Abstract
Interest in the years of reproductive changes for women with epilepsy (WWE), specifically perimenopause, menopause and postmenopause has been emerging in the epilepsy community. This article discusses evidence for changes in seizure frequency during perimenopause and postmenopause. Further, a catamenial epilepsy pattern during the reproductive years may be a hallmark for the observed seizure frequency change during these years; that is, an increase at perimenopause but a decrease at menopause. This finding implies that a subset of WWE are particularly susceptible to endogenous reproductive hormonal changes. An adverse effect on seizure frequency with the use of hormone replacement therapy (HRT) during postmenopause for WWE was reported in questionnaires, and was later borne out in a clinical trial. The laboratory counterpart of this human trial, HRT in ovariectomized rodent seizure models, shows that estrogen and progesterone are neuroprotective and do not uniformly increase seizure frequency. Possible reasons for the discrepancy between “the lab and the clinic” are presented. Strategies for managing HRT in symptomatic postmenopausal WWE using estrogenic and progestogenic compounds that may be less likely to promote seizures are discussed.
Keywords: epilepsy, perimenopause, menopause, postmenopause, catamenial, hormone replacement therapy
Introduction
Women with epilepsy (WWE) in perimenopause, menopause and perimenopause present a practical clinical challenge as well a complex hormonal brain environment in which to explore the relationships between hormones and epilepsy. In this brief review, associations between these life changes in WWE, seizure frequency and the use of hormone replacement therapy (HRT) will be presented. The laboratory evidence either supporting or refuting the changes in seizure frequency found in these studies will be discussed. Finally, suggestions for management of mature WWE will be put forth.
Hormone replacement therapy in Women with Epilepsy
The idea that HRT could affect seizure activity in WWE was first suggested in a questionnaire study intended to obtain preliminary information about the effect of menopause and perimenopause on the course of epilepsy. A secondary aim of the questionnaire was to assess whether a history of a catamenial seizure pattern would influence this course [1]. Questionnaires were sent to WWE using a mailing list from the local Epilepsy Foundation chapter and only those responses from women currently in menopause or perimenopause who did not have antepileptic drug (AED) changes during these life epochs, and who gave clear and legible responses were used. They provided information regarding the course of their epilepsy and treatment, seizure type, relationship of seizures to menses during their reproductive years, specifically the occurrence of seizures in the week before menses and at the onset of menses (catamenial seizure pattern type 1 [2]), and the use of HRT. The relationship of HRT to seizure occurrence was not specifically queried in this questionnaire. Statistical analysis was performed by using Pearson chi-square with 95% confidence limits.
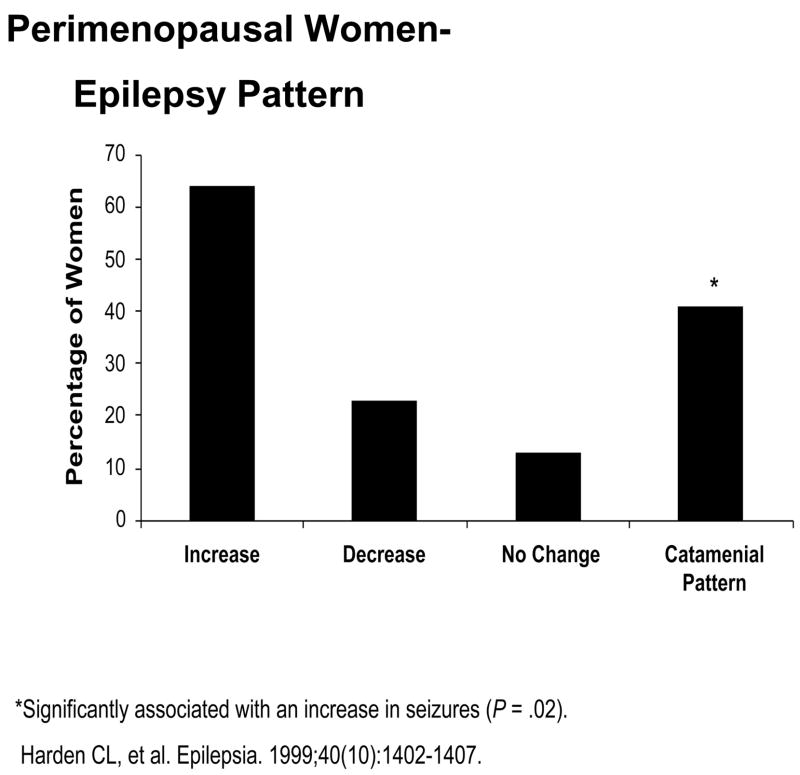
Thirty-nine perimenopausal women (ages 38–55 years) as defined by a recent change in menstrual pattern and the occurrence of “hot flushes” responded. Nine subjects reported no change in seizures at perimenopause, five reported a decrease in seizure frequency, and 25 reported an increase. Eight (15%) subjects took synthetic HRT, and 28 (72%) reported having a catamenial seizure pattern before menopause. HRT had no significant effect on seizures; however, a history of catamenial seizure pattern was significantly associated with an increase in seizures at perimenopause (p = 0.02) (see Figure 1). Considering that estrogen is generally proconvulsant and progesterone is anticonvulsant in animal seizure models [3], it is possible that the reproductive hormonal changes in perimenopause could contribute to increased seizures. During perimenopause, estrogen levels remain unchanged or may rise with age until the onset of menopause, presumably in response to the elevated FSH levels. However, the cyclic progesterone elevation during the luteal phase of the menstrual cycle gradually becomes less frequent throughout perimenopause, resulting in increasing rates of anovulatory cycles [4]. The elevation of the estrogen-to-progesterone ratio may contribute to the increase in seizure frequency at perimenopause for some WWE.
Figure 1.
Perimenopausal Women-Epilepsy Pattern
*Significantly associated with an increase in seizures (P = .02).
Harden CL, et al. Epilepsia. 1999; 40(10): 1402–1407.
Forty-two postmenopausal women (ages 41–86 years) as defined as one year without menses responded. Twelve subjects reported no change in seizures at menopause, 17 reported a decrease in seizure frequency, and 13 reported an increase. Sixteen (38%) took synthetic HRT. Sixteen (38%) additional subjects (having some overlap with the HRT group) reported having a catamenial seizure pattern before menopause. HRT was significantly associated with an increase in seizures during perimenopause (p = 0.001). A history of catamenial seizure pattern was significantly associated with a decrease in seizures at menopause (p = 0.013) (see Figure 2).
Figure 2.
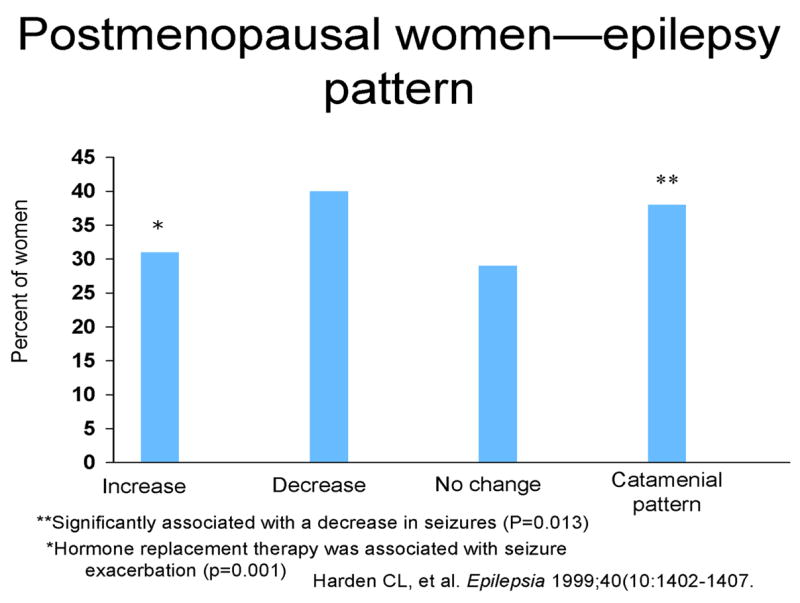
Postmenopausal women-epilepsy pattern
**Significantly associated with a decrease in seizures (P = 0.013).
*Hormone replacement therapy was associated with seizure exacerbation (p = 0.001)
Harden CL, et al. Epilepsia 1999; 40 (10: 1402–1407.
These pilot data suggest that WWE may be more likely than not to have an increase in seizures at perimenopause and therefore may need more careful monitoring at this time for AED adjustment or AED change. Further, these findings indicate that catamenial seizure pattern may be associated with seizure decrease during menopause but with an increase during perimenopause. This is consistent with the idea that a subset of WWE are especially sensitive to endogenous hormonal changes, associated with increased seizures in response to hormonal fluctuations, but decreased seizures when reproductive hormones stabilize. Finally, the results suggest that synthetic HRT may be associated with an increase in seizure frequency in menopausal WWE. The important implications of this finding prompted further study whether such an effect of endogenous hormones on WWE was present.
The study of HRT in WWE was designed to determine whether adding HRT to the medication regimen of postmenopausal WWE was associated with an increase in seizure frequency [5]. The study design was a randomized, double-blind, placebo-controlled trial of the effect of HRT on seizure frequency in postmenopausal WWE, taking stable doses of antiepileptic drugs (AEDs), and within 10 years of their last menses. After a 3-month prospective baseline, subjects were randomized to placebo, Prempro (0.625 mg of conjugated equine estrogens plus 2.5 mg of medroxyprogesterone acetate or CEE/MPA) daily, or double-dose CEE/MPA daily for a 3-month treatment period. This particular form of HRT was chosen for the trial because it was a very popular and widely used form of HRT at the time. Further, it was the treatment in the large, long-term, placebo-controlled The Women’s Health Initiative (WHI) study which looked at multiple medical outcomes. Therefore, it was thought that the use of this complex equine-derived estrogen and this synthetic progestin in the WWE study would be appropriate in the WWE trial because the results could be extrapolated to the general population of WWE. Further, this was the most commonly used form of HRT reported in the questionnaire study. The results were analyzed by chi-square for trend, comparing the numbers of subjects whose seizure frequency increased on treatment compared to baseline versus the number of subjects whose seizures did not increase across treatment arms.
Unfortunately, the first report of the WHI results in 2002 was the that HRT in the form of CEE/MPA was associated with an increased risk for breast cancer and stroke [6]. These results were surprising in the light of the long use of this compound, and lead to early termination of the WHI trial as well as the HRT in WWE study due to safety concerns.
Therefore, only twenty-one subjects were randomized after completing baseline; the results are discussed herein. The subjects’ ages ranged from 45 to 62 years (mean, 53 years; SD, +/−5), and the number of AEDs used ranged from none to three (median, one). There were no differences in baseline characteristics including age, age at menopause, number of AEDs and complex partial and generalized seizure frequency between the three treatment arms. However, the double dose CEE/MPA arm had significantly more simple partial seizures during baseline than the other treatment arms. Most subjects had no seizures of any type during baseline.
On HRT treatment compared to placebo, there was a significant trend toward increased seizures in a dose-related manner using several seizure frequency analyses. Five (71%) of seven subjects taking double-dose CEE/MPA had a worsening seizure frequency of at least one seizure type, compared with four (50%) of eight taking single-dose CEE/MPA and one (17%) of six taking placebo (p = 0.05). An increase in seizure frequency of the subject’s most severe seizure type was associated with increasing CEE/MPA dose (p = 0.008). An increase in complex partial seizure frequency also was associated with increasing CEE/MPA dose (p = 0.05) (see Table 1). Total seizure number approached significance with increasing CEE/MPA (p = 0.10).
Table 1.
Number of Subjects with Increase in Seizure Frequency by Treatment Arm (N=21)
| Treatment Group | Simple Partiala | Complex Partialb | Secondarily Generalized | Total # Szc | Any Sz Typed | Most severe Sz Typee | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Placebo (n=6) | 1 | (16.7) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (16.7) | 0 | (0.0) |
| Single CEE/MPA (n=8) | 3 | (37.5) | 1 | (12.5) | 0 | (0.0) | 3 | (37.5) | 4 | (50.0) | 3 | (37.5) |
|
| ||||||||||||
| Double CEE/MPA (n=7) | 1 | (14.3) | 3 | (42.9) | 1 | (14.3) | 3 | (42.9) | 5 | (71.4) | 5 | (71.4) |
CEE = conjugated equine estrogens; MPA = medroxyprogesterone acetate.
P = .88 by chi-square test for trend.
P = .05 by chi-square test for trend.
P = .10 by chi-square test for trend.
P = .05 by chi-square test for trend.
P = .008 by chi-square test for trend.
Two subjects taking lamotrigine had a decrease in lamotrigine levels of 25–30% while taking CEE/MPA. This is likely due induction of uridine diphosphate–glucuronosyltransferase (UGT) 1A4 by ethinyl estradiol, which is the main metabolic enzyme for lamotrigine, as occurs with hormonal contraceptives [7]. Therefore, although the small numbers of subjects in this study may limit its generalizability, the results indicate that CEE/MPA is associated with a dose-related increase in seizure frequency in postmenopausal WWE. Further, the lamotrigine clearance may be increased with HRT as well.
Menopause and HRT in animal seizure models
Interestingly, although these two clinical reports have consistent outcomes of adverse effects on seizure, there are not supportive laboratory counterparts of these findings. Ovariectomy in adult female rats, as an analogous state to menopause, is associated with increased seizure activity in using standard seizure-producing techniques. Using the pilocarpine model, ovariectomized rats showed a significantly more rapid progression to status epilepticus compared with intact animals [5]. With an NMDA seizure-induction model, ovariectomized rats had a significantly increased total seizure number and more severe hippocampal damage compared with age-matched intact female animals [6].
As an analogous state to HRT during menopause, is there laboratory evidence for how hormone replacement affects seizure activity in a rodent postmenopausal model? Again, contrary to the findings of the recent human studies, several experiments have shown an overall neuroprotective effect of estrogen and progesterone, but little effect on seizure frequency. In a kainate-induced model, estrogen pretreatment had no effect on seizure severity but significantly decreased “spread,” neuronal loss, and mortality in ovariectomized rats compared with ovariectomized rats without pretreatment. Progesterone pretreatment in this model had a slightly different effects; it decreased seizure severity and hippocampal damage [7]. In the NMDA-induced model, estrogen pretreatment decreased total seizure number in ovariectomized rats compared with ovariectomized rats without pretreatment; and in fact, estrogen replacement restored seizure number to that of the intact state [6]. In the lithium–pilocarpine model of status epilepticus, estrogen pretreatment is neuroprotective in ovariectomized rats compared with sham-treated ovariectomized controls [8].
Several factors may explain the seeming difference in these laboratory results to the effects of HRT on menopausal WWE. First, ovariectomized rats liley do not have an analogous hormonal brain milieu to naturally menopausal women. The rats were not in surgical postmenopause for a long period of time prior to the experiments, a matter of days in most experiments, in contrast to postmenopausal women who had years to develop changes to neurons and glia following hormone withdrawal. Second, the doses of HRT used by menopausal women are relatively higher than the doses used in these laboratory experiments. Finally, it is possible that the synthetic progestin, MPA, used in human studies could account for the adverse effects on seizures. Little is known regarding the effects of MPA on brain excitability. It is widely accepted that estrogen generally facilitates seizure activity, while progesterone (through the action of its reduced metabolite, allopregnanolone) has anticonvulsant properties [3]. However, MPA clearly has a different profile of activity in the brain, is not metabolized to allopregnanolone and is not neuroprotective in rodents [12]. In one study of ovariectomized rats with and without estrogen replacement, the effect of progesterone versus MPA pretreatment on kainate-induced seizures was evaluated with quite differing results for the two compounds. Both progesterone and MPA blocked the neuroprotective effects of estrogen in these experiments (a result differing from previous experiments for progesterone), and seizure severity was worse but not significantly so in the MPA-treated group [13]. Therefore, several factors, including the possibility of an adverse effect of MPA on seizures, may account for the inability to readily translate this paradigm from the lab to the clinic, or in this case, from the clinic back to the lab.
Since progesterone in its natural form in the FDA-approved form of Prometrium. is readily available, it is a reasonable option as the progestin component when HRT is needed for WWE, especially since there is evidence that it has active anticonvulsant properties [14]. For the estrogen component, a simplified estrogen compound, such as compound, 17-β-estradiol could be considered as well. Clinicians must consider these options since HRT will certainly be needed for some WWE; sleep deprivation related to “hot flushes” can have an adverse effect on seizure frequency and in such cases, HRT may be beneficial by permitting adequate sleep [15].
Conclusion
A risk of increased seizures with HRT consisting of CEE and MPA appears to be present in postmenopausal WWE. This was found in a cross-sectional questionnaire as well as a randomized, double-blinded, placebo-controlled clinical trial. Yet WWE will need to take HRT at times, for symptomatic relief and to allow adequate sleep when “hot flushes” are disruptive. The author suggests that a combination of a single estrogenic compound such as 17-β-estradiol along with natural progesterone be considered in this clinical scenario.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harden CL, Pulver MC, Jacobs AR. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia. 1999;40:1402–1407. doi: 10.1111/j.1528-1157.1999.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 2.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38(10):1082–8. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 3.Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47(9):1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–75. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 5.Harden CL, Herzog AG, Nikolov BG, et al. Hormone replacement therapy in women with epilepsy: a randomized, double-blind, placebo-controlled study. Epilepsia. 2006;47(9):1447–51. doi: 10.1111/j.1528-1167.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Anderson GL, Prentice RL, et al. the Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy post-postmenopausal women: principal results of the Women’s Health Initiative randomized, controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Harden CL, Leppik I. Optimizing therapy of seizures in women who use oral contraceptives. Neurology. 2006;67(12 Suppl 4):S56–8. doi: 10.1212/wnl.67.12_suppl_4.s56. [DOI] [PubMed] [Google Scholar]
- 8.Scharfman HE, Goodman JH, Rigoulot MA, Berger RE, Walling SG, Mercurio TC, Stormes K, Maclusky NJ. Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp Neurol. 2005;196(1):73–86. doi: 10.1016/j.expneurol.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalkbrenner KA, Standley CA. Estrogen modulation of NMDA-induced seizures in ovariectomized and non-ovariectomized rats. Brain Res. 2003;964(2):244–249. doi: 10.1016/s0006-8993(02)04065-9. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman GE, Moore N, Fiskum G, Murphy AZ. Ovarian steroid modulation of seizure severity and hippocampal cell death after kainic acid treatment. ExpNeurol. 2003;182(1):124–134. doi: 10.1016/s0014-4886(03)00104-3. [DOI] [PubMed] [Google Scholar]
- 11.Galanopoulou AS, Alm EM, Velísková J. Estradiol reduces seizure-induced hippocampal injury in ovariectomized female but not in male rats. Neurosci Lett. 2003;342(3):201–205. doi: 10.1016/s0304-3940(03)00282-9. [DOI] [PubMed] [Google Scholar]
- 12.Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66:916–928. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- 13.Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099(1):206–210. doi: 10.1016/j.brainres.2006.03.127. [DOI] [PubMed] [Google Scholar]
- 14.Herzog AG. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology. 1999;52:1917–1918. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- 15.Peebles CT, McAuley JW, Moore JL, Malone HJ, Reeves AL. Hormone replacement therapy in a postmenopausal woman with epilepsy. Ann Pharmacother. 2000;34(9):1028–31. doi: 10.1345/aph.19417. [DOI] [PubMed] [Google Scholar]