Abstract
Defects in preimplantation embryonic development, uterine receptivity, and implantation are the leading cause of infertility, pregnancy problems and birth defects. Significant progress has been made in our basic understanding of these processes using the mouse model, where implantation is ovarian estrogen-dependent in the presence of progesterone. However, an animal model where implantation is progesterone-dependent must also be studied to gain a full understanding of the embryo and uterine events that are required for implantation. In this regard, the hamster is a useful model and this review summarizes the information currently available regarding mechanisms involved in synchronous preimplantation embryo and uterine development for implantation in this species.
Keywords: Hamster, Mouse, Embryo, Uterus, Implantation
Introduction: pathways to implantation
The implantation process is dependent on precisely orchestrated steps that cannot be hurried. Although the details of implantation types and events vary among species, it is generally accepted that both the uterus and the embryo undergo synchronized restructuring and maturation processes to attain implantation competency. As a result of embryo-uterine interaction, the mother recognizes her pregnancy and triggers host immune-surveillance to accommodate the fetal allograft. Thus, implantation is possibly the most eventful stage of pregnancy and the most vulnerable point for pregnancy failure. This review provides a brief overview of the mechanisms of embryo implantation in various animal models as well as recent information suggesting that the hamster is a valuable model for dissection of implantation-specific mechanisms.
Early embryo development initiates the journey to implantation
Following the union of male and female gametes (fertilization) at the ampullary segment of the oviduct, a diploid zygote is formed. The one-cell zygote then divides mitotically to 2-cell, 4-cell, 8-cell, morula and blastocyst stages as they pass through the rest of the ampulla and isthmus to reach the uterus. While the 8-cell stage embryo enters the uterus in hamsters by 62 h post coitus [1], the late morula or early blastocyst stage enters the uterus at about 72 post coitus in mice [2] Although blastocyst formation (cavitation) occurs within the uterus in most species, blastocyst formation in hamsters occurs after the 4th cleavage (16-cell stage) compared to 5th cleavage (32-cell stage) in mice.
During blastocyst formation, the outer layer of polar trophectoderm (TE) surrounds a small cluster of nonpolar inner cell mass (ICM) cells alongside the blastocoel cavity. Cells in the mouse embryo can differ in their developmental fate and potential as early as the four-cell stage. As the 4-cell embryos divide further, a gradual increase in intercellular adhesion occurs and the embryos become compacted to form the morula possibly due to junction formation between some of the blastomeres. E-cadherin and zonula occludens-1 (ZO-1) first start to assemble at cell contact sites in the 8-cell stage embryos [3;4]. However, embryos lacking either its own or maternal E-cadherin undergo compaction. Thus, the definite role of E-cadherin in polarization of blastomeres is still not clear. The role of ZO-1 and ZO-2 in blastomere polarization is also unclear since these genes have not been mutated in embryos. The role of junctional proteins in morula-to-blastocyst transformation and the mechanism of fluid accumulation inside the hamster embryo are yet to be elucidated. However, our preliminary results suggest the presence of E-cadherin and F-actin proteins in the cell surface of hamster embryos at the morula and blastocyst stages (Fig. 1).
Figure 1. Membranous E-cadherin and F-actin localization in the morula and blastocyst stage embryos of hamsters.
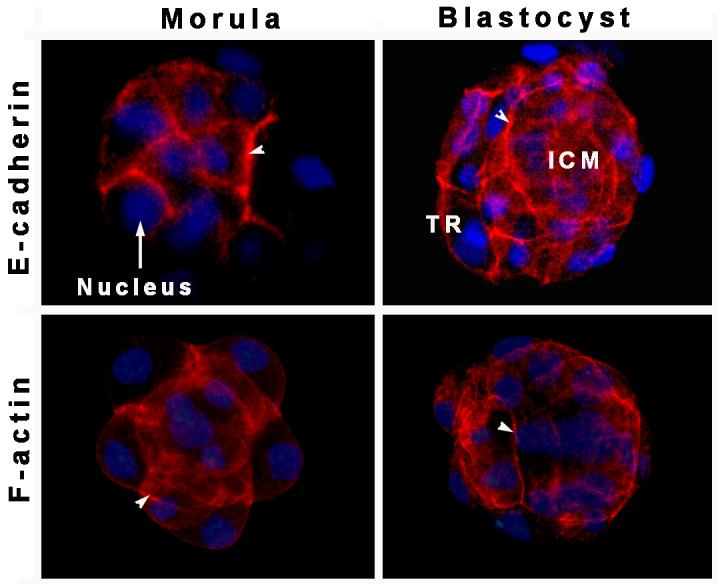
Arrowheads indicate E-cadherin (TRITC, red) and F-actin localization at cell-cell contact sites between blastomeres. A rat monoclonal E-cadherin antibody and Texas red-labeled phalloidin were used for E-cadherin and F-actin staining, respectively. Nuclei were stained with DAPI (blue). ICM, inner cell mass; TR, trophectoderm
Blastocyst competency: Embryonic preparation for implantation
After blastocyst formation, escape (hatching) from the zona pellucida is the next pivotal step towards achieving implantation competency. Studies have suggested that in vitro grown blastocysts hatch zona pellucida either by exerting pressure due to their expansion or by secretion of a trypsin-like protease from the trophectoderm. Several investigators have also proposed that zona hatching in vivo involves either uterine zona lysin or lysin from both the embryo and uterus [rev. in: 5]. Methods for zona escape in hamster, however, are different from other species. Gonzales and Bavister [5] demonstrated that blastocysts in vitro escape from the zona pellucida through a hole at the site of trophecdodermal projections. Hamster blastocysts in vivo do not undergo expansion and they hatch by gradual zona thinning and complete dissolution [5;6]. Mishra and Seshagiri [7] demonstrated that possibly embryonic, but not uterine, lysin is involved in lysis of zona pellucida in hamsters since 1-8 cell stage embryos from hamsters, but not mice, rats, sheep, or humans, shed their zona pellucida when cultured with zona-escaping blastocysts.
Implantation
Ovarian progesterone (P4) is required for uterine receptivity, implantation, and maintenance of early pregnancy in all mammals. However, while in mice [8;9] and rats [10] both maternal P4 and estrogen are critical to implantation, in the majority of species including hamsters [11-13], guinea pigs [14], rabbits [15], and pigs [16] implantation can occur in the presence of P4 alone; ovarian estrogen is not necessary. Furthermore, observations suggest that uterine receptivity as defined by surface morphology occurs primarily with P4 in the human and rhesus monkey uterus. There are also reports that luteal phase estrogen is not a requirement for establishment of pregnancy in rhesus monkeys and women [17;18]. In some of these P4-dependent species, estrogen may be required for implantation, but the source of this nidatory estrogen may be the blastocyst. In this regard, it has been demonstrated that the blastocyst of rabbits [15], pigs [16], hamsters [19] and humans [20] may be capable of producing estrogen. We also show here the presence of aromatase protein in the hamster blastocyst (Fig. 2).
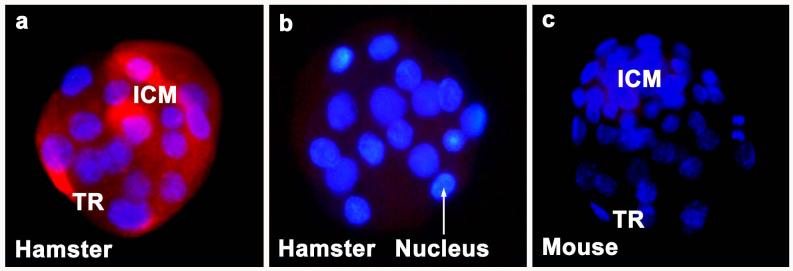
Figure 2. Immunofluorescence of cytochrome P450 aromatase (Cyp19) protein in day 4 hamster blastocysts.
Blastocysts of hamsters (a) and mice (c) were incubated with polyclonal anti-human aromatase antibody. As a negative control, hamster blastocysts (b) were stained without primary antibody. In comparison, mouse blastocysts (c) show no expression of Cyp19. Simultaneous staining was performed for Cyp19 antigen (TRITC, red) and nuclei (DAPI, blue). ICM, inner cell mass; TR, trophectoderm.
Embryo implantation in hamsters takes place late in the morning of day 4 of pregnancy and blastocysts can no longer be flushed from the uterus. However, apparent increase in vascular permeability at the implantation site after an intravenous injection of blue dye is not clearly observed until the evening (1600-1800 h) of day 4 (Fig. 3), suggesting that permeability change at the site of implantation takes longer in hamsters than mice. Implantation is induced in ovariectomized-adrenalactomized hamsters with P4 alone; exogenous estradiol-17β is not a requirement [12]. However, there are also profound differences in the response of rats and hamsters to exogenous estrogens or progestins. Estradiol-17β and diesthylstilbesterone are only 4% to 5% as active in the hamster as uterotrophic or contraceptive compounds compared with the rat. This correlates with significantly higher levels of circulating estrogen in pregnant hamsters than pregnant rats [13]. In contrast, the hamster is exquisitely sensitive to small amounts of P4 compared to rats and mice. While hamsters ovariectomized on day 2 of pregnancy need only 62 μg of daily injection of P4 to initiate implantation [11], mice ovariectomized on day 3 and 4 of pregnancy need at least 1-3 mg of daily P4 when superimposed with 10-25 ng of estradiol-17β [8;21]. Thus, P4 is the most sensitive steroid in hamsters, and its circulating levels steadily increase from day 1 to 14 of pregnancy. During early pregnancy in the hamster, ovarian venous plasma P4 levels were fivefold lower than in the rat [13]. Similarly, venous estrogen concentration also increases slowly during early pregnancy in hamsters and its concentration in ovarian venous effluent on day 4 of pregnancy is approximately 20-fold greater than in the rat [22].
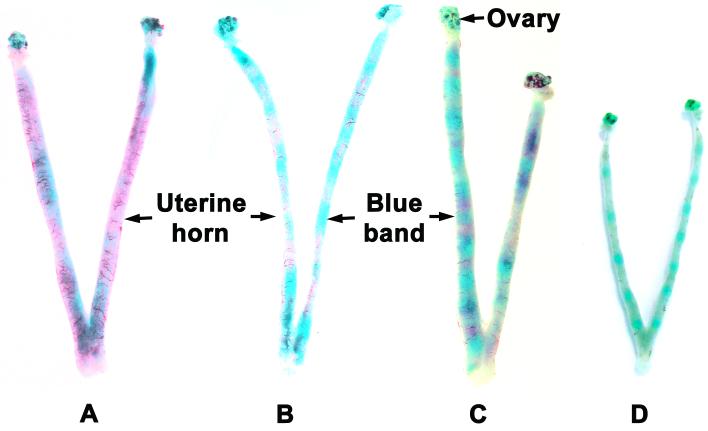
Figure 3. Implantation sites on days 4 and 5 of pregnancy as detected by intravenous injection of Chicago blue B dye injection.
Blue bands along the uterine horn of hamsters (A, day 4 1200 h; B, day 4 1800 h; C, day 5 0900 h) and mice (D, day 5, 0800 h) are indicators of implantation sites.
Uterine morphological changes in preparation for implantation
Specialized protrusions on the apical surface of uterine epithelial cells, termed pinopodes or uterodomes, appear in mice, humans and other species at the time of implantation [23]. The appearance of pinopodes is also associated with expression of implantation-specific factors including Lif, HB-EGF, the homeobox gene Hoxa-10, and αvβ3 integrin, and the mid-luteal increase in P4 levels in women. The function of pinopodes remains unclear, but when they appear, uterine epithelial apical microvilli begin to retract providing a smoothened endometrial surface for embryo-uterine interaction [24]. In contrast to mice and rats, where the epithelial surface becomes flattened and microvilli are no longer visible, epithelial microvilli never completely disappear during implantation in hamsters, but remain until phagocytosed by the trophoblast [6;25]. Beyond pinopode formation and microvilli retraction, the uterine lumen of most of species is lined with glycoproteins such as integrins, selectins, galectins, heparan sulfate proteoglycans (HSPGs), the extracellular matrix molecules osteopontin and laminin, and the trophinin-tastin-bystin complex [26] that can act as cell surface adhesion molecules to position the blastocyst over the implantation site. Mucins are another component of the uterine glycocalyx that function primarily in an anti-adhesive role. MUC-1 is the best-studied member of the reproductive tract mucin family, and is present in the apical border during the pre-receptive state but reduced or absent during receptivity in most species [27]. However, it has been shown that uterine MUC1 persists in women and both MUC-1 mRNA and protein showed several fold increase from the proliferative to the mid-secretory phase in humans [28]. This expression may inhibit the blastocyst attachment process until shedding via interaction with local factors or removal at specific sites such as pinopodes. Similar to women, we observed very little or no reduction of MUC-1 from the uterine epithelium of hamsters (Fig. 4). Furthermore, we observed strong accumulation of MUC-1 at the antimesometrial embryo-uterine contact site on day 5 of pregnancy (Fig. 4). Thus further research is necessary to better understand the precise role of MUC-1 in hamster and human implantation.
Figure 4. MUC-1 immunostaining of the luminal epithelium of hamsters on days 1-5 of pregnancy.

CT-1, a rabbit polyclonal antibody, was a generous gift from Dr. Daniel D. Carson, University of Delaware, DE, and used to detect MUC-1. CT-1 was the antibody of choice since it is not species specific nor affected by the glycosylation state of MUC-1 and recognizes all MUC-1 isoforms except the secreted form lacking the cytoplasmic tail. Immunostaining was performed using Zymed kit for rabbit primary antibody. Sections were lightly counterstained with hematoxylin. Arrows indicate apical MUC-1 staining. bl, blastocyst; ge, glandular epithelium; le, luminal epithelium; s, stroma.
Morphological Types of Implantation
Based on the type of blastocyst-uterine interaction, implantation is classified as eccentric in rats, mice and hamsters where blastocysts are small and become lodged in a uterine fold [24]. Initiation of implantation is generally considered a three-phase process that includes blastocyst apposition, adhesion and trophoblast penetration. While the majority of uterine epithelial cells remain normal and intact until invasion by the trophoblast in hamsters, autolysis of uterine epithelial cells precede the invasion of the trophoblast in mice [24]. Ultrastructural studies examining implantation in mice and rats indicated that some epithelial cells become dissociated from their underlying basal lamina before trophoblast penetration. The hamster differs from mice and rats in that trophoblast processes penetrate directly into epithelial cells, phagocytosing portions of these cells. Thus, it appears that trophoblast of hamsters is more invasive than that of mouse and rat [25]. The formation of microfilaments along the apical surface of the trophoblast helps to form the trophoblastic processes which extend, invade and engulf the epithelium.
Factors involved in implantation
Molecular mediators that govern the implantation process operate within a complex cellular and hormonal milieu. Factors that are expressed within a narrow temporal window are spatially restricted to the implantation site, and are either hormone- or embryo-responsive suggesting their involvement in implantation. In addition, the ability to pharmacologically or genetically perturb their function has led to the identification of genes and pathways that are critical for various implantation events. Emerging information on factors that regulate implantation in the hamster has begun to shed light on the value of this model for implantation studies.
Cytokines
The diverse functions of cytokine family members in proliferation, differentiation, inflammation and immunomodulation imply a potential contribution to uterine preparation, implantation or decidualization. Numerous human and animal studies support roles for interleukin-1 (IL-1), IL-6, IL-15, SOCS3, TNFα, Lif, GM-CSF and CSF-1 in uterine preparation, implantation or decidualization [29;30]. Although gene deletion studies in mice fail to illicit an implantation-specific phenotype for many of these factors, the possibility of redundancy or compensatory function by other family members may mask their contribution. However, signaling of two members of the IL-6 family, Lif and IL-11, appear to be critical for implantation and decidualization [31;32]. Lif is implicated in uterine cyclicity, endometrial function, and the human implantation process and has similar roles in other species. However, the mechanism and definitive site of Lif actions in the uterus remains elusive. Lif mRNA and protein expression in hamsters is variable during days 1-6 of pregnancy [33]. Our unpublished results suggest that Lif expression is significantly higher on days 1 and 4 of pregnancy. On day 4, Lif is mainly localized in glandular epithelial cells, although low levels were also observed in luminal epithelial cells. While Lif-r expression is only observed in luminal epithelium, gp130 expression was observed in both the luminal and glandular epithelium on day 4 of pregnancy. As the implantation process proceeds from days 5 to 8 of pregnancy, Lif expression ceased, but Lif-r and gp130 extended to decidual cells. We also observed that while Lif was induced by estrogen, Lif-r and gp130 were induced by P4 in the ovariectomized hamster uterus [34]. Lif enhances human embryo development [35] and hamster blastocyst hatching in vitro [33]. In addition, we have shown that an intraluminal injection of Lif antibody significantly reduces blastocyst implantation sites in hamsters. Taken together, these studies suggest that Lif signaling is also important for blastocyst hatching and implantation in hamsters.
Growth factors
Cell-specific alterations in differentiation, proliferation, and cell turnover are mediated in part by growth factors acting through endocrine, paracrine, or autocrine mechanisms. The knockout phenotype of members of the TGF-β superfamily and their associated intracellular signaling molecules emphasize the importance of this family in decidualization, placentation, and host immune response. In this regard, a recent study showed that mice with conditional ablation of BMP2 from the uterus are infertile due to failure of stromal cell decidualization [36]. However, inhibin-induced inhibition of implantation is attributed to inhibition of estrogen or P4 production in mice and hamsters, respectively [37;38]. The insulin-like family of growth factors (IGFs) and their binding proteins are also involved in implantation but may be more important for decidualization and placentation [39], and have significant impact on the establishment of pregnancy and feto-placental growth in women, non-human primates, domestic animals, and most laboratory species [40].
Members of the EGF family of growth factors also play a significant role in embryo-uterine signaling during implantation. In mice, TGFα, HB-EGF, amphiregulin (Ar), betacellulin (BTC), epiregulin (Er), and several neuregulins are expressed in the peri-implantation uterus and regulated by ovarian hormones [41]. The most compelling evidence for implantation-specific roles of the EGF family comes from HB-EGF. HB-EGF is expressed in the mouse uterus prior to the onset of embryo attachment in response to an undefined embryonic stimulus [42]. Soluble HB-EGF, in turn, stimulates proliferation, zona-hatching, trophoblast outgrowth and phosphorylation of target receptors in the blastocyst of mice, human and hamsters [33;42;43]. HB-EGF is expressed in human endometrium during the peri-implantation period [44;45] and fluctuates in response to cycle stage and ovarian steroids [46;47]. HB-EGF is also expressed on the surface of pinopodes and is able to stimulate other biomarkers of uterine receptivity in women [47;48]. The actions of HB-EGF are mediated by erbB1 and erbB4, which are expressed in both the uterus and embryo [42;49-53]. A recent study also showed that HB-EGF may improve implantation by stimulating expression of integrin αvβ3 in preimplantation mouse embryos [54]. In hamsters, HB-EGF is produced by both the blastocyst and uterus and its expression is not observed in uterine decidual cells after initiation of implantation [33;53;55]. Expression of amphiregulin, on the other hand, is quite different in hamsters and mice. It is not expressed in the uterine epithelium around the time of implantation and is also not regulated by P4. Instead, it is primarily expressed in decidual cells adjacent to the implanted embryo from day 5 to day 8 of pregnancy [56]. While HB-EGF induces stromal cell proliferation and differentiation in mice [57], amphiregulin induces stromal cell proliferation in hamsters [56]. Taken together, it appears that HB-EGF and amphiregulin have species-specific actions in hamsters, and that a better understanding of these differences may give broader insight into the roles these growth factors play in the implantation process.
Vasoactive mediators
The presence of increased vascular permeability at the site of implantation [58] is a common feature among diverse species [59]. Numerous vasoactive substances are present at the implantation site, including platelet activating factor (PAF), various kinins, histamine, vascular endothelial growth factor (VEGF), angiopoeitins, nitric oxide, prostaglandins and other eicosanoids, as well as direct hormone-induced changes in vascular architecture [60]. Histamine and prostaglandins have long been considered as important mediators of embryo-uterine interactions. Normal implantation in mast cell deficient mice [61] and virtual absence of mast cells from the mouse endometrium and deciduum [62] argue against the role of mast cell-derived histamine in implantation. Histidine decarboxylase (HDC) is the rate-limiting enzyme in histamine formation and it is a P4-responsive epithelial gene in the mouse uterus [63]. In contrast to mice, the expression of HDC in hamster uterus occurs only on day 4 of pregnancy in the glandular epithelium. Its expression in the hamster uterus is not regulated by P4 or estrogen. Furthermore, its expression in the day 4 uterus requires the presence of embryos, suggesting influence of the embryo in uterine preparation for implantation [56]. It is possible that local uterine histamine may be required for uterine relaxation [64] in hamsters to provide ample time and uterine surface for blastocyst-uterine interaction and attachment for implantation.
Prostaglandins are proinflammatory mediators derived from membrane-associated lipid precursors via phospholipase (cPLA2) and the Cox-1 and Cox-2 enzymes. Impaired fertility following treatment with inhibitors of prostaglandin synthesis first suggested the importance of prostaglandins during implantation in multiple species including hamsters [65-68]. Deletion of cPLA2 alters the timing and orderly spacing of implantation, whereas Cox-2 deletion results in impaired ovulation, fertilization, implantation, and decidualization [69;70]. Evidence indicates that Cox-2-derived prostacyclin (PGI2) is important for implantation in mice [71]. Cox-1 deleted mice have no implantation problem, although Cox-1 assists in uterine preparation for implantation, and Cox-1 and Cox-2 can compensate for the absence of the opposite isoform to sustain the implantation process [72;73]. Other lipid-derived vasoactive mediators, including lipoxygenase products [74;75] and lysophospholipids [76;77] appear to have significant roles in implantation. Both the Cox-1 and Cox-2 enzymes are expressed in the hamster preimplantation uterus in a pattern similar to mice [68]. A reduction of Cox-1 expression from uterine epithelial cells and a gradual induction of Cox-2 expression exclusively in luminal epithelium and adjacent decidual cells occurred at the implantation site of the hamsters. Most interestingly, however, Cox-2 expression is mainly observed in decidual cells immediately surrounding the implantation chamber on day 6. In contrast to mice, PGE2, but not PGI2, is the preferential product of cyclooxygenases in hamsters [68]. Because PGE2 is a potent inflammatory prostaglandin, our finding suggests that this lipid mediator is involved in local implantation-associated changes in the uterus at the site of implantation in hamsters.
Morphogens and developmental patterning genes
Efforts to better understand the implantation process led to the identification of genes with recognized roles in early embryo development and body plan organization, including the HOX genes, a family of developmental stage-specific transcription factors. Mice lacking Hoxa10 have altered segmental identity of the utero-tubal region, implantation and decidualization failure, and impaired endometrial responses to P4 [78-81]. HOXA11 null mice are also infertile, with reduced endometrial development and diminished LIF production [82;83]. Human studies reveal similarly important functions for HOXA10 and HOXA11 in embryo development, uterine differentiation, and fertility [84]. Wnt genes including Wnt2, Wnt4, Wnt5a, Wnt7a and others, also appear to have important functions in the implantation process across species [85-88].
Among several of the Hedgehog (hh) family of morphogens (Sonic hh, Desert hh, and Indian hh), Ihh is the sole member expressed in the peri-implantation mouse uterus [89;90], and is regulated by P4 or the presence of blastocysts or growth factor-saturated beads [90;91]. Global deletion of Ihh results in mice with dwarfism and perinatal lethality, whereas mice with conditional Ihh deletion are infertile due to implantation and decidualization failure [92]. Similarly, conditional deletion of COUP-TFII, an epithelial Ihh-regulated gene, was found to cause implantation failure [93]. These findings suggest that P4 action via induction of epithelial Ihh production plays an essential role in induction of implantation in mice. Ihh is also a P4-regulated gene in hamsters that is critical for uterine preparation for implantation. Its expression during early pregnancy closely follows the pattern observed in mice [56].
Hamsters as preferred models for implantation studies
The Syrian or golden hamster, Mesocricetus auratus, was once widely used in animal research because of their tolerance to tissue transplants as well as human tumors, viruses and bacteria. Although the relative use of hamsters in research has declined over the years, they are uniquely suited for reproductive studies. Female hamsters are polyestrous, and their sexual maturity begins around 4 weeks of age [94]. In mice, both uterine horns open in a common vagina [95], but a dual vagina is present in rats, rabbits, guinea pigs and hamsters [96]. While vaginal cytology shows regular 4 day estrous cycles (estrus, metestrous, diestrus and proestrous) in hamsters, the average individual cycle length in mice lasts 4-5 days, but this is highly variable and easily influenced by environment [97]. In hamsters, the day of estrus is easily predictable by external copious vaginal discharge that is not observed in mice. In addition, a female’s receptivity for mating in laboratory mice is not limited to estrous but may occasionally take place during proestrous or metestrous. Among all eutherian mammals, the gestation period of golden hamsters lasts only 16 days [98] while in mice it lasts for 19-21 days [97]. Ovulation time is highly predictable in hamsters and the female shows rapid development and regression of corpora lutea during a single cycle, unlike the mouse and rat where there is retention of several sets of corpora lutea from previous cycles [99]. Like mice, superovulation can be performed in hamsters by exogenous gonadotrophins. Although preimplantation embryos of mice, humans and hamsters can be cultured in vitro, hamster embryos are extremely sensitive to culture conditions [33]. Thus, hamster embryos can be used to test media components to improve the quality of the blastocyst. Early cleavage of hamster embryos in vivo is very similar to mice but hamster embryos enter into the uterus at the 8-cell stage on day 3 of pregnancy approximately 60 h after fertilization [53], while in mice, embryos enter into the uterus at the late morula and early blastocyst stage early on the morning of day 4 approximately 72 h after fertilization [2]. Although blastocyst formation mostly occurs inside the uterus in all mammals, unlike mice where blastocysts undergo expansion before implantation, blastocysts of hamsters do not undergo expansion in vivo [5]. While in hamsters initiation of implantation begins around late morning of day 4 [100], implantation begins in mice around midnight of day 4 or early morning on day 5 [42]. In addition, early implanted embryos cannot be flushed out from the uterus of hamsters suggesting firm trophoblast attachment and invasion compared to those of mice and rats. Pseudopregnant and pregnant hamsters also undergo stromal cell decidualization in response to artificial stimuli or implanting blastocysts like mice and rats [101]. Finally, hamsters might be considered as the species of choice for early pregnancy research considering the fact that implantation in this species is absolutely dependent on ovarian P4, but not estrogen, similar to rabbits, guinea pigs, agricultural animals, primates, and possibly humans. Thus, important new information might be generated studying the mechanisms of embryo development and implantation using the hamster model.
Where do we go from here?
Finding the means to bridge and extrapolate data from animal implantation models to the setting of human implantation is a daunting challenge. Implantation is not only a multiphase but also a species-specific event. Use of morphological, pharmacological, biochemical and molecular techniques has enabled us to gain greater understanding of the signaling pathways involved in implantation. However, multiple redundant mechanisms exist to ensure the success of pregnancy. Moreover, studies in various animal models suggest that common pathways may be involved between divergent species. Thus, the importance of a molecule might only be established by identifying its role in several species. Although animal models have their own limitations, they represent an important and indispensable tool to derive a better understanding of the underlying molecular and genetic mechanisms of implantation. Furthermore, comparative studies can help to answer a variety of questions about species specificity of the signals, the components of signaling systems and the differing functions that the communication system may have in the establishment of pregnancy.
Acknowledgements
B. C. Paria is supported by National Institutes of Health (NIH) grant HD 044741 and UO1 HD042636. We are grateful to Dr. Qian Zhang for Muc1 immunostaining.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Trejo CA, Navarro MC, Ambriz GD, Rosado A. Effect of maternal age and parity on preimplantation embryo development and transport in the golden hamster (Mesocricetus auratus) Lab Anim. 2005;39:290–297. doi: 10.1258/0023677054306999. [DOI] [PubMed] [Google Scholar]
- [2].Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the mouse embryo. Cold Spring Harbor Laboratory Press; New York: 2003. [Google Scholar]
- [3].Vestweber D, Kemler R. Identification of a putative cell adhesion domain of uvomorulin. EMBO J. 1985;4:3393–3398. doi: 10.1002/j.1460-2075.1985.tb04095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sheth B, Fesenko I, Collins JE, Moran B, Wild AE, Anderson JM, Fleming TP. Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha+ isoform. Development. 1997;124:2027–2037. doi: 10.1242/dev.124.10.2027. [DOI] [PubMed] [Google Scholar]
- [5].Gonzales DS, Bavister BD. Zona pellucida escape by hamster blastocysts in vitro is delayed and morphologically different compared with zona escape in vivo. Biol Reprod. 1995;52:470–480. doi: 10.1095/biolreprod52.2.470. [DOI] [PubMed] [Google Scholar]
- [6].Parkening TA. An ultrastructural study of implantation in the golden hamster. I. Loss of the zona pellucida and initial attachment to the uterine epithelium. J Anat. 1976;121:161–184. [PMC free article] [PubMed] [Google Scholar]
- [7].Mishra A, Seshagiri PB. Evidence for the involvement of a species-specific embryonic protease in zona escape of hamster blastocysts. Mol Hum Reprod. 2000;6:1005–1012. doi: 10.1093/molehr/6.11.1005. [DOI] [PubMed] [Google Scholar]
- [8].Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McCormack JT, Greenwald GS. Evidence for a preimplantation rise in oestradiol-17beta levels on day 4 of pregnancy in the mouse. J Reprod Fertil. 1974;41:297–301. doi: 10.1530/jrf.0.0410297. [DOI] [PubMed] [Google Scholar]
- [10].Psychoyos A. Nidation in the rat and the necessary dose of estrogen. C R Hebd Seances Acad Sci. 1961;253:1616–1617. [PubMed] [Google Scholar]
- [11].Orsini MW, Meyer RK. Effect of varying doses of progesterone on implantation in the ovariectomized hamster. 110 ed. 1962. pp. 713–715. [Google Scholar]
- [12].Harper MJ, Dowd D, Elliott AS. Implantation and embryonic development in the ovariectomized-adrenalectomized hamster. Biol Reprod. 1969;1:253–257. doi: 10.1095/biolreprod1.3.253. [DOI] [PubMed] [Google Scholar]
- [13].Greenwald GS. Endocrinology of the pregnant hamster. Plenum Publishing Corp; NY: 1985. pp. 53–72. [Google Scholar]
- [14].Deanesly R. Normal implantation in ovariectomized guinea pigs. Nature. 1960;186:327–328. doi: 10.1038/186327b0. [DOI] [PubMed] [Google Scholar]
- [15].George FW, Wilson JD. Estrogen formation in the early rabbit embryo. Science. 1978;199:200–201. doi: 10.1126/science.579477. [DOI] [PubMed] [Google Scholar]
- [16].Perry JS, Heap RB, Amoroso EC. Steroid hormone production by pig blastocysts. Nature. 1973;245:45–47. doi: 10.1038/245045a0. [DOI] [PubMed] [Google Scholar]
- [17].Ghosh D, De P, Sengupta J. Luteal phase ovarian oestrogen is not essential for implantation and maintenance of pregnancy from surrogate embryo transfer in the rhesus monkey. Hum Reprod. 1994;9:629–637. doi: 10.1093/oxfordjournals.humrep.a138561. [DOI] [PubMed] [Google Scholar]
- [18].Zegers-Hochschild F, Altieri E. Luteal estrogen is not required for the establishment of pregnancy in the human. J Assist Reprod Genet. 1995;12:224–228. doi: 10.1007/BF02211803. [DOI] [PubMed] [Google Scholar]
- [19].Sholl SA, Orsini MW, Hitchins DJ. Estrogen synthesis and metabolism in the hamster blastocyst, uterus and liver near the time of implantation. J Steroid Biochem. 1983;19:1153–1161. doi: 10.1016/0022-4731(83)90410-7. [DOI] [PubMed] [Google Scholar]
- [20].Edgar DH, James GB, Mills JA. Steroid secretion by human early embryos in culture. Hum Reprod. 1993;8:277–278. doi: 10.1093/oxfordjournals.humrep.a138037. [DOI] [PubMed] [Google Scholar]
- [21].Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shaikh AA, Birchall K, Saksena SK. Steroids in the ovarian venous plasma and F prostaglandins in the peripheral plasma during pseudopregnancy and days 1-9 of pregnancy in the golden hamster. Prostaglandins. 1973;4:17–30. doi: 10.1016/0090-6980(73)90052-x. [DOI] [PubMed] [Google Scholar]
- [23].Nikas G. Endometrial receptivity: changes in cell-surface morphology. Semin Reprod Med. 2000;18:229–235. doi: 10.1055/s-2000-12561. [DOI] [PubMed] [Google Scholar]
- [24].Schlafke S, Enders AC. Cellular basis of interaction between trophoblast and uterus at implantation. Biol Reprod. 1975;12:41–65. doi: 10.1095/biolreprod12.1.41. [DOI] [PubMed] [Google Scholar]
- [25].Parkening TA. An ultrastructural study of implantation in the golden hamster. II. Trophoblastic invasion and removal of the uterine epithelium. J Anat. 1976;122:211–230. [PMC free article] [PubMed] [Google Scholar]
- [26].Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- [27].Aplin JD, Hey NA, Graham RA. Human endometrial MUC1 carries keratan sulfate: characteristic glycoforms in the luminal epithelium at receptivity. Glycobiology. 1998;8:269–276. doi: 10.1093/glycob/8.3.269. [DOI] [PubMed] [Google Scholar]
- [28].Thathiah A, Carson DD. MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem J. 2004;382:363–373. doi: 10.1042/BJ20040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dimitriadis E, Stoikos C, Stafford-Bell M, Clark I, Paiva P, Kovacs G, Salamonsen LA. Interleukin-11, IL-11 receptoralpha and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. J Reprod Immunol. 2006;69:53–64. doi: 10.1016/j.jri.2005.07.004. [DOI] [PubMed] [Google Scholar]
- [30].Makrigiannakis A, Minas V, Kalantaridou SN, Nikas G, Chrousos GP. Hormonal and cytokine regulation of early implantation. Trends Endocrinol Metab. 2006;17:178–185. doi: 10.1016/j.tem.2006.05.001. [DOI] [PubMed] [Google Scholar]
- [31].Charnock-Jones DS, Sharkey AM, Fenwick P, Smith SK. Leukaemia inhibitory factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at this time. J Reprod Fertil. 1994;101:421–426. doi: 10.1530/jrf.0.1010421. [DOI] [PubMed] [Google Scholar]
- [32].Stewart CL. The role of leukemia inhibitory factor (LIF) and other cytokines in regulating implantation in mammals. Ann N Y Acad Sci. 1994;734:157–165. doi: 10.1111/j.1749-6632.1994.tb21743.x. [DOI] [PubMed] [Google Scholar]
- [33].Seshagiri PB, Mishra A, Ramesh G, Rao RP. Regulation of peri-attachment embryo development in the golden hamster: role of growth factors. J Reprod Immunol. 2002;53:203–213. doi: 10.1016/s0165-0378(01)00086-9. [DOI] [PubMed] [Google Scholar]
- [34].Ding T, Song H, Wang X, Khatua A, Paria BC. Leukemia inhibitory factor ligand-receptor signaling is important for uterine receptivity and implantation in golden hamsters (Mesocricetus auratus) Reproduction. 2007;135:1–14. doi: 10.1530/REP-07-0013. [DOI] [PubMed] [Google Scholar]
- [35].Dunglison GF, Barlow DH, Sargent IL. Leukaemia inhibitory factor significantly enhances the blastocyst formation rates of human embryos cultured in serum-free medium. Hum Reprod. 1996;11:191–196. doi: 10.1093/oxfordjournals.humrep.a019016. [DOI] [PubMed] [Google Scholar]
- [36].Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nandedkar TD, Vijayalashmi S, Sarvamangala BS, Moodbidri SB, Munshi SR, Sheth AR. Effect of inhibin on ovulation and implantation in mice. Contraception. 1981;24:695–703. doi: 10.1016/0010-7824(81)90020-2. [DOI] [PubMed] [Google Scholar]
- [38].Bapat BV, Nandedkar TD, Sheth AR. Reversal of the anti-implantation effect of inhibin with progesterone in the hamster. Int J Fertil. 1986;31:71–76. [PubMed] [Google Scholar]
- [39].Zhou J, Dsupin BA, Giudice LC, Bondy CA. Insulin-like growth factor system gene expression in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1994;79:1723–1734. doi: 10.1210/jcem.79.6.7527408. [DOI] [PubMed] [Google Scholar]
- [40].Lathi RB, Hess AP, Tulac S, Nayak NR, Conti M, Giudice LC. Dose-dependent insulin regulation of insulin-like growth factor binding protein-1 in human endometrial stromal cells is mediated by distinct signaling pathways. J Clin Endocrinol Metab. 2005;90:1599–1606. doi: 10.1210/jc.2004-1676. [DOI] [PubMed] [Google Scholar]
- [41].Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- [42].Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- [43].Martin KL, Barlow DH, Sargent IL. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum Reprod. 1998;13:1645–1652. doi: 10.1093/humrep/13.6.1645. [DOI] [PubMed] [Google Scholar]
- [44].Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet. 1997;21:102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [45].Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84:3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- [46].Chobotova K, Muchmore ME, Carver J, Yoo HJ, Manek S, Gullick WJ, Barlow DH, Mardon HJ. The mitogenic potential of heparin-binding epidermal growth factor in the human endometrium is mediated by the epidermal growth factor receptor and is modulated by tumor necrosis factor-alpha. J Clin Endocrinol Metab. 2002;87:5769–5777. doi: 10.1210/jc.2002-020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J. Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev. 2002;62:446–455. doi: 10.1002/mrd.10129. [DOI] [PubMed] [Google Scholar]
- [48].Stavreus-Evers A, Aghajanova L, Brismar H, Eriksson H, Landgren BM, Hovatta O. Co-existence of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol Hum Reprod. 2002;8:765–769. doi: 10.1093/molehr/8.8.765. [DOI] [PubMed] [Google Scholar]
- [49].Das SK, Tsukamura H, Paria BC, Andrews GK, Dey SK. Differential expression of epidermal growth factor receptor (EGF-R) gene and regulation of EGF-R bioactivity by progesterone and estrogen in the adult mouse uterus. Endocrinology. 1994;134:971–981. doi: 10.1210/endo.134.2.7507841. [DOI] [PubMed] [Google Scholar]
- [50].Paria BC, Tsukamura H, Dey SK. Epidermal growth factor-specific protein tyrosine phosphorylation in preimplantation embryo development. Biol Reprod. 1991;45:711–718. doi: 10.1095/biolreprod45.5.711. [DOI] [PubMed] [Google Scholar]
- [51].Paria BC, Das SK, Andrews GK, Dey SK. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation. Proc Natl Acad Sci U S A. 1993;90:55–59. doi: 10.1073/pnas.90.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 1996;122:637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- [53].Wang X, Wang H, Matsumoto H, Roy SK, Das SK, Paria BC. Dual source and target of heparin-binding EGF-like growth factor during the onset of implantation in the hamster. Development. 2002;129:4125–4134. doi: 10.1242/dev.129.17.4125. [DOI] [PubMed] [Google Scholar]
- [54].Lim JJ, Lee DR, Song HS, Kim KS, Yoon TK, Gye MC, Kim MK. Heparin-binding epidermal growth factor (HB-EGF) may improve embryonic development and implantation by increasing vitronectin receptor (integrin alphanubeta3) expression in peri-implantation mouse embryos. J Assist Reprod Genet. 2006;23:111–119. doi: 10.1007/s10815-006-9021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mishra A, Seshagiri PB. Heparin binding-epidermal growth factor improves blastocyst hatching and trophoblast outgrowth in the golden hamster. Reprod Biomed Online. 2000;1:87–95. doi: 10.1016/s1472-6483(10)61945-1. [DOI] [PubMed] [Google Scholar]
- [56].Khatua A, Wang X, Ding T, Zhang Q, Reese J, DeMayo FJ, Paria BC. Indian hedgehog, but not histidine decarboxylase or amphiregulin, is a progesterone-regulated uterine gene in hamsters. Endocrinology. 2006;147:4079–4092. doi: 10.1210/en.2006-0231. [DOI] [PubMed] [Google Scholar]
- [57].Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol. 2004;265:181–195. doi: 10.1016/j.ydbio.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Psychoyos A. The decidual reaction is preceded by early changes in the capillary permeability of the uterus. C R Seances Soc Biol Fil. 1960;154:1384–1387. [PubMed] [Google Scholar]
- [59].Pakrasi PL, Tiwari A. Evidence of increased endometrial vascular permeability at the time of implantation in the short-nosed fruit bat, Cyanopterus sphinx. Anim Reprod Sci. 2007;101:179–185. doi: 10.1016/j.anireprosci.2006.11.017. [DOI] [PubMed] [Google Scholar]
- [60].Rogers PA. Early endometrial microvascular response during implantation in the rat. Reprod Fertil Dev. 1992;4:261–264. doi: 10.1071/rd9920261. [DOI] [PubMed] [Google Scholar]
- [61].Wordinger RJ, Jackson FL, Morrill A. Implantation, deciduoma formation and live births in mast cell-deficient mice (W/Wv) J Reprod Fertil. 1986;77:471–476. doi: 10.1530/jrf.0.0770471. [DOI] [PubMed] [Google Scholar]
- [62].Salamonsen LA, Jeziorska M, Newlands GF, Dey SK, Woolley DE. Evidence against a significant role for mast cells in blastocyst implantation in the rat and mouse. Reprod Fertil Dev. 1996;8:1157–1164. doi: 10.1071/rd9961157. [DOI] [PubMed] [Google Scholar]
- [63].Paria BC, Das N, Das SK, Zhao X, Dileepan KN, Dey SK. Histidine decarboxylase gene in the mouse uterus is regulated by progesterone and correlates with uterine differentiation for blastocyst implantation. Endocrinology. 1998;139:3958–3966. doi: 10.1210/endo.139.9.6173. [DOI] [PubMed] [Google Scholar]
- [64].Patnaik GK, Dhawan BN. Histamine H2 receptor mediated relaxation of hamster (Mesocricetus auratus) uterus. Indian J Exp Biol. 1991;29:78–79. [PubMed] [Google Scholar]
- [65].Terranova PF, Dey SA. Indomethacin delays zona-shedding and implantation in the ovariectomized progesterone-treated hamster. Prostaglandins. 1982;24:165–172. doi: 10.1016/0090-6980(82)90142-3. [DOI] [PubMed] [Google Scholar]
- [66].Evans CA, Kennedy TG. The importance of prostaglandin synthesis for the initiation of blastocyst implantation in the hamster. J Reprod Fertil. 1978;54:255–261. doi: 10.1530/jrf.0.0540255. [DOI] [PubMed] [Google Scholar]
- [67].Diao HL, Zhu H, Ma H, Tan HN, Cong J, Su RW, Yang ZM. Rat ovulation, implantation and decidualization are severely compromised by COX-2 inhibitors. Front Biosci. 2007;12:3333–3342. doi: 10.2741/2316. [DOI] [PubMed] [Google Scholar]
- [68].Wang X, Su Y, Deb K, Raposo M, Morrow JD, Reese J, Paria BC. Prostaglandin E2 is a product of induced prostaglandin-endoperoxide synthase 2 and microsomal-type prostaglandin E synthase at the implantation site of the hamster. J Biol Chem. 2004;279:30579–30587. doi: 10.1074/jbc.M400573200. [DOI] [PubMed] [Google Scholar]
- [69].Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- [70].Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, Bonventre JV, Dey SK. Cytosolic phospholipase A2alpha is crucial [correction of A2alpha deficiency is crucial] for ‘on-time’ embryo implantation that directs subsequent development. Development. 2002;129:2879–2889. doi: 10.1242/dev.129.12.2879. [DOI] [PubMed] [Google Scholar]
- [71].Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Reese J, Brown N, Paria BC, Morrow J, Dey SK. COX-2 compensation in the uterus of COX-1 deficient mice during the pre-implantation period. Mol Cell Endocrinol. 1999;150:23–31. doi: 10.1016/s0303-7207(99)00033-7. [DOI] [PubMed] [Google Scholar]
- [73].Wang H, Ma WG, Tejada L, Zhang H, Morrow JD, Das SK, Dey SK. Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeup. J Biol Chem. 2004;279:10649–10658. doi: 10.1074/jbc.M312203200. [DOI] [PubMed] [Google Scholar]
- [74].Pakrasi PL, Becka R, Dey SK. Cyclooxygenase and lipoxygenase pathways in the preimplantation rabbit uterus and blastocyst. Prostaglandins. 1985;29:481–495. doi: 10.1016/0090-6980(85)90106-6. [DOI] [PubMed] [Google Scholar]
- [75].Li Q, Cheon YP, Kannan A, Shanker S, Bagchi IC, Bagchi MK. A novel pathway involving progesterone receptor, 12/15-lipoxygenase-derived eicosanoids, and peroxisome proliferator-activated receptor gamma regulates implantation in mice. J Biol Chem. 2004;279:11570–11581. doi: 10.1074/jbc.M311773200. [DOI] [PubMed] [Google Scholar]
- [76].Liu Z, Armant DR. Lysophosphatidic acid regulates murine blastocyst development by transactivation of receptors for heparin-binding EGF-like growth factor. Exp Cell Res. 2004;296:317–326. doi: 10.1016/j.yexcr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- [77].Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13:1005–1017. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- [79].Satokata I, Uchiyama M. Mice carrying null mutations of the homeotic genes Hoxa10 and Msx1. Tanpakushitsu Kakusan Koso. 1995;40:2134–2143. [PubMed] [Google Scholar]
- [80].Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- [81].Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- [82].Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K, Potter SS. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995;121:1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- [83].Gendron RL, Paradis H, Hsieh-Li HM, Lee DW, Potter SS, Markoff E. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in Hoxa-11 null mice. Biol Reprod. 1997;56:1097–1105. doi: 10.1095/biolreprod56.5.1097. [DOI] [PubMed] [Google Scholar]
- [84].Daftary GS, Taylor HS. Implantation in the human: the role of HOX genes. Semin Reprod Med. 2000;18:311–320. doi: 10.1055/s-2000-12568. [DOI] [PubMed] [Google Scholar]
- [85].Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- [86].Tulac S, Nayak NR, Kao LC, Van WM, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E, Giudice LC. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab. 2003;88:3860–3866. doi: 10.1210/jc.2003-030494. [DOI] [PubMed] [Google Scholar]
- [87].Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A. 2005;102:8579–8584. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hayashi K, Spencer TE. WNT pathways in the neonatal ovine uterus: potential specification of endometrial gland morphogenesis by SFRP2. Biol Reprod. 2006;74:721–733. doi: 10.1095/biolreprod.105.049718. [DOI] [PubMed] [Google Scholar]
- [89].Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci U S A. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol. 2002;245:280–290. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- [91].Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16:2338–2348. doi: 10.1210/me.2001-0154. [DOI] [PubMed] [Google Scholar]
- [92].Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- [93].Petit FG, Jamin SP, Kurihara I, Behringer RR, DeMayo FJ, Tsai MJ, Tsai SY. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci U S A. 2007;104:6293–6298. doi: 10.1073/pnas.0702039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Selley RM. Hamster sexually mature at twenty-eight days of age. Science. 1945;102:485–486. doi: 10.1126/science.102.2654.485. In. [DOI] [PubMed] [Google Scholar]
- [95].Leppi TJ. A study of the uterine cervix of the mouse. Anat Rec. 1964;150:51–65. doi: 10.1002/ar.1091500106. [DOI] [PubMed] [Google Scholar]
- [96].Ludwig TE, Lane M, Bavister BD. Differential effect of hexoses on hamster embryo development in culture. Biol Reprod. 2001;64:1366–1374. doi: 10.1095/biolreprod64.5.1366. [DOI] [PubMed] [Google Scholar]
- [97].Bronson FH, Dagg CP, Snell GD. Reproduction. In: Green EL, editor. Biology of the Laboratory Mouse. McGraw-Hill, Inc.; New York: 1966. pp. 187–204. [Google Scholar]
- [98].Czyba JC, Dams R, Laurent JL. Data on the normal evolution of gestation of golden hamsters (Mesocricetus auratus) in the laboratory. C R Seances Soc Biol Fil. 1970;164:2267–2269. [PubMed] [Google Scholar]
- [99].Bivin Ws, Olsen GH, Murray KA. Morphophysiology. In: Van Hoosier GL, McPherson CW, editors. Laboratory Hamsters. Academic Press, Inc; New York: 1987. pp. 9–41. [Google Scholar]
- [100].Orsini MW. Study of ovo-implantation in the hamster, rat, mouse, guinea-pig and rabbit in cleared uterine tracts. J Reprod Fertil. 1962;3:288–293. doi: 10.1530/jrf.0.0030288. [DOI] [PubMed] [Google Scholar]
- [101].Lukaszewska JH, Greenwald GS. Comparison of luteal function in pseudopregnant and pregnant hamsters. J Reprod Fertil. 1969;20:185–187. doi: 10.1530/jrf.0.0200185. [DOI] [PubMed] [Google Scholar]