Abstract
Yersinia Pestis outer proteins, plasminogen activator protease and Yop secretion protein F are necessary for the full virulence of Yesinia pestis and have been proposed as potential protective antigens for vaccines against plague. In the current study, we used DNA immunization as a tool to study the relative protective immunity of these proteins with a standardized intranasal challenge system in mice. While the natural full-length gene sequences for most of these Y. pestis proteins did not display a good level of protein expression in vitro when delivered by a DNA vaccine vector, the overall immunogenicity of these wild type gene DNA vaccines was low in eliciting antigen-specific antibody responses and gene sequence modifications improved both of these parameters. However, even modified YopD, YopO and YscF antigens were only able to partially protect immunized mice at various levels against lethal challenge with Y. pestis KIM 1001 strain while no protection was observed with either the YopB or Pla antigens. These results demonstrate that DNA immunization is effective in screening, optimizing and comparing optimal antigen designs and immunogenicity of candidate antigens for the development of a subunit-based plague vaccine.
Keywords: Y. pestis antigens, Immunogenicity, DNA vaccine
Introduction
Plague is one of the most feared infectious diseases in humans. It has killed over 200 million humans throughout history and still remains endemic in certain areas of the world. The causative agent of plague, Yersinia pestis (Y. pestis), is also a concern for its potential use as an agent of biological warfare and biological terrorism. Although killed whole-cell vaccines are available for human use in certain countries, poor efficacy has limited their use for prevention of natural or human-inflicted outbreaks [1], [2], [3]. Up to this point, the effort to develop next generation plague vaccines has focused mainly on the use of two well-studied protective antigens, F1 and V (LcrV), in the form of subunit-based recombinant protein vaccines [4], [5], [6], [7], [8]. However, as shown in a recent study conducted in humans with subunit-based plague vaccines, the immunogenicity of the current vaccine composition is quite weak [9]. Therefore, the importance of identifying additional protective antigens is clear, especially for the highly virulent pneumonic plague.
Previous studies have shown that antibodies to some of the Yersinia outer proteins (Yops) are present in convalescence sera from patients infected by Y. pestis and in mice that survived experimental challenge [10], [11]. Therefore, it was hypothesized that these proteins may serve as protective antigens. Along with F1 and LcrV, Yops and the plasminogen activator protease (Pla) are necessary for the full virulence of Y. pestis [12], [13] and are encoded by the virulence plasmids [13], [14], [15]. Among the 12 secreted Yops, YopB and YopD are known as translocators in the Ysc-Yop type III secretion system involved in the translocation of the Yop effectors across the eukaryotic cell plasma membrane into the target cells [16], [17]. The type III secretion apparatus of Y. pestis is a conserved mechanism to deliver virulent factors into mammalian host cells [15], [18]. Both YopB and YopD are transmembrane proteins and interact with LcrV to form the pore of the Type III secretion apparatus through which the Yop effectors are translocated [13], [19], [20]. YopO/YpkA is a 81.7 kDa protein kinase with the ability to catalyze autophosphorylation on a serine residue, as shown by in vitro experiments, and is targeted to the inner surface of the plasma membrane inside the cells [21].
Recent reports have demonstrated that mice immunized with recombinant YscF, which is a surface localized protein, can produce YscF-specific antibody responses and partial protective immunity against Y. pestis by intravenous or subcutaneous challenge [22], [23]. These particular results suggest that the antibody responses induced by YscF protein immunization are useful for protection against bubonic plague although the protection mechanism is not quite clear. There is no evidence whether YscF-specific antibody responses are protective for mucosal infection including the pneumonic plague.
Pla is an integral outer membrane protein and a surface protease. It belongs to the omptin family of enterobacterial surface proteins and derives its name from the fact that it can activate the mammalian plasma proenzyme plasminogen into plasmin [24], [25], [26]. The importance of Pla for bacterial virulence has been demonstrated in vivo. The LD50 for a Pla-negative mutant strain was close to 107 bacteria, as compared to less than 50 bacteria for the isogenic Pla-positive strain [12]. This protein is responsible for the highly efficient invasion of plague bacterium from the subcutaneous infection site into circulation [24]. Furthermore, recent data have shown that intranasal infection with a Y. pestis strain lacking Pla resulted in the development of a deadly plague infection after 7 days in only 50% of the infected mice when compared to a 100% fatality rate less than 4 days post-inoculation in the wild type strain indicating that Pla has a significant involvement in the fatal outcome following respiratory infection with Y. pestis [27].
The immunogenicity and potential for protection have not been well characterized for the Yop and Pla proteins. In one study using recombinant Yop proteins expressed in Escherichia coli (E. coli) and purified by the His-tag approach, the recombinant YopO protein significantly prolonged the mean survival time but did not increase the overall survival of mice that had received a subcutaneous challenge of a virulent, non-encapsulated isogenic variant of Y. pestis [28]. In this same study, YopD was also shown to be partially protective against death in mice challenged with the non-encapsulated strain but not the wild-type encapsulated Y. pestis [28]. No detailed data were provided regarding the antibody responses to individual Yop proteins. The authors also noted that several Yop proteins were denatured before their use as immunogens which may have contributed to the poor protection with Yop proteins.
In recent years, the DNA vaccine approach has been used as an effective tool to study the immunogenicity of candidate antigens against a variety of infectious agents due to its potential to express protective antigens in vivo. For example, DNA immunization was used to quickly confirm the Spike protein as the protective antigen against the Severe Acute Respiratory Syndrome – associated coronavirus (SARS-CoV) and to map the protective domains on this Spike protein [29], [30]. DNA immunization was also used to effectively identify individual protective antigens in order to formulate subunit-based vaccines against smallpox infection [31]. In a previous study from our laboratory, we successfully established a mouse intranasal challenge model coupled with DNA immunization technology to test the protection efficacy of modified Y. pestis antigens against lethal plague challenge [32]. This system provided a standard assay process to screen and score the relative effectiveness of known or novel Y. pestis antigens.
In the current study, a molecular antigen engineering approach was used to improve the expression and immunogenicity of Yops, YscF and Pla proteins. Our previous studies, which used DNA vaccines against plague and other infectious agents, have demonstrated that a proper leader sequence is important in eliciting a high level of antibody responses and protective immunity [30], [32], [33]. Since yopB, yopD and yopO genes do not encode typical signal peptides, the tPA leader sequence was engineered with each of these yop genes individually to produce modified yop gene inserts for DNA immunization. In addition, certain intragene hydrophobic regions were removed to further improve the immunogenicity of Yop antigens. Our results demonstrate that antigen engineering was effective in improving the immunogenicity of the above Yop, Pla and YscF antigens and that these modified antigens in general induced higher antibody responses than the wild-type gene inserts. In addition, modified YopD, YopO and YscF proteins improved the level of protection. Overall, the protection efficacies with these Y. pestis proteins were still low and incomplete when compared to the known protective antigen LcrV, indicating that a search for additional protective antigens against Y. pestis is needed.
Materials and methods
Bacterial strains
Y. pestis strain KIM 1001, fully virulent and containing all known virulent factors [12] was prepared by growing inocula for 18 h at 37 °C on Tryptose Blood Agar Base (Difco) supplemented with 2.5 mM CaCl2 but without the addition of blood. Bacteria were removed from the plate with an inoculating loop and resuspended in injection-grade PBS. The bacteria count in the suspension was correlated to its optical density (OD600). The number of bacteria in the final inocula was confirmed by colony counts.
Construction of plague DNA vaccines
Y. pestis genes (yopB, yopD, yopO, pla and yscF) were first amplified with pfu polymerase (Strategene, CA) from plasmids pCD1 or pPCP1. The yopB, yopD and yopO/ypkA are encoded by the 70 kb plasmid, pCD1 [13] and the pla is encoded by the 9.5 kb plasmid, pPCP1 [34], [35]. Primer pairs are shown in Table 1 . Two DNA vaccine constructs for each primer pair were made for expressing either YopB, YopD or YopO and three constructs for Pla.
Table 1.
Primers used in the study
| Primers | Oligonucleotide sequencea | Description |
|---|---|---|
| YopB-1 | gtcgctcc GCTAGCNheI ATGAGTGCGTTGATAACCCATGAC | yopB, sense |
| YopB-2 | agtcac GGATCCBamHI TTAAACAGTATGGGGTCTGCC | yopB, anti-sense |
| YopB-3 | gtcgctcc GCTAGCNheIAAGCTTHindIII GGTGCTAACACCGCAAG | yopB, sense |
| YopB-4 | agtcac GGATCCBamHI TCA AAGCTTHindIIITAGTGCGATGCCGGATTTG | yopB, anti-sense |
| YopD-1 | gtcgctcc GCTAGCNheI ATGACAATAAATATCAAGACAGAC | yopD, sense |
| YopD-2 | agtcac GGATCCBamHI TCAGACAACACCAAAAGCGGC | yopD, anti-sense |
| YopD-3 | acaa CTG CAGPstI TCTATAGCGAAAGAGGTGAAAATAG | yopD, sense |
| YopD-4 | agtcac GGATCCBamHI TCA CTGCAGPstI TTTTGCACCGCTGACCATC | yopD, anti-sense |
| YopO-1 | p-CATGAAAAGCGTGAAAATCATGGG | yopO, sense |
| YopO-2 | agtcac TGATCABclI TCACATCCATTCCCGCTCCAACCG | yopO, anti-sense |
| Pla-1 | gtcgctcc AAGCTTHindIIIGCTAGCNheI ATGAAGAAAAGTTCTATTGTGGC | pla, sense |
| Pla-2 | gagc AGGCC TStuICAGAAGCGATATTGCAGACCCGC | pla, anti-sense |
| Pla-3 | gtcgctcc GCTAGCNheI TCCGGGAGTGCTAATGCAGCATC | pla, sense |
| YscF-1 | gtcgctcc AAGCTTHindIII ATGAGTAACTTCTCTGGATTTACG | yscF, sense |
| YscF-2 | agtcac GGATCCBamHI TTA TGGGAACTTCTGTAGGATGCC | yscF, anti-sense |
| YscF-3 | gtcgctcc GCTAGCNheI AGTAACTTCTCTGGATTTACG | yscF, sense |
| YscF-4 | atgg ACTAGTSpeI ggcggc TGGGAACTTCTGTAGGATGCC | yscF, anti-sense |
| YscF-5 | atgg ACTAGTSpeI ggcggc AGTAACTTCTCTGGATTTACG | yscF, sense |
The lower case letters indicate the non-specific sequences; the upper case letters indicate the specific sequences matching the target genes. The italicized sequences are the restriction enzyme sites.
For YopB constructs, the full-length yopB (wt.yopB) gene was amplified with primers YopB-1 and YopB-2. The truncated yopB (yopB.dTM) gene, following removal of the hydrophobic regions (aa128–aa258), was first amplified as two gene fragments: the 5′ yopB gene and the 3′ yopB gene fragments with primer pairs YopB-1/YopB-4 and YopB-3/YopB-2 respectively and these two fragments were ligated together by inducing an HindIII site in the junction.
For YopD DNA vaccine constructs, the full-length yopD (wt.yopD) gene was amplified with primers YopD-1 and YopD-2. The truncated yopD (yopD.dTM) gene, following removal of the hydrophobic regions (aa128–aa149), was first amplified as two gene fragments: the 5′ yopD gene and the 3′ yopD gene fragments with primer pairs YopD-1/YopD-4 and YopD-3/YopD-2 respectively, and these two fragments were ligated together by inducing a PstI site in the junction.
While there is a hydrophobic region at the N-terminus of the Pla protein, it does not represent a natural signal peptide. Several versions of Pla DNA vaccines were constructed both with and without the removal of this N-terminal hydrophobic region. The full-length pla gene and d-pla gene, following removal of the hydrophobic region at N-terminus of Pla, were amplified with primer pairs Pla-1/Pla-2 and Pla-3/Pla-2, respectively. Each of the above gene inserts was directly sub-cloned into the previously described DNA vaccine vector, pJW4303, immediately downstream of the tPA leader sequence [32], [33], which is in-frame with such sub-cloned inserts.
The full-length yopO gene was amplified with primers YopO-1 and YopO-2 and cloned into pJW4303 with or without tPA leaders sequence and produced wt.YopO and tPA.YopO DNA vaccines, respectively.
Three YscF DNA vaccines were constructed: single copy of yscF gene with and without tPA leader sequence, and two copies of yscF gene in tandem. The wild type full-length yscF gene was amplified with primers YscF-1 and YscF-2. The fragments of two-copy yscF tandem gene insert were amplified with primer pairs YscF1/YscF-4 and YscF-5/YscF-2, respectively, and these two fragments were ligated together by inducing a SpeI site and a linker (Ala-Ala-Thr-Ser-Thr-Ser-His-Val-Asp) in the junction. The wild type yscF and two-copy yscF tandem inserts were subcloned into pJW4303 vector at HindIII and BamHI sites without using any leader sequence. The tPA-yscF insert was amplified with primers YscF-3 and YscF-2 and subcloned into the pJW4303 vector immediately downstream of tPA leader sequence. Sufficient amounts of the DNA vaccines were prepared by the Qiagen Mega plasmid purification kit (Valencia, CA) for in vitro transfection and animal immunization applications.
DNA vaccines for plague antigens, V and F1, were as constructed as previously reported [32] and were used as a positive control in the intranasal challenge study as they have been previously shown to provide protection in a mucosal challenge model in mice [32].
DNA immunization of Balb/C mice
Six to eight week old female Balb/C mice (9–10 mice/group) were purchased from Taconic Farms (Germantown, NY) and housed in the Department of Animal Medicine at the University of Massachusetts Medical School (UMMS) in accordance with IACUC approved protocols. The animals were immunized with a Helios gene gun (Bio-Rad), using helium gas pressure at 300 psi according to manufacturer's instruction. Each mouse received four monthly immunizations with six DNA shots of 1 μg each at each immunization. Blood samples were collected periorbitally prior to each immunization and 2 weeks after the last immunization.
In vitro expression of Y. pestis antigens and Western-blot analysis
The expression of the YopB, YopD, YopO, Pla and YscF DNA vaccines were tested in transiently transfected 293T cells by a calcium phosphate co-precipitation method using 10 μg of plasmid DNA for 2 × 106 cells on a 60-mm dish. The cells were harvested 72 h after transfection. The transiently expressed YopB, YopD, YopO, Pla or YscF antigens were analyzed by SDS-10% polyacryamide gel electrophoresis (SDS-PAGE), then transferred onto PVDF membranes, and blocked overnight at 4 °C in blocking buffer (0.2% I-block, 0.1% Tween-20 in 1X PBS). Membranes were incubated with a 1:200 dilution of group pooled mouse sera immunized with corresponding DNA vaccines. The mouse sera against YopB, YopD, YopO, Pla or YscF were generated by YopB.dTM, YopD.dTM, tPA.YopO, tPA.dPla or YscF-2 DNA vaccine, respectively. After being washed, blots were incubated with alkaline phosphatase-conjugated goat anti-mouse IgG at 1:5000 dilution and signals were detected using a chemiluminescence Western-light Kit (Tropix, Bedford, MA).
ELISA
Enzyme-linked immunosorbent assay (ELISA) was performed to measure the titers of specific IgG against each individual Y. pestis antigens in pooled mouse sera of each group (equal amount of serum from each mouse in a group were first pooled before further dilution and testing). The YopB, YopD or YopO antigens used for ELISA were supernatants from 293T cells transfected with YopB.dTM, YopD.dTM or tPA.YopO DNA vaccines, respectively. Non-purified Pla or YscF antigen used for ELISA was from 293T cell lysate transfected with tPA-Pla or YscF-2 DNA vaccine, respectively. Ninety-six-well microtiter plates were coated with 100 μl of one of the above antigens harvested from transfected 293T cells at 1 μg/ml and incubated overnight at 4 °C. Serially diluted mouse sera were added to the plates and assayed in duplicate. The plates were incubated with biotinylated anti-mouse IgG diluted at 1:1000, followed by horseradish peroxidase-conjugated streptavidin diluted at 1:2000 and finally, developed with 3,3′,5,5′-tetramethybenzidine (TMB) solution. The reactions were stopped by adding 25 μl of 2 M H2SO4 and the plates were read at OD450.
Intranasal challenge with lethal doses of Y. pestis
Challenge was performed by an intranasal instillation of 50 μl saline containing lethal doses of Y. pestis (KIM 1001 strain) to ketamine-anesthetized mice. KIM 1001 is a fully virulent Y. pestis strain and contains all known virulent factors [12]. This challenge method leads to a rapid infection and is lethal to 100% of non-immunized mice within 3–4 days [32]. The LD50 (median lethal dose) of this challenge model was determined by a previous study: 5000 cfu is equal to approximately 15 LD50 [32]. Individual mice were challenged 1 week after the fourth immunization and observed twice daily to monitor both morbidity and mortality. Studies were conducted in a Biosafety Level 3 containment facility at the Department of Animal Medicine, UMMS.
Statistical analyses
Fisher's exact test was used to compare the protection efficacies against Y. pestis infection between different vaccination groups.
Results
Construction and expression of DNA vaccines expressing YopB, YopD, YopO, Pla and YscF antigens
For Y. pestis antigens YopB and YopD, two versions of the DNA vaccine inserts were designed (Figs. 1A and 2A , respectively). Both Yop proteins contain at least one hydrophobic region, a potential transmembrane (TM) segment, in the middle of polypeptide sequences. Therefore, one modified version of the DNA insert (“dTM”) had the hydrophobic region(s) removed while the wild type version (wt.yopB and wt.yopD) retained the full-length sequence. A human tissue plasminogen activator leader sequence (tPA) was included in both versions of the inserts to facilitate the expression of DNA vaccine antigens in mammalian cells, as previously reported [36]. For the YopO, which does not contain any significant hydrophobic segments, only the tPA leader was added to the full-length gene insert and a control yopO gene insert was produced with the wild type sequence without any identifiable leader sequences (Fig. 3A). These yop gene inserts were individually cloned into the DNA vaccine vector, pJW4303, which is driven by a cytomegalovirus (CMV) promoter, as previous reported [37] and was used effectively for a LcrV DNA vaccine [32].
Figure 1.
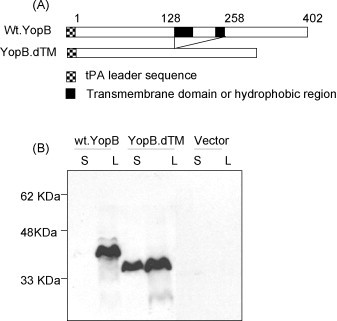
(A) Schematic diagram of yopB gene inserts in DNA vaccine constructs. Weaved and solid boxes represent the added tPA-leader sequence and hydrophobic domains, respectively. The numbers indicate the amino acid positions. (B) Western-blot analysis on the expression of either the full-length YopB protein or the YopB protein without the hydrophobic domains coded in two YopB DNA vaccines, plus empty vector control, in supernatant (S) and lysate (L) of transiently transfected 293T cells. Pooled sera from mice immunized with YopB.dTM DNA vaccine was used at 1:200 dilution as the detecting antibody.
Figure 2.
(A) Schematic diagram of yopD gene inserts in DNA vaccine constructs. Weaved and solid boxes indicate the tPA-leader sequence and hydrophobic domains, respectively. The numbers indicate the amino acid positions. (B) Western-blot analysis of YopD protein expression by DNA vaccines encoding either the full-length YopD protein or the YopD protein without the hydrophobic domain, plus empty vector, in supernatant (S) and lysate (L) of transiently transfected 293T cells. Pooled sera from mice immunized with YopD.dTM DNA vaccine was used at 1:200 dilution as the detecting antibody.
Figure 3.
(A) Schematic diagram of yopO gene inserts in DNA vaccine constructs. Weaved box represents the tPA-leader sequence. The numbers indicate the amino acid positions. (B) Western-blot analysis of expression of the wild type YopO protein or the YopO protein with a new tPA leader, plus empty vector, in supernatant (S) and lysate (L) of transiently transfected 293T cells. Pooled sera from mice immunized with the tPA.YopO DNA vaccine was used at 1:200 dilution as the detecting antibody.
Mammalian cell expression of the above Yop DNA vaccines was confirmed by using a transiently transfected 293T cell system in vitro [32]. Both cell lysate and culture supernatant were collected after the transfection and examined by Western-blot analysis. YopB antigen expression was observed for both full-length yopB and modified yopB gene inserts (Fig. 1B) but only the modified yopB insert, with the removal of hydrophobic regions, displayed a good level of expression in both cell lysate and supernatant. Therefore, the removal of 130 aa in the middle of yopB gene greatly enhanced the secretion of YopB protein (Fig. 1B). On the other hand, the impact of removing a much smaller 21 aa hydrophobic sequence on the expression of YopD was limited. Both full-length YopD and modified YopD DNA vaccines were able to express comparable levels of antigen in both cell lysate and supernatant (Fig. 2B).
The role of the leader sequence in the expression of YopO protein was significant. Compared to the YopO DNA vaccine without leader sequence (wt.YopO), the YopO construct with the tPA leader (tPA.YopO) was able to produce a larger amount of YopO protein in the supernatant, supporting the utility of tPA leader for expressing secreted proteins (Fig. 3B). Interestingly, the addition of a tPA leader also led to an overall higher level of expression for YopO protein even though the exact mechanism for such a role has not been well elucidated.
Our previous data demonstrated that DNA immunization with the pla gene in its wild type sequence was not immunogenic despite the possibility of there being a putative leader sequence with 21 hydrophobic amino acid residues at the N-terminus of Pla. Although the addition of a tPA leader to the wild type pla sequences was able to achieve better expression of Pla protein in transiently transfected 293T cells, it was still not immunogenic [32]. In the current study, another modified Pla DNA vaccine was constructed by removing the natural hydrophobic sequence while keeping the tPA leader (Fig. 4A). Transient expression of Pla was confirmed with this new DNA vaccine plasmid. Both versions of Pla DNA inserts were able to express Pla antigen only in cell lysate (Fig. 4B).
Figure 4.
(A) Schematic diagram of pla gene inserts in DNA vaccine constructs. The wt-Pla, tPA-Pla and tPA-dPla, respectively, represent the constructs with full-length pla gene alone, full-length pla gene with addition of tPA leader sequence, and pla gene with deletion of the N-terminal hydrophobic sequences and addition of the tPA leader sequence. Weaved and solid boxes indicate the added tPA-leader sequence and hydrophobic domains, respectfully. The numbers indicate the amino acid positions. (B) Western-blot analysis of the Pla protein expression by DNA vaccines encoding wt-Pla, tPA-Pla, tPA-dPla proteins, plus empty vector, in supernatant (S) and lysate (L) of transiently transfected 293T cells. Pooled sera from mice immunized with the tPA-dPla DNA vaccine was used at 1:200 dilution as the detecting antibody.
Three versions of DNA vaccine inserts were designed for YscF antigen (Fig. 5A). YscF is a small protein without any leader sequence. Therefore, one construct with full-length wild type YscF (wt.YscF) and another modified YscF with tPA leader sequence (tPA-YscF) were made. To improve the expression and immunogenicity of YscF, the third YscF DNA vaccine with two-copy tandem YscF antigen (YscF-2) was designed and constructed. These yscF gene inserts were individually cloned into the DNA vaccine vector pJW4303. The transient expression of three YscF DNA vaccine constructs in 293T cells demonstrated that both wt.YscF and tPA.YscF did not induce detectable YscF antigen expression (Fig. 5B). Although the addition of the tPA leader sequence could improve the overall expression of many other antigens, it did not improve the YscF expression in the current tPA.YscF DNA vaccine design. Interestingly, YscF-2 antigen was well expressed as a dimeric YscF protein with expected size (16 kDa) (Fig. 5b). There is a non-specific band just above the 25 kDa Marker across all transfected samples including vector control, which was not seen in other vector control samples (Figure 1, Figure 2, Figure 3, Figure 4). Depending on the cell conditions, we have observed that mouse sera sometimes may show non-specific reactivity against cell lysate samples (unpublished observation).
Figure 5.
(A) Schematic diagram of yscF gene inserts in DNA vaccine constructs: wt-YscF, tPA-YscF and YscF-2, respectively, represent the full-length yscF gene alone, full-length yscF gene with addition of tPA leader sequence, and the two-copy yscF gene in tandem. (B) Western-blot analysis of the YscF protein expression by DNA vaccines encoding wt-YscF, tPA-YscF and YscF-2 proteins, plus empty vector, in supernatant (S) and lysate (L) of transiently transfected 293T cells. Pooled sera from mice immunized with the YscF-2 DNA vaccine was used at 1:200 dilution as the detecting antibody. Arrow points to the dimeric form of YscF.
Serum IgG responses in mice immunized with individual YopB, YopD, YopO, Pla and YscF DNA vaccines
Y. pestis antigen-specific antibody responses were measured by ELISA using pooled mice sera from each immunization group (9–10 mice/group), at 2 weeks after the third monthly DNA vaccination. While the YopB.dTM DNA vaccine induced positive anti-YopB IgG responses, the full-length YopB DNA vaccine did not induce detectable antibody responses (Fig. 6A). Anti-YopD IgG responses were elicited with both the full-length and the modified (i.e., removal of the hydrophobic domain) DNA vaccines. However, the latter was more immunogenic as indicated by the elicitation of higher anti-YopD IgG when compared to the full-length YopD insert (Fig. 6B). Therefore, the deletion of hydrophobic regions improved the immunogenicity of YopB and YopD DNA vaccines. YopO DNA vaccine with the tPA leader (tPA.YopO) was also able to induce higher titer anti-YopD antibody responses than the wild type YopO DNA vaccine (wt.YopO) (Fig. 6C). Based on the reciprocal end-titration titers, modified YopD and YopO constructs were able to induce higher levels of antibody responses (in the range of 1:20,000–1:70,000) than the modified YopB DNA vaccine (∼1:8000).
Figure 6.
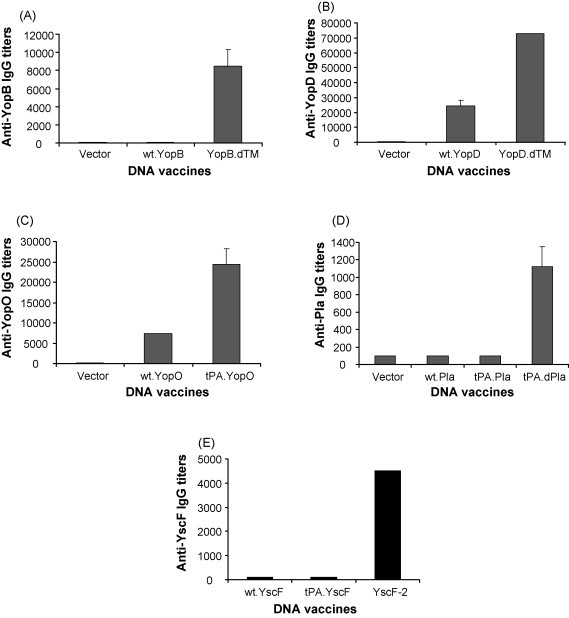
Antigen-specific IgG responses induced by different YopB, YopD, YopO, Pla and YscF DNA vaccines in Balb/C mice after the third DNA immunization. The levels of antibody responses were measured by ELISA against autologous antigens expressed from 293T cells transfected with the DNA vaccines YopB.dTM, YopD.dTM, tPA.YopO, tPA-Pla and YscF-2 for Panels A, B, C, D and E, respectively. The bars show the average antibody titers (the reciprocal of sera dilutions) of three duplicated assays using pooled mouse sera from each group as indicated (9 mice/group for Panels A, B and C and 10 mice per group for Panels D and E). Standard error bars are shown if applicable.
The tPA-dPla DNA vaccine, which removed the N-terminal hydrophobic region, was clearly able to induce positive anti-Pla IgG responses (Fig. 6D) even though the final titers were still relatively low (∼1:900). This low immunogenicity of the Pla DNA vaccine was not completely unexpected given that none of the modified Pla DNA vaccine constructs were able to induce a significant level of secreted Pla protein in supernatant fractions from transfected 293T cells (Fig. 4b). No significant anti-Pla IgG antibody responses were detected from mice immunized with either wt-Pla or tPA-Pla DNA vaccines, as previously reported [32]. It is interesting to note that while the tPA-dPla construct was more immunogenic than the tPA-Pla construct, they both showed a similar pattern of Pla protein expression. Indeed, this would imply that the N-terminal hydrophobic region had a negative effect on the immunogenicity of Pla protein when delivered via DNA vaccine vector.
YscF-specific antibody responses were produced in mice that received the YscF-2 DNA vaccine (Fig. 6E) but not in those that received the wt.YscF or tPA.YscF DNA vaccines. The results are consistent with the finding that only YscF-2 DNA vaccine had detectable YscF protein expression (Fig. 5B). The YscF-specific IgG titers were 1:4000–1:5000 after three monthly DNA immunizations in YscF-2 immunized mice.
In the current study, both IgG1 and IgG2a subclasses are present in the above sera analysis with the ratio of IgG2a/IgG1 less than 1 (data not shown), as expected based on well-established information in literature that gene gun mediated DNA immunization mainly induces a Th2 type of antibody responses (IgG2a/IgG1 ratio < 1) [32].
Protection of immunized mice against intranasal challenge with lethal Y. pestis
Mice (9–10 mice/group) received another boost with the same DNA vaccines in week 12, and 1 week later, they were challenged with a lethal dose of the KIM 1001 strain of Y. pestis at 5000 cfu (∼15 LD50), delivered intranasally. Only three DNA vaccines with modified Y. pestis gene inserts, YopD.dTM, tPA.YopO and YscF-2, were able to provide partial protection, as measured by survival during 2 weeks’ observation (Fig. 7 ). In YopD.dTM and tPA.YopO immunized groups, 22.2% of mice survived the intranasal challenge. No statistical difference was observed by Fisher's exact test possibly because there was not enough statistical power (9 mice/group) to compare an observed 20–25% difference. In the YscF-2 immunized group, 60% of mice survived the intranasal challenge, which was significant as compared to either wt.YscF or tPA.YscF DNA vaccines according to Fisher's exact test (p < 0.05). No protection was observed with the wt.YopD, wt.YscF or tPA.YscF DNA vaccines or any of the YopB and Pla DNA vaccines (Fig. 7) although wt.YopO, wt.YopD and YopB.dTM DNA vaccines induced good levels of antibody responses (Fig. 6).
Figure 7.
In vivo protection of mice immunized with different DNA vaccines, as indicated. Balb/C mice were challenged with a lethal dose of 5000 cfu Y. pestis (KIM 1001 strain) by intranasal inoculation 1 week after the 4th DNA immunization. Cumulative survival curves were plotted to show the protection for each group (9 mice/group for Panels A–C and 10 mice/group for Panel D–F). A vector group was included in each plot as the negative control.
The mock DNA immunization group (received empty DNA vector without any Y. pestis antigen insert) quickly developed easily observable signs of sickness (e.g., rough coats, hunched or huddled posture, shivering, labored breathing and lethargy) within the first 24–36 h post-challenge and all of them died within 5 days (Fig. 7). Mice immunized with any of the YopB or Pla DNA vaccines did not show any improvement over the mock (empty vector control) DNA immunization group (Fig. 7). The positive control group immunized with the combination of V and F1 DNA vaccines achieved 100% protection (Fig. 7).
Discussion
The current study compared the relative immunogenicity of wild type and modified antigens for three Yop antigens, the Pla antigen and the YscF antigen to demonstrate that DNA vaccines can be used as an effective tool to study the relative immunogenicity of candidate protective antigens, to optimize the design of such antigens and to screen for new antigens against Y. pestis. Overall, YopB, YopD, YopO, Pla and YscF DNA vaccines were not very immunogenic when the wild type gene sequences were delivered by DNA immunization. Production of these antigens in the mammalian expression system is limited by the lack of proper leader sequences and/or by the presence of hydrophobic segments in the middle of their coding sequences. Antigen engineering approaches, such as the use of tPA leader sequences and the removal of intra-polypeptide hydrophobic regions, were effective in improving the expression of coded Yop antigen, resulting in higher levels of antigen expression, with some in secreted form of antigenic proteins.
In general, modified Yop DNA inserts led to better immunogenicity when compared to their wild type counterparts. For YopB, a 41.8 kDa protein with two putative hydrophobic domains and YopD, a 33.3 kDa protein with a 31-amino acid hydrophobic region in the middle of the protein, removal of the hydrophobic regions clearly improved the immunogenicity of these proteins although the modified YopD insert had a similar level of antigen expression as the full-length YopD insert. Modified DNA vaccine inserts also became better protective antigens for YopD and YopO proteins.
Information on the immunogenicity and protection potential of Yop antigens is limited in the literature. Studies that have been conducted show that YopD and YopO are only partially protective against mostly non-capsulated Y. pestis strains in a subcutaneous challenge model [28]. In certain cases, the effect of protection can be shown only by extended length of survival but not improved percentage of survival [28]. The current study attempts to link antigen expression, immunogenicity of specific antibody induction and final protection of animals immunized against plague in a mucosal challenge model. Our results demonstrate that antigen engineering is effective in improving antigen expression, immunogenicity and final protection against the Yop proteins that were included in this study. The quality of protection induced by modified YopD or YopO antigens was better than that previously reported as these antigens provided better survival than their wild type antigens against mucosal challenge with a highly virulent Y. pestis KIM 1001 strain. However, the current designs are still not sufficient to elicit full protection when used alone.
To our knowledge, this is the first study showing that anti-Pla antibody responses could be induced by DNA immunization. This effect was achieved by removing the N-terminal hydrophobic region, making this strategy different from two previous designs of Pla DNA vaccines that were not immunogenic at all [32]. These results suggest that the N-terminal hydrophobic region for bacterial proteins may not function as a signal peptide leader sequence as it does for many viral proteins. This information is important for the design of bacterial vaccines that are using gene-based (i.e. DNA or viral vector) vaccination approaches for human applications.
YscF is an 7 kDa surface localized protein encoded by the 70 kb plasmid pCD1. The polymerized YscF has been shown to form a needle structure (referred to as an ‘injectosome’) on the type III secretion system [20], [38]. At least six Yop effectors: YopH, YopE, YopM, YpkA, YopK, and YopJ, are delivered by YscF injectosome into target eukaryotic cells. Based on the fact that YscF is a surface-expressed protein and is required for virulence in Y. pestis, it was assumed that YscF might be a candidate as a protective antigen. Recent studies have showed that immunization with a purified YscF protein could induce limited protective immunity against experimental plague infection [22], [23]. However, more conventional YscF DNA vaccines with the wild type gene insert and even with the addition of a tPA leader sequence could not induce detectable antibody responses or protective immunity against lethal Y. pestis challenge. Interestingly, the YscF-2 DNA vaccine, constructed to produce a YscF dimer protein, induced YscF-specific antibody responses and a significant level of protection (60%). It is known that the natural polymerization of YscF protein forms the injectosome of the type III secretion system [20]. The dimeric designed YscF-2 DNA vaccine might produce antibody responses against the polymerized YscF conformation, which may be critical for the induction of protective immunity by the YscF-DNA vaccine, in addition to overall improved YscF protein expression and immunogenicity. In the current study, we did not test the design with both tPA leader and dimeric YscF-2 components because putting two changes together will be hard to see whether they may interfere with each other. But future studies can include such design to further optimize the immunogenicity of YscF antigen. Results from this current report suggest that antigen conformation is critical for any candidate protective antigen, including those against Y. pestis. These results also provide important information to aid in the design of other forms of subunit-based vaccines, including recombinant protein vaccines.
Among the five Y. pestis antigens that were included in the current analysis, three antigens (YopD, YopO and YscF) were able to provide only partial protection and induce significant levels of antigen-specific antibody responses above a titer of 1:1000 when optimally designed. Although the modified YopB DNA vaccine induced a high level of antibody responses, it did not protect the mice against intranasal Y. pestis challenge. Furthermore, the Pla DNA vaccine induced only low levels of antibody responses and also did not provide any protection against lethal Y. pestis challenge. Given the need to identify new protective antigens for improved plague vaccines, the antigen optimization strategy and testing system reported here offers an efficient and reproducible platform to screen for new protective Y. pestis antigens. The use of DNA immunization to modify and optimize the immunogenicity of candidate Y. pestis antigens can be used to accelerate such an effort. In addition, optimized DNA vaccines expressing Y. pestis antigens can also be used as part of a DNA prime-protein boost regimen that may be more immunogenic than the protein alone approach in eliciting high quality protective antibody responses as shown in HIV vaccine studies [39].
Acknowledgements
This work was supported in part by the NIH grant U01 AI 05636 (S Lu). The project also used core facility resources at the University of Massachusetts Medical School supported by NIH grant 5P30DK32520 from the NIDDK. The authors thank Dr. Jill M. Grimes-Serrano for critical reading of the manuscript.
References
- 1.Marshall J.D., Jr., Cavanaugh D.C., Bartelloni P.J., Meyer K.F. Plague immunization. 3. Serologic response to multiple inoculations of vaccine. J Infect Dis. 1974;129(May (Suppl.)):S26–S29. doi: 10.1093/infdis/129.supplement_1.s26. [DOI] [PubMed] [Google Scholar]
- 2.Marshall J.D., Jr., Bartelloni P.J., Cavanaugh D.C., Kadull P.J., Meyer K.F. Plague immunization. II. Relation of adverse clinical reactions to multiple immunizations with killed vaccine. J Infect Dis. 1974;129(May (Suppl.)):S19–S25. doi: 10.1093/infdis/129.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 3.Williamson E.D., Eley S.M., Stagg A.J., Green M., Russell P., Titball R.W. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against pneumonic plague. Vaccine. 1997;15(July):1079–1084. doi: 10.1016/s0264-410x(96)00303-9. [DOI] [PubMed] [Google Scholar]
- 4.Powell B.S., Andrews G.P., Enama J.T., Jendrek S., Bolt C., Worsham P. Design and testing for a nontagged F1-V fusion protein as vaccine antigen against bubonic and pneumonic plague. Biotechnol Prog. 2005;21(September–October):1490–1510. doi: 10.1021/bp050098r. [DOI] [PubMed] [Google Scholar]
- 5.Anderson G.W., Jr., Leary S.E., Williamson E.D., Titball R.W., Welkos S.L., Worsham P.L. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect Immun. 1996;64(November):4580–4585. doi: 10.1128/iai.64.11.4580-4585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson E.D., Eley S.M., Stagg A.J., Green M., Russell P., Titball R.W. A single dose sub-unit vaccine protects against pneumonic plague. Vaccine. 2000;19(October (4–5)):566–571. doi: 10.1016/s0264-410x(00)00159-6. [DOI] [PubMed] [Google Scholar]
- 7.Elvin S.J., Williamson E.D. The F1 and V subunit vaccine protects against plague in the absence of IL-4 driven immune responses. Microb Pathog. 2000;29(October (4)):223–230. doi: 10.1006/mpat.2000.0385. [DOI] [PubMed] [Google Scholar]
- 8.Titball R.W., Williamson E.D. Vaccination against bubonic and pneumonic plague. Vaccine. 2001;19(July (30)):4175–4184. doi: 10.1016/s0264-410x(01)00163-3. [DOI] [PubMed] [Google Scholar]
- 9.Williamson E.D., Flick-Smith H.C., Lebutt C., Rowland C.A., Jones S.M., Waters E.L. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect Immun. 2005;73(June (6)):3598–3608. doi: 10.1128/IAI.73.6.3598-3608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benner G.E., Andrews G.P., Byrne W.R., Strachan S.D., Sample A.K., Heath D.G. Immune response to Yersinia outer proteins and other Yersinia pestis antigens after experimental plague infection in mice. Infect Immun. 1999;67(4):1922–1928. doi: 10.1128/iai.67.4.1922-1928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazza G., Karu A.E., Kingsbury D.T. Immune response to plasmid- and chromosome-encoded Yersinia antigens. Infect Immun. 1985;48(June (3)):676–685. doi: 10.1128/iai.48.3.676-685.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sodeinde O.A., Subrahmanyam Y.V., Stark K., Quan T., Bao Y., Goguen J.D. A surface protease and the invasive character of plague. Science. 1992;258(November (5084)):1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 13.Cornelis G.R., Boland A., Boyd A.P., Geuijen C., Iriarte M., Neyt C. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62(December (4)):1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelis G.R. The Yersinia Yop virulon, a bacterial system to subvert cells of the primary host defense. Folia Microbiol (Praha) 1998;43(3):253–261. doi: 10.1007/BF02818610. [DOI] [PubMed] [Google Scholar]
- 15.Perry R.D., Fetherston J.D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10(1):35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakansson S., Schesser K., Persson C., Galyov E.E., Rosqvist R., Homble F. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15(November (21)):5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 17.Boland A., Sory M.P., Iriarte M., Kerbourch C., Wattiau P., Cornelis G.R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D,N delivery apparatus. EMBO J. 1996;15(October (19)):5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 18.Torruellas J., Jackson M.W., Pennock J.W., Plano G.V. The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol Microbiol. 2005;57(September (6)):1719–1733. doi: 10.1111/j.1365-2958.2005.04790.x. [DOI] [PubMed] [Google Scholar]
- 19.Hakansson S., Bergman T., Vanooteghem J.C., Cornelis G., Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993;61(January (1)):71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelis G.R. The Yersinia Ysc-Yop virulence apparatus. Int J Med Microbiol. 2002;291(February (6–7)):455–462. doi: 10.1078/1438-4221-00153. [DOI] [PubMed] [Google Scholar]
- 21.Hakansson S., Galyov E.E., Rosqvist R., Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20(May (3)):593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 22.Swietnicki W., Powell B.S., Goodin J. Yersinia pestis Yop secretion protein F: purification, characterization, and protective efficacy against bubonic plague. Protein Expr Purif. 2005;42(July (1)):166–172. doi: 10.1016/j.pep.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Matson J.S., Durick K.A., Bradley D.S., Nilles M.L. Immunization of mice with YscF provides protection from Yersinia pestis infections. BMC Microbiol. 2005;5(1):38. doi: 10.1186/1471-2180-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahteenmaki K., Kuusela P., Korhonen T.K. Bacterial plasminogen activators and receptors. FEMS Microbiol Rev. 2001;25(December (5)):531–552. doi: 10.1111/j.1574-6976.2001.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 25.Lahteenmaki K., Kukkonen M., Korhonen T.K. The Pla surface protease/adhesin of Yersinia pestis mediates bacterial invasion into human endothelial cells. FEBS Lett. 2001;504(August (1–2)):69–72. doi: 10.1016/s0014-5793(01)02775-2. [DOI] [PubMed] [Google Scholar]
- 26.Kukkonen M., Korhonen T.K. The omptin family of enterobacterial surface proteases/adhesins: from housekeeping in Escherichia coli to systemic spread of Yersinia pestis. Int J Med Microbiol. 2004;294(July (1)):7–14. doi: 10.1016/j.ijmm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Lathem W.W., Price P.A., Miller V.L., Goldman W.E. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science (New York, NY) 2007;315(Jan (5811)):509–513. doi: 10.1126/science.1137195. [DOI] [PubMed] [Google Scholar]
- 28.Andrews G.P., Strachan S.T., Benner G.E., Sample A.K., Anderson G.W., Jr., Adamovicz J.J. Protective efficacy of recombinant Yersinia outer proteins against bubonic plague caused by encapsulated and nonencapsulated Yersinia pestis. Infect Immun. 1999;67(March (3)):1533–1537. doi: 10.1128/iai.67.3.1533-1537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(April (6982)):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S., Chou T.H., Sakhatskyy P.V., Huang S., Lawrence J.M., Cao H. Identification of two neutralizing regions on the severe acute respiratory syndrome coronavirus spike glycoprotein produced from the mammalian expression system. J Virol. 2005;79(February (3)):1906–1910. doi: 10.1128/JVI.79.3.1906-1910.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooper J.W., Thompson E., Wilhelmsen C., Zimmerman M., Ichou M.A., Steffen S.E. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78(May (9)):4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S., Heilman D., Liu F., Giehl T., Joshi S., Huang X. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine. 2004;22(September (25–26)):3348–3357. doi: 10.1016/j.vaccine.2004.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu S., Wyatt R., Richmond J.F., Mustafa F., Wang S., Weng J. Immunogenicity of DNA vaccines expressing human immunodeficiency virus type 1 envelope glycoprotein with and without deletions in the V1/2 and V3 regions. AIDS Res Hum Retroviruses. 1998;14(January (2)):151–155. doi: 10.1089/aid.1998.14.151. [DOI] [PubMed] [Google Scholar]
- 34.Sodeinde O.A., Sample A.K., Brubaker R.R., Goguen J.D. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988;56(October (10)):2749–2752. doi: 10.1128/iai.56.10.2749-2752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sodeinde O.A., Goguen J.D. Genetic analysis of the 9.5-kilobase virulence plasmid of Yersinia pestis. Infect Immun. 1988;56(October (10)):2743–2748. doi: 10.1128/iai.56.10.2743-2748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S., Farfan-Arribas D.J., Shen S., Chou T.H., Hirsch A., He F. Relative contributions of codon usage, promoter efficiency and leader sequence to the antigen expression and immunogenicity of HIV-1 Env DNA vaccine. Vaccine. 2006;24(May (21)):4531–4540. doi: 10.1016/j.vaccine.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 37.Lu S., Manning S., Arthos J. Lowrie D., Walen R., editors. Antigen engineering in DNA immunizationMethods Mol Med. 1998;29:355–374. doi: 10.1385/1-59259-688-6:355. [DOI] [PubMed] [Google Scholar]
- 38.Swietnicki W., O’Brien S., Holman K., Cherry S., Brueggemann E., Tropea J.E. Novel protein-protein interactions of the Yersinia pestis type III secretion system elucidated with a matrix analysis by surface plasmon resonance and mass spectrometry. J Biol Chem. 2004;279(September (37)):38693–38700. doi: 10.1074/jbc.M405217200. [DOI] [PubMed] [Google Scholar]
- 39.Wang S., Arthos J., Lawrence J.M., Van Ryk D., Mboudjeka I., Shen S. Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J Virol. 2005;79(June (12)):7933–7937. doi: 10.1128/JVI.79.12.7933-7937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


