Abstract
Adolescents differ from adults in their acute sensitivity to several drugs of abuse, but little is known about the long-term neurobehavioral effects of adolescent drug exposure. To explore this further, we evaluated the locomotor responses to repeated cocaine administration in adolescent and adult male DBA/2J mice and alterations in extracellular levels of dopamine (DA) and glutamate (GLU) in the nucleus accumbens (NAc) in response to a subsequent cocaine challenge. Adolescent and adult mice were treated daily with saline or cocaine (10 mg/kg i.p) for 9 consecutive days. Ten days following the last injection, animals were implanted with microdialysis probes and 24 hr later microdialysis samples were collected before and after an acute cocaine challenge. Adolescents but not adults demonstrated development of behavioral sensitization to cocaine. Microdialysis procedures revealed that cocaine-treated mice displayed greater peak increases in extracellular DA in response to a subsequent cocaine challenge as compared to saline-treated mice, in contrast with lower peak increases in extracellular GLU. While adults exhibited greater peaks in extracellular DA in response to cocaine than adolescents, adolescent mice presented a more rapid onset of peak extracellular DA levels than adults. Our results indicate differences in the behavioral and neurochemical responses to cocaine in adolescent versus adult mice, which may be relevant to the increased risk of developing addiction in humans who are exposed to drugs of abuse during adolescence.
Keywords: adolescent, cocaine, sensitization, glutamate, dopamine, nucleus accumbens
1. Introduction
It has been suggested that personality characteristics manifested especially in adolescents, such as high levels of impulsivity and risk-taking, contribute to a greater vulnerability to the development of drug abuse (reviewed in Spear, 2000 and Chambers et al, 2003). Drug use is often initiated during adolescence, with cocaine use often being initiated as early as 12 years of age (1999 National Household Survey on Drug Abuse), when the central nervous system is still undergoing developmental rearrangements (Huttenlocher, 1984; Insel et al, 1990). Interestingly, adults having first experienced cocaine at a young age are more likely to be dependent on this drug than adults who initiated use at a later age (Anthony and Petronis, 1995).
The progressive increase in locomotor activity following repeated administration to cocaine, known as behavioral sensitization, is a very well established phenomenon used as a model of addiction (Epstein and Altshuler, 1978; Post et al, 1981; Hinson and Poulos, 1981; Partridge and Schenk, 1999). Behavioral sensitization to cocaine has been demonstrated in periadolescents rats (Laviola et al, 1995), inclusively with age-related variabilities in acute and chronic responses to psychoactive drugs (Spear and Brick, 1979; Laviola et al, 1995; 2001; Adriani et al, 1998; Bolanos et al, 1998; Cruz et al, 2005).
There are, however, inconsistencies in the reported age-dependent changes in behavioral and neurochemical sensitization to psychostimulants. For example, adolescent rats, but not adult rats, sensitize to amphetamine-induced nigrostriatal dopamine (DA) release (Laviola et al, 2001). In addition, some studies have revealed that periadolescent rodents sensitize to the locomotor-stimulating effects of cocaine (Laviola et al, 1995; Niculescu et al, 2005), while others show that this age group does not display behavioral sensitization to cocaine (Collins and Izenwasser, 2002; Guerriero et al, 2006).
An increasing body of literature has emerged describing the differential sensitivity to cocaine in adolescent and adult rodents (Laviola et al, 1995; Philpot and Kirstein, 1999; Collins and Izenwasser, 2002; Ehrlich et al, 2002; Stansfield and Kirstein, 2005; Frantz et al, 2007). For example, adolescent rats given an acute challenge with cocaine demonstrate a greater dopaminergic responses in the NAc that adult rats in a manner that is dependent on the degree of novel object exploration, a putative measure of impulsivity (Stansfield and Kirstein, 2005). Repeated cocaine exposure has been reported to produce a leftward shift in DA responsiveness in NAc of adolescent rats as compared to acute cocaine administration (Philpot and Kirstein, 1999; Badanich et al, 2006). However, with regards to the effects of repeated cocaine exposure, not all studies have produced similar results. For example, Frantz et al (2007) compared the motor and reinforcing effects of cocaine in adolescents and adults Wistar rats and measured extracellular DA in the NAc of both groups following a cocaine challenge. Although these investigators found age-related differences in behavioral activity, no differences were observed in increases in extracellular DA levels in the NAc produced by cocaine. On the other hand, Cao et al. (2007) showed that adolescent rats were less sensitive to the ability of cocaine to elevate extracellular levels of DA in the NAc as compared with adults.
Although the neurochemical variations that contribute to the behavioral differences between adolescents and adults are not well known, some studies propose these differences to be primarily mediated by a differential degree of maturation of dopaminergic systems in the brain (Shalaby et al, 1981; Spear and Brake, 1983; Kalsbeek et al, 1988; Teicher et al, 1995). Specifically, DA content, fiber density and DA receptors reach peak levels during adolescence and undergo pruning in several brain regions (Hohn and Wuttke, 1979; Kalsbeek et al, 1988; Lidow et al, 1991; Teicher et al, 1995; Tarazi et al, 1999). Not only do dopaminergic systems undergo complex changes during adolescence, but also other neurotransmitter systems involved in decision taking and cognitive processes. For instance, there is a massive loss of glutamatergic input to the PFC during adolescence in humans, monkeys and rats (Huttenlocher, 1984; Insel et al, 1990). The importance of this ontogenetic rearrangement resides on the fact that glutamatergic afferents from the PFC, together with those from amygdala and hippocampus, transmit executive, affective and memory information to the NAc, an important nucleus implicated in the rewarding effects of many drugs of abuse. During the period of adolescence, the PFC (responsible for impulsive behavior control) is still maturing. Considering that adolescents are guided by more impulsive behaviors and are more vulnerable to drug addiction, the neural basis of this may be a result of developmental changes in dopaminergic and glutamatergic systems in the forebrain (for review, see Chambers et al, 2003).
The aim of this study was to verify whether adolescent cocaine exposure would induce differential behavioral and neurochemical effects when these animals were re-exposed to a challenge cocaine dose. To achieve this goal, we first analyzed the locomotor response to cocaine in both adolescent and adult mice. We then investigated the effects of a subsequent challenge of cocaine on extracellular DA and glutamate (GLU) release in the NAc of both age groups using in vivo microdialysis techniques.
2. Results
2.1. Locomotor response to cocaine in adolescent and adult mice
Figure 1 shows the effect of repeated cocaine or saline treatment on locomotor activity in adolescent and adult mice. Three-way ANOVA (age × treatment × days) for repeated measures (days) revealed an age effect [F(1,87) = 4.16; p < 0.05], with adolescents displaying higher levels of locomotor activity than adults. A treatment effect [F(1,87) = 536.87; p < 0.01] showed that cocaine-treated mice displayed higher levels of locomotor activity when compared to saline groups. An age × days interaction [F(2,174) = 3.28; p < 0.05], treatment × days interaction [F(2,174) = 21.73; p < 0.01] and an age × treatment × days interaction [F(2,174) = 4.02; p < 0.01] were also observed. Post-hoc analysis of these interactions showed that no differences were found between adolescent and adult mice treated with saline. On the other hand, cocaine-treated adolescents displayed higher levels of locomotor activity as compared to the respective adult group on day 9 of treatment. Furthermore, while repeated cocaine increased locomotor activity in adolescent mice, adult mice did not sensitize to the locomotor stimulant effects of cocaine. Behavioral sensitization to the stimulant effects of cocaine appeared following the second injection in adolescent mice. A two-way ANOVA performed for data from day 18 revealed a treatment effect [F(1,26) = 57.6; p < 0.01] and a treatment × age interaction [F(1,26) = 5.27; p < 0.05]. Regardless of age, cocaine-treated mice displayed higher levels of locomotor activity as compared to saline-treated mice, which can be attributed to the stimulant effect of cocaine. In addition, cocaine sensitized adolescent mice displayed higher levels of locomotor activity as compared to the respective adult group.
Fig. 1.

(A) Effects of repeated treatment with cocaine (10 mg/kg i.p.) or saline on locomotor activity in adolescent and adult DBA/2J mice. Cocaine-treated adolescent mice (Adol-Coc) displayed higher levels of locomotor activity on days 2 and 9 as compared with day 1 of treatment (*, p<0.05), indicating the development of behavioral sensitization. On day 9, Adol-Coc mice displayed increased locomotor activity as compared with Adult-Coc mice (+, p<0.05). Saline-treated adolescent (Adol-Sal) and adult mice (Adult-Sal) showed no changes in locomotor data across all days tested. Data are presented as mean ± SEM. N=18 for all groups. (B) Following recovery from surgical implantation of microdialysis guide cannula, a subsequent cocaine challenge (10 mg/kg i.p.) was given on Day 18 to cocaine-pretreated mice to assess the expression of behavioral sensitization. Cocaine-pretreated adolescent mice displayed greater locomotor activity in response to this cocaine challenge as compared with cocaine-pretreated adult mice (*, p<0.05). Data are presented as mean ± SEM.
2.2. The effects of cocaine on extracellular DA and GLU release in the NAc of adolescent and adult mice
To assess possible changes in neurochemical systems involved in motivational circuitry that might result from adolescent cocaine exposure, we analyzed extracellular DA and GLU levels in the NAc in response to a subsequent cocaine challenge after repeated daily treatment with cocaine during adolescence or adulthood. Figure 3 shows the histological placement of dialysis probe membranes in the NAc. As can be seen, probes were found to be placed in both the shell and core subregions of the NAc.
Fig. 3.

Coronal diagrams of the mouse brain showing placement of microdialysis probes into the NAc, as estimated from histological sections. Numbers indicate distance (in mm) of each section from bregma. Drawings were taken from atlas of Franklin and Paxinos (2001).
Figure 2A shows the effect of a 10 mg/kg cocaine challenge on extracellular DA in the NAc of saline- and cocaine-treated adolescent and adult mice.
Fig. 2.
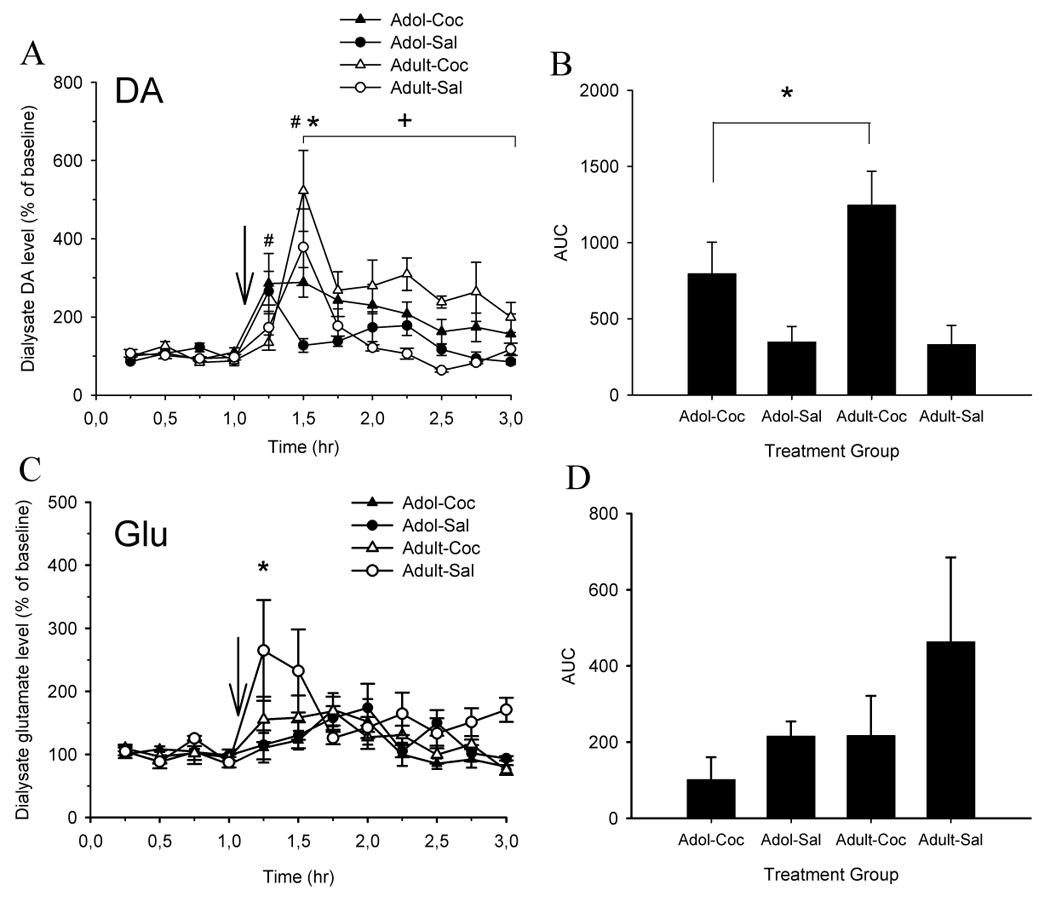
Effects of repeated cocaine or saline treatment on cocaine-induced increases in extracellular levels of dopamine (DA) and glutamate (GLU) in the NAc of adolescent and adult mice. (A, left panel) Cocaine-pretreated adult mice (Adult-Coc, n=10) displayed greater peak extracellular DA levels in the NAc as compared to cocaine-pretreated adolescent mice (Adol-Coc, n=11) at 30 min post cocaine injection (*,p<0.05). In addition, cocaine-induced increases in extracellular DA in Adult-Coc were higher throughout the post-injection period as compared with Adol-Coc mice (+,p<0.05). Peak cocaine-induced increases in dialysate DA content were observed within 15 min of injection in Adol-Coc and saline-pretreated adolescent mice (Adol-Sal, n=10). In contrast, peak cocaine-induced increases in dialysate DA content were observed within 30 min of injection in Adult-Coc and saline-pretreated adult mice (Adult-Sal, n=7) (#, p<0.05). (B, right panel) Area-under-the-curve (AUC) analysis revealed that cocaine-pretreated mice displayed greater overall increase in extracellular DA during the 2 hr post-injection period as compared with saline-pretreated mice (*, p<0.05). (C, left panel) Saline-treated Adult mice (n=7) displayed greater peak increases in extracellular Glu levels in the NAc as compared to saline-treated Adol mice (n=7) at 15 min post-injection (*, p<0.05). (D, right panel) (p>0.05). No differences in peak dialysate levels of Glu or AUC were observed between Adol-Coc (n=9) and Adult-Coc (n=7). For all panels, first four microdialysis samples represent baseline samples. Data are presented as mean ± SEM.
Average of the absolute DA levels in the first four samples (baseline) for adolescent (saline and cocaine) and adult (saline and cocaine) groups are, respectively, 94.33 ± 6.28, 99.11 ± 11.48, 106.96 ± 9.65, 99.89 ± 5.93. A three-way ANOVA (age × treatment × time) performed for the four baseline samples for each group showed no differences among groups [F(3,102) = 1.72; p > 0.05]. Overall, cocaine-pretreated mice presented larger increases in dialysate concentrations of DA in NAc compared to saline-pretreated mice [treatment effect: F(1,34) = 18.81; p < 0.01]. A main effect of time [F(11,374) = 11.88; p < 0,01] indicated that DA levels increased over baseline levels post-cocaine injection. A significant age × time interaction [F(11,374) = 4.23; p < 0,01] and a treatment × time [F(11,374) = 2.46; p < 0.01] were found. Follow-up analysis of these interactions demonstrated that adult mice displayed greater peak dialysate DA levels than adolescents at 30 min post-injection (Tukey, p < 0.05).
One–way analysis for repeated measures showed peaks in extracellular DA after cocaine challenge at 30 min for saline and cocaine-treated adult mice [F(11,66) = 5.19; p < 0.01 and F(11,88) = 8.17 p < 0.01, respectively]; and at 15 min for saline and cocaine-treated adolescent mice [F(11,99) = 5.45; p < 0.01 and F(11,110) = 2.68, p < 0.01, respectively].
Figure 2B shows the AUC of extracellular DA in NAc calculated after cocaine challenge. Statistics revealed a treatment effect [F(1,34) = 12.82; p < 0.01]. Cocaine-treated mice displayed greater overall increase in extracellular DA during the 2 hr post-injection period as compared with saline-treated mice.
The effects of cocaine on extracellular GLU in the NAc of saline and cocaine-treated adolescent and adult mice were also investigated (Fig. 2C). Average of the absolute GLU levels in the first four samples (baseline) for adolescent (saline and cocaine) and adult (saline and cocaine) groups are, respectively, 125.42 ± 5.02, 92.27 ± 8.83, 104.85 ± 6.02, 88.66 ± 5.94. A three-way ANOVA (age × treatment × time) performed for the four baseline samples for each group showed no differences among groups [F(3,78) = 1.2; p > 0.05]. Adults had a greater glutamatergic response following cocaine injection than adolescents [age effect: F(1,26) = 4.72; p < 0.05]. Cocaine significantly increased extracellular concentrations of GLU in NAc compared to the basal levels [time effect: F(11,286) = 5.64; p < 0.05], with saline-pretreated mice presenting higher change from baseline compared to cocaine-pretreated mice [treatment effect: F(1,26) = 4.30; p < 0.05]. A significant age × time interaction [F(11, 286) = 2.89; p < 0.01] was obtained. Follow-up analysis of this interaction demonstrated that saline-treated adult mice displayed greater peaks in extracellular GLU than saline-treated adolescent mice at 15 min post-injection (Tukey, p < 0.05).
One–way analysis for repeated measures showed peak levels of extracellular GLU after cocaine challenge at earlier times for adult mice as compared to adolescent mice. There were peaks in extracellular GLU after cocaine challenge at 15 min for saline and cocaine-pretreated adult mice [F(11,66) = 2.79; p < 0.01 and F(11,66) = 3.1 p < 0.01, respectively]; at 45 min for cocaine-pretreated adolescent mice [F(11,88) = 2.59, p < 0.01] and 60 min for saline-pretreated adolescent mice [F(11,66) = 1.85; p < 0.05].
AUC of extracellular GLU in NAc after cocaine challenge is shown in Figure 2D. No statistical changes were found among groups.
3. Discussion
Behavioral sensitization has been linked to the loss of control over the consumption of drugs and establishment of drug dependence (reviewed in Robinson and Becker, 1986). However, few studies have been made concerning behavioral sensitization in response to psychostimulant drugs in adolescents (Laviola et al, 1995; Adriani et al, 1998; Laviola et al, 2001; Collins and Izenwasser, 2002; Planeta and Marin, 2002), with reports of different profile of behavioral sensitization to cocaine in adolescents compared to adults (Laviola et al, 1995; Collins and Izenwasser, 2002; Frantz et al, 2007). Our findings showed that the development of behavioral sensitization after repeated cocaine administration was observed in adolescents; but there was only a trend toward behavioral sensitization in adult mice. Although behavioral sensitization to cocaine in adults has been extensively shown, this phenomenon relies on the treatment regimen employed (Stewart and Badiani, 1993). Since adult mice did not develop sensitization to the locomotor stimulant effect induced by cocaine, it is possible that a longer treatment regimen would be necessary to induce behavioral sensitization in these animals.
In the current study, we showed that adolescent and adult mice treated repeatedly with cocaine expressed sensitization to the cocaine-induced increase in extracellular DA overflow. However, there appears to be a dissociation between the locomotor effects of repeated cocaine exposure and subsequent cocaine-induced alterations in extracellular DA in the NAc. While sensitized adolescent mice displayed greater locomotor responses to repeated cocaine compared with adult mice, they had lower DA peak and overall increases in DA in the NAc in response to cocaine challenge. On the other hand, adolescent mice presented maximal DA elevations at earlier time points than adult mice. It was surprising to find an age-dependent dissociation between the magnitude of locomotor sensitizing response and the subsequent sensitizing response to the cocaine-induced release in extracellular DA in NAc. In relation to the locomotor sensitization, our results contradict studies showing that periadolescent rats exhibited lower locomotor sensitization to cocaine than adult counterparts (Frantz et al, 2007). In addition, these authors found no age-difference in cocaine-induced DA release in rats, which is also in opposition to our findings. This discrepancy may be explained by the fact that these authors used a different species as subjects as compared to our study (i.e., rats versus mice). Besides, differences between the present study and Frantz et al (2007) may very likely be due to differences in probe placements between these two studies.
Dopamine neurons in NAc are very well known for their role in rewarding processes, but these neurons are also involved in motivation, goal-direct behavior and attention (Schultz et al, 1997). Since the rewarding cocaine effects are due, at least in part, by the prolonging influence of DA on the NAc and PFC (Wise and Hoffman, 1992), the higher DA overflow in sensitized animals may suggest a neurochemical correlate of drug expectancy, although this is very speculative. Indeed, brain imaging studies in humans have shown a correlation between increased dopaminergic activity in NAc and striatum and reports of liking and craving (Risinger et al, 2005; Volkow et al, 2002). Data from AUC confirms that cocaine-treated mice, regardless of age, had greater DA overflow in response to a cocaine challenge as compared to naïve mice. The properties of DA system in the NAc are consistent with a role in the expression of motivation and action (Chambers et al, 2003).
Another interesting result is that adolescent mice presented a more rapid cocaine-induced increase in extracellular DA levels than adult mice, in contrast to more delayed increases in extracellular GLU levels as compared to adult mice. As pointed by Philpot and Kirstein (1999), an earlier onset of DA overflow may reflect a blend of the cocaine effect plus the overflow induced by expectancy of the drug. Our results suggest that this neurochemical expectancy response is age-dependent, with adolescent animals having an earlier anticipatory dopaminergic response than adult animals.
We also found a differential neurochemical pattern in relation to cocaine induced-changes in extracellular DA as compared to GLU in the NAc. While repeated cocaine treatment sensitized the dopaminergic response, this treatment induced a lower GLU response to a subsequent cocaine challenge as compared to saline treated mice. Naïve mice presented greater overall increase in extracellular GLU as compared to cocaine-treated mice following a cocaine challenge, regardless of age. This is in contrast with previous studies that showed a higher increase in GLU in the NAc of cocaine-sensitized rats compared with saline-treated rats (Pierce et al, 1996, Reid and Berger, 1996). The differences found between those studies and ours could be explained by the use of different species (rats vs mice). For example, implantation of probes in specific sub-region of the NAc (core or shell) is possible when the experimental subjects are rats, but this methodological sophistication is not possible to be reached in a very accurate way in mice. However, this discrepancy may not be only a matter of the used specie. The study of McFarland et al (2003), implicating DA and GLU in cocaine-induced reinstatement, showed a rise in extracellular GLU levels in the core of NAc only when extinguished cocaine self-administration was reinstated by a cocaine-priming injection, but not when yoked cocaine or saline rats were administered a cocaine prime. On the other hand, extracellular DA levels were increased independently of behavior. This study suggests that differently of DA, GLU levels are not always increased after acute or repeated cocaine administration. Moreover, it has been shown that repeated cocaine administration attenuates group I mGluR function in NAc. This diminished function might be associated with decreased level of extracellular Glu, given the capacity of group I mGluRs to stimulate GLU release (Nakazawa e cols., 1997; Swanson et al, 2001).
The analysis of the temporal curves of GLU levels (Fig. 2C) showed that cocaine increased GLU levels in NAc for all groups, which is in agreement with previous studies performed in rats (Smith et al, 1995; Reid et al, 1997). Glutamatergic inputs to NAc modulate the expression of behavioral sensitization, as demonstrated by several studies, through the blockade of behavioral sensitization to psychostimulants (Pierce et al, 1996) and ethanol (Camarini et al, 2000) by AMPA and NMDA antagonists. Nevertheless, a reduced responsiveness of NAc spiny cells to iontophoretic glutamate following repeated cocaine administration has also been demonstrated (White et al, 1995). The NAc receives excitatory glutamatergic projections from several brain regions including the basolateral amygdala, ventral subiculum of the hippocampus and the PFC. The dorsal region of the PFC, which sends excitatory amino acid inputs to the NAc, appears to be critically involved in the expression of behavioral sensitization to cocaine (Pierce et al, 1998).
It is not clear if the opposite effects we found for dopaminergic and glutamatergic responsiveness in cocaine-sensitized mice are connected. This is because the augmentation of GLU release induced by cocaine in NAc seems to be dependent on DA neurotransmission, given that DA antagonists block this effect (Reid et al, 1997). Studies have demonstrated that neostriatal synaptic responses mediated by NMDA receptors were potentiated by DA while those mediated by non-NMDA receptors were attenuated (Levine et al, 1996). The present results suggest that the blunted glutamatergic response we found in sensitized animals compared to saline-treated animals may reflect the influence of enhanced extracellular DA levels. However, modulatory effects of other neurotransmitters or neuromodulators, such as adenosine (Floresco et al., 2001; Quarta et al., 2004; Borycz et al., 2007), endogenous opioids (Fusa et al., 2005) or glycine (Leonetti et al., 2006) on these DA-GLU interactions cannot be ruled out.
One potential limitation of our study is the possibility that stress induced by transportation of the adolescent animals during shipment to our animal facility may have produced lasting changes in neural function and development, which in turn could have confounded the results obtained. Additional studies using adolescent animals bred within our animal facility (as opposed to being shipped at a specific age from a commercial vendor) are necessary to eliminate this possibility.
In summary, our results point to the behavioral and neurochemical differences between adolescent and adults DBA/2J mice in response to cocaine. Clearly, additional studies in other rodent species and strains are needed to determine the generality of these effects. However, if our results eventually become relevant to human drug addiction, it could be hypothesized that cocaine exposure during adolescence produces behavioral and neurochemical changes that are qualitatively different than those observed following cocaine exposure only in adulthood. The differential degree of CNS development during adolescence associated with the neurochemical differential response to the drug may explain partly these divergences.
4. Experimental procedures
4.1. Animals
Male adolescent DBA/2J mice (postnatal day 30 at the start of the experiments, weighing 17.5 ± 0.5 at the beginning of the experiments and 20.9 ± 0.4 at the end) and male adult mice (postnatal day 60 at the start of the experiments, weighing 24.6 ± 0.9 at the beginning of the experiment and 22.9 ± 0.4 at the end) were obtained from Jackson Laboratories (Bar Harbor, ME). Considering the difficulty to define the absolute margins of adolescence, we adopted the age range from PND 30–39 to treat the adolescent groups because the adolescence is certainly comprised into this period of time. According to Spear (2000) the age range from PND 28–42 is considered the age range which rodents exhibit adolescent-typical neurobehavioral and hormonal characteristics, although Laviola et al (2003) consider the adolescent period from weaning (PND 21) to adulthood (PND 60). Animals were housed 4 per cage in standard polycarbonate cages, with food and water freely available under a 12-h light/dark cycle with lights off at 1000 hr. All studies were performed in the dark phase of the light-dark cycle. Mice were allowed to acclimate to housing conditions for 7 days before initiation of the experiments. Animals were handled and injected with saline before starting the experiment to minimize stress induced by experimenter handling and injection procedures. All experimental procedures were conducted under National Institutes of Health Guidelines for the Care and use of Laboratory Animals (NIH Publication 80-23), and with the approval with an Institutional Animal Care and Use Committee.
4.2. Drugs
Cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in physiological saline (0.9% w/v sodium chloride) and administered via the intraperitoneal (i.p.) route in a volume of 1 ml per 100 g body weight at a dose of 10 mg/kg.
4.3. Locomotor activity testing
Locomotor activity was measured in 40 × 40 cm clear Plexiglas open field testing chambers (Hamilton-Kinder, San Diego, CA) equipped with infrared photobeams that were interfaced to a PC computer for automated monitoring of horizontal locomotor activity at 100 ms resolution. Activity chambers were housed individually in sound-attenuating cubicles equipped with a house light and exhaust fan to minimize external noises and odors. Mice received one injection of saline prior to the beginning of treatment. For the next 9 consecutive days, mice were injected with either saline or cocaine (10 mg/kg i.p.). On day 17, mice underwent surgical procedures (see below). On the following day, mice received an injection of either saline or cocaine, according to their respective treatment groups and locomotor activity was measured. Locomotor activity was monitored following drug administration for 15 min on days 1, 2, 9 and 18. Dose and trial duration was based on the study of Phillips et al. (1998). The schedule of all procedures, from pre-treatment to microdialysis sample collection, is shown in Table 1.
Table 1.
Summary of the experimental schedule
| Day | 0 | 1–2 | 3–8 | 9 | 10–16 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|
| Treat | |||||||||
| Sal | S | S | S | S | HC | Surgery | S | Probe impl | SC,C |
| Coc | S | C | C | C | HC | Surgery | C | Probe impl | SC,C |
| Activity | |||||||||
| Monitoring | Yes | Yes | Yes |
S, saline injections; C, cocaine injections; HC, home cage; Surgery, implantation of microdialysis guide cannula; Probe impl, implantation of microdialysis probe; SC, samples collection (4 samples prior cocaine injections-baselines-and 8 samples following cocaine injection). Locomotor activity was monitored on days 1, 2, 9 and 18.
4.4. Surgical and microdialysis procedures
Mice were anesthetized using isoflurane vaporized in medical grade breathing air and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) with mouse ear bar adapters. The isoflurane concentration was 5% for induction of anesthesia and maintained at 1–2% throughout the remainder of the surgery. The head was shaved and an incision was made over the skull. Two holes were drilled in the skull approximately 3.0 mm lateral and 3.0 mm posterior to bregma where miniature skull screws were inserted and fixed with dental acrylic resin to anchor the guide cannula to the skull. A hole was drilled directly above the NAc, +1.7 mm anterior and +0.9 mm lateral to bregma according to the atlas of Franklin and Paxinos (2001). A guide cannula 15 mm in length was stereotaxically lowered 5.0 mm below skull surface and secured with dental acrylic resin. A dummy probe was then inserted into the guide cannula to prevent obstruction, and the wound was then closed with sutures. At the end of surgery, mice were placed individually in cylindrical Plexiglas microdialysis cages (Instech Laboratories, Plymouth Meeting, PA) with ad libitum access to food and water. Three days after surgery, mice were lightly re-anesthetized with isoflurane as described above and microdialysis probes (CMA/7, 1 mm cuprophane membrane, CMA Microdialysis, North Chelmsford, MA) were inserted into the microdialysis guide cannula. Artificial cerebrospinal fluid (aCSF, Harvard Apparatus, Holliston, MA) was pumped through a dual channel liquid swivel (Instech Laboratories) in a syringe pump (Harvard Apparatus) that was connected to the probe via fluorinated ethylene-propylene tubing (FEP, 0.005” ID) at a flow rate of 0.5 µl/min overnight. Composition of the aCSF was as follows (in mM): 150 Na+, 3 K+, 1 P, 0.8 Mg2+, 1.4 Ca2+, 155 Cl−, pH=7.4. Following overnight recovery from probe implantation, the flow rate was adjusted to 2.0 µl/min and after a 1 hr re-equilibration period, dialysis samples were collected at 15 min intervals. Four baseline samples were collected, followed by administration of 10 mg/kg cocaine i.p., after which post-injection samples were collected for an additional 120 min. All microdialysis samples were collected into polyethylene tubes containing 5.0 µl of 0.5 M perchloric acid to prevent oxidation of DA. Immediately following collection, samples were split in two 15 µl aliquots and stored at −80°C until analysis of neurotransmitter content by HPLC (see below).
4.5. Analysis of Dialysate Dopamine and Glutamate Levels
DA and GLU were analyzed by HPLC electrochemical detection as described previously (Olive et al., 2000; Griffin and Middaugh, 2006) with slight modifications. Briefly, for DA analysis, 10 µl of each sample was injected onto a SPER C18 reverse-phase narrowbore column (100 × 2.1 mm, Princeton Chromatography, Cranbury, NJ) using an Alcott Model 718 AL Autosampler (Norcross, GA). Flow rate through the column was 0.23 ml/min and controlled by a Model LC1120 isocratic pump (GBC Scientific, Hampshire, IL). A Decade Amperometric Electrochemical Detector (Antec Leyden, The Netherlands) was set to a working potential of +400 mV. Mobile phase consisted of 6% methanol, 65 mg/l octane sulfonic acid, 40 mg/l EDTA, 0.05 M phosphoric acid, 0.05 M citric acid; pH=3.0. Data were quantified by comparing peak areas against those of a four-point calibration of DA standards (0, 1, 5 and 10 pg/µl) run every 10–20 samples. For GLU analysis, immediately prior to injection, 10 µl of dialysis sample were derivatized with 0.7 mg/ml o-phthaldehyde in the presence of β-mercaptoethanol in sodium borate buffer (0.1 M, pH = 9.3) via a refrigerated autosampler (ESA, North Chelmsford, MA). Fifteen µl of the derivatized sample was then injected onto an 80 × 3.2 mm reversed phase C18 column (HR-80, ESA). Mobile phase, consisting of 0.1 M Na2HPO4, 25% methanol, pH = 6.75, was pumped at a flow rate of 0.6 ml/min. Derivatized GLU was detected by a post-column Model 5014B electrochemical detector cell (ESA, electrode 1 = +150 mV, electrode 2 = +550 mV) connected to computer via a CoulArray interface (ESA). Glutamate levels from microdialysis samples were quantified by comparing computer-integrated peak areas of samples with those of L-glutamate standards using a three-point calibration curve (0, 50 and 250 pg/µl) run every 20 samples. The detection limit was approximately 0.1 pg/µl for DA and 5 pg/µl for GLU.
4.6. Histological Verification of Probe Placement
Following experimentation, mice were euthanized by cervical dislocation. Brains were removed and placed in 10% formalin at 4°C for 1 week prior to being cut at 30 µm sections on a cryostat. Probe placement in the nucleus accumbens was histologically verified according to the atlas of Franklin and Paxinos (2001). Only data from mice with probe placement found to be within the NAc were included in the statistical analysis (Fig 3).
4.7. Microdialysis data analyses
Microdialysis data were analyzed by percent of basal DA or GLU following cocaine administration. The mean baseline value was calculated by averaging the concentration of the four basal dialysate samples. The variation of concentrations in each sample was then expressed as a percentage of the baseline value. The AUC (area under the curve) was calculated by taking the percent change in DA or GLU release from baseline (i.e., percent of baseline minus 100) for each microdialysis sample following cocaine injection and summing those values for each individual animal.
4.8. Statistical analyses
Statistical analyses of data for behavioral sensitization and microdialysis data were evaluated using a three-way analysis of variance (ANOVA) (age × treatment × days for behavioral and age × treatment × time for microdialysis) with days or time as repeated measures. Two-way ANOVAs (age × treatment) were performed when necessary for both behavioral and microdialysis analysis, followed by post hoc comparison with Tukey’s test. A one-way ANOVA for repeated measures was used to determine differences in the temporal curve of neurotransmitter concentrations for each group.
Acknowledgements
Supported by the Center for Drug and Alcohol Programs at the Medical University of South Carolina, FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo - 05/56739-8) and CCInt-USP (Comissão de Cooperação Internacional da Universidade de São Paulo).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Petronis KR. Early-onset use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Borycz J, Pereira MF, Melani A, Rodrigues RJ, Köfalvi A, Panlilio L, Pedata F, Goldberg SR, Cunha RA, Ferré S. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem. 2007;101:355–363. doi: 10.1111/j.1471-4159.2006.04386.x. [DOI] [PubMed] [Google Scholar]
- Camarini R, Frussa-Filho R, Monteiro MG, Calil HM. MK-801 blocks the development of behavioral sensitization to ethanol. Alcohol Clin Exp Res. 2000;24:285–290. [PubMed] [Google Scholar]
- Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007;32:2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Cruz FC, DeLucia R, Planeta CS. Differential behavioral and neuroendocrine effects of repeated nicotine in adolescent and adult rats. Pharmacol Biochem Behav. 2005;80:411–417. doi: 10.1016/j.pbb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Ehrlich ME, Sommer J, Canas E, Unterwald EM. Periadolescent mice show enhanced ΔFosB upregulation in response to cocaine and amphetamine. J Neurosci. 2002;22:9155–9159. doi: 10.1523/JNEUROSCI.22-21-09155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein PN, Altshuler HL. Changes in the effects of cocaine during chronic treatment. Res Commun Chem Pathol Pharmacol. 1978;22:93–105. [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Fusa K, Takahashi I, Watanabe S, Aono Y, Ikeda H, Saigusa T, Nagase H, Suzuki T, Koshikawa N, Cools AR. The non-peptidic delta opioid receptor agonist TAN-67 enhances dopamine efflux in the nucleus accumbens of freely moving rats via a mechanism that involves both glutamate and free radicals. Neuroscience. 2005;130:745–755. doi: 10.1016/j.neuroscience.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Griffin WC, Middaugh LD. The influence of sex on extracellular dopamine and locomotor activity in C57BL/6J mice before and after acute cocaine challenge. Synapse. 2006;59:74–81. doi: 10.1002/syn.20218. [DOI] [PubMed] [Google Scholar]
- Guerriero R, Hayes M, Dhaliwal S, Ren J, Kosofsky B. Preadolescent methylphenidate versus cocaine treatment differ in the expression of cocaine-induced locomotor sensitization during adolescence and adulthood. Biol Psychiatry. 2006;60:1171–1180. doi: 10.1016/j.biopsych.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Hinson RE, Poulos CX. Sensitization to the behavioral effects of cocaine: modification by Pavlovian conditioning. Pharmacol Biochem Behav. 1981;15:559–562. doi: 10.1016/0091-3057(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Hohn KG, Wuttke W. Ontogeny of catecholamine turnover rates in limbic and hypothalamic structures in relation to serum prolactin and gonadotropin levels. Brain Res. 1979;179:281–293. doi: 10.1016/0006-8993(79)90444-x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Mental Defic. 1984;88:488–496. [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard LE. The ontogeny of excitatory amino acid receptors in rat forebrain: I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Behav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitization to D-amphetamine in periadolescent but not in adult rats. Pharmacol Biochem Behav. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Leonetti M, Desvignes C, Bougault I, Souilhac J, Oury-Donat F, Steinberg R. 2-Chloro-N-[(S)-phenyl [(2S)-piperidin-2-yl] methyl]-3-trifluoromethyl benzamide, monohydrochloride, an inhibitor of the glycine transporter type 1, increases evoked-dopamine release in the rat nucleus accumbens in vivo via an enhanced glutamatergic neurotransmission. Neuroscience. 2006;137:555–564. doi: 10.1016/j.neuroscience.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Levine MS, Li Z, Cepeda C, Cromwell HC, Altemus KL. Neuromodulatory actions of dopamine on synaptically-evoked neostriatal responses in slices. Synapse. 1996;24:65–78. doi: 10.1002/syn.890240102. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proc Natl Acad Sci U S A. 1991;88:10218–10221. doi: 10.1073/pnas.88.22.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Mikawa S, Ito M. Persistent phosphorylation parallels long-term desensitization of cerebellar Purkinje cell AMPA-type glutamate receptors. Learn Mem. 1997;3:578–591. doi: 10.1101/lm.3.6.578. [DOI] [PubMed] [Google Scholar]
- Niculescu M, Ehrlich ME, Unterwald EM. Age-specific behavioral responses to psychostimulants in mice. Pharmacol Biochem Behav. 2005;82:280–288. doi: 10.1016/j.pbb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Hodge CW. Microdialysis in the mouse nucleus accumbens: a method for detection of monoamine and amino acid neurotransmitters with simultaneous assessment of locomotor activity. Brain Res Protoc. 2000;5:16–24. doi: 10.1016/s1385-299x(99)00054-9. [DOI] [PubMed] [Google Scholar]
- Partridge B, Schenk S. Context-independent sensitization to the locomotor-activating effects of cocaine. Pharmacol Biochem Behav. 1999;63:543–548. doi: 10.1016/s0091-3057(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Kirstein CL. Repeated cocaine exposure: effects on catecholamines in the nucleus accumbens septi of periadolescent animals. Pharmacol Biochem Behav. 1999;62:465–472. doi: 10.1016/s0091-3057(98)00198-1. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Huson MG, McKinnon CS. Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. J. Neurosci. 1998;18:3023–3034. doi: 10.1523/JNEUROSCI.18-08-03023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Reeder DC, Hicks J, Morgan ZR, Kalivas PW. Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience. 1998;82:1103–1114. doi: 10.1016/s0306-4522(97)00366-7. [DOI] [PubMed] [Google Scholar]
- Planeta CS, Marin MT. Effects of cocaine on periadolescent rats with or without early maternal separation. Braz J Med Biol Res. 2002;35:1367–1371. doi: 10.1590/s0100-879x2002001100015. [DOI] [PubMed] [Google Scholar]
- Post RM, Lockfeld A, Squillace KM, Contel NR. Drug-environment interaction: context dependency of cocaine-induced behavioral sensitization. Life Sci. 1981;28:755–760. doi: 10.1016/0024-3205(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Quarta D, Borycz J, Solinas M, Patkar K, Hockemeyer J, Ciruela F, Lluis C, Franco R, Woods AS, Goldberg SR, Ferré S. Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation. J Neurochem. 2004;91:873–880. doi: 10.1111/j.1471-4159.2004.02761.x. [DOI] [PubMed] [Google Scholar]
- Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- Reid MS, Hsu K, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shalaby IA, Dendel PS, Spear LP. Differential functional ontogeny of dopamine presynaptic receptor regulation. Brain Res. 1981;227:434–439. doi: 10.1016/0165-3806(81)90082-1. [DOI] [PubMed] [Google Scholar]
- Smith JA, Mo Q, Guo H, Kunko PM, Robinson SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brick J. Cocaine-induced behavior in the developing rat. Behav Neural Biol. 1979;26:401–415. doi: 10.1016/s0163-1047(79)91410-9. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Neurochemical effects of cocaine in adolescence compared to adulthood. Dev Brain Res. 2005;159:119–125. doi: 10.1016/j.devbrainres.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–313. [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley P, Kalivas PW. Repeated cocaine administration attenuates Group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J. Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol. 2002;13:371–377. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- Wise RA, Hoffman DC. Localization of drug reward mechanisms by intratecal injections. Synapse. 1992;10:247–263. doi: 10.1002/syn.890100307. [DOI] [PubMed] [Google Scholar]


