Abstract
Phosphorylation of the organic matrix proteins of dentin is important for the initiation of mineralization, but its relevance in later mineralization stages is controversial. The objective of this study was to analyze changes in the total matrix phosphate content during dentin development and to identify their origin. Amino acid and total matrix phosphate analyses of microdissected developing mantle and circumpulpal fetal bovine dentin specimens were performed. The amino acid composition showed few changes during mantle and circumpulpal dentin maturation. However, the total matrix phosphate content showed a significant, positive correlation with tissue maturation in both mantle and circumpulpal dentin, with a two- and a three-fold increase, respectively, being observed. The data indicate that changes occur in the pattern of phosphorylation of matrix proteins during dentin maturation, which we suggest may play a functional role in later stages of tooth mineralization.
Keywords: bovine, dentin, development, matrix, phosphorylation
Highly phosphorylated acidic proteins constitute a major part of the non-collagenous component of dentin matrices. They have been postulated to have critical and diverse functions in dentin mineralization, including initiating mineral nucleation and modulating mineral crystal growth (1-7). The in vitro functions of three abundant dentin phosphorylated proteins [dentin phosphoprotein (DPP), dentin sialoprotein (DSP), and dentin matrix protein 1 (DMP1)] have been shown to depend, at least in part, on the phosphorylation of their residues (8-10). While the number and sequence of phosphorylated residues is different among these proteins, study of the overall dentin matrix phosphorylation during dentin mineralization can provide insight into mineralization mechanisms. Earlier results describing variations in total matrix protein phosphorylation in dentin are conflicting, as both a decrease (11, 12) and an increase (13) in matrix phosphorylation have been described. Data from these earlier studies are diffcult to interpret because of the high variability of the structure and composition of analyzed dentin as a result of sampling the whole tooth crown, which presents high histological variability (14, 15), and because of the lack of normalization of the matrix phosphate changes observed (number of matrix protein residues phosphorylated vs. relative phosphorylated protein content in the matrix).
The purpose of the present study was to test the hypothesis that there are variations in the organic matrix phosphate levels during dentin maturation. Dentin maturation, for the purposes of this study, was defined as the age-dependent processes occurring after deposition of the organic matrix at the mineralization front, including formation and growth of the mineral crystals, degradation of matrix proteins, changes in collagen cross-linking and other possible modifications of the organic matrix. To test the above-stated hypothesis, mantle and circumpulpal dentin specimens from various developmental stages were localized on fetal bovine incisors based on a previously described developing dentin model (16, 17). The previous spectroscopic studies showed spatially resolved changes in dentin mineral properties, which correlated with tissue age, as well as suitability of the developmental stage of the particular bovine incisors used (16, 17). In these fetal bovine incisors, changes in dentin mineral properties exhibited a wide range from initial rapid changes to a lack of further change in older parts of the crown. As a result of differences in the matrix compositions, in mineral properties, and in the initiation of mineralization mechanism (18, 19), also confirmed in our previous studies, mantle and circumpulpal dentin were analyzed separately, although comparisons were made between the two regions.
The specimens were microdissected longitudinally and their matrix phosphorylation levels and amino acid residue profile were determined to assess changes in the matrix phosphate levels and whether these changes represented modification of the extent of phosphorylation or altered phosphoprotein concentration. The concentration of phosphoproteins is highly correlated to the relative content of aspartic acid (Asp) and serine (Ser), which account for 40–90% of the residues in the phosphoproteins (3, 20). In addition to these analyses, we performed similar measurements using dentin at the same level of the crown in different stages of maturation, by analyzing transverse sections, across the tooth from the pulp to the dentin–enamel junction (DEJ).
Material and methods
For the longitudinal analyses, the third and fourth lateral incisors (I3 and I4) from seven third-trimester fetal cows were examined; samples were kept at −80°C until used. Specifically, seven incisors were used for total amino acid analysis and seven for determination of total matrix phosphate. Additionally, one central (I1) and one first lateral (I2) incisor were examined to assess the effect of tooth type on changes in matrix phosphorylation. For the transverse analyses, two first central incisors (I1) were used for amino acid analysis and four were used for organic phosphate determination, from a total of four 6-month-old animals.
For the longitudinal analyses, sections 1 mm thick for amino acid analysis and 800 μm thick for organic phosphate analysis were acquired by serial sectioning perpendicular to the long axis of the crown, using a diamond wafer disc on an automated sectioning device (Isomet 5000; Buehler, Lake Bluff, IL, USA), starting from the most cervical end of the crown and proceeding to the incisal aspect (Fig. 1A). A total of 6–10 sections were acquired per incisor. Microdissection using a dissecting microscope was subsequently performed on the sections with scalpel blades. Mantle and circumpulpal dentin specimens, respectively, were separated from the mesial and distal halves of the same teeth. For mantle dentin specimens, a dentin zone ≈80 μm adjacent to the DEJ was selected, and another 80 μm zone, located 20–40 μm away from mantle dentin, provided the circumpulpal dentin specimens (Fig. 1C). Specimens, stored in a desiccator at 4°C overnight, were weighed, giving a typical yield of 0.3–1.2 mg. In the mineralization front to the DEJ distribution group, four 200 μm wide segments of dentin were microdissected from a section located at the middle of the incisor crown. These segments were located immediately beyond the mineralization front (0 μm) and ≈500, 1000, 1,500, and 2,000 μm (2,000 μm in only one incisor) from the mineralization front.
Fig. 1.
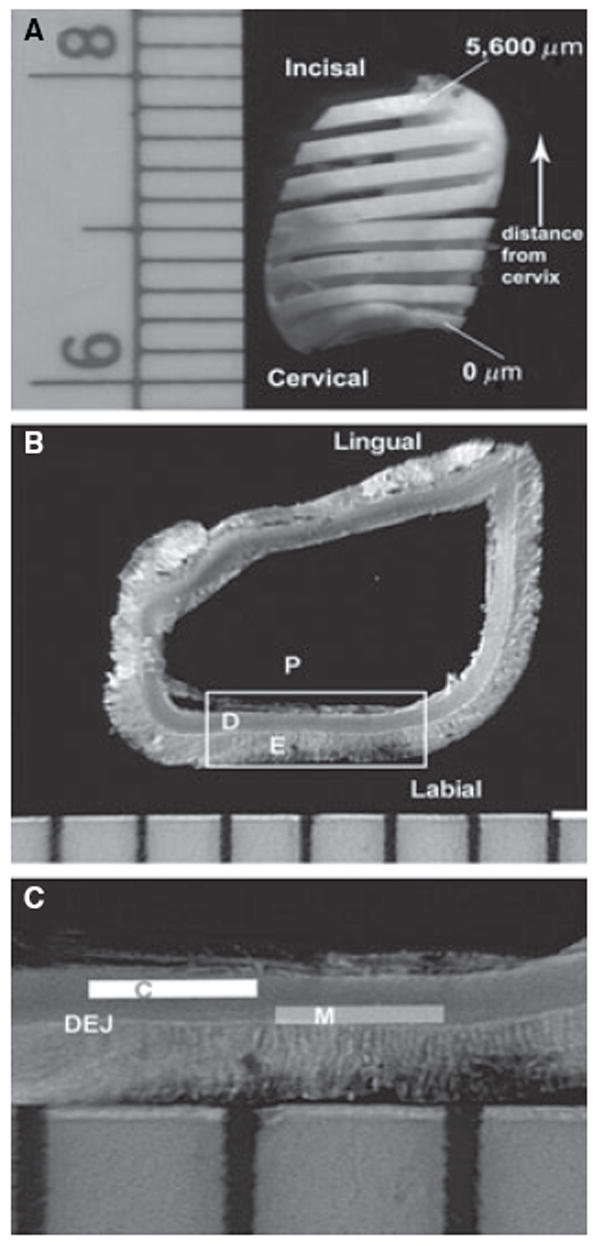
Microdissection of dentin specimens. (A) Incisor cut longitudinally at 800-μm intervals. Sections were numbered based on the distance from the cervix. (B) Section used for dentin microdissection. Labial and lingual aspects of incisor. D, dentin; E, enamel; P, pulp. (C) Schematic of areas used for microdissection on detail (framed) from the section in (B). C, circumpulpal dentin; DEJ, dentin–enamel junction; M, mantle dentin.
Amino acid analysis
The dried specimens were hydrolyzed with 300 μl of 6 M HCl in vacuo, after flushing with N2, at 110°C for 22 h, dried by speed vacuum centrifugation (Thermo Electron Corporation, Milford, MA, USA), dissolved in distilled water, filtered through a 0.22-μm membrane (Costar, Corning Inc., NY, USA), and subjected to amino acid analysis. The analysis was performed on a Varian 9050/9012 high-performance liquid chromatography (HPLC) system (Varian, Walnut Creek, CA, USA) configured as an amino acid analyzer with a cation exchange column (AA-911; Transgenomic, Omaha, NE, USA) (21,22). Amino acid analysis was performed on a total of 79 mantle or circumpulpal dentin samples, and the quantities of relevant amino acids were expressed as mmol per mmol of collagen based on a value of 300 residues of hydroxyproline per collagen molecule. In the mineralization front to the DEJ distribution analysis part, 9 samples in total were analyzed. No corrections were made for the partial degradation of Ser and threonine (Thr) by acid hydrolysis.
Organic phosphate determination
Samples were initially demineralized individually in 6-well tissue culture plates by continuous stirring in several changes of 3 ml of 0.5 M HCl. Aliquots (20 μl) from every extract were used to monitor the progress of demineralization by a phosphate colorimetric reaction (see below). The demineralization was terminated after two consecutive phosphate analyses matched values in the 0.05 M HCl control solutions. Serial extracts were combined, lyophilized to reduce the volume, transferred to 3.5 kDa molecular weight cut-off dialysis cassettes (Slide-A-Lyzer; Pierce, Rockford, IL, USA) with the addition of distilled water, and dialyzed exhaustively against 0.05 M HCl to remove the dissolved inorganic phosphate. The removal of inorganic phosphate was monitored in the dialysis solution as described above. Extracts were relyophilized after dialysis, dissolved in 500 μl of 1 M NaOH, transferred to 2-ml heat-resistant, screw-capped Teflon tubes, and combined with the original demineralized dentin samples. Dissolved extracts and demineralized pieces in the tubes were subsequently subjected to alkaline hydrolysis at 110°C for the release of organic phosphate from phosphorylated proteins and of hydroxyproline from collagen. Using the optimal time known from pilot experiments, a 300-μl aliquot was taken from each tube at 12 h for organic phosphate analysis and a 150-μl aliquot was taken at 18 h for hydroxyproline determination. Aliquots for both the organic phosphate and the hydroxyproline analysis were first neutralized by the addition of 6 M HCl. Colorimetric determination of organic phosphate from phosphorylated protein residues was performed using a modified malachite green method (23). Results were normalized to collagen (hydroxyproline) content (24). The total number of analyzed dentin specimens from I3 and I4 incisors was 85. Thirty-three additional samples from I1 and I2 incisors were analyzed to examine the effect of tooth type in matrix organic phosphate changes. Sixteen samples in total were analyzed to examine the organic phosphate changes as a function of distance from the mineralization front to the DEJ.
Statistical analysis
Analysis of bivariate and partial correlations between amino acid or organic phosphate/hydroxyproline ratios and distance of sample location from the tooth cervix were performed for the mantle and circumpulpal dentin distributions of matrix phosphate values as a function of the specimens cervical to incisal location on the crown. Linear regression based on least squares was applied. One-way analysis of variance was performed on the matrix phosphate values as a function of the mineralization front to the DEJ location on mid-crown sections from 6-month-old animals. To determine whether there were significant differences between organic phosphate/hydroxyproline ratios in the transverse analyses, a Tukey-Kramer post hoc multiple comparison was applied between the groups of distances from the mineralization front to identify significant differences between groups. spss 14.0 (spss, Chicago, IL, USA) software was used for the statistical processing.
Results
Amino acid analysis
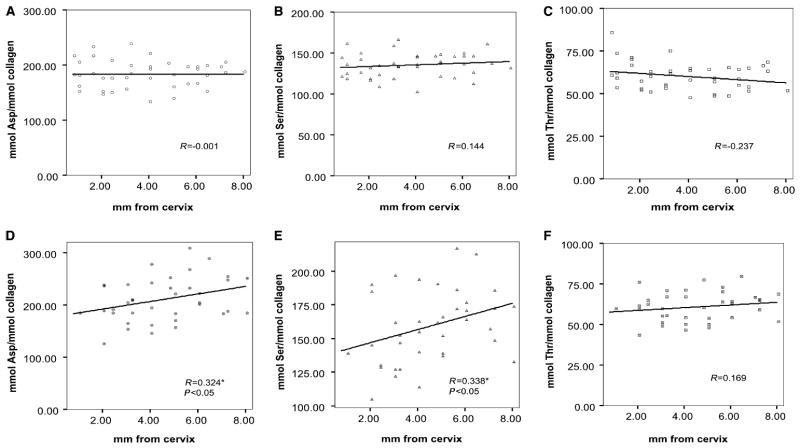
The analytical data for Asp, Ser, and Thr (mmol per mmol of collagen) in various maturational stages of mantle and circumpulpal dentin specimens are presented in Fig. 2A –F. No significant changes were observed in the content of any of the three amino acids relative to location distributions (distance from cervix) in mantle dentin (Fig. 2A–C). In the case of circumpulpal specimens, the relative contents of both Asp and Ser were significantly (P < 0.05; R = 0.324 and 0.338, respectively) correlated with distance of specimen location from the incisor cervix (Fig. 2D,E). Although the correlation was statistically significant, the overall changes in the Asp or Ser levels were relatively small, as predicted by the linear regression, in the order of ≈ 20% (Ser) to 30% (Asp). The relative Thr content did not show any significant changes (Fig. 2F). There were also no significant differences if the same location in circumpulpal and mantle dentin was compared.
Fig. 2.
Relative content of amino acids (mmol per mmol of collagen) in dentin matrix as a function of distance from the cervix. (A) Aspartic acid (Asp), (B) serine (Ser), and (C) threonine (Thr) from mantle dentin specimens. (D) Asp, (E) Ser, and (F) Thr from circumpulpal dentin. Pearson’s coeffcient (R) and statistical significance of relative content correlation with distance from cervix (where correlation is significant) are noted for each amino acid. Linear regression is shown. *Indicates statistical significance.
Organic phosphate determination
The results for organic phosphate content normalized to hydroxyproline of the microdissected mantle and circumpulpal dentin specimens are presented in Fig. 3. The data from one series of circumpulpal dentin specimens were discarded, as the assessed phosphate levels were very high (more than three standard deviations of the distribution of other samples at same distances) for all the samples in the series, probably as a result of contamination during processing.
Fig. 3.
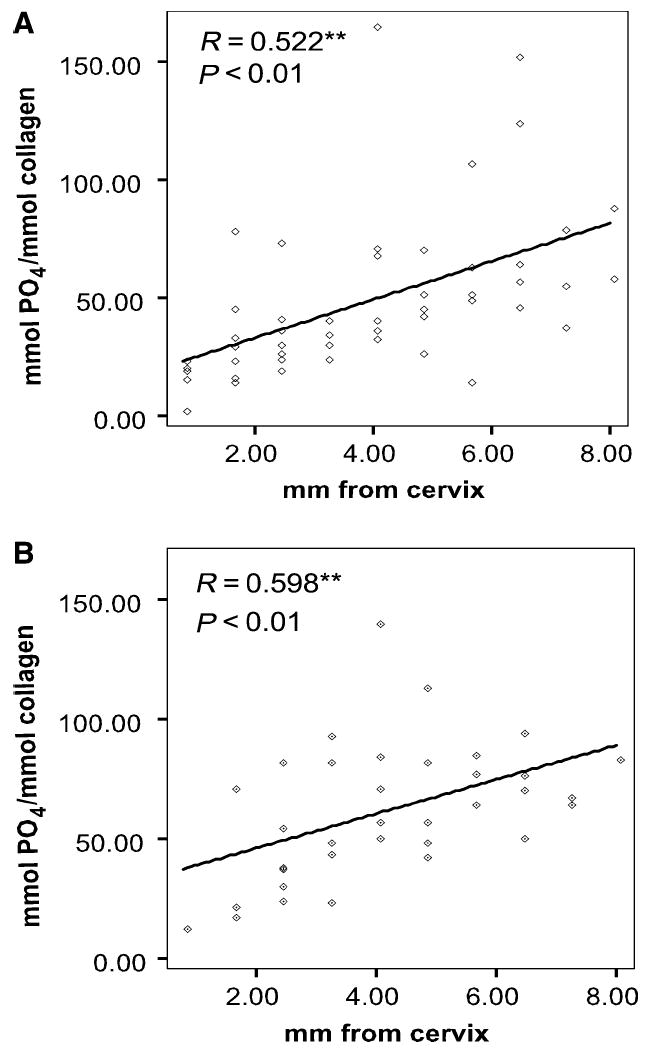
Relative matrix phosphate content in dentin as a function of distance from the cervix. (A) Mantle dentin and (B) circumpulpal dentin from incisor specimens I3 and I4. Pearson’s coeffcient (R) and statistical significance of relative content correlation with distance from the cervix are noted. Linear regression is shown. **Indicates statistical significance.
A highly significant (P < 0.01) increase of the relative amount of matrix phosphate was observed in both mantle dentin (Fig. 3A) and circumpulpal dentin (Fig. 3B) through the maximum distance of dentin specimens from the cervix (late stages of maturation). Despite the considerable variation between teeth in the matrix phosphate levels at any given distance of specimen location from the cervix, the Pearson’s correlation coeffcients were relatively high (R = 0.52 for mantle and R = 0.59 for circumpulpal dentin). The increase in organic phosphate, as estimated by linear regression, was approximately three-fold in mantle dentin and more than twice the initial values in circumpulpal dentin. Similar results were obtained from the specimens dissected out of the I1 and I2 fetal incisors (Fig. 4A,B), although statistical analysis was not attempted because of the small sample size.
Fig. 4.
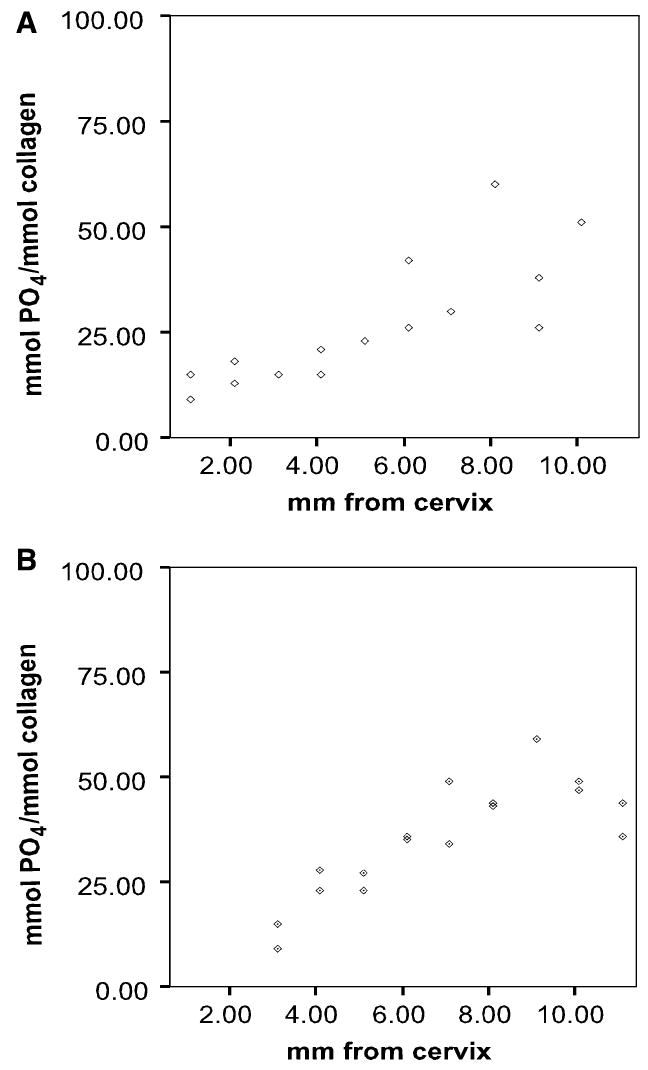
Relative matrix phosphate content in dentin as a function of distance from the cervix. (A) Mantle dentin and (B) circumpulpal dentin. Incisors I1 and I2.
Mineralization front to DEJ distribution: amino acids and organic phosphate
The acidic amino acid/hydroxyproline ratios in the transverse sections showed an overall decrease from the mineralization front to the DEJ (Fig. 5A). However, the organic phosphate/hydroxyproline ratios measured in transverse sections were significantly different, increasing from the mineralization front to the 500-μm specimen group and then gradually decreasing to values significantly lower than the initial value near the DEJ (Fig. 5B).
Fig. 5.
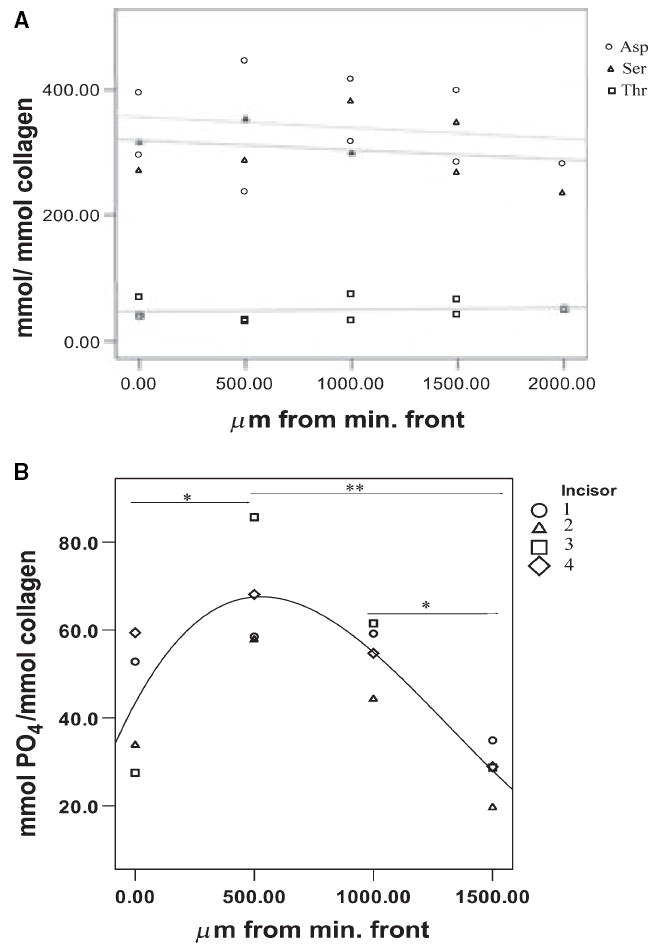
Relative amino acid and phosphate content of matrix as a function of distance from the mineralization front. (A) Aspartic acid (Asp), serine (Ser), and threonine (Thr) relative content in dentin matrix. (B) Relative matrix phosphate content. The presence of an asterisk between groups indicates statistical significance (*, P < 0.05, **, P < 0.01). min. front, mineralization front.
Discussion
In this study, changes in the relative matrix phosphate content were analyzed in dentin as a function of specimen distance from the incisor cervix, as this distance correlates with tissue maturation (16,17). To examine any contribution of changes in the relative content of total phosphorylated proteins to the changes in the extent of protein phosphorylation [e.g. because of the progressive accumulation of peritubular dentin, which is higher in anionic phosphorylated proteins than in intertubular dentin (25)], the maturing dentin matrix amino acid profile was also determined. As the levels of Asp and Ser in dentin collagen are approximately 10 times lower than those of DPP, the major dentin phosphoprotein (26, 27), changes in the concentration of phosphoproteins relative to that of collagen were assumed to be reflected by the ratio of these amino acids relative to hydroxyproline. The matrix content in Asp, Ser, and Thr (another amino acid that can be phosphorylated) relative to collagen content only showed minor changes during tissue maturation (cervical to incisal regions). In the analysis of the transverse sections (the mineralization front to the DEJ) there was a continuous small decrease in this ratio. These results indicated no change in the relative amount of total phosphorylated proteins in the developing mantle dentin, a small increase (20–30%) in developing circumpulpal dentin as a function of tissue age, and a slightly higher increase from the DEJ to the mineralization front. This last increase can be attributed to the reported higher concentration of peritubular dentin closer to the mineralization front and would probably be expected to be steeper in an older incisor (when the formation of peritubular dentin is completed).
The organic phosphate/hydroxyproline ratios exhibited a marked increase – two (mantle) or three (circumpulpal) times the initial levels – with dentin maturation and a variable pattern in a mineralization front to the DEJ distribution. In addition to the lateral incisors I3 and I4 analyzed, this increase was observed in the small sample of I1 and I2 incisors, although in the latter a plateau was being reached, probably because they start developing before I3 and I4 incisors. These data show that there is extensive modification of the phosphorylation of dentin matrix during maturation; however, the origin of these changes is unknown. There are several possibilities that might explain the increase in phosphorylation. One possibility is in situ phosphorylation of proteins already apposited at the mineralization front. The casein kinase I or II activity that would be necessary for this has been demonstrated in tooth slices (without odontoblast and predentin layers) incubated in the presence of phosphate donors (28). Similar casein kinase activity has been observed in mineralized bone (29). A vast network of cellular processes exist within dentinal tubules, through which small ATP and GTP donors can be delivered (30). Although much of the extracellular matrix must be sequestered within the mineral, exact information regarding accessibility of matrix proteins in the tissue does not exist, as immunolocalization studies are most commonly conducted on decalcified dentin. Another reason for the apparent increase in dentin matrix phosphorylation would be a change in the tissue composition, such as formation of large amounts of peritubular dentin after the initiation of mineralization, although this was not indicated by the amino acid analyses. An additional possibility is that during the mineralization process more highly phosphorylated matrix proteins bind to the dentin mineral crystals and become entrapped within them. There is a known spatial distribution in the types of phosphorylated matrix proteins accumulated in dentin (31), but verification of this would require detailed protein separation from each distinct tissue slice as a function of tissue age, analyses beyond the scope of the current study. It is also possible that the well-documented age-dependent degradation of phosphorylated proteins (11, 32, 33) creates a difference in the release of the phosphate from different tissue age matrices. Under the conditions of this study, on the other hand, all matrix phosphate should be released during alkaline hydrolysis.
The results of the matrix phosphate analyses of sequential areas in a mineralization front to the DEJ direction did not help to resolve the question of mechanism, but provided an interesting insight into the information derived from the longitudinal study. The transverse results clearly showed a non-monotonic pattern of change in the relative organic matrix content. This pattern was most suggestive of an increase of the matrix phosphorylation with tissue age around the mineralization front (where dentin maturation is active), followed by a significant decrease in the areas further away from the mineralization front. That decrease may either be the result of the described gradient of peritubular dentin concentration (lower concentration and consequently phosphoprotein relative content away from the pulp) or of degradation of phosphoproteins and loss of phosphate moieties in the older tissue (as is the tissue further away from the mineralization front). The decrease in the relative peritubular dentin concentration partially agrees with the amino acid results, although the latter indicated only minor changes in the phosphoprotein/hydroxyproline content. In any case, the statistically significant and extensive increase of matrix phosphate in the mineralization front area is compatible with the in situ phosphorylation possibility, while at the same time it cannot be explained by described histology factors or degradation of the matrix alone.
It is diffcult to place the results of this study in the context of previous reports from dentin studies, as previous results have been inconsistent. A significant decrease was described for the amount of matrix phosphate, analyzed either in total matrix isolated from 3–45-yr-old human teeth (12) or in chromatographically purified fractions of fetal calf dentin matrix (11). Another study (13) described the opposite effect, with an increase in matrix phosphorylation relative to tissue age. In that study, crown dentin of young permanent incisors in 2-yr-old cows was compared with that from crowns (or crowns and roots) at a later stage of formation. A high variability in the origin of dentin analyzed was introduced, because at every development point examined, the whole crown (or part of roots as well) was used for dentin analysis. While the conclusions of that study are generally in agreement with those of the present study on the same species, the histological variability in the samples used prevents a direct comparison with our data. Also, as already described, in this last study whether the changes in the former reflected continued phosphorylation of existing proteins or changes in the concentration of phosphorylated proteins was not determined. Finally, in another report (34), highly phosphorylated DPP was reported to be the only phosphorylated protein in developing bovine dentin. As a result of the lack of information of the particular age of the animals examined and the stage of the crown completion, and information on other phosphoproteins known to be present in the dentin (3), it is not possible to compare these results with ours. Our results agreed with those from a similar study of in vivo allogenic bone implant (demineralized bone matrix) mineralization (35), where dramatic changes in the phosphorylation state of the bone major phosphoproteins osteopontin (OPN) and bone sialoprotein (BSP) were observed. As estimated by the mole phosphoserine/mole BSP and mole phosphoserine/mole OPN ratios (determined through modified amino acid analysis and affnity HPLC), an approximately two-fold and three-fold, respectively, increase was observed in the number of phosphorylated residues in BSP and OPN of demineralized matrix implants placed in rat calvaria. This increase took place at about the same time as the major stage of mineral deposition (10 days), after which it stopped or, notably as in our mineralization front to DEJ distribution results, decreased. It has to be noted that, because a homogeneous mass of acid demineralized bone matrix and not whole bone or dentin, histological variability was not a limiting factor in this study, as in the present study.
Our findings, which failed to show significant differences between matrix phosphorylation in mantle and in circumpulpal dentin, were in contrast with existing data on phosphoprotein distribution in dentin in rat incisors (36) and in human premolars (37). In the latter (based on immunostaining) studies, DPP or highly phosphorylated proteins as a group were reported to be absent from mantle dentin. Technical issues, such as limited accessibility of the epitopes in the non-decalcified sections or lack of staining specificity (20), may explain the differences between these results and ours. Under the present study conditions, essentially all the matrix phosphate from the microdissected specimens was recovered after demineralization and analyzed without interference from the mineral. A certain part of the mantle dentin matrix phosphate found in the present study may have also originated in phospholipids that are present in mantle dentin matrix vesicles (38).
It is commonly accepted that acidic matrix proteins – mainly DPP, DSP, and DMP1 in dentin – determine where the initial deposition of mineral occurs on the collagen matrix. Notably, however, dentin from animals null in the dspp and dmp1 genes do not fail to mineralize, although in the case of dspp the resulting mineral was described to be less organized than the one in the normal animals (39, 40). This is a result, in part, of the redundant expression of these proteins and of the fact that not only phosphorylated proteins but also other macromolecules (such as biglycan and decorin) and the formation of matrix vesicles affect the initiation of mineralization. It has also been proposed that the same proteins may regulate crystal growth and determine crystal size and shape. The possibility of a dual role has been shown in vitro for phosphoproteins through experiments of mineral induction capacity, where binding to different surfaces and at different concentrations was studied (10, 41). The importance of phosphorylation in defining the functions and properties of these proteins, including affnity to the collagen fibers, has also been demonstrated (8-10). If a continued phosphorylation of matrix proteins indeed takes place, regulation of overall phosphorylation or phosphorylation of specific sequences could make modulation of the phosphoproteins’ functions possible. In any case, the differentiation of the pattern of phosphorylation in phosphoproteins observed here implies a functional role for them at later stages of dentin mineralization, in addition to initial mineral nucleation (4).
It is conceivable that through this mechanism the odontoblasts could modulate the extent of matrix protein phosphorylation, and possibly dephosphorylation, altering, in turn, the functions of phosphorylated proteins and retaining in this way a regulatory function after dentin is first formed. The results of this study demonstrate that there are variations in the extent of total matrix protein phosphorylation as dentin matures. Determining which proteins are affected will require a more detailed proteomics analysis.
Acknowledgments
This work is based on a thesis (KV) submitted to the graduate faculty, University of North Carolina at Chapel Hill, in partial fulfillment of the requirements for the PhD degree in Oral Biology. This work was supported by NIH DE Mentored Scientist Award K08467, and by DE04141, DE10489, and AR046121. This investigation was conducted in a facility constructed with support from Research Facilities Improvement grant C06-RR12538 from the National Center for Research Resources, NIH, USA.
References
- 1.Veis A. Mineral–matrix interactions in bone and dentin. J Bone Miner Res. 1993;8:S493–S497. doi: 10.1002/jbmr.5650081312. [DOI] [PubMed] [Google Scholar]
- 2.Boskey AL. Biomineralization: conflicts challenges, opportunities. J Cell Biochem (Suppl) 1998;30–31:83–91. [PubMed] [Google Scholar]
- 3.Qin C, Baba O, Butler WT. Post-translational modifications of SIBLING proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 4.Addadi L, Moradian-Oldak J, Furedi-Milhofer H, Weiner S, Veis A. Stereochemical aspects of crystal regulation in calcium phosphate-associated mineralized tissues. In: Slavkin HC, Price PA, editors. Chemistry and biology of mineralized tissues. 1. New York, NY: Excerpta Media; 1992. pp. 153–162. [Google Scholar]
- 5.Boskey AL. The role of extracellular matrix components in dentin mineralization. Crit Rev Oral Biol Med. 1991;2:369–387. doi: 10.1177/10454411910020030501. [DOI] [PubMed] [Google Scholar]
- 6.George A, Bannon L, Sabsay B, Dillon JW, Malone J, Veis A, Jenkins NA, Gilbert DJ, Copeland NG. The carboxyl-terminal domain of phosphophoryn contains unique extended triplet amino acid repeat sequences forming ordered carboxyl–phosphate interaction ridges that may be essential in the biomineralization process. J Biol Chem. 1996;271:32869–32873. doi: 10.1074/jbc.271.51.32869. [DOI] [PubMed] [Google Scholar]
- 7.Moradian-Oldak J, Frolow F, Addadi L, Weiner S. Interactions between acidic matrix macromolecules and calcium phosphate ester crystals: relevance to carbonate apatite formation in biomineralization. Proc R Soc Lond B Biol Sci. 1992;247:47–55. doi: 10.1098/rspb.1992.0008. [DOI] [PubMed] [Google Scholar]
- 8.He G, Ramachandran A, Dahl T, George S, Schultz D, Cookson D, Veis A, George A. Phosphorylation of phosphophoryn is crucial for its function as a mediator of biomineralization. J Biol Chem. 2005;280:33109–33114. doi: 10.1074/jbc.M500159200. [DOI] [PubMed] [Google Scholar]
- 9.Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279:18115–18120. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- 10.Saito T, Arsenault AL, Yamauchi M, Kuboki Y, Crenshaw MA. Mineral induction by immobilized phosphoproteins. Bone. 1997;21:305–311. doi: 10.1016/s8756-3282(97)00149-x. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Kossiva D, Glimcher MJ. Phosphoproteins of bovine dentin: evidence for polydispersity during tooth maturation. Biochemistry. 1983;22:2596–2601. doi: 10.1021/bi00280a001. [DOI] [PubMed] [Google Scholar]
- 12.Masters PM. In vivo decomposition of phosphoserine and serine in noncollagenous proteins from human dentin. Calcif Tissue Int. 1985;37:236–241. doi: 10.1007/BF02554869. [DOI] [PubMed] [Google Scholar]
- 13.Fujisawa R, Kuboki Y. Increase of dentin phosphophoryn with dentin formation. Connect Tiss Res. 1988;17:231–238. doi: 10.3109/03008208809017474. [DOI] [PubMed] [Google Scholar]
- 14.Jones SJ, Boyde A. Ultrastructure of dentin and dentinogenesis. In: Linde A, editor. Dentin and Dentinogenesis. Vol. 1. Boca Raton, FL: CRC Press; 1984. pp. 81–134. [Google Scholar]
- 15.Pashley DH. Dentin: a dynamic substrate – a review. Scanning Microsc. 1989;3:161–176. [PubMed] [Google Scholar]
- 16.Verdelis K, Crenshaw MA, Paschalis EP, Doty S, Atti E, Boskey AL. Spectroscopic imaging of mineral maturation in bovine dentin. J Dent Res. 2003;82:697–702. doi: 10.1177/154405910308200908. [DOI] [PubMed] [Google Scholar]
- 17.Verdelis K, Lukashova L, Wright JT, Mendelsohn R, Peterson MGE, Doty S, Boskey AL. Maturational changes in dentin mineral properties. Bone. 2007;40:1399–1407. doi: 10.1016/j.bone.2006.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tjaderhane L, Hietala EL, Larmas M. Mineral element analysis of carious and sound rat dentin by electron probe microanalyzer combined with back-scattered image. J Dent Res. 1995;74:1770–1774. doi: 10.1177/00220345950740110901. [DOI] [PubMed] [Google Scholar]
- 19.Tagaki Y, Sasaki S. Histological distribution of phosphophoryn in normal and pathological human dentins. J Oral Pathol. 1986;15:463–467. doi: 10.1111/j.1600-0714.1986.tb00659.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Unterbrink A, Kadapakkam S, Dong J, Gu TT, Dickson J, Chuang HH, MacDougall M. Regulation of the cell type-specific dentin sialophosphoprotein gene expression in mouse odontoblasts by a novel transcription repressor and an activator CCAAT-binding factor. J Biol Chem. 2004;279:42182–42191. doi: 10.1074/jbc.M402476200. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi M, Katz EP, Mechanic GL. Intermolecular cross-linking and stereospecific molecular packing in type I collagen fibrils of the periodontal ligament. Biochemistry. 1986;25:4907–4913. doi: 10.1021/bi00365a027. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi M, Shiiba M. Lysine hydroxylation and crosslinking of collagen. Methods Mol Biol. 2002;194:277–290. doi: 10.1385/1-59259-181-7:277. [DOI] [PubMed] [Google Scholar]
- 23.Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassays. Anal Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 24.Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1949;184:299–306. [PubMed] [Google Scholar]
- 25.Weiner S, Veis A, Beniash E, Arad T, Dillon JW, Sabsay B, Siddiqui F. Department of Structural Biology, Weizmann Institute of Science, Rehovot 76100. Peritubular dentin formation: crystal organization and the macromolecular constituents in human teeth. J Struct Biol. 1999;126:27–41. doi: 10.1006/jsbi.1999.4096. [DOI] [PubMed] [Google Scholar]
- 26.Veis A, Sharkey M, Dickson I. Noncollagenous proteins of bone and dentin extracellular matrix and their role in organized mineral deposition. In: Wasserman RH, Corradino RA, Crafoli E, Kretsinger RH, Mclennan DH, Siegel FL, editors. Calcium binding proteins and calcium function. North-Holland, NY: Elsevier; 1977. pp. 409–418. [Google Scholar]
- 27.Linde A. Noncollagenous proteins and proteoglycans in dentinogenesis. In: Linde A, editor. Dentin and dentinogenesis. II. Boca Raton, FL: CRC Press; 1984. pp. 55–92. [Google Scholar]
- 28.Suzuki Y, Yamaguchi A, Ikeda T, Kawase T, Saito S, Mikuni-Takagaki Y. In situ phosphorylation of bone and dentin proteins by the casein kinase II-like enzyme. J Dent Res. 1998;77:1799–1806. doi: 10.1177/00220345980770100701. [DOI] [PubMed] [Google Scholar]
- 29.Mikuni-Takagaki Y, Glimcher J. Post-translational processing of chicken bone phosphoprotein. Identification of bone phosphoprotein kinase. Biochem J. 1990;268:593–597. doi: 10.1042/bj2680593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mjor IA, Nordahl I. The density and branching of dentinal tubules in human teeth. Arch Oral Biol. 1996;41:401–412. doi: 10.1016/0003-9969(96)00008-8. [DOI] [PubMed] [Google Scholar]
- 31.Mckee MD, Zalzal S, Nanci A. Extracellular matrix in tooth cementum and mantle dentin: localization of osteopontin and other noncollagenous proteins, plasma proteins, and glycoconjugates by electron microscopy. Anat Rec. 1996;245:293–312. doi: 10.1002/(SICI)1097-0185(199606)245:2<293::AID-AR13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Butler WT, Brown M, Dimuzio MT, Linde A. Noncollagenous proteins of dentin. Isolation and partial characterization of rat dentin proteins and proteoglycans by a three-step preparation method. Coll Relat Res. 1981;1:187–199. doi: 10.1016/s0174-173x(81)80019-2. [DOI] [PubMed] [Google Scholar]
- 33.Jontell M, Pertoft H, Linde A. Disagreement in molecular weight determination of dentin phosphoproteins. Biochim Biophys Acta. 1982;705:315–320. doi: 10.1016/0167-4838(82)90253-9. [DOI] [PubMed] [Google Scholar]
- 34.Huq NL, Cross KJ, Talbo GH, Riley PF, Loganathan A, Crossley MA, Perich JW, Reynolds EC. N-terminal sequence analysis of bovine dentin phosphophoryn after conversion of phosphoseryl to S-propylcysteinyl residues. J Dent Res. 2000;79:1914–1919. doi: 10.1177/00220345000790111701. [DOI] [PubMed] [Google Scholar]
- 35.Salih E, Wang J, Mah J, Fluckiger R. Natural variation in the extent of bone phosphoproteins as a function of in vivo new bone formation induced by demineralized bone matrix in soft tissue and bony environments. Biochem J. 2002;364:465–474. doi: 10.1042/BJ20011272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahima M, Tsay TG, Andujar M, Veis A. Localization of phosphophoryn in rat incisor dentin using immunocytochemical techniques. J Histochem Cytochem. 1988;36:153–157. doi: 10.1177/36.2.3335773. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura O, Gohda E, Ozawa M, Senba I, Miyazaki H, Murakami T, Daikuhara Y. Immunohistochemical studies with a monoclonal antibody on the distribution of phosphophoryn in predentin and dentin. Calcif Tissue Int. 1985;37:491–500. doi: 10.1007/BF02557832. [DOI] [PubMed] [Google Scholar]
- 38.Katchburian E. Membrane-bound bodies as initiators of mineralization of dentine. J Anat. 1973;116:285–302. [PMC free article] [PubMed] [Google Scholar]
- 39.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, Wright JT, Macdougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 40.Ye L, Macdougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 41.Boskey AL, Maresca M, Doty S, Sabsay B, Veis A. Concentration-dependent effects of dentin phosphophoryn in the regulation of in vitro hydroxyapatite formation and growth. Bone Miner. 1990;11:55–65. doi: 10.1016/0169-6009(90)90015-8. [DOI] [PubMed] [Google Scholar]