels did not correlate with PMI (r = 0.04, p > 0.45; Fig. 2C).
Figure 2.
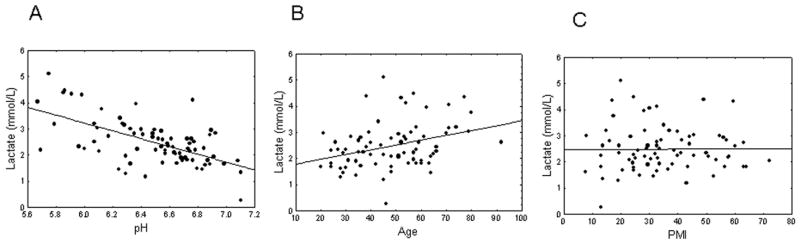
(A) Lactate levels are highly correlated with pH (r = −0.53), (B) weakly correlated with age (r = 0.29), (C) and not significantly correlated with postmortem interval (PMI; r = 0.04).
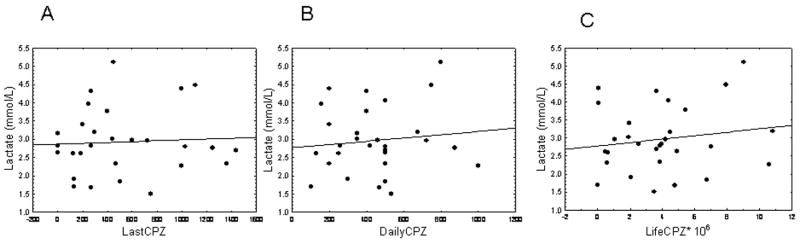
In patients, lactate did not correlate with any measure of antipsychotic treatment, i.e, last (r = 0.03; Fig. 3A), daily (r = 0.03; Fig. 3B), or lifetime doses (r = 0.14; Fig. 3C) expressed in chlorpromazine (CPZ) equivalents in milligrams. Moreover, lactate levels were not different between groups of patients with schizophrenia showing positive toxicological results for antipsychotic medication at the time of death (n = 19) and those showing negative toxicology results (n = 13), p > 0.3, suggesting that most recent medication intake vs recorded treatment did not differentially predict lactate levels.
Figure 3.
Lactate levels are not significantly correlated with last (A), daily (B), or lifetime (C) chlorpromazine (CPZ) equivalents (0 < r < 0.1).
Although as mentioned above in multiple regression analysis, lactate but not smoking was a determining factor in the prediction of the cerebellar pH, we additionally tested whether smoking affected lactate levels differently in patients and controls. In a two-way ANCOVA with smoking and diagnosis as independent factors, age as a covariate and lactate as a dependent variable, we found that there was a significant effect of diagnosis (F = 13.6, p = 0.0004), no significant effect of smoking and no significant smoking by diagnosis interaction (both F values < 1, p values > 0.5), suggesting that smoking was not affecting lactate levels. Similarly, in two-way ANOVAs with diagnosis and either agonal state or manner of death, diagnosis was significant (F > 5.0, p < 0.01) but neither agonal state or manner of death had significant effects on lactate levels (F < 1.0, p > 0.5).
Cerebellar Lactate Levels, RNA quality and Gene Expression
In order to test whether lactate levels predicted quality of RNA and mRNA expression, we used archival data from the white and grey matter dorsolateral prefrontal cortex (DLPFC) and hippocampus (methods and data collection described in Lipska et al 2006). We found significant negative correlations between cerebellar lactate levels and RNA integrity number (RIN) in the hippocampus (r = − 0.4, p = 0.001) and white matter DLPFC (r = − 0.31, p = 0.009) but not grey matter DLPFC (r = − 0.17, p 0.19). Expression of two housekeeping genes, porphobilinogen deaminase (PBGD) in the hippocampus and β-glucuronidase (GUSB) in grey matter DLPFC, correlated negatively with cerebellar lactate levels (r = − 0.30 and − 0.24, respectively, p < 0.01). Other housekeeping genes (β-actin ACTB, β-2-microglobulin B2M) in the DLPFC or hippocampus did not show significant correlations, suggesting that the effects may be tissue and transcript-specific.
Lactate levels in rats treated with antipsychotics
ANOVA revealed a significant effect of antipsychotic treatment on lactate concentrations F(2,12) = 11.1, p = 0.002; Fig. 4). Post hoc analysis showed that both drugs, haloperidol at 0.08 mg/kg (by 31%, p = 0.0005) and clozapine at 5mg/kg (by 22%, p = 0.01), significantly increased lactate concentrations in the frontal cortex as compared to vehicle (Fig. 4).
Figure 4.
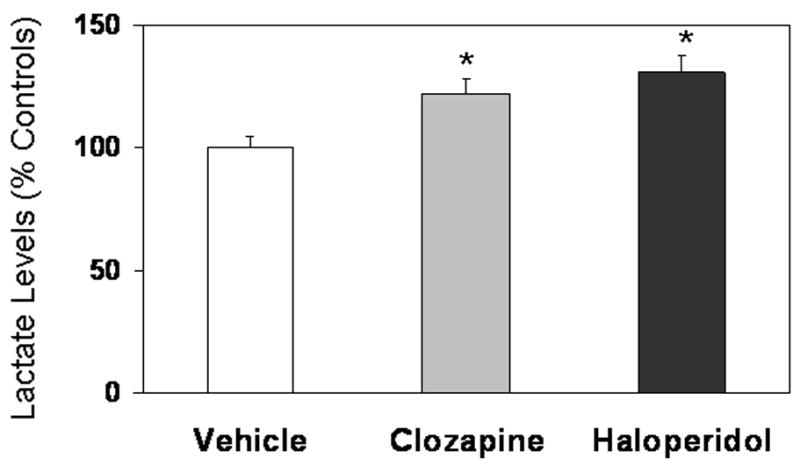
Lactate concentrations (mean ± SD) in the frontal cortex of rats treated with clozapine (5 mg/kg), haloperidol (0.8 mg/kg), or vehicle (0.1% lactic acid) for 28 days. Chronic intraperitoneal injections of haloperidol or clozapine significantly increased post mortem lactate levels relative to vehicle-treated animals (*p < 0.01, significantly different from vehicle control).
Discussion
We have expanded a previous finding of increased lactate levels in patients with schizophrenia to the cerebellum (Prabakaran et al 2004). Moreover, we found that lactate levels were highly correlated with pH, particularly in brains of patients with schizophrenia. Our findings of lactate increases in the cerebellum indicate that the changes are more widespread than previously suggested. Although we cannot rule out that these changes are related to primary metabolic abnormalities in the prefrontal cortex of schizophrenics, we think that it is very likely that they are secondary to antipsychotic treatment because of the increased lactate levels in the frontal cortex of rats treated with antipsychotics.
The question arises as to how antipsychotics may lead to increased postmortem brain lactate concentrations. It has been known for quite some time that monoamines regulate carbohydrate metabolism not just in liver and heart (Sutherland and Rall 1960) but in brain (Hoffman et al 1973). Also, blocking or depleting monoamines results in increased brain glycogen (Hoffmann et al 1973, Hutchins and Rogers 1971, 1973), although there are also reports suggesting more complex relationships, especially in vivo (Nahorski & Rogers 1973). Thus, antipsychotics, insofar as they block dopaminergic transmission, can be expected to increase brain glycogen levels, which upon death will be converted rapidly to lactate. Despite a clear effect of both clozapine and haloperidol on lactate concentrations in the rat frontal cortex, we have not found, however, a correlation between lactate levels and chlorpromazine equivalents in patients, calculated either as a last dose, daily dose or lifetime medication exposure. There are a number of possible reasons for this lack of correlation, including the accuracy of the CPZ estimation. Perhaps, more relevant is that the time course of the effect of antipsychotics on lactate levels is unknown both in terms of the onset and the duration of the effects. Moreover, the effect may not be linear. Given the fact that occupancy of at least 65% of dopamine D(2) receptors is needed for clinical response to antipsychotics (Tauscher and Kapur 2001), it would not be surprising that a majority of patients treated with antipsychotics will have elevated levels of brain glycogen while alive and subsequently increased lactate levels postmortem.
The correlation of lactate levels with age is also noteworthy. Given that brain glycogen is almost exclusively localized to astrocytes (Suh et al 2007), one would expect that postmortem lactate concentrations would increase with age as do the numbers of astrocytes. This is precisely what has been seen in our study. Insofar as advanced age and antipsychotic treatment may be associated with high lactate concentrations, the effect of antipsychotics on lactate levels may appear particularly exaggerated in elderly patients with schizophrenia.
Our pervious data strongly suggested that smoking status was associated with lower pH (Lipska et al. 2006). We have thus previously interpreted lower pH in schizophrenia as the result of increased incidence of smoking in patients. Our lactate data suggest, however, that increased lactate levels, and not smoking, is the best predictor of low pH. Smoking may still contribute to the effect but this contribution appears to be small and did not reach significance in this study when lactate data were also included in the multiple regression analysis.
Brain pH, along with other factors including agonal state and RNA integrity measures, is used as an indicator of tissue quality (Bahn et al 2001; Harrison et al 1995; Johnston et al 1997; Tomita et al 2004; Lipska et al 2006). A number of postmortem studies using brains of patients with schizophrenia have found reduced brain pH. Our results suggest that lower brain pH is associated with postmortem increases in lactate, and that both seem to be secondary to antipsychotic treatment. Given previous data that pH predicts mRNA expression (Atz et al 2007), and that cerebellar lactate levels are inversely correlated with the integrity of RNA and the expression of a number of genes in other brain regions, including the hippocampus and prefrontal cortex, these measures represent a significant confound that needs to be identified and co-varied for in future studies.
In summary, our results suggest that medication-induced increase in lactate concentration may account for lower postmortem pH observed in patients with schizophrenia. Further studies are required to elucidate the mechanism of antipsychotic-induced changes in carbohydrate metabolism and subsequent changes in postmortem lactate levels.
Acknowledgments
This work was inspired by Professors A. Heller and the late P.C. Hoffmann, Dr Kleinman’s Ph.D. advisors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atz M, Walsh D, Cartagena P, Li J, Evans S, Choudary P, Overman K, Stein R, Tomita H, Potkin S, Myers R, Watson SJ, Jones EG, Akil H, Bunney WE, Vawter MP Members of National Institute of Mental Health Conte Center and Pritzker Neuropsychiatric Disorders Research Consortium. Methodological considerations for gene expression profiling of human brain. J Neurosci Methods. 2007;163:295–309. doi: 10.1016/j.jneumeth.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y, Young TA, Bullard J, Yokoe H, Webster MJ, Knable MB, Brockman JA. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. doi: 10.1016/j.biopsych.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Bahn S, Augood SJ, Ryan M, Standaert DG, Starkey M, Emson PC. Gene expression profiling in the post-mortem human brain--no cause for dismay. J Chem Neuroanat. 2001;22:79–94. doi: 10.1016/s0891-0618(01)00099-0. [DOI] [PubMed] [Google Scholar]
- Bettinger TL, Mendelson SC, Dorson PG, Crismon ML. Olanzapine-induced glucose dysregulation. Ann Pharmacother. 2000;34:865–867. doi: 10.1345/aph.19327. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Bradley RJ, Kablinger AS, Freeman AM., 3rd Glucose metabolism in relation to schizophrenia and antipsychotic drug treatment. Ann Clin Psychiatry. 2001;13:103–113. doi: 10.1023/a:1016671725396. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr Res. 2005;73:159–172. doi: 10.1016/j.schres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Ferno J, Raeder MB, Vik-Mo AO, Skrede S, Glambek M, Tronstad KJ, Breilid H, Lovlie R, Berge RK, Stansberg C, Steen VM. Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: a novel mechanism of action? Pharmacogenomics J. 2005;5:298–304. doi: 10.1038/sj.tpj.6500323. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200:151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- Hoffmann PC, Toon R, Kleinman J, Heller A. The association of lesion-induced reductions in brain monoamines with alterations in striatal carbohydrate metabolism. J Neurochem. 1973;20:69–80. doi: 10.1111/j.1471-4159.1973.tb12105.x. [DOI] [PubMed] [Google Scholar]
- Holmes E, Tsang TM, Huang JT, Leweke FM, Koethe D, Gerth CW, Nolden BM, Gross S, Schreiber D, Nicholson JK, Bahn S. Metabolic profiling of CSF: evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS Med. 2006;3:e327. doi: 10.1371/journal.pmed.0030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JT, Leweke FM, Tsang TM, Koethe D, Kranaster L, Gerth CW, Gross S, Schreiber D, Ruhrmann S, Schultze-Lutter F, Klosterkötter J, Holmes E, Bahn S. CSF metabolic and proteomic profiles in patients prodromal for psychosis. PLoS ONE. 2007;2:e756. doi: 10.1371/journal.pone.0000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins DA, Rogers KJ. Effect of receptor blocking drugs on the depletion of brain glycogen by amphetamine. Br J Pharmacol. 1971;43:504–513. doi: 10.1111/j.1476-5381.1971.tb07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins DA, Rogers KJ. Effect of depletion of cerebral monoamines on the concentration of glycogen and on amphetamine-induced glycogenolysis in the brain. Br J Pharmacol. 1973;48:19–29. doi: 10.1111/j.1476-5381.1973.tb08218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston NL, Cervenak J, Shore AD, Torrey EF, Yolken RH. Multivariate analysis of RNA levels from postmortem human brains as measured by three different methods of RT-PCR. Stanley Neuropathology Consortium. J Neurosci Methods. 1997;77:83–92. doi: 10.1016/s0165-0270(97)00115-5. [DOI] [PubMed] [Google Scholar]
- Kapur S, Wadenberg ML, Remington G. Are animal studies of antipsychotics appropriately dosed? Lessons from the bedside to the bench. Can J Psychiatry. 2000;45:241–246. doi: 10.1177/070674370004500302. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Deep-Soboslay A, Weickert CS, Hyde TM, Martin CE, Herman MM, Kleinman JE. Critical factors in gene expression in postmortem human brain: Focus on studies in schizophrenia. Biol Psychiatry. 2006;60:650–658. doi: 10.1016/j.biopsych.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Marbach EP, Weil MH. Rapid enzymatic measurement of blood lactate and pyruvate. Clin Chem. 1967;13:314–325. [PubMed] [Google Scholar]
- Minet-Ringuet J, Even PC, Valet P, Carpene C, Visentin V, Prevot D, Daviaud D, Quignard-Boulange A, Tome D, de Beaurepaire R. Alterations of lipid metabolism and gene expression in rat adipocytes during chronic olanzapine treatment. Mol Psychiatry. 2007;12(6):562–571. doi: 10.1038/sj.mp.4001948. [DOI] [PubMed] [Google Scholar]
- Nahorski SR, Rogers KJ. In vivo effects of amphetamine on metabolites and metabolic rate in brain. J Neurochem. 1973;21:679–686. doi: 10.1111/j.1471-4159.1973.tb06012.x. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Abnormalities of glucose metabolism associated with atypical antipsychotic drugs. J Clin Psychiatry. 2004;65 Suppl 18:36–46. [PubMed] [Google Scholar]
- Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry. 2006;51:480–491. doi: 10.1177/070674370605100803. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Haupt DW, Fucetola R, Melson AK, Schweiger JA, Cooper BP, Selke G. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry. 2002;59:337–345. doi: 10.1001/archpsyc.59.4.337. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–697. 643. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Wengenroth M, Lockstone HE, Lilley K, Leweke FM, Bahn S. 2-D DIGE analysis of liver and red blood cells provides further evidence for oxidative stress in schizophrenia. J Proteome Res. 2007;6:141–149. doi: 10.1021/pr060308a. [DOI] [PubMed] [Google Scholar]
- Romanczyk TB, Weickert CS, Webster MJ, Herman MM, Akil M, Kleinman JE. Alterations in trkB mRNA in the human prefrontal cortex throughout the lifespan. Eur J Neurosci. 2002;15:269–80. doi: 10.1046/j.0953-816x.2001.01858.x. [DOI] [PubMed] [Google Scholar]
- Suh SW, Bergher JP, Anderson CM, Treadway JL, Fosgerau K, Swanson RA. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316, 819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmethyl)propyl]-1H-indole-2-carboxamide) J Pharmacol Exp Ther. 2007;321(1):45–50. doi: 10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- Sutherland EW, Rall TW. Formation of adenosine-3,5-phosphate (cyclic adenylate) and its relation to the action of several neurohormones or hormones. Acta Endocrinol Suppl (Copenh) 1960;34(Suppl 50):171–4. doi: 10.1530/acta.0.xxxivs171. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Kapur S. Choosing the right dose of antipsychotics in schizophrenia: lessons from neuroimaging studies. CNS Drugs. 2001;15(9):671–8. doi: 10.2165/00023210-200115090-00001. [DOI] [PubMed] [Google Scholar]
- Tomita H, Vawter MP, Walsh DM, Evans SJ, Choudary PV, Li J, Overman KM, Atz ME, Myers RM, Jones EG, Watson SJ, Akil H, Bunney WE., Jr Effect of agonal and postmortem factors on gene expression profile: quality control in microarray analyses of postmortem human brain. Biol Psychiatry. 2004;55:346–352. doi: 10.1016/j.biopsych.2003.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Torres-Ramos M, Melone M, Conti F, Matute C. Clozapine reduces GLT-1 expression and glutamate uptake in astrocyte cultures. Glia. 2005;50:276–279. doi: 10.1002/glia.20172. [DOI] [PubMed] [Google Scholar]