Abstract
Background
Sleep-disordered breathing has been implicated in hypertension, but whether daytime breathing is a factor in blood pressure regulation has not been investigated to date. The present study sought to determine the role of breathing pattern in salt sensitivity of blood pressure.
Methods and Results
Thirty-six women, ages 40–70, were placed on a six-day low sodium/low potassium diet followed by a six day high sodium/low potassium diet. Breathing pattern at rest and 24-hr ambulatory blood pressure were monitored at baseline and after each six-day diet period. Respiratory rate (but not tidal volume or minute ventilation) was an inverse predictor of systolic (r = −0.50 p <.001) and diastolic (r = = −0.59; p <.001) blood pressure sensitivity to high sodium intake. Respiratory rate was positively associated with hemoglobin (r = +0.38; p <.01), and the salt-induced change in hemoglobin was associated with salt-induced change in blood pressure (r= −0.35; p <.05).
Conclusion
These findings indicate that a pattern of slow breathing not compensated by increased tidal volume is associated with salt sensitivity of blood pressure in women. Breathing patterns could play a role in the hypertensive response via sustained effects on blood gases and acid-base balance, and/or be a marker for other biological factors mediating the cardiovascular response to dietary salt intake.
Keywords: blood pressure, hypertension, respiration, sodium
INTRODUCTION
Clinical trials demonstrate dose-dependent changes in blood pressure across a broad range of sodium intakes1. Well-recognized heterogeneity exists in individual blood pressure responses to sodium intake, with individuals who exhibit greater changes in blood pressure in response to change in sodium intake being considered “salt sensitive”2,3. Genetic, dietary factors, and co-morbid conditions which predict individual salt sensitivity include African-American race, older-age, hypertension, and presence of kidney disease 4. While certain genetic and phenotypic characteristics predict groups of individuals who may exhibit salt sensitivity, biochemical or physiological characteristics which predict an individual’s blood pressure response to sodium intake remain to be clarified.
We previously reported animal models of sodium-induced experimental hypertension which described a cascade of physiological responses that occur to increase blood pressure in genetically-normotensive, behaviorally-conditioned dogs and pigs5,6. We documented that intermittent behavioral stress evoked an altered breathing pattern that was associated with a mild respiratory acidosis, expansion of plasma volume, and effects on circulating concentrations of natriuretic hormones. An inhibited breathing pattern (decreased respiratory rate) predicted an elevation in 24-hr blood pressure in response to increased sodium intake.
Although these studies with laboratory animals suggest that respiratory patterns are modifiable by behavioral stress and predict blood pressure salt sensitivity, this association has not been established in human studies. Difficulties in accurate assessment of respiratory patterns in adults in outpatient settings have limited the utility of this “vital sign” as a risk factor in epidemiological studies. Previous studies have associated respiratory patterns with individual differences in blood gases7,8, and both pCO29 and acid-base balance10 have been implicated in salt-induced hypertension in humans with normal or prehypertensive blood pressure, as categorized by The JNC 7 Report11. However, direct measurements of respiratory rate as a predictor of human blood pressure sensitivity to dietary sodium intake have not been made to date.
We undertook this clinical feeding study to investigate questions relevant to the extent to which respiratory rate is associated with salt sensitivity of blood pressure in humans with normal or prehypertensive blood pressure. Do respiratory patterns predict blood pressure response to increased sodium intake? We hypothesized that reduced respiratory rate would be associated with blood pressure salt sensitivity. Are other variables, related to respiration and associated physiological systems, also predictors of salt sensitivity? The extent to which respiratory patterns are an index of salt sensitivity is a critical issue in understanding the biological mechanisms that contribute to salt sensitivity.
METHODS
Study Design
This was a single center clinical feeding study testing the effects of increased sodium intake on blood pressure in women. All enrolled participants were fed a low-sodium and low-potassium diet for six days followed by six days on a high-sodium and low-potassium diet. Automated determinations of respiratory patterns and blood pressure (oscillometric) were used to assure blinded measurement of the outcome variables at the end of the low-salt and high-salt periods.
Participants
Eligibility for the study was limited to Caucasian women since race and gender have been reported to confound the association between sodium regulation, blood pressure, and salt sensitivity12. Participants were recruited by local advertisements and personal referrals. Major exclusion criteria were self-reported respiratory, cardiac, liver or kidney disease, diabetes, or hypertension. Other exclusion criteria included the use of medications including diuretics, steroids, hormone replacement medications, or central nervous system agents. Recent cigarette smokers and women with body mass index > 30 kg/m2 were also excluded. The protocol was approved by the Medstar Research Institute Institutional Review Board, and was conducted in the National IA Clinical Research Unit at Harbor Hospital in Baltimore, Maryland.
Experimental design
Participants were screened for medical eligibility and willingness to complete all dietary intervention and data collection procedures. At baseline and before dietary intervention, blood pressure, respiratory patterns and socio-demographic variables were collected. At the start of the intervention, participants were fed an isocaloric diet that had a low sodium (1 mmol/kg/day) content, followed immediately by six days on the same diet but containing high sodium (4 mmol/kg/day). Both diets had low potassium (1 mmol/kg/day). The high sodium diet included supplementation with enteric-coated sodium chloride capsules (Slo-Sodium, Novartis, Basel, Switzerland) ingested with each meal and at bedtime. All meals were provided and compliance with meals was monitored by trained staff. Participants were allowed up to one cup of a caffeinated beverage per day. Participants were instructed to maintain normal daily activities throughout the 12-day period.
Outcome variables
At baseline and at the end of each feeding period, blood pressure was measured in the seated position following a five-min rest period by oscillometric measurement and a standardized protocol. At the end of each feeding period, 24-hr hour blood pressures were determined by ambulatory monitoring, as described below. Also as described below, breathing patterns were recorded during seated rest in the clinic setting. Blood and 24-hr urine samples were collected following each diet period.
Blood pressure
Blood pressure and heart rate were obtained via an inflatable cuff attached to an automated oscillometric device (Spacelabs, Model 90207, Redmond, WA). Mean 24-hr blood pressure at baseline, and after six days on low salt and high salt diet, were calculated from the hourly measurements for 24 hr in the natural environment, including the last four measurements made in seated rest at the onset of the 24-hr period. (Exclusion of the clinic measurements from the 24-hr means did not affect either the 24-hr means or relationships to breathing patterns.) Salt sensitivity was defined as a significant increase in mean 24-hr systolic blood pressure on the high salt diet compared to the low salt diet. Salt insensitivity was defined as an insignificant increase in 24-hr systolic blood pressure between the low and high salt diet sessions.
Respiratory Patterns
Breathing frequency, tidal volume, and minute ventilation were recorded continuously during 25 min seated rest via the Lifeshirt (Vivometrics, Ventura, CA), an elasticized vest that detects chest and abdominal motion via inductive plethysmography, connected to a microcomputer that recorded breathing parameters on a breath-to-breath basis13. Electrocardiographic apparatus in the Lifeshirt provided continuous measurement of heart rate. Tidal volume was calibrated for each individual before each session by exhaling a fixed volume of air into an inflatable bag. Median breathing frequency, tidal volume, and minute ventilation was determined for the last 20 min of each session from the successive inter-breath intervals. Medians, rather than means were selected, because they were closer to modal values, enabling better exclusion of outliers due to movement artifact.
Biochemical Measurements
Venous blood samples were obtained before and after each diet period for measurement of hemoglobin and a basic metabolic profile including sodium, potassium, calcium, and magnesium in the Clinical Core Laboratory of Harbor Hospital (Johnson & Johnson Vitros 950). Acute changes in hemoglobin levels with a dietary salt challenge are proposed as a way to assess changes in intravascular volume14. Acute reductions in hemoglobin are expected to reflect hemodilution from increased intravascular volume that contributes to higher blood pressures.
Adherence
Adherence with feeding was assessed by attendance at the clinic, daily telephone contact with the study coordinator, and completion of food diaries. Adherence to the diets was confirmed by determination of urine sodium and potassium levels from 24-hr urine samples collected during the last day on each diet. Urines were collected in a three-liter plastic container and portable cooler, beginning after the first morning void. The urine samples were transported the next day to the clinic setting, and 24-hr sodium and potassium excretion measured via dry slide (Johnson and Johnson Clinical Diagnostics, Vitros 250).
Analytical Considerations
The target sample size of 40 was calculated to provide 80% power to detect a five mmHg change in systolic blood pressure. Correlation coefficients were determined by Pearson’s correlation analyses. Predictors of change in blood pressure, breathing patterns and hemoglobin from the low-salt to high-salt diets were determined via univariate and multivariate linear regression analyses, using successive backwards elimination of non-significant variables. All statistics were performed using SPSS, Version 11 (SPSS Inc. Chicago, Illinois).
RESULTS
Of the 60 women who signed a consent form, 38 women (63%) were accepted into and completed the study. Two of the 38 were excluded from the data analysis when respiratory rate indicated hyperventilatory breathing in the clinic setting. Baseline characteristics of the remaining 36 participants are shown in Table 1. At baseline, none were hypertensive, 18 were overweight (BMI 25–30 kg/m2), and no participants were obese (BMI>30 kg/m2).
Table 1.
Means and standard deviations of demographic, cardiovascular, hematologic and respiratory variables at study onset for the group, and salt-sensitive and salt-insensitive subgroups.
| Group (n=36) | Na-Sensitive (n=13) | Na-Insensitive (n=23) | p | |
|---|---|---|---|---|
| Age (yr) | 52.6(8.4) | 57.1(8.4) | 50.0(7.3) | .012 |
| Body weight (kg) | 67.4(8.7) | 71.5(8.7) | 65.1(7.9) | .031 |
| BMI (kg/m2) | 25.3(3.3) | 26.1(3.0) | 24.9(3.5) | .269 |
| Systolic BP (mmHg) | 117.4(10.2) | 121.6(10.4) | 115.1(9.6) | .067 |
| Mean BP (mmHg) | 87.6(7.3) | 90.5(7.2) | 86.0(6.9) | .073 |
| Diastolic BP (mmHg) | 71.9(6.7) | 74.5(6.3) | 70.5(6.6) | .085 |
| Heart rate (bpm) | 71.7(8.7) | 71.7(7.7) | 71.7(9.4) | .999 |
| Hemoglobin (gm/dl) | 13.5(0.7) | 13.5(0.8) | 13.5(0.7) | .999 |
| Breaths/min | 14.2(2.6) | 12.5(2.4) | 15.3(2.1) | .000 |
| Tidal volume (ml) | 330(136) | 353(129) | 316(141) | .441 |
| Min vent (L/min) | 4.59(2.03) | 4.14(1.38) | 4.86(2.32) | .316 |
Adherence
Results of 24-hour urine sodium analyses confirmed excellent adherence to the diets. At the end of the low-sodium diet, mean 24-hr urine sodium levels for the group were 38.3 ± 23.0 mmol/day compared with the sodium provided (mean 67 mmol/day), while at the end of the high-salt diet period, 24-hr sodium was (236.6 ± 51.5 mmol/day), compared to the 272 mmol/day provided. Urine potassium levels were equivalent between the low-salt (62 ± 19 mmol day) and high-salt (57 ± 19 mmol day) diet periods, confirming the a priori study design goals to provide 1mmol/kg/day in both diets.
Sodium loading effects on cardiovascular, respiratory and other variables
Baseline characteristics of participants stratified by salt sensitivity are presented in Table 1, which shows demographic and physiological measures for the group as a whole, and in salt-sensitive and salt-insensitive subgroups. Salt-sensitive persons were older, weighed more, and had lower respiratory rate than salt-insensitive persons. The differences in blood pressure approached statistical significance.
Table 2 shows means and standard deviations of each variable at the end of the low- and high- salt intake periods, and changes in each variable, for salt-sensitive and salt-insensitive subgroups. On high salt diet, the salt-sensitive subgroup showed increases in body weight (p <.05) and urinary sodium excretion ( p <.01) as well as all blood pressure measures, and decreases in hemoglobin ( p <.01). The salt-insensitive subgroup increases in urine volume (p <.01) and urinary sodium (p <.01) on high salt diet, and decreases in hemoglobin (p <.01), diastolic pressure (p <.05), and heart rate (p <.05). High- salt diet had no significant effect on any respiratory measure.
Table 2.
Means and standard deviations of cardiovascular, hematologic and respiratory measures after low and high sodium diet for salt-sensitive and salt-insensitive subgroups.
| Salt-Sensitive (n=13) | Salt-Insensitive (n=23) | Between Group Δ(95% CI) | p | |||||
|---|---|---|---|---|---|---|---|---|
| Body weight (kg) | 70 (8.6) | 71.9 (8.9) | 1.2 (1.3)* | 64.5 (7.8) | 64.9 (8.1) | 0.4 (1.2) | 0.8 (−0.4,1.0) | 0.062 |
| Systolic BP (mmHg) | 115.2 (8.9) | 124.5 (10.9) | 9.5 (5.2)** | 113.9(10.3) | 113.9(10.8) | 0.1 (3.9) | 9.3 (6.1,12.4) | <.001 |
| Mean BP (mmHg) | 87.1 (5.7) | 93.1 (7.2) | 6.0 (4.2)** | 86.1 (6.9) | 84.9 (6.8) | −1.2 (3.2) | 7.2 (4.7,9.7) | <.001 |
| Diastolic BP (mmHg) | 71.8 (5.6) | 76.2 (5.8) | 4.4 (4.0)** | 71.0 (6.9) | 69.1 (6.2) | −1.9 (3.5)* | 6.3 (3.7,9.0) | <.001 |
| Heart Rate (bpm) | 71.5 (7.7) | 70.2 (8.7) | −1.3(4.1) | 73.7 (8.7) | 71.7 (6.8) | −3.0 (5.4)* | 1.7 (−2.4,5.8) | 0.400 |
| Hemoglobin (gm/dl) | 13.8 (0.7) | 13.0 (0.7) | −0.9(0.4)** | 13.8 (0.7) | 13.1 (0.7) | −0.7(0.6)** | −0.2 (−0.6,0.2) | 0.371 |
| Resp Rate (/min) | 12.4 (2.1) | 12.9 (3.1) | 0.5 (1.8) | 14.9 (2.7) | 15.3 (2.4) | 0.5 (1.7) | −0.1 (−1.2,1.1) | 0.924 |
| Tidal Volume (ml) | 315(114) | 355 (140) | 24 (121) | 315 (150) | 307 (152) | −12 (95) | 31 (−37,100) | 0.365 |
| Minute Vent (L/min) | 4.31 (1.17) | 4.55 (1.70) | 0.24 (1.50) | 4.70 (2.27) | 4.40 (2.34) | −0.32(2.14) | −0.44 (0.95,1.84) | 0.522 |
| Urine Volume (ml) | 2141 (883) | 2533 (643) | 392 (936) | 1950 (95) | 2618 (698) | 669(769)** | −276 (−907,354) | 0.377 |
| Urinary Na (mmol) | 41 (31) | 229 (37) | 188 (50)** | 37 (18) | 252 (49) | 205 (60)** | −17 (−60,25) | 0.422 |
BP = blood pressure, Resp Rate= Respiration Rate, Minute Vent = Minute Ventilation.
= p <0.05;
= p <0.01
The two right-hand columns of Table 2 show that the increase in body weight was marginally greater in the salt-sensitive than the salt-insensitive subgroup (p <0.062). However, even though heart rate and urine volume effects were significant in salt-insensitive and non salt-sensitive subgroups, the differences in those effects were not significant.
Effects of decreases in sodium intake on blood pressure
In order to determine whether these results were specific to the dietary sequence, we calculated the changes in 24-hr systolic blood pressure for each subject from the “no-salt control” baseline to the low-salt control condition. The previously designated salt-sensitive subgroup showed larger decreases in systolic BP after six days of low salt diet than the salt-insensitive subgroup (i.e. −11.1 ± 8.9 vs −4.6 ± 6.6 mmHg; t = 2.50; p <.009). Thus, the “salt-sensitive” and “salt-insensitive” subgroups showed consistency of response whether dietary sodium was increased or decreased.
Effects of change in sodium intake on breathing measures
Table 2 also shows means and standard deviations of breathing frequency, tidal volume, and minute ventilation after six days on the low and high salt diets. The changes in salt intake had no significant effects on breathing frequency, tidal volume, or minute ventilation.
Table 3 shows correlation coefficients for individual median breathing frequency, tidal volume, and minute ventilation on low- and high-salt diets. Respiratory rate on low salt was positively correlated with respiratory rate on high salt. Respiratory rate on high salt (but not low salt) was inversely related to tidal volume on low and high salt, but not to minute ventilation on any diet. Tidal volume and minute ventilation were positively correlated with each other on both low and high salt diets.
Table 3.
Correlations coefficients between respiration rate (breaths/min), tidal volume (ml) and minute ventilation (ml/min) on low salt and high salt diets.
| Respiration rate | Tidal volume | Minute Ventilation | |||||
|---|---|---|---|---|---|---|---|
| Low Salt | High Salt | Low Salt | High Salt | Low Salt | High Salt | ||
| Respiration | Low Salt | 0.83** | −0.26 | −0.23 | 0.14 | 0.01 | |
| Rate | High Salt | −0.40** | −0.33* | −0.07 | 0.04 | ||
| Tidal | Low Salt | 0.74** | 0.91** | 0.53** | |||
| Volume | High Salt | 0.66** | 0.92** | ||||
| Minute | Low Salt | 0.53** | |||||
| Ventilation | High Salt | ||||||
= p <0.05;
= p <0.01
Association of respiratory rate with blood pressure response
Figure 1 shows that individual median respiratory rate was inversely correlated with systolic (r = −0.50; p <.001) and diastolic (r = −0.59; p <.001) blood pressure change from low to high salt diet. When a multiple regression of respiratory rate, age, body mass index, median tidal volume, median minute ventilation, baseline systolic blood pressure, and baseline heart rate on systolic blood pressure change was performed, only breathing frequency and baseline systolic pressure remained significant independent determinants (F2,33 = 11.02; p <.000; r2 = 0.40). When a multiple regression of the same variables (including baseline diastolic blood pressure) on diastolic pressure change was performed, only respiratory rate remained a significant independent determinant (F1,28 = 17.99; p <.000; r2 = 0.35).
Figure 1.
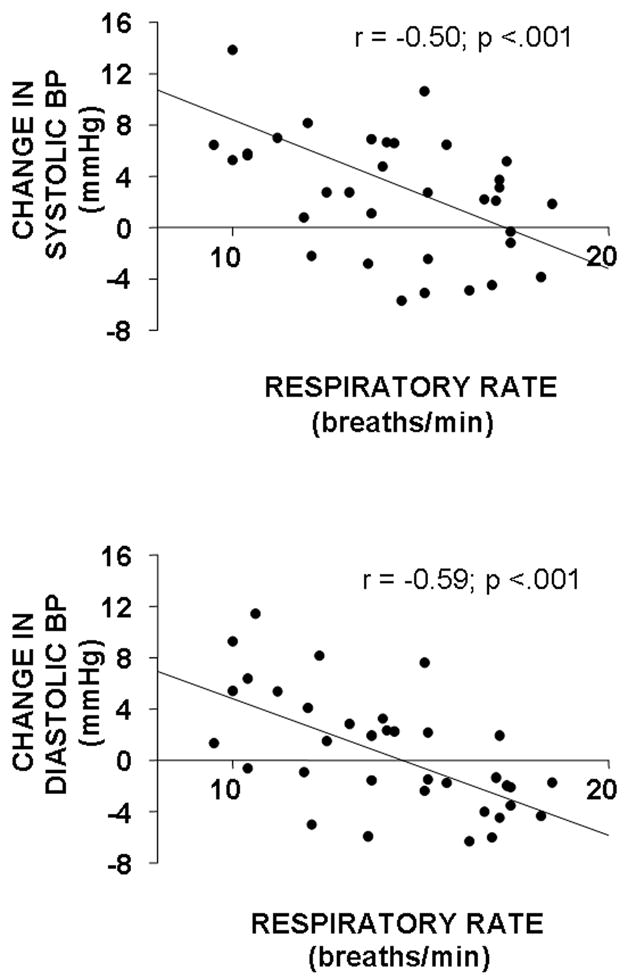
Scatter plots of individual breathing frequency versus high-salt induced change in systolic and diastolic blood pressure for 36 women.
No significant associations of either tidal volume or minute ventilation with blood pressure change were observed (nor were they observed when either respiratory measure was corrected for individual differences in body surface area).
Associations of respiratory rate with hemoglobin
Acute changes in hemoglobin levels with a dietary salt challenge are proposed as a way to assess changes in intravascular volume13. Acute reductions in hemoglobin are expected to reflect hemodilution from increased intravascular volume. Accordingly, Table 2 shows that hemoglobin decreased on high salt diet in both salt-sensitive and salt-insensitive groups.
Figure 2 shows scatter plots of individual median respiratory rate with individual hemoglobin measured on low- and high-salt diets. In each session, a significant positive association of respiratory rate with hemoglobin was observed. No such associations of tidal volume or minute ventilation (absolute or corrected for body surface area) with hemoglobin were observed.
Figure 2.
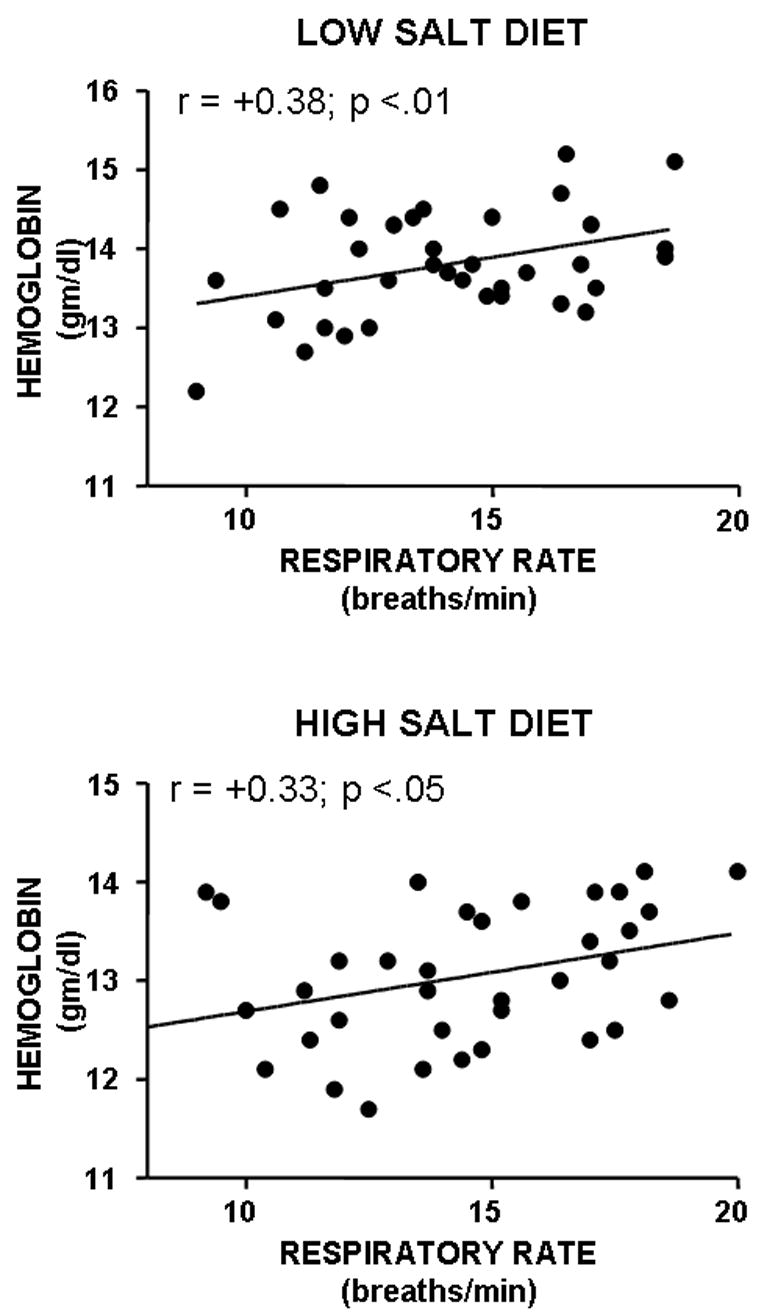
Scatter plots of individual breathing frequency versus hemoglobin levels on low and high salt diet for 36 women.
Association of change in blood pressure with change in hemoglobin on high salt diet
Figure 3 shows that the magnitude of increase in blood pressure and decrease in hemoglobin owing to high salt diet were significantly associated. This finding suggests that salt sensitivity was a positive function of hemodilution in response to the high-salt diet.
Figure 3.
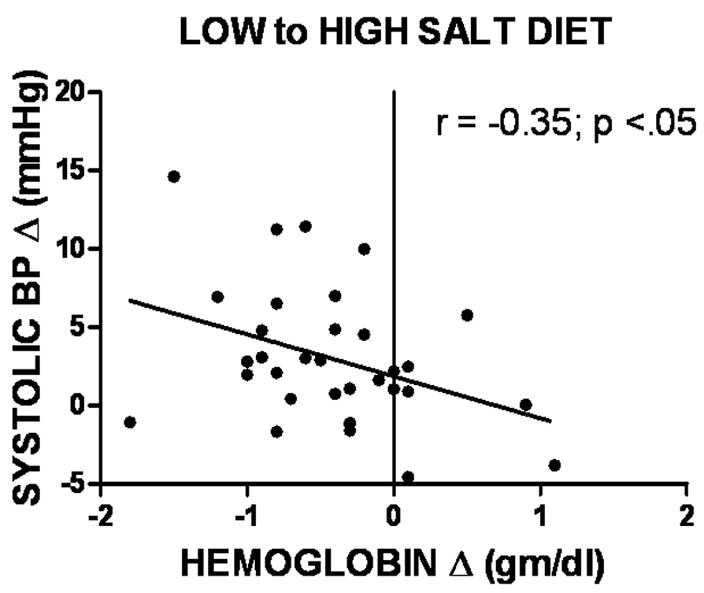
Scatter plot of high salt-induced change in hemoglobin versus change in systolic blood pressure for 36 women.
DISCUSSION
The primary finding of this study is that respiratory rate was associated with salt sensitivity of blood pressure; that is, those women who breathed slowly were more likely to be salt sensitive than those who breathed more rapidly. A correlated finding was that women who breathed more slowly tended to have lower hemoglobin levels at baseline than those who breathed more rapidly.
Individual differences in respiratory rates were generally stable across monitoring sessions, averaged 14 per minute, and varying between 10 and 18 per minute. This finding is consistent with the results of previous studies of the stability of individual human breathing pattern over intervals as long as several years15. Faster resting respiratory rate is positively correlated with greater trait anxiety8. By contrast, tidal volume varies with the energetic needs of the individual, being greater in aerobic exercise and lower in sleep than at rest, with respiratory rate remaining constant unless volumetric capacity is exceeded16. The inverse relationship between respiratory rate and tidal volume on high-salt diet is also consistent with previous research that differentiated two general patterns of breathing: one with faster rates and lower tidal volume, and another with slower rates and greater tidal volume 7,8. Whether persons who are slow breathers are characterized by emotional inhibition needs to be investigated, but personality studies of hypertensive patients have often found them to be characterized by repressed emotion17 or alexithymia18.
Fast breathers tend to have lower resting end tidal CO2 (which is correlated with pCO2), while slow breathers tend to have higher resting end tidal CO27. It is known that changes in blood gases can influence renal sodium regulation, with mild hypoxia increasing, and hypercapnia decreasing sodium excretion19. More severe hypoxia can also decrease renal sodium retention20. The primary finding in the present study combined with the results of previous studies showing that renal sodium excretion is slowed in salt sensitive individuals21 suggests that breathing pattern could be an index of renal sodium regulation. In the present study, no significant differences in renal excretion of sodium between salt-sensitive and salt-insensitive subjects were observed, but the urine samples were taken after six days on each diet, and any differences in renal sodium excretion and sodium balance might have been expected to stabilize by that time. In that regard, high-salt diet increased body weight significantly only in salt-sensitive subjects, and urine volume increased significantly only in salt-insensitive subjects.
The role of breathing pattern in the mediation of salt sensitivity remains to be clarified. One previous study of normotensive persons found that low plasma pH was a predictor of salt sensitivity10. That study concluded that individual differences in pH were due to metabolic factors, but breathing suppression could also mediate decreased sodium excretion via effects on pCO2 and plasma pH. Clearly, other factors that prevent compensatory vasodilation in salt-sensitive persons on high-salt diets must also be involved in the salt-induced blood pressure elevations. Among these are endogenous sodium pump inhibitors, such as those that have been shown to be stimulated by breathing suppression in laboratory animals22 and human subjects23.
No previous evidence of a correlation between individual differences in respiratory rate and hemoglobin could be found in the literature, though both are, of course, involved in oxygen transport. Salt sensitivity is consistently associated with low plasma renin activity4, and one previous study reported that hemoglobin was lower in hypertensive patients with low than high plasma renin activity than in others with high plasma renin activity24. An interesting implication is that slow breathing pattern might also be associated with low plasma renin activity.
The findings in the present study may be relevant to only a subset of “salt sensitive” normotensive or prehypertensive persons, and may not be relevant to salt sensitivity in hypertensive persons, since the mechanism of salt sensitivity in hypertensive subjects may be distinct from that in normotensive or prehypertensive subjects. Studies have suggested differences between salt sensitive normotensives and hypertensives in both forearm blood flow25 and plasma catecholamines26, although the conditions under which these differences are observed remain to be clarified19,27. We suggest that the role of salt sensitivity in the pathogenesis of hypertension may be best studied in normotensive or prehypertensive persons, since the structural changes that constitute hypertension may themselves create a different form of salt sensitivity. One of these differences may, in fact, be in respiration, since chronic hypertension has been reported to be associated with a mild hyperventilation28.
Another limitation of this study is that the generality of the present findings to men and other racial groups needs to be established. In addition, whether the breathing patterns observed in the clinical research setting are representative of breathing in the natural environment needs to be investigated. Better control over diet and urine collection procedures could have been obtained in an inpatient study. However, the provision of all food and the availability of 24-hr urinary output under both dietary conditions provided some assurance about adherence to the diets.
In conclusion, the findings of the present study strongly support the view that below average respiratory rate is a risk factor for salt sensitivity of blood pressure in women with normal or prehypertensive blood pressure. It remains to future research to determine whether this breathing pattern is a marker of other characteristics that mediate the hypertensive response, or whether breathing pattern itself plays a mediating role in salt sensitivity and the development of some forms of chronic hypertension.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH National Institute on Aging. The authors wish thank D.L. Longo for advice on study design, data analysis, and manuscript critique, C. Swanson for medical support, and M.A. Chesney for technical support and manuscript critique.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001 Jan 4;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 2.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25(3 Suppl):247S–255S. doi: 10.1080/07315724.2006.10719574. [DOI] [PubMed] [Google Scholar]
- 3.Luft FC, Weinberger MH. Heterogeneous responses to changes in dietary salt intake: the salt-sensitivity paradigm. Am J Clin Nutr. 1997;65(2 Suppl):612S–617S. doi: 10.1093/ajcn/65.2.612S. [DOI] [PubMed] [Google Scholar]
- 4.Muntzel M, Drueke T. A comprehensive review of the salt and blood pressure relationship. Am J Hypertens. 1992;5:1S–42S. doi: 10.1093/ajh/5.4s.1s. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DE. Behavioral Factors in Hypertension. Vol. 9. New York: Elsevier; 1987. Experimental behavioral hypertension in laboratory animals. Handbook of Hypertension; pp. 226–245. [Google Scholar]
- 6.Anderson DE, Fedorova OV, French AW. Preavoidance hypercapnia and decreased hematocrit in micropigs. Physiol & Behav. 1995;59:857–861. doi: 10.1016/0031-9384(95)02165-5. [DOI] [PubMed] [Google Scholar]
- 7.Grossman P. Respiration, stress, and cardiovascular function. Psychophysiology. 1983;20:284–299. doi: 10.1111/j.1469-8986.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer KE. Respiratory pattern and respiratory response to CO2. J. Appl. Physiol. 1958;13:1–14. doi: 10.1152/jappl.1958.13.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DE, Parsons DJ, Scuteri A. End tidal CO is an independent determinant of systolic blood pressure in women. J Hypertens. 1999;17:1073–1080. doi: 10.1097/00004872-199917080-00005. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Sharma M, Kribben A, Schattenfroh S, Cetto C. A Distler Salt sensitivity in humans is associated with abnormal acid-base regulation. Hypertension. 1990;16:407–413. doi: 10.1161/01.hyp.16.4.407. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. The JNC 7 Report. J Am Med Assn. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz S, Fisher ND, Ferri C, Hopkins PN, Williams GH, Hollenberg NK. Controlled analysis of blood pressure sensitivity to sodium intake: interactions with hypertension type. J Hypertens. 2003;21:869–870. doi: 10.1097/00004872-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm FH, Roth WT, Sackner MA. The LifeShirt. An advanced system for ambulatory measurement of respiratory and cardiac function. Behav Modif. 2003 Oct;27(5):671–91. doi: 10.1177/0145445503256321. [DOI] [PubMed] [Google Scholar]
- 14.Speedy DB, Thompson JMD, Rodgers I, Collins M, Sharwood K. Oral salt supplementation during ultradistance exercise. Clin J Sport Med. 2002;12:279–284. doi: 10.1097/00042752-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Benchetrit Benchetrit G, Shea SA, Dinh TP, Bodocco S, Baconnnier P, Guz A. Individuality of breathing patterns in adults assessed over time. Respir Physiol. 1989;75:199–209. doi: 10.1016/0034-5687(89)90064-9. [DOI] [PubMed] [Google Scholar]
- 16.Otis AB. The work of breathing. In: Fenn OW, Rahn HR, editors. Handbook of Physiol. Vol. 99. Washington DC: American Physiological Society; 1961. pp. 463–476. [Google Scholar]
- 17.Mann SJ. The mind-body link in essential hypertension: time for a new paradigm. Altern Ther Health Med. 200;6:39–45. [PubMed] [Google Scholar]
- 18.Jula A, Salminen JK, Saarijarvi S. Alexithymia: a facet of essential hypertension. Hypertension. 1999;33:1057–1061. doi: 10.1161/01.hyp.33.4.1057. [DOI] [PubMed] [Google Scholar]
- 19.Honig A. Peripheral arterial chemoreceptors and reflex control of sodium and water homeostasis. Am J Physiol Integ Comp Physiol. 1989;257(26):R1282–R1302. doi: 10.1152/ajpregu.1989.257.6.R1282. [DOI] [PubMed] [Google Scholar]
- 20.Neylon M, Marshall J, Johns EJ. The role of the renin-angiotensin system in the renal response to moderate hypoxia in the rat. J Physiol. 1996;491(pt 2):479–488. doi: 10.1113/jphysiol.1996.sp021232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27(part 2):481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 22.Fedorova OV, French AW, Anderson DE. Inhibition of Na+, K+ ATPase inhibition during anticipatory hypoventilation in micropigs. Amer J Hypertens. 1996;9:1126–1131. doi: 10.1016/0895-7061(96)00194-x. [DOI] [PubMed] [Google Scholar]
- 23.Bagrov AY, Fedorova OV, Austin-Lane JL, Dmitrieva RI, Anderson DE. Endogenous marinobufagenin-like immunoreactive factor and Na+, K+ ATPase inhibition during voluntary hypoventilation. Hypertension. 1995 Nov;26(5):781–8. doi: 10.1161/01.hyp.26.5.781. [DOI] [PubMed] [Google Scholar]
- 24.Drayer JI, Weber MA, Sealey JE, Laragh JH. Low and high renin hypertension: a comparison of clinical and biochemical characteristics. Am J Med Sci. 1981;282(3):135–142. doi: 10.1097/00000441-198105000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Mark AL, Lawton AW, Abboud FM, Fitz AE, Connor WE, Heistad DD. Effects of high and low sodium intake on arterial pressure and forearm vascular resistance in borderline hypertension: A preliminary report. Circ Res. 1975;36(suppl I):I-95–I-98. doi: 10.1161/01.res.36.6.194. [DOI] [PubMed] [Google Scholar]
- 26.Campese VM, Romoff MS, Levitan J, Saglikes Y, Friedier R, Masary SG. Abnormal relationship between sodium intake and plasma norepinephrine in patients with essential hypertension. Kidney Int. 1982;21:371–378. doi: 10.1038/ki.1982.32. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertens. 1991;17(Suppl I):I-61–I-68. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 28.Habeck JO. Peripheral arterial chemoreceptors and hypertension. J Auton Nerv Syst. 1991;34:1–7. doi: 10.1016/0165-1838(91)90003-l. [DOI] [PubMed] [Google Scholar]


