Abstract
Highly chromogenic 18-crown-6-dipyrrolylquinoxaline coordinates primary amines of peptides, forming non-covalent complexes that can be transferred to the gas phase by electrospray ionization. The appended chromogenic crown ether facilitates efficient energy transfer to the peptide upon ultraviolet irradiation in the gas phase, resulting in diagnostic peptide fragmentation. Collisional activated dissociation (CAD) and infrared multiphoton dissociation (IRMPD) of these non-covalent complexes results only in their disassembly with the charge retained on either the peptide or crown ether, yielding no sequence ions. Upon UV photon absorption the intermolecular energy transfer is facilitated by the fast activation time scale of UVPD (< 10 ns) and by the collectively strong hydrogen bonding between the crown ether and peptide, thus allowing effective transfer of energy to the peptide moiety prior to disruption of the intermolecular hydrogen bonds.
Keywords: Electrospray ionization, UVPD, Peptide sequencing, Crown ethers, Ion activation
Introduction
The ongoing need for improved methods for characterizing biological molecules is driving efforts to develop new ion activation and dissociation approaches in mass spectrometry (MS) [1,2]. Currently, collisional activated dissociation (CAD) remains the most popular technique used to produce diagnostic fragment ions [3,4]. However, this MS/MS method is subject to a number of shortcomings such as insufficient or inefficient energy deposition. Accordingly, a number of other techniques, including surface induced dissociation (SID) [5], electron capture dissociation (ECD) [6,7], electron transfer dissociation (ETD) [8], and photodissociation (PD) [9–22] have been developed in recent years. Of these, photodissociation appears particularly attractive because it offers the possibility of tuning the level of energy deposition and providing high energy input without collisional scattering effects; it is also compatible with both time-of-flight and ion trapping platforms [9–22]. Unfortunately, as currently practiced photodissociation requires that the ions of interest absorb the specific wavelength to induce fragmentation. This is often problematic since many biological molecules display regions of low absorptivity over much of the UV/Vis spectral range. To overcome this deficiency, efforts have been made to append external chromophores to molecules of interest, and this is an approach that we [23,24] and others are exploring [25]. However, far more appealing and potentially more general is the idea of attaching chromophores via non-covalent interactions as an attractive alternative because of its simplicity and potential versatility. On the other hand, non-covalent interactions are relatively weak. This makes it a challenge to design chromogenic receptors that can not only bind to various target ions of interest but also allow energy transfer following photoexcitation. In this report we describe a simple first generation chromogenic molecule (18-crown-6-dipyrrolylquinoxaline, 1) [26] that binds non-covalently to peptides via hydrogen bonding interactions and allows effective energy transfer upon UV irradiation of the resulting complex in the gas phase.
Experimental
Mass Spectrometry
A Spectra-Physics GCR-11 Quanta-Ray Nd:YAG laser with a HG-2 harmonics generator (Mountain View, CA) was coupled to a Thermo Finnigan LCQ Deca XP (San Jose, CA) for UVPD experiments as previously described in more detail [24]. The vacuum manifold was modified to incorporate a CF viewport flange to hold an anti-reflective quartz window (CVI laser optics; Albuquerque, NM) to allow transmission of the laser beam through a 5 mm hole drilled into the ring electrode for irradiation of the ion cloud. The laser control unit was modified to integrate an operational amplifier to increase the TTL output from the mass spectrometer up to the necessary 15 V to gate the laser. The laser was operated at full energy with the triple harmonic at 355 nm produced approximately 60 mJ/pulse whereas the fourth harmonic at 266 nm produced approximately 30 mJ/pulse based upon manufacturer specifications. IRMPD was conducted on the same LCQ Deca XP instrument using a 48–5 Synrad 50W CO2 continuous wave laser (Mukilteo, WA) with a ZnSe window in place of the quartz window for IR transmission. A more detailed description has been previously disclosed [21]. 1:1 solution mixtures of compound 1 and lysine-containing peptides purchased from Bachem (King of Prussia, PA) were diluted in 50/50/2 H2O/MeOH/HoAc at 10 μM and infused at 3 μl/min with a Harvard Apparatus PhD 2000 syringe pump (Holliston, MA). A qz value of 0.10 was used for UVPD experiments to provide a significant reduction in the low-mass cut off for the retention and observation of low m/z fragment ions. The standard qz value of 0.25 was used for CAD experiments.
Results and Discussion
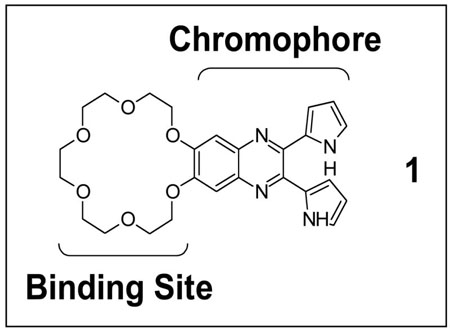
Primary amines in peptides, such as the N-terminus or basic amino acid side chains, afford appropriate hydrogen-bond donor sites suitable for coordination by crown ethers. The resulting stable non-covalent complexes can be transferred to the gas phase by electrospray ionization (ESI) for MS analysis [27–31]. Gas phase spectroscopic studies of small molecule/atom complexes have been studied with UV excitation [32]; however, the inherent complexation properties of crown ethers have not yet been exploited in the context of photodissociation and mass spectrometry. To achieve this latter goal, it is necessary to generate a chromophore-modified crown ether. One such modified crown ether is 18-crown-6-dipyrrolylquinoxaline (1), a potential ditopic receptor that we have recently synthesized [26]. In addition to a crown either moiety suitable for non-covalent complexation with the N-terminus or the lysine side chain of peptides, it contains a chromogenic dipyrrolylquinoxaline (DPQ) functionality which exhibits high absorptivity at both 355 nm and 266 nm, the third and fourth harmonics of the Nd:YAG laser that are useful for UVPD [26]. It is thus attractive as a potential non-covalent receptor for peptides because it might promote their photodissociation via a combination of recognition and UV/Vis photoexcitation/energy transfer.
UVPD of Peptide Crown Ether Complexes
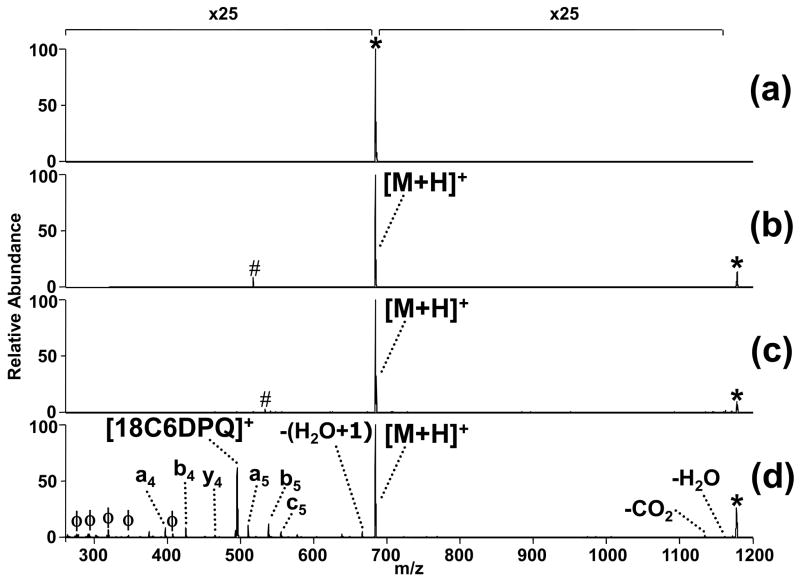
Solutions containing a Lys-containing peptide, YGGFLK at 10 μM, with and without 1, also at 10 μM, were analyzed by ESI-MS/MS. UVPD of the protonated peptide alone was evaluated first to provide a benchmark for the PD efficiency in the absence of the chromogenic crown ether. As shown in Figure 1a, protonated YGGFLK does not undergo any PD at 355 nm even after exposure to 20 laser pulses. This result indicates that the protonated peptide does not absorb at 355 nm in the gas phase. Collisional activation of the protonated 1:1 YGGFLK/1 complex (Figure 1b) yields only a single product ion, the protonated peptide via disassembly of the complex. Another low energy activation method, IRMPD, also causes disassembly of the non-covalent complex (Figure 1c). Figure 1d illustrates the 355 nm UVPD mass spectrum of the complex, with several diagnostic a, b and y ions evident that are comparable to ones produced upon CAD of the protonated peptide (CAD spectrum not shown), albeit at much lower abundance in the UVPD spectrum. This data suggests that not only is 1 responsible for UV photoabsorption and energy accumulation but also that a sufficiently large amount of internal energy is redistributed within the non-covalent complex to cause fragmentation of the peptide prior to disruption of the non-covalent intermolecular bonds that link the crown ether and peptide.
Figure 1.
ESI-product ion spectra of (a) singly protonated YGGFLK activated by 355 nm UV irradiation with 20 pulses at 10 Hz; and singly protonated (YGFFLK + 1) complex energized by (b) collisional activation (0.78 V at 30 ms); (c) IR irradiation [50 W, 2.5 ms]; and (d) 355 nm UV irradiation with 2 pulses at 10 Hz. Fragment ions of compound 1 are labeled with a phi (ϕ) symbol and noise peaks are denoted by a pound (#) symbol. The magnification scale applies to all spectra shown and the precursor ion is denoted with an asterisk (*). The peptide is represented by M.
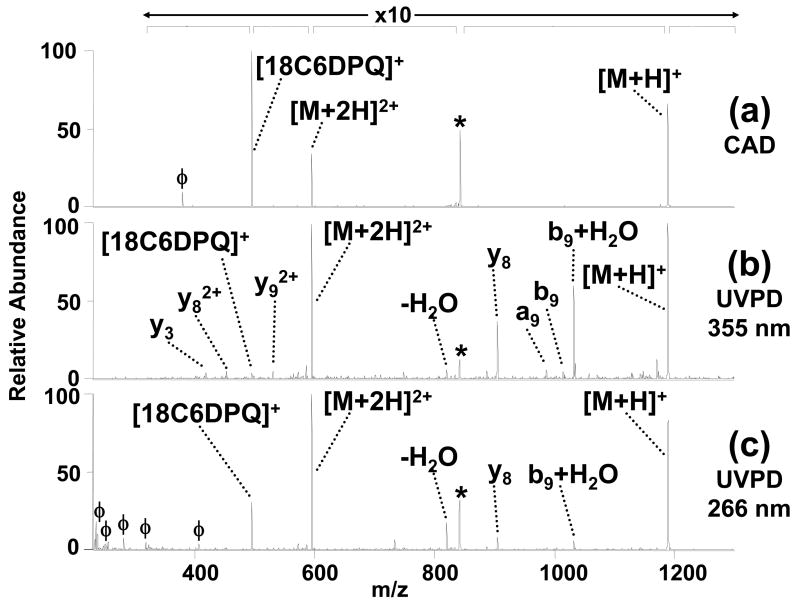
Another example of this intermolecular energy transfer through non-covalent bonds is shown for the complex containing another lys-containing peptide (KRPPFGSPFR) with 1. Upon collisional activation (Figure 2a), both the singly and doubly protonated 1:1 complexes simply disassemble, resulting in the protonated peptide and protonated crown ether partners or the doubly protonated peptide (and non-detectable neutral crown ether). In contrast, UV activation at 355 nm or 266 nm generates a variety of diagnostic sequence ions (Figures 2b and 2c). Interestingly, UV irradiation at 355 nm results in more efficient dissociation of the peptide than observed at 266 nm by nearly a factor of 10, in terms of the conversion efficiency of precursor complexes to diagnostic fragment ions. We speculate that this contrast in PD efficiency stems from a combination of three factors: 1) differences in absorption efficiency of compound 1 at 266 nm versus 355 nm in the gas phase, 2) varying extents of relaxation via fluorescence, and 3) lower laser power at 266 nm versus 355 nm. The first two factors are not directly supported based on the UV absorbance and excitation data of 1 in solution (Supplemental Figure S1) which indicates that the absorptivity of 1 is three times higher at 266 nm than 355 nm in methanol, as well as having a lower fluorescence yield at 266 nm; however, solution data is not necessarily a good predictor of gas-phase absorption/emission properties because of solvent effects and the charged nature of the complex in the gas phase. Our gas-phase UVPD data is consistent with a model wherein localized photoabsorption by the DPQ functionality is followed by energy transfer through the hydrogen bonds between the crown ether and peptide.
Figure 2.
ESI-product ion spectra of the [KRPPFGSPFR + 2H +1]2+ complex produced by (a) collisional activation [0.68V, 30 ms], (b) 355 nm UV irradiation [2 pulses at 60 mJ/pulse, 10 Hz] and (c) 266 nm UV irradiation [10 pulses at 30 mJ/pulse, 10 Hz]. The magnification scale bar applies to all three spectra over the indicated mass range. The precursor ion is represented with an asterisk (*) and characteristic fragments of 1 are labeled with a (ϕ). The peptide is represented by M.
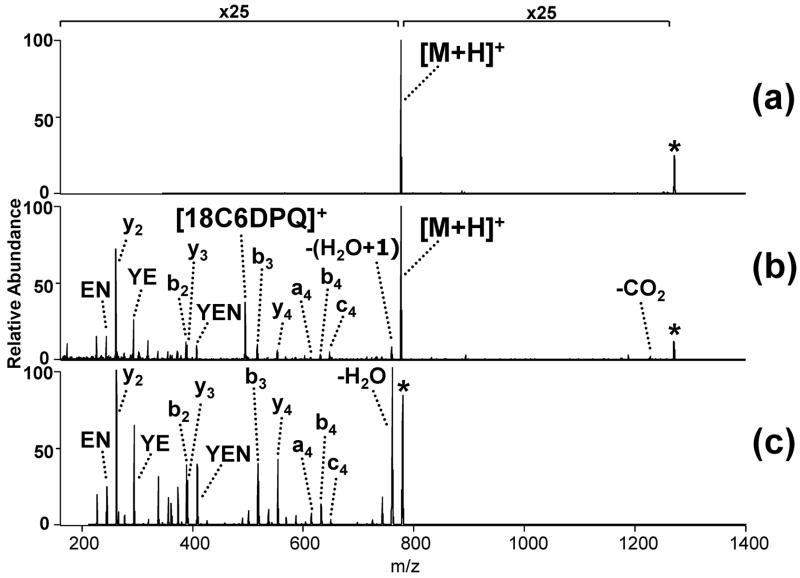
A third comparison illustrating UV-induced dissociation of these non-covalent complexes is shown in Figure 3 for the 1:1 complex containing pyroGlu-LYENK. As seen for other complexes, collisional activation solely results in disruption of the non-covalent interactions, yielding the protonated peptide as the only product ion (Figure 3a). UV irradiation at 355 nm produces an array of peptide b and y ions (Figure 3b). For comparison, a CAD spectrum of the singly charged peptide alone is shown in Figure 3c. The peptide fragment ions observed in Figure 3b are remarkably similar to those obtained in Figure 3c, suggesting comparable dissociation pathways are accessed by both activation methods and that vibrational energy transfer through the non-covalent complex mimics energization by CAD.
Figure 3.
ESI-product ion spectra of (a) the [pyroGlu-LYENK + H + 1]1+ complex produced by collisional activation [1.02 V, 30 ms], (b) the [pyroGlu-LYENK + H + 1]1+ complex produced by 355 nm UV irradiation [2 pulses at 60 mJ/pulse, 10 Hz] and (c) protonated pyro Glu-LYENK by collisional activation [1.35 V, 30 ms]. The magnification scale bar applies only to the top two spectra over the indicated mass range; the bottom spectrum (c) is not magnified. The peptide is represented by M.
The specific mechanism whereby energy transfer occurs from 1 to the peptide is difficult to decipher by mass spectrometry alone. We speculate that UV excitation of 1 is followed by energy redistribution through the intermolecular hydrogen bonds on a time scale faster than dissociation. This process is dependent on strong hydrogen bonds and is consistent with the multi-site coordination of the 18-crown-6 moiety with the protonated lysine side chain of the peptide. Additionally, the time scale of ion activation in CAD and IRMPD is on the order of milliseconds whereas energy accumulation in UVPD occurs in less than ten nanoseconds which could play a significant role in the observed peptide fragmentation. Second, the absorption of 355 nm (3.5 eV) and 266 nm (4.7 eV) photons may cause greater energy deposition compared to CAD and IRMPD which may in turn enhance energy transfer and fragmentation of the peptide backbone relative to disassembly of the complex.
Conclusions
The present study represents the first time a chromogenic receptor is used to initiate photodissociation of a non-covalent gas-phase complex and production of diagnostic fragment ions in the context of MS/MS analysis. Specifically, we have shown that the DPQ-functionalized crown ether (1) may be used to effect the UV-induced cleavage of covalent bonds in non-chromogenic peptide ions and concomitant generation of diagnostic sequence ions. Although the present first generation system results in only a modest degree of UVPD of the peptide, the possibility of enhancing the photoabsorption efficiency through the simple addition of a chromophoric complexation reagent to an analyte prior to ESI-MS is an approach that we think shows considerable promise and one that warrants further exploration and optimization through stronger complexation and energy transfer.
Supplementary Material
Acknowledgments
Funding from the Welch Foundation (F1155 and F1018), the National Science Foundation (CHE-0718320), an NSF/IGERT grant that supported JJW (D6E-03333080), and the NIH (GM58907) are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McLuckey S, Goeringer D. Slow heating methods in tandem mass spectrometry . J Mass Spectrom. 1997;32:461–474. [Google Scholar]
- 2.Sleno L, Volmer D. Ion activation methods for tandem mass spectrometry . J Mass Spectrom. 2004;39:1091–1112. doi: 10.1002/jms.703. [DOI] [PubMed] [Google Scholar]
- 3.Wells J, McLuckey S. Collision-induced dissociation (CID) of peptides and proteins. Method Enzymol. 2005;402:148–185. doi: 10.1016/S0076-6879(05)02005-7. [DOI] [PubMed] [Google Scholar]
- 4.Laskin J, Futrell J. Collisional activation of peptide ions in FT-ICR mass spectrometry . Mass Spectrom Rev. 2003;22:158–181. doi: 10.1002/mas.10041. [DOI] [PubMed] [Google Scholar]
- 5.Dongre A, Somogyi A, Wysocki V. Surface-induced dissociation: An effective tool to probe structure, energetics and fragmentation mechanisms of protonated peptides . J Mass Spectrom. 1996;31:339–350. doi: 10.1002/(SICI)1096-9888(199604)31:4<339::AID-JMS322>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Zubarev R. Electron-Capture Dissociation Tandem Mass Spectrometry . Curr Opin Biotechnol. 2004;15:12–16. doi: 10.1016/j.copbio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Cooper H, Hakansson K, Marshall A. The role of electron capture dissociation in biomolecular analysis . Mass Spectrom Rev. 2005;24:201–222. doi: 10.1002/mas.20014. [DOI] [PubMed] [Google Scholar]
- 8.Syka J, Coon J, Schroeder M, Shabanowitz J, Hunt D. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry . P Natl Acad Sci USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi K, Yoon S, Sun M, Oh J, Moon J, Kim M. Characteristics of photodissociation at 193 nm of singly protonated peptides generated by matrix-assisted laser desorption ionization (MALDI) . J Am Soc Mass Spectr. 2006;17:1643–1653. doi: 10.1016/j.jasms.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Flora J, Muddiman D. Gas-phase ion unimolecular dissociation for rapid phosphopeptide mapping by IRMPD in a penning ion trap: An energetically favored process . J Am Chem Soc. 2002;124:6546–6547. doi: 10.1021/ja0261170. [DOI] [PubMed] [Google Scholar]
- 11.Fung Y, Kjeldsen F, Silivra O, Chan T, Zubarev R. Facile disulfide bond cleavage in gaseous peptide and protein cations by ultraviolet photodissociation at 157 nm . Angew Chem Int Edit. 2005;44:6399–6403. doi: 10.1002/anie.200501533. [DOI] [PubMed] [Google Scholar]
- 12.Gabryelski W, Li L. Photo-induced dissociation of electrospray generated ions in an ion trap/time-of-flight mass spectrometer . Rev Sci Instrum. 1999;70:4192–4199. [Google Scholar]
- 13.Kim T, Thompson M, Reilly J. Peptide photodissociation at 157 nm in a linear ion trap mass spectrometer . Rapid Commun Mass Sp. 2005;19:1657–1665. doi: 10.1002/rcm.1969. [DOI] [PubMed] [Google Scholar]
- 14.Morgan J, Russell D. Comparative studies of 193-nm photodissociation and TOF-TOFMS analysis of bradykinin analogues: The effects of charge site(s) and fragmentation timescales . J Am Soc Mass Spectr. 2006;17:721–729. doi: 10.1016/j.jasms.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Payne A, Glish G. Thermally assisted infrared multiphoton photodissociation in a quadrupole ion trap . Anal Chem. 2001;73:3542–3548. doi: 10.1021/ac010245+. [DOI] [PubMed] [Google Scholar]
- 16.Polfer N, Valle J, Moore D, Oomens J, Eyler J, Bendiak B. Differentiation of isomers by wavelength-tunable infrared multiple-photon dissociation-mass spectrometry: Application to glucose-containing disaccharides . Anal Chem. 2006;78:670–679. doi: 10.1021/ac0519458. [DOI] [PubMed] [Google Scholar]
- 17.Raspopov S, El-Faramawy A, Thomson B, Siu K. Infrared multiphoton dissociation in quadrupole time-of-flight mass spectrometry: Top-down characterization of proteins . Anal Chem. 2006;78:4572–4577. doi: 10.1021/ac052248i. [DOI] [PubMed] [Google Scholar]
- 18.Thompson M, Cui W, Reilly J. Fragmentation of singly charged peptide ions by photodissociation at lambda=157 nm . Angew Chem Int Edit. 2004;43:4791–4794. doi: 10.1002/anie.200460788. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Schubothe K, Li B, Russell S, Lebrilla C. Infrared multiphoton dissociation of O-linked mucin-type oligosaccharides . Anal Chem. 2005;77:208–214. doi: 10.1021/ac0489824. [DOI] [PubMed] [Google Scholar]
- 20.Crowe M, Brodbelt J. Differentiation of phosphorylated and unphosphorylated peptides by high-performance liquid chromatography-electrospray ionization-infrared multiphoton dissociation in a quadrupole ion trap . Anal Chem. 2005;77:5726–5734. doi: 10.1021/ac0509410. [DOI] [PubMed] [Google Scholar]
- 21.Wilson J, Brodbelt J. Infrared multiphoton dissociation for enhanced de novo sequence interpretation of N-terminal sulfonated peptides in a quadrupole ion trap . Anal Chem. 2006;78:6855–6862. doi: 10.1021/ac060760d. [DOI] [PubMed] [Google Scholar]
- 22.Wilson J, Brodbelt J. Infrared multiphoton dissociation of duplex DNA/drug complexes in a quadrupole ion trap . Anal Chem. 2007;79:2067–2077. doi: 10.1021/ac061946f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pikulski M, Wilson J, Aguilar A, Brodbelt J. Amplification of infrared multiphoton dissociation efficiency in a quadruple ion trap using IR-active ligands . Anal Chem. 2006;78:8512–8517. doi: 10.1021/ac061472k. [DOI] [PubMed] [Google Scholar]
- 24.Wilson J, Brodbelt J. MS/MS Simplification by 355 nm Ultraviolet Photodissociation of Chromophore-Derivatized Peptides in a Quadrupole Ion Trap . Anal Chem. 2007;79:7883–7892. doi: 10.1021/ac071241t. [DOI] [PubMed] [Google Scholar]
- 25.Tecklenburg R, Miller M, Russell D. Laser Ion-Beam Photodissociation Studies of Model Amino-Acids and Peptides . J Am Chem Soc. 1989;111:1161–1171. [Google Scholar]
- 26.Kirkovits G, Zimmerman R, Huggins M, Lynch V, Sessler J. Synthesis, Structural Characterization and Complexation Properties of the First “Crowned” Dipyrrolylquinoxalines. Eur J Org Chem. 2002:3768–3778. [Google Scholar]
- 27.Crowe M, Brodbelt J. Evaluation of noncovalent interactions between peptides and polyether compounds via energy-variable collisionally activated dissociation . J Am Soc Mass Spectr. 2003;14:1148–1157. doi: 10.1016/S1044-0305(03)00452-5. [DOI] [PubMed] [Google Scholar]
- 28.Julian R, Akin M, May J, Stoltz B, Beauchamp J. Molecular recognition of arginine in small peptides by supramolecular complexation with dibenzo-30-crown-10 ether . Int J Mass Spectrom. 2002;220:87–96. [Google Scholar]
- 29.Julian R, Beauchamp J. Site specific sequestering and stabilization of charge in peptides by supramolecular adduct formation with 18-crown-6 ether by way of electrospray ionization . Int J Mass Spectrom. 2001;210:613–623. [Google Scholar]
- 30.Julian R, May J, Stoltz B, Beauchamp J. Molecular mousetraps: Gas-phase studies of the covalent coupling of noncovalent complexes initiated by reactive carbenes formed by controlled activation of diazo precursors . Angew Chem Int Edit. 2003;42:1012–1015. doi: 10.1002/anie.200390258. [DOI] [PubMed] [Google Scholar]
- 31.Ly T, Julian R. Using ESI-MS to probe protein structure by site-specific noncovalent attachment of 18-crown-6 . J Am Soc Mass Spectr. 2006;17:1209–1215. doi: 10.1016/j.jasms.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Pratt DW. High Resolution Spectroscopy in the Gas Phase: Even Large Molecules Have Well-Defined Shapes. Annu Rev Phys Chem. 1998;49:481–530. doi: 10.1146/annurev.physchem.49.1.481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.