Abstract
While clozapine is the acknowledged superior pharmacotherapeutic for the treatment of schizophrenia, the side effect profile, which includes potentially fatal complications, limits its usefulness. Central administration of clozapine directly into the brain could circumvent many of the side effect issues due to the dramatic reduction in dose and the limitation of the drug primarily to the CNS. The present study demonstrates that clozapine can be formulated as a stable solution at physiological pH, which does not have in vitro neurotoxic effects at concentrations which may be effective at treating symptoms. Acute central administration improved auditory gating deficits in a mouse model of schizophrenia-like deficits. Assessment of behavioral alterations in rats receiving chronic central infusions of clozapine via osmotic minipump were performed with the open field and elevated plus mazes. Neither paradigm revealed any detrimental effects of the infusion. While these data represent only an initial investigation, they none-the-less suggest that central administration of clozapine may be a viable alternate therapeutic approach for schizophrenia patients which may be effective in symptom reduction without causing behavioral or neurotoxic effects.
Keywords: intracerebroventricular, rats, DBA/2 mice, elevated plus maze, open field maze, auditory gating
1. Introduction
A primary reason for the ineffective treatment of schizophrenia is the numerous drawbacks of atypical antipsychotics, as currently used. Ineffective treatment is a consequence of medication side effects, failure to achieve therapeutic dose, and problems with patient compliance. Prospective studies, with up to 20 years of follow-up, have demonstrated that 50-70% of schizophrenia patients have a persistent course of illness and only 20-30% of patients are able to lead somewhat normal lives (Fleischhaker et al, 2005).
Clozapine has long been known as a superior medication, as highlighted in the recently published NIH-sponsored CATIE trial for the treatment of schizophrenia. This study demonstrated that clozapine is superior to other atypical antipsychotics as well as the typical antipsychotics (McEvoy et al, 2006). Older studies showed that clozapine is one of the most effective oral atypical antipsychotics due to 1) superior improvement in positive and negative symptoms, 2) efficacy in the treatment of refractory schizophrenia, and 3) reduction in patient suicide risk (Reid et al, 1998, Volvavka et al, 2002, Azorin et al 2001, Buchanan et al, 1998). Even when observational studies are considered, as opposed to clinical trials, clozapine has been shown to have superior efficacy (Brambilla et al 2002) particularly with refractory patients. However, clozapine has a 1% incidence rate of agranulocytosis and a 3% incidence rate of neutropenia (Atkin et al, 1996, Alvir et al, 1993), potentially lethal systemic side effects which limit its use. It has been reported that even among the patients who will consent to use clozapine, 17% discontinue use due to systemic side effects (Iqbal et al, 2003). Design of a less-toxic clozapine has been a pharmaceutical development goal for the last 25 years.
Central administration of clozapine directly into the ventricular space via a chronically implanted catheter can potentially meet the criterion of reduced toxicity and, when combined with implantation of a programmable pump system such as those currently used for intraspinal drug delivery, has the potential to significantly benefit patient adherence. Ventricular administration of opiates has been shown to have minimal and accepted complications in a Cochrane review of 250 published cases of cancer related pain (Ballantyne et al, 2006). Intramuscular (IM) formulations (e.g. rispiradone, olanzapine and haloperidol) do not significantly avoid systemic exposure and are also limited by the inability to halt medication absorption after injection. Transdermal atypical antipsychotics currently under development may improve compliance, eliminate the pain of IM injections, and permit abrupt discontinuation, but are still limited by constant dosing, and more importantly, unaltered peripheral side effect profile. These side effects are the most profoundly limiting issue in antipsychotic administration, reducing patient acceptance of the medication, potentially resulting in patient illness (e.g., liver toxicity and cardiac conduction deficits) (Lublin et al 2005; Iqbal et al 2003; Miller 2000) and mortality (e.g., bone marrow failure).
The development of formulations for clozapine for central administration is relatively unexplored. As noted in the Kapur study (Kapur et al 2003), it is difficult to attain the concentrations required for central administration with drugs as insoluble as clozapine. The present studies assessed formulation of clozapine using hydroxypropyl-β–cyclodextrin (HPBCD), a compound with demonstrated acceptable toxicity when administered intrathecally (Yaksh et al 1991, Jang et al, 1992). In vitro toxicology was performed to establish an indication of the level of neurotoxicity of direct application of HPBCD alone and clozapine formulated with HPBCD. Efficacy of centrally administered clozapine was assessed in the DBA/2 mouse model of deficient auditory gating in schizophrenia (Stevens et al, 1996), an accepted and widely used animal model for schizophrenia drug development (Stevens et al, 1998; Hashimoto et al, 2005; Radek et al, 2006). Finally, behavioral tests (open field and elevated plus mazes) were performed to determine if central administration of clozapine produced any overt behavioral alterations.
2. Methods
2.1 Solubility of formulated clozapine
Clozapine is insoluble at neutral pH, requiring either organic solvents or a low pH in order to be solubilized (Merck Index, 2004). Because it is desirable to formulate drugs at physiological pH (∼7.4), and because degradation accelerates under acidic conditions (Hasan et al 2002, Yaksh 1999), HPBCD was used to solubilize clozapine at pH 7.4. Clozapine (1 mg/ml) was solubilized under acidic conditions (pH ∼2.0 with HCl), increasing mole ratios of HPBCD (0-4) added and the solutions titrated back toward neutral pH with NaOH. Since precipitation causes clozapine to form insoluble particles, light scattering (absorbance at 500 nm in a Hitachi UV/VIS spectrophotometer; model U-2001) can be used to monitor the loss of solubility as the formulation is alkalinized. After each addition of NaOH, the pH was determined and the solution was transferred to a quartz cuvette to quantify clozapine precipitation via light scattering. All formulations used in subsequent experiments utilized a HBPCD:clozapine molar ration of 4:1, and were filter sterilized (0.22 μm) prior to ICV administration
2.2 In vitro neurotoxicology
A preliminary assessment of the toxicity of HBPCD, with or without clozapine, in primary mouse cortical neuron cultures was performed. Primary cortical cultures were obtained from fetal (E15) C57BL/6J mice as previously published (Donohue et al 2006). After dissection, and cellular dissociation, the cells were washed with Dulbecco's Modified Eagle's Medium with 10% fetal bovine serum. Following centrifugation, the cells were resuspended in plating medium, counted with trypan blue and plated at a constant density of 6.5 ×104 cells per well in a 96-well pre-coated plate. The plating media was 2% B27, 0.5 mM L-glutamine and 25 mM glutamic acid in NEUROBASAL medium (Invitrogen, Carlsbad, CA). On the 4th day, half the media was replaced with fresh media that did not contain glutamic acid. The cultures were maintained at 37° C in a humidified atmosphere of 5% CO2. On the 7th day of culture incubation, half the media (40 m1) was replaced with media containing various concentrations of the clozapine-HPBCD formulation or HPBCD alone. The cultures were incubated and cell toxicity assayed at 24, 48 and 72 hours. Viability was assessed by the MTT (3-(4,5 diethylthiazol-2-yl)-2,5) diphenyltetrazolium bromide) assay, CellTiter 96 Non-radioactive cell Proliferation Assay (Promega, Madison, WI) and by visual examination.
2.3 Auditory gating in DBA/2 mice with central clozapine administration
In order to assess the ability of centrally administered clozapine to improve deficient auditory gating, DBA/2 male mice (20-25 g, Harlan Sprague Dawley, San Diego, CA) were anesthetized with chloral hydrate (400 mg/kg, ip) and pyrazole (400 mg/kg, ip) to inhibit metabolism of the chloral hydrate. The mice were then placed in a mouse adapter for the stereotaxic instrument and the scalp incised. A burr hole was opened over the dorsal hippocampus and another over the contralateral anterior cortex. A stainless steel, teflon-coated recording electrode was lowered to the CA3 region of the dorsal hippocampus; final placement was determined by the presence of complex spike activity typical of hippocampal pyramidal neurons (Miller and Freedman, 1995). An identical electrode was placed on dura over the anterior cortex to act as a reference. A third burr hole was opened over the lateral anterior ventricle on one side and a 26 gauge needle connected to a Hamilton microliter syringe was lowered to the anterior-lateral ventricular space. Miniature earphones attached to hollow ear bars, placed at the externalization of the aural canal, delivered the auditory stimuli. EEG responses to paired click stimuli (3000 Hz, 10 msec, 70 dB SPL, presented 0.5 sec apart, with 9 sec between pairs) were amplified 1000 times with bandpass filtering at 1-500 Hz and led to a computer for storage and analysis. Data were collected and analyzed using the SciWorks acquisition system (DataWave, Berthoud, CO). The responses to 16 pairs of stimuli were collected and averaged at 5-minute intervals. The maximum negativity between 20 and 60 msec after the stimulus was selected and measured relative to the preceding positivity. This composite component has been shown to be less variable than either component alone (Hashimoto et al 2005). Three parameters were measured per record: conditioning amplitude—the magnitude of the response to the first stimulus, test amplitude—the magnitude of the response to the second stimulus, and TC ratio—the ratio of the test amplitude/conditioning amplitude. A TC ratio of 0.4 or less is evidence of normal auditory sensory inhibition. Four or five baseline records were obtained per mouse prior to intracerebroventricular (ICV) clozapine administration (0.1, 0.5 or 1 μg in 1 μl) and records were obtained at 5 minute intervals for 90-95 minutes after administration.
2.4 In vivo behavioral toxicity studies in rats
Male Sprague Dawley rats (250-300 g, Harlan Sprague Dawley, San Diego, CA) were implanted with Alzet osmotic minipumps connected to brain infusion cannula (Durect, Cupertino, CA) aimed at the lateral anterior ventricle. The pumps delivered saline, 10 or 17 mg/ml clozapine formulated in HPBCD (HPBCD:clozapine molar ratio = 4) at a rate of 0.25 μl/hr for 28 days. The rats were permitted to recover for 7 days prior to behavioral testing. On the 7th day, one half of the rats in each group were tested in the elevated plus maze, while the other half were tested in the open field maze. One week later (day 14 after pump implantation) the groups were reversed. The elevated plus maze has been described elsewhere (Overstreet et al 2003). Briefly, the study consisted of placing the rat in the center region of a black Plexiglas + shaped platform with 13 cm wide arms. Two of the arms were enclosed by 32 cm high walls while the remaining 2 arms were open. The maze was elevated 51 cm off the floor. The movements of the rat were computer tracked for the 5 minutes of the paradigm and the percent time spent in each type of arm and distance traveled in each arm (open, closed or in the center square) computer analyzed (Limelight 2, Actimetrics, Wilmette, IL). The open field maze has also been described elsewhere (Overstreet et al 2003). The open field consisted of a 104 × 104 cm square of black Plexiglas with 39 cm high walls. The rat was placed near one side wall of the field and allowed to explore at will for 5 minutes during which its activity was computer monitored (Limelight 2, Actimetrics, Wilmette, IL). The field was divided into 3 regions (3 concentric squares), the outer region (adjacent to the walls), a middle region and the center square. The percent time and distance traveled in each region were analyzed.
2.5 Statistical analysis
The sensory gating data were analyzed by repeated measures multivariate analysis of variance. The open field and elevated plus maze data were analyzed with analysis of variance (SPSS, Inc, Chicago, IL). Where statistical significance was found, Fisher's LSD a posteriori analysis was performed.
3. Results
3.1 Clozapine Solubility
Because clozapine is an organic compound that is “practically insoluble” in water, it was necessary to use an excipient to improve the solubility at physiological pH. Preliminary studies indicated that HPBCD could provide this improved solubility. Figure 1 shows the level of light scattering for solutions of clozapine formulated at different HPBCD-to-clozapine molar ratios over a pH range of 2 to 12. As the solutions were titrated from acidic conditions toward physiological pH, the clozapine formulation lacking HPBCD exhibited a sharp increase in light scattering between pH 6 and 7, indicative of precipitation (Figure 1). Formulation of clozapine at increasing molar ratios of HPBCD maintained solubility at progressively higher pH, and molar ratios ≥ 3 completely prevented precipitation even under very alkaline conditions (pH ∼ 12). Upon visual inspection, the solutions formulated with adequate levels of HPBCD maintained a clear, yellow appearance indicating that the clozapine remained fully solubilized. Mole ratios of HPBCD-to-clozapine that maintained solubility at pH ∼12 were capable of maintaining clozapine solubility at physiological pH for two months at 37°C.
Figure 1.
Clozapine (1 mg/ml) was solubilized under acidic conditions, and the solution pH was titrated up toward physiological pH. In the absence of HPBCD, clozapine precipitation at approximately pH 6.5 is indicated by a dramatic increase in light scattering. Formulation of clozapine at increasing HPBCD-to-clozapine mole ratios maintains solubility at progressively higher pH, and precipitation is completely eliminated at mole ratios of 3:1 and 4:1. The ability of HPBCD to maintain clozapine solubility, even under highly alkaline conditions (pH ∼12), is an indicator of how effectively the drug is solubilized under these conditions.
3.2 In vitro toxicology
Assessment of in vitro neuronal toxicity demonstrated that HPBCD, alone at percent concentrations ranging from 0.002 to 0.1, there was no significant reduction in cell viability (Figure 2A) consistent with previous studies (Yaksh et al. 1991, Jang et al., 1992). When clozapine at various concentrations ranging from 0 to 150 μM was formulated with HPBCD, cells remained viable up to a clozapine concentration of 10 μg/ml (Figure 2B); approximately 100-fold higher than that required for efficacy in the mouse auditory gating paradigm (see section 3.3).
Figure 2.

A. This graph shows the cell viability at 24 hours at dilutions of HPBCD in culture media from 0.002% to 0.1%. There was no significant reduction in neuronal viability with the HPBCD solutions used to solubilize clozapine. B. When clozapine was formulated with HPBCD and added to the cells, significant toxicity was observed at final clozapine concentrations of 10 μg/ml (30 μM) or higher. This toxicity is similar to that reported to occur to human neutrophils and monocytes (Gardner et al. 1998).
3.3 Auditory gating in DBA/2 mice with central clozapine administration
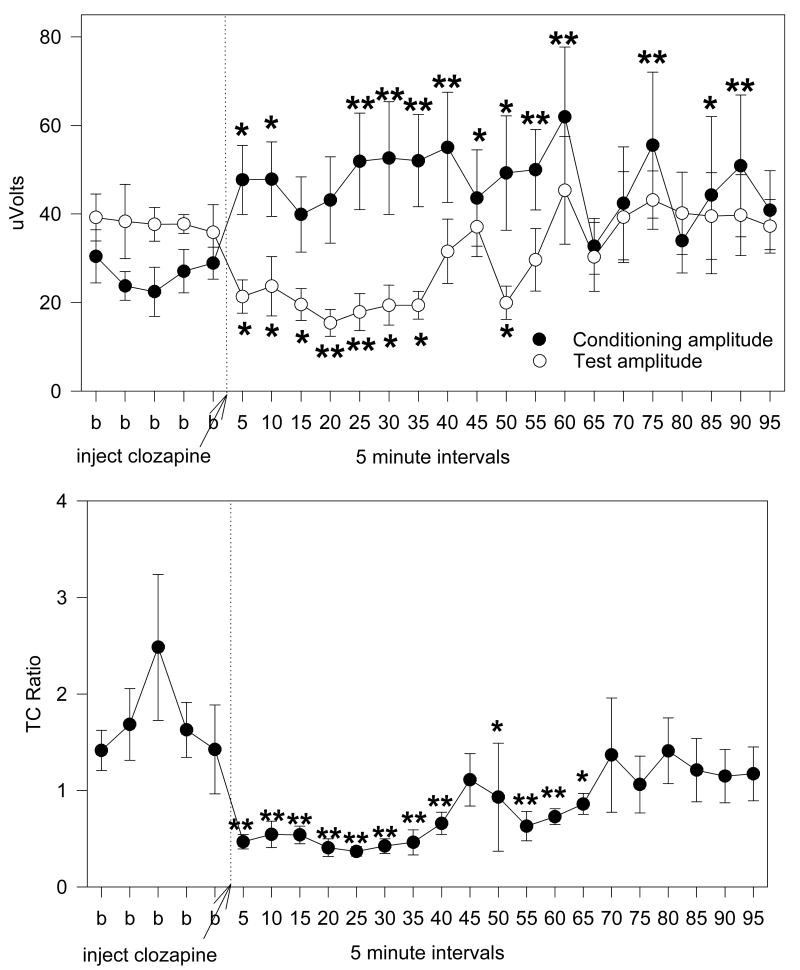
ICV clozapine improved auditory gating in the DBA/2 mouse in a dose dependent manner. The lowest dose (0.1 μg) failed to show any significant change in conditioning (F(21,147)=1.28, p=0.196) or test amplitude (F(21,147)=0.50, p=0.968) or in TC ratio (F(21,147)=0.94, p=0.541) (Figure 3). In contrast, both the 0.5 and 1.0 μg doses showed significant improvement in TC ratio (F(23,184)=3.07, p<0.001; F(23,115)=3.08, p<0.001, respectively) (Figures 4 and 5). Fisher's LSD a posteriori analyses showed extended periods of decreased TC ratio for both doses. When the conditioning and test amplitudes for the 0.5 μg dose were analyzed, the conditioning amplitude was found not to change significantly (F(23,184)=1.48, p=0.083) while significant decreases were observed in the test amplitude (F(23,184)=2.80, p<0.001) (Figure 4), though only two time points attained significance in a posteriori analyses. Analyses of the amplitudes of the conditioning and test responses for the 1.0 μg revealed significant changes for both responses (F(23,115)=2.50, p<0.001; F(23,115)=2.58, p=0.001, respectively). Fisher's LSD found significant increases in conditioning amplitude for most of the 95 minutes of recording post injection, while the test amplitude was significantly reduced from 5-35 minutes post injection (Figure 5).
Figure 3.
The 0.1 μg clozapine in 1.0 μl injection of clozapine into the anterior lateral ventricle of male DBA/2 mice did not significantly alter any of the auditory gating variables tested. Four baseline records (b) were obtained prior to ICV administration of the clozapine (at the arrow) and records were obtained at 5 minute intervals for 90 minutes after injection. Data are mean ± SEM, n=8.
Figure 4.
The 0.5 μg clozapine in 1.0 μl injection of clozapine into the anterior lateral ventricle of male DBA/2 mice caused a significant reduction in test amplitude which produced a significant reduction in TC ratio. While in increase in conditioning amplitude can be seen in the graph, it failed to attain significance. Five baseline records (b) were obtained prior to ICV injection of clozapine (at the arrow) and records were taken at 5 minute intervals for 95 minutes after injection. Data are mean ± SEM; n=8; * p <0.05, ** p <0.01, Fishers' LSD.
Figure 5.
The 1.0 μg clozapine in 1.0 μl injection of clozapine into the anterior lateral ventricle of male DBA/2 mice produced significance increases in conditioning amplitude and decreases in test amplitude which combined to produce a sustained decrease in TC ratio. Five baseline records (b) were obtained prior to ICV injection of clozapine (at the arrow) and records were taken at 5 minute intervals for 95 minutes after injection. Data are mean ± SEM; n=8; * p <0.05, ** p <0.01, Fishers' LSD.
3.4 In vivo behavioral studies in rats
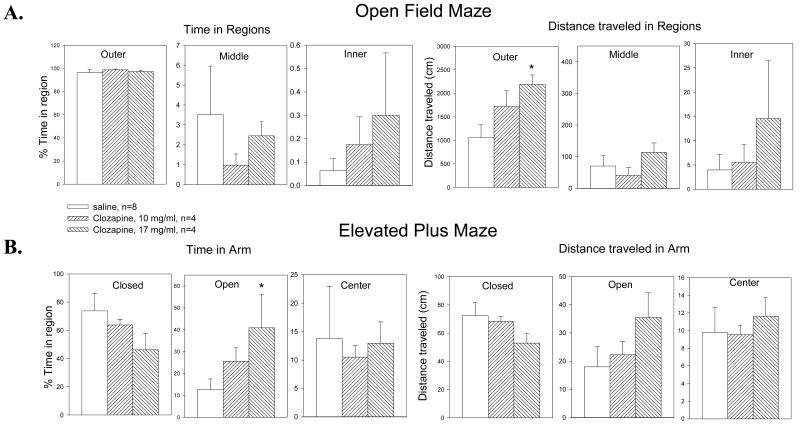
Rats with chronic ICV administration of clozapine (10 or 17 mg/ml) in HPBCD were tested in both the open field and elevated plus mazes to determine if there were any behavioral alterations produced by the intervention. The data were compared to data from rats which had received only saline ICV administration. Analysis of variance for the open field study, analyzing percent time spent in specific regions and total distance traveled, showed significant differences only in the distance traveled in the outer square of the maze (Table 1). Fisher's LSD showed that the distance for the 17 mg/ml dose was significantly increased over that for saline (Figure 6A). When the data for the elevated plus maze were similarly analyzed, the percent time spent in the open arms of the maze was significantly different (Table 1) and a posteriori analyses again showed that the percent time for the 17 mg/ml dose was significantly elevated compared to the saline injection (Figure 6B).
Table 1.
ANOVA Results for Behavioral Paradigms
| Paradigm | Variable | Region | F(2,15) | p |
|---|---|---|---|---|
| Open Field | Percent time in region | Outer | 0.441 | 0.653 |
| Middle | 0.489 | 0.624 | ||
| Center | 0.887 | 0.435 | ||
| Distance traveled in region | Outer | 5.177 | 0.022 | |
| Middle | 1.201 | 0.332 | ||
| Center | 0.891 | 0.434 | ||
| Elevated Plus | Percent time in arm | Closed | 1.770 | 0.209 |
| Open | 3.483 | 0.061 | ||
| Center | 0.058 | 0.943 | ||
| Distance traveled in arm | Closed | 1.637 | 0.232 | |
| Open | 1.749 | 0.212 | ||
| Center | 0.209 | 0.814 |
Figure 6.
Male Sprague-Dawley rats were implanted with an osmotic minipump delivering either 10 or 17 mg/ml clozapine (0.25 μl/hr) directly into one anterior lateral ventricle. A. In open field testing, the only significant change observed was an increase in distance traveled over the 5 minutes of testing, in the outer region of the open field maze suggesting that the clozapine did not produce any sedation in these animals. B. When tested in the elevated plus maze, the rats only showed significant changes in the amount of time spent in the open arms of the maze, suggesting some anxiolytic effects of the centrally administered clozapine. Together these data demonstrate that chronic central administration of clozapine did not produce any detrimental behavioral effects in these rats. Data are mean ± SEM; n=4 per group; * p<0.05, Fisher's LSD.
4. Discussion
The present studies demonstrate that clozapine can be formulated to be soluble in aqueous solution at pH 7.4 and remain fully dissolved for at least 2 months. Previous studies in both humans (Light et al 2000; Nagamoto et al 1996; 1999) and mice (Simosky et al 2003) have demonstrated that systemic administration of clozapine improves auditory gating. The present studies show that when administered directly into the ventricles of the brain, clozapine shows the same efficacy. The improvement in auditory gating at the highest dose was produced by both an increase in the conditioning amplitude and a decrease in the test amplitude. This dual action was most likely the result of indirect release of acetylcholine in the hippocampus (Shirazi-Southall et al 2002) possibly through blockade of 5HT-3 receptors (Adler et al 1998). Previous studies have demonstrated that stimulation of α7 nicotinic receptors reduces the test amplitude (Stevens et al 1997) and stimulation of α4β2 nicotinic receptors increases conditioning amplitude (Radek et al 2006). The combination of these two actions results in a significantly reduced TC ratio as observed in the present studies. The in vitro neurotoxicology studies suggest that, at the doses needed for improvement in auditory gating, the clozapine/HPBCD combination is not toxic to neurons. An ICV injection of 1 μg of a clozapine preparation would be diluted in a ventricular CSF fluid compartment of about 0.1 ml in rats and thus the total clozapine ventricular concentration is expected to be below the toxicity level determined in vitro. Moreover, the in vitro values are obtained by adding drug to a pure preparation of cells in culture with no barriers to transport or distribution. Therefore we would expect that the in vivo concentrations that could achieve similar toxicity would have to be higher than what was used in vitro. The behavioral testing suggests that there may be minimal negative behavioral consequences. In the present studies, the increased activity observed in the open field maze suggests that central clozapine does not induce sedation and the increased entry into the open arms of the elevated plus suggests that, rather than anxiogenic, central clozapine may be somewhat anxiolytic.
The recently published Catie study (McEvoy et al 2006) supports previous studies (Brambilla et al 2002; Breier et al 1994; Claghorn et al 1987; Essock et al 1995) confirming clozapine as the most efficacious drug for the treatment of schizophrenia, especially refractory cases. However, clozapine has numerous side effects which limit its usefulness. These include very serious complications such as agranulocytosis, cardiovascular/respiratory arrest and seizures, as well as sedation, tachycardia, hyper-and hypotension, weight gain, hepatic effects, constipation and fever (Miller 2006). The majority of these are peripheral in nature and would be reduced or eliminated if administration of clozapine could be confined to the central nervous system. Though side effects such as seizure and sedation would not be expected to be significantly altered by central administration since it is thought that these side effects are centrally mediated and the concentration at the synapse should be similar, if efficacy has been achieved, regardless of the route of administration.
The data presented here using a rodent model of deficient auditory gating, demonstrate the feasibility of administering clozapine centrally to produce improvements in sensory processing in schizophrenia patients. The severity of auditory gating deficits has been correlated to positive (Croft et al 2001), negative (Erwin et al 1998) and cognitive (Cullum et al 1993) symptoms of schizophrenia. Further, improvements in auditory gating in patients have been correlated with improvements in symptomotology of schizophrenia (Nagamoto et al 1999).
While these studies are only preliminary, they suggest that centrally administered clozapine could be effective in human patients. The DBA/2 mouse model has proven efficacy in predicting human improvement in auditory gating (Stevens et al 1998; Olincy et al 2006) and is currently being used by several pharmaceutical companies in the development of nicotinic agonists for schizophrenia. Clozapine itself is known to be an effective medication (Brambilla et al 2002; McEvoy et al 2006) and if equivalent central delivery in humans can be developed, there is a high likelihood of effectiveness. In support of this theory, a recent study showed effective treatment of epilepsy with central administration of valproic acid (Serralta et al. 2006). Rather than spinal administration, as is used in treatment for chronic pain and spacticity (Dones 2007; Koulousakis et al 2007; Richard and Menei 2007) it proposed that clozapine be administered directly into the anterior lateral ventricles via an indwelling catheter. The catheter would be connected to a standard abdominally implanted, programmable, refillable pump similar to those currently used for intrathecal drug delivery (Levy 1997). Delivery into the ventricles places the drug in relative proximity to the brain regions thought to be associated with the symptoms of schizophrenia.
Although the present data are encouraging, there are factors which suggest that these studies may not be predictive of human efficacy. These include the relative limitation of the auditory gating model for drug testing, the limitations of all animal models of schizophrenia including differences in metabolic rates and the unknown distribution in the large size human brain compared with the rodent brain studied in this manuscript. The auditory gating model focuses specifically on the gating deficit in schizophrenia and is most predictive of this particular aspect of schizophrenia; although a recent study revealed improvement in some cognitive features of schizophrenia concomitant with improvements in auditory gating (Olincy et al 2006). All animal models of schizophrenia have inherent limitations compared with the disorder in humans (Powell et al 2006). Brain size of rodents is so dramatically smaller than humans that if diffusion is a key limiting factor, brain size may be a very important barrier to central administration. An approach to assess this would be to determine relative receptor occupancy rates (Tauscher et al 2004). None-the-less, the present studies suggest that central administration of clozapine for certain schizophrenia patients may be a reasonable new treatment option which bears further testing.
Acknowledgments
The authors would like to thank Raymond Bunch MD for his contribution to the initial concept, and Andrew Gano for his consistent support and encouragement. This work supported by a UCDHSC Proof-of-Concept grant to TJA, a State of Colorado and UCDHSC Technology Transfer grant and NIMH RO1 MH73725 to KES.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvir JM, Lieberman JA, Saffermanm AZ, Schwimmer JL, Schaaf JA. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162–7. doi: 10.1056/NEJM199307153290303. [DOI] [PubMed] [Google Scholar]
- Atkin K, Kendall F, Gould D, Freeman H, Liberman J, O'Sullivan D. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169(4):483–8. doi: 10.1192/bjp.169.4.483. [DOI] [PubMed] [Google Scholar]
- Azorin JM, Spiegel R, Remington G, Vanelle JM, Pere JJ, Giguere M, Bourdeix I. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry. 2001;158(8):1305–13. doi: 10.1176/appi.ajp.158.8.1305. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, Carwood CM. Comparative efficacy of epidural, subarachnoid, and intracerebroventricular opioids in patients with pain due to cancer. Cochrane Database Syst Rev. 2005;25(1):CD005178. doi: 10.1002/14651858.CD005178. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Barale F, Caverzasi E, Tognoni T, Barui C. clozapine-treated subjects with treatment-resistant schizophrenia: a systemic review of experimental and observational studies. Int Clin Psychopharmacol. 2002;17(4):189–195. doi: 10.1097/00004850-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Brier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, Carpenter WT. Effects of clozapine in schizophrenic outpatients: effects on positive and negative symptoms. Arch Gen Psychiatry. 1994;151(1):20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT., Jr Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry. 1998;155(6):751–60. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- Claghorn J, Honigfeld G, Abuzzahab FS, Wang R, Steinbook R, Tuason V, Klerman G. The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol. 1987;7(6):377–384. [PubMed] [Google Scholar]
- Croft RJ, Lee A, Bertolot J, Gruzelier JH. Associations of P50 suppression and desensitization with perceptual and cognitive features of “unreality” in schizotypy. Biol Psychiat. 2001;50(6):441–446. doi: 10.1016/s0006-3223(01)01082-4. [DOI] [PubMed] [Google Scholar]
- Cullum CM, Harris JG, Waldo MC, Smeroff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res. 1993;10(2):131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- Dones I. Intrathecal baclofen for the treatment of spasticity. Acta Neurochir Suppl. 2007;97(Pt 1):185–8. doi: 10.1007/978-3-211-33079-1_25. [DOI] [PubMed] [Google Scholar]
- Donohue M, Wei W, Wu J, Zawia NH, Hyd N, De Jesus V, Schmechel D, Hettick JM, Beezhold DH, Vesper S. Characterization of nigerlysin, hemolysisn produced by Apergillus niger, and effect on mouse neuronal cells in vitro. Toxicol. 2006;219(13):150–155. doi: 10.1016/j.tox.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Turetsky BI, Moberg P, Gur RC, Gur RG. P50 abnormalities in schziophrenia: relationsip to clinical and neuropsychological indices of attention. Schizophr Res. 1998;33(3):157–167. doi: 10.1016/s0920-9964(98)00075-9. [DOI] [PubMed] [Google Scholar]
- Essock SM, Hargreaves WA, Covell NH, Goethe J. Clozapine's effectiveness for patients in state hospitals: results from a randomized trial. Psychopharmacol Bull. 1996;32(4):683–697. [PubMed] [Google Scholar]
- Fleischhaker C, Schulz E, Tepper K, Martin M, Hennighausen K, Remschmidt H. Long-Term Course of Adolescent Schizophrenia. Schizophr Bull. 2005;31(3):769–780. doi: 10.1093/schbul/sbi014. [DOI] [PubMed] [Google Scholar]
- Gardner I, Leeder JS, Chin T, Zahid N, Uetrecht JP. A comparison of the covalent binding of clozapine and olanzapine to human neutrophils in vitro and in vivo. Mol Pharmacol. 1998;53(6):999–1008. [PubMed] [Google Scholar]
- Hasan NY, Elkawy MA, Elzeany BE, Wagieh NE. Stability indicating methods for the determination of clozapine. J Pharm Biomed Analysis. 2002;30(1):35–47. doi: 10.1016/s0731-7085(02)00125-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Iyo M, Freedman R, Stegens KE. Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of α7 nicotinic acetylcholine receptors. Psychopharmacol. 2005;183(1):13–19. doi: 10.1007/s00213-005-0142-0. [DOI] [PubMed] [Google Scholar]
- Iqbal MM, Rahman A, Husain Z, Mahmud SZ, Ryan WG, Feldman JM. Clozapine: a clinical review of adverse effects and management. Ann Clin Psychiatry. 2003;15(1):33–48. doi: 10.1023/a:1023228626309. [DOI] [PubMed] [Google Scholar]
- Jang J, Yaksh TL, Hill HF. Use of 2-hydroxypropyl-beta-cyclodextrin as an intrathecal drug vehicle with opioids. J Pharmacol Exp Ther. 1992;261(2):592–600. [PubMed] [Google Scholar]
- Kapur S, Vanderspek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing inpreclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305(2):625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Koulousakis A, Kuchta J, Bayarassou A, Sturm V. Intrathecal opioids for intractable pain syndromes. Acta Neurochir Suppl. 2007;97(pt 1):43–48. doi: 10.1007/978-3-211-33079-1_5. [DOI] [PubMed] [Google Scholar]
- Levy R. Neurosurgical Management of Pain. Chapter 19. New York, NY: Springer-Verlag; 1997. Implanted Drug Delivery Systems for Control of Chronic Pain. [Google Scholar]
- Lublin H, Eberhard J, Levander S. Current therapy issues and unmet clinical needs in the treatment of schizophrenia: a review of the new generation antipsychotics. Int Clin Psychopharmacol. 2005;20(4):183–98. doi: 10.1097/00004850-200507000-00001. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600–10. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- Miller DD. Review and management of clozapine side effects. J Clin Psychiat. 2000;61 8:14–17. [PubMed] [Google Scholar]
- Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neurosci. 1995;69:371–81. doi: 10.1016/0306-4522(95)00249-i. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, McRae KA, Heuttl P, Cawthra E, Gerhardt G, Hea R, Griffith J. Auditory P50 in schizophrenics on clozapine: Improved gating parallels cliical improvement and changes in plamsa 3-methoxy-hydroxyphenylglycol. Neuropsychobiol. 1999;39(1):10–17. doi: 10.1159/000026553. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Commissaris RC, De La Garza R, File SE, Knapp DJ, Seiden LS. Involvement of 5-HT1A receptors in animal tests of anxiety and depression: evidence from genetic models. Stress. 2003;6(2):101–10. doi: 10.1080/1025389031000111311. [DOI] [PubMed] [Google Scholar]
- Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiat. 2006;59(12):1198–207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek RJ, Miner HM, Bratcher NA, Decker MW, Goplalakrishnan M, Bitner RS. Alpha4beta2 nicotinic receptor stimulation contributes to the effects of nicotine in the DBA/2 mouse model of sensory gating. Psychopharmacol (Berl) 2006;187(1):47–55. doi: 10.1007/s00213-006-0394-3. [DOI] [PubMed] [Google Scholar]
- Reid WH, Mason M, Hogan T. Suicide Prevention Effects Associated With Clozapine Therapy in Schizophrenia and Schizoaffective Disorder. Psychiatr Serv. 1998;49(8):1029–1033. doi: 10.1176/ps.49.8.1029. [DOI] [PubMed] [Google Scholar]
- Richard I, Menei P. Intrathecal baclofen in the treatment of spasticity, dystonia and vegetative disorders. Acta Neurochir Suppl. 2007;97(Pt 1):213–8. doi: 10.1007/978-3-211-33079-1_29. [DOI] [PubMed] [Google Scholar]
- Serralta A, Barcia JA, Ortiz P, Duran C, Hernandez ME, Alos M. Effect of intracerebroventricular continuous infusion of valproic acid versus single i.p. and i.c.v. injections in the amygdala kindling epilepsy model. Epilepsy Res. 2006;70(1):15–26. doi: 10.1016/j.eplepsyres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacol. 2002;165(4):386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Kem WR, Freedman R. Intragastric DMXB-A, an α7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biol Psychiat. 2001;50(7):493–500. doi: 10.1016/s0006-3223(01)01093-9. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neurospychopharmacol. 1996;15(2):152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha-7 nicotinic agonists normalize inhibition of auditory response in DBA/2 mice. Psychopharmacol. 1998;136(4):320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry. 2002;159(2):255–62. doi: 10.1176/appi.ajp.159.2.255. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Spinal systems and pain processing: development of novel analgesic drugs with mechanistically defined models. Trends Pharmacol Sci. 1999;20(8):329–37. doi: 10.1016/s0165-6147(99)01370-x. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Jang JD, Nishiuchi Y, Braun KP, Ro SG, Goodman M. The utility of 2-hydroxypropyl-beta-cyclodextrin as a vehicle for the intracerebral and intrathecal administration of drugs. Life Sci. 1991;48(7):623–33. doi: 10.1016/0024-3205(91)90537-l. [DOI] [PubMed] [Google Scholar]