Abstract
Dendritic spines are highly dynamic protuberances that are thought to be crucial for learning and memory. Although it is well known that actin filaments and membrane dynamics regulate spine plasticity, how these two events are linked locally is less clear. Here, we provide evidence that Citron-N (CIT-N), a binding partner of the small GTPase RhoA, is associated with the actin filaments and Golgi compartments of dendritic spines. We also show that CIT-N is required for recruiting F-actin and Golgi membranes at spines of in vitro-grown neurons. Studies in knockout mice show that this protein is essential for the maturation of dendritic spines. We suggest that CIT-N might function as a scaffold protein in spine organization through its ability to bind to Golgi membranes and by affecting actin remodelling.
Keywords: dendritic spines, Golgi, actin, Citron, RhoA
Introduction
Dendritic spines are small protrusions on the surface of dendrite shafts that develop at excitatory synapses and compartmentalize the biochemical events activated by synaptic transmission (Tada & Sheng, 2006). The biogenesis and rearrangements of dendritic spines are driven primarily by the actin cytoskeleton (Dillon & Goda, 2005) and by the localized delivery of post-synaptic membrane components to the site of axo-dendritic contacts (Kennedy & Ehlers, 2006). Recent studies have indicated that the secretory machinery shows a strong local organization in the spines. Membrane compartments labelled by Golgi markers, but discontinuous with somatic Golgi, have been identified in both dendritic shafts and spines (Kennedy & Ehlers, 2006).
However, the mechanisms by which these membrane compartments are recruited to and retained at spines have not yet been described. Considering that actin represents the main cytoskeletal force in dendritic spines, one could suggest that proteins that interact with the actin regulatory machinery and Golgi membranes might mediate the association of Golgi compartments with these sites. One plausible candidate for this is Citron-N (Cit-N), a brain-specific isoform of the regulator of cytokinesis Citron kinase (Cit-K; Di Cunto et al, 2000). Cit-N has been described to localize at excitatory synapses and spines of hippocampal neurons (Zhang & Benson, 2006), and to represent a prominent component of the post-synaptic scaffold (Zhang et al, 1999). Furthermore, we found previously that Cit-N is also localized to the somatic neuronal Golgi and that it is able to regulate its organization through the local recruitment of actin filaments (Camera et al, 2003).
Here, we directly address the role of Cit-N in dendritic spines by using embryonic rat hippocampal neurons in culture and in genetically modified mice. We found that Cit-N is required for the maturation and maintenance of dendritic spines. Furthermore, we show that these functions might depend on the ability of Cit-N to recruit actin and to interact with Golgi membranes.
Results
Cit-N associates with Golgi at spines
To investigate the functional role of Cit-N in dendritic spines and spine-associated Golgi, we used embryonic rat hippocampal neurons in primary culture (PHN), a system used extensively to study the mechanisms underlying the formation and maintenance of dendritic spines. We started by analysing the subcellular distribution of endogenous Cit-N at 14 days in vitro (DIV), a time point at which neurons are polarized but synaptogenesis is not complete, and at 21 DIV, when neurons are fully mature and show a large number of dendritic spines. Neurons were immunolabelled for Cit-N and dendritic spines were visualized with phalloidin (Schubert et al, 2006). In 14 DIV cells, we observed Cit-N puncta in the dendritic shaft, and few points of colocalization with F-actin (Fig 1A). However, colocalization was much more evident at 21 DIV (Fig 1B). At this stage, 65% of the Cit-N/F-actin clusters colocalized with postsynaptic density 95 (Psd95), thus confirming that most of these structures are associated with excitatory synapses (supplementary Fig 1A online). Although in agreement with previous observations (Zhang & Benson, 2006), these data clarify that Cit-N is progressively recruited to dendritic spines during synaptogenesis.
Figure 1.
Localization of Citron-N to dendritic spines and spine-associated Golgi. (A) Cultured hippocampal neurons (14 DIV) were immunostained with a Citron antibody (Cit-N) and Texas red isothiocyanate-conjugated phalloidin (PHD). Cit-N is localized in discrete clusters that are distributed along the dendritic shaft. A few of them colocalized with actin-rich protrusions (arrows). (B) In 21 DIV neurons processed as in (A), most of the Cit-N-reactive clusters colocalized with peripheral actin-rich structures (arrows). Scale bars, 10 μm. (C) High-magnification images of a 21 DIV primary hippocampal neuron dendrite co-stained for Cit-N (green), F-actin (blue) and TGN38 (red). The arrows indicate some of the structures in which all the analysed proteins colocalize. (D) A 50 μg portion of different fractions obtained from the adult mouse brain were analysed for levels of the indicated proteins by western blotting. Rib-II, ribophorin-II; T, total homogenate; P, low-speed pellet; S, low-speed supernatant; NF, nuclear pellet; Syn, crude synaptosomes; Cyt, cytosol. (E) A 1 mg portion of the crude synaptosomal fraction was immunoprecipitated (IP) with Cit-N antibodies or preimmune serum (Pre), and co-precipitation of the indicated proteins was analysed by western blotting. Data are representative of four independent experiments. Cit-N, Citron-N; DIV, days in vitro.
Next, we studied whether Cit-N is associated with Golgi membranes in dendrites and at spines, by analysing its distribution with respect to F-actin and the two well-characterized Golgi markers GM130 and TGN38. Interestingly, 60% of the Cit-N clusters positive for F-actin colocalized with TGN38 (Fig 1C), which has been previously reported to label the spine apparatus (Gardiol et al, 1999). Accordingly, colocalization was particularly evident in the proximity of peripheral actin accumulations (Fig 1C). Although Cit-N/F-actin clusters also colocalized with GM130, their association was significantly lower (supplementary Fig 1B,C online).
In accordance with the morphological data, Cit-N and TGN38 were markedly enriched in crude synaptosomal preparations from adult brain, in which they co-fractionated with Psd95 (Fig 1D). Immunoprecipitation experiments from crude synaptosomes showed that Cit-N forms molecular complexes with Psd95, RhoA, RockII and actin, as expected (Camera et al, 2003), and also with TGN38 (Fig 1E).
These data show that Cit-N is accumulated progressively at dendritic spines in maturing neurons and that it is abundantly associated with TGN38-positive synaptosomal membranes.
Cit-N is required for the maintenance of spine Golgi
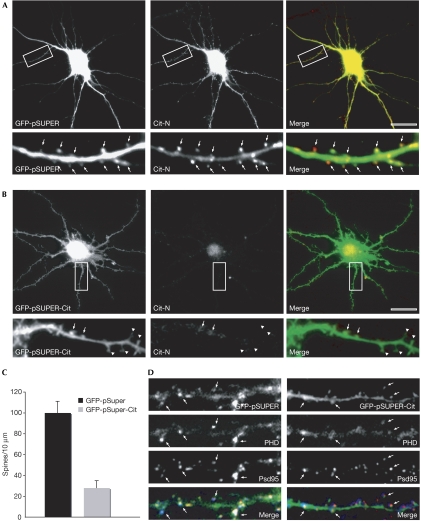
To investigate the role of Cit-N in dendritic spines, we depleted the protein in 21 DIV PHN by RNA interference (RNAi). We used two independent constructs against Citron that had been tested previously in PC12 cells (Berto et al, 2007). In control neurons transfected with unrelated green fluorescent protein (GFP)-pSUPER plasmid and thus expressing cytosolic GFP, endogenous Cit-N showed a subcellular distribution pattern, similar to that in untransfected cells, accumulating in dendritic spine heads (Fig 2A, spines detected with cytosolic GFP labelling). As expected, neurons expressing the specific RNAi constructs showed a marked reduction in endogenous Cit-N protein levels, as determined by fluorescence intensity (Fig 2B). In these cells, the linear density of mature, mushroom-shaped dendritic spines was reduced to a great extent (Fig 2B,C). Furthermore, RNAi-treated neurons showed many dendritic protrusions characterized by a morphology compatible with that of immature spines (Fig 2B). An analysis of F-actin in these cells showed a slight reduction throughout the dendritic shafts and a much greater decrease in the dendritic protrusions of RNAi-treated neurons (Fig 2D). By contrast, the distribution of Psd95-positive clusters was not altered by Cit-N knockdown (Fig 2D).
Figure 2.
Citron-N knockdown results in a decreased density of mature spines. Cultured hippocampal neurons (21 DIV) were transfected with (A) a control green fluorescent protein (GFP)-pSuper vector or (B) a GFP-pSuper-Cit-N short-interfering RNA construct. Two days after transfection, cells were processed for immunofluorescence microscopy with Cit-N antibodies. The arrows indicate Cit-N immunoreactivity associated with mature spines, whereas the arrowheads indicate immature dendritic protrusions. (C) Quantitative analysis of the linear density of mushroom spines in dendrites of control cells compared with RNA interference-treated cells. Error bars, standard error; P<0.01, Student's t-test. (D) High-magnification images of dendrites from a 21 DIV PHN treated as in panels (A) and (B), respectively, stained with Psd95 antibodies (red) and phalloidin (PHD; blue). The arrows indicate the colocalization of Psd95/actin. Cit-N, Citron-N; DIV, days in vitro; PHN, primary hippocampal neuron. Scale bar, 10 μm.
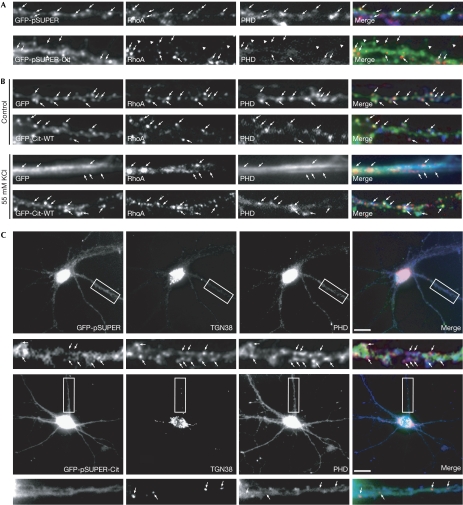
As the organization of F-actin at spines has been shown previously to depend on the localized activation of RhoA (Schubert et al, 2006), we determined the distribution of active RhoA in the dendrites of control and Cit-N-depleted neurons. As shown in Fig 3A, the localization of RhoA to spines of RNAi-treated cells decreased consistently, especially in those characterized by abnormal morphology. Conversely, overexpression of Cit-N efficiently prevented the relocalization of F-actin and RhoA from the spines to the dendritic shaft (Fig 3B), which is normally elicited in neurons by KCl-induced depolarization (Schubert et al, 2006).
Figure 3.
Functional requirement of Citron-N for F-actin, RhoA and Golgi accumulation at dendritic spines. (A) Hippocampal neurons (21 DIV) were transfected with control green fluorescent protein (GFP)-pSuper (upper panels) and GFP-pSuper-Cit (lower panels), and processed to show active RhoA and F-actin. The arrowheads indicate abnormal spines. (B) Hippocampal neurons (21 DIV) were transfected with vectors expressing GFP or GFP-Cit-N. At 48 h after transfection, the coverslips were exposed to control or 55 mM KCl-containing medium for 3 min and processed as in (A). The arrows in (A) and (B) indicate the position of RhoA clusters. (C) Neurons treated as in (A) were stained with TGN38 antibodies (red) and phalloidin (PHD; blue). The arrows indicate some of the TGN38-positive clusters. Scale bars, 10 μm. Cit-N, Citron-N; DIV, days in vitro.
These data indicate that Cit-N is required and sufficient for maintaining the accumulation of active RhoA and F-actin at dendritic spines.
Next, we asked whether downregulation of Cit-N had an effect on the assembly of Golgi compartments in dendrites. As expected (Camera et al, 2003), in RNAi-treated neurons, somatic TGN38-positive structures were more dispersed than in controls (Fig 3C). However, a more marked effect was observed in dendrites, which showed a strong reduction in TGN38-positive clusters (Fig 3C). In addition, the remaining clusters were mostly localized to the dendritic shaft and did not colocalize with actin-labelled protrusions (Fig 3C). By contrast, the distribution of GM130-positive clusters was not significantly affected (data not shown).
Hence, apart from regulating spine actin, Cit-N is also required for the organization of TGN38-positive dendritic Golgi membranes and for their maintenance at dendritic spines.
Domains of Cit-N required for its function at spines
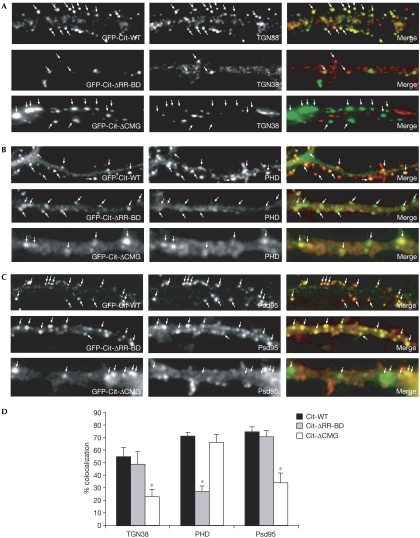
Previously, we have shown that the Rho-binding domain of Cit-N mediates the recruitment of actin in the Golgi apparatus of developing neurons, whereas its carboxy-terminal portion, containing a CMG domain, binds to Golgi membranes (Camera et al, 2003). These results, together with the present findings, indicate that Cit-N could promote spine maintenance by keeping together TGN stacks and actin at spines. To investigate this hypothesis, we transiently overexpressed GFP-Cit-N fusion constructs (supplementary Fig 2A online), lacking either the Rho-binding domain (Cit-ΔRR-BD) or the CMG domain (Cit-ΔCMG), and compared them with full-length GFP-Cit-N-expressing cells.
Cells expressing GFP-Cit-N showed a TGN38 distribution comparable with that of untransfected controls, with TGN38 accumulating in the periphery of the dendrite (Fig 4A). Analysis of the merged image showed that these clusters colocalized with GFP-Cit-N. Cells expressing Cit-ΔRR-BD showed disperse labelling for TGN38 with few clusters when compared with neurons expressing wild-type Cit-N (Fig 4A). Statistical analysis showed that approximately 50% of the Cit-ΔRR-BD clusters colocalized with TGN38 (Fig 4D). These results indicate that the Rho-binding domain of Cit-N is important for the compactness of the Golgi apparatus, but that it is not essential for the targeting of the protein to this organelle. In contrast to the first mutant, neurons expressing Cit-ΔCMG showed prominent clusters of TGN38 within the dendritic shaft, which did not colocalize with the mutant protein (Fig 4A), indicating that the CMG domain is required for targeting Cit-N to Golgi membranes.
Figure 4.
Domains of Citron-N required for its function. (A–C) Hippocampal neurons (21 DIV) were transfected with the indicated constructs and stained after 48 h for (A) TGN38, (B) F-actin and (C) Psd95. High-magnification micrographs show the distribution of the overexpressed proteins (arrows). (D) Quantitative analysis of the colocalization between the wild-type and mutant green fluorescent protein (GFP)-Cit-N fusions, and the indicated proteins. The asterisks indicate results that are statistically different from control (P<0.01, t-test). Error bars, standard error. Cit, Citron; DIV, days in vitro; PHD, phalloidin.
Cells expressing wild-type GFP-Cit-N showed the typical F-actin distribution found in dendrites of mature neurons (Fig 4B). Furthermore, the recombinant protein was targeted to Psd95-positive structures, similar to the endogenous protein (Fig 4C). By contrast, the expression of Cit-ΔRR-BD markedly reduced the signal intensity of F-actin in the dendrites (Fig 4B). Furthermore, most of the clusters formed by this mutant protein did not colocalize with F-actin (Fig 4D), but were still targeted to excitatory synapses (Fig 4C,D). The expression of Cit-ΔCMG also led to a reduction in the signal intensity of F-actin in the dendrites, although to a lesser extent, and with some remaining prominent clusters (Fig 4B). An analysis of the merged images showed that Cit-ΔCMG colocalized with these F-actin clusters, but did not colocalize with Psd95 (Fig 4C,D), indicating that the mutant protein is not targeted to synapses. Thus, the associated actin accumulations might represent ectopic F-actin structures, owing to recruitment of the actin-binding machinery (Schubert et al, 2006) rather than dendritic spines. Accordingly, in these overexpression experiments, RhoA was recruited to the Cit-ΔCMG clusters (supplementary Fig 2B online).
These data indicate that the CMG domain of Cit-N is required for synaptic targeting of the protein and also that correct spine morphology might require Cit-N in a two-step mechanism involving its interaction with Golgi structures, through the CMG domain, and with the actin cytoskeleton, through the Rho-binding domain.
Cit-N is required for maturation of spine in vivo
Cit-K and Cit-N are produced by a complex transcription unit through an alternative transcriptional initiation mechanism. Previously, we generated a mutant mouse line (Cit-K−/−) showing specific loss of the Cit-K isoform. Cit-K−/− mice are affected by severe microcephaly, caused by the cytokinesis block of specific neuronal precursors, followed by massive apoptosis (Di Cunto et al, 2000). To investigate the function of Cit-N in vivo, we performed a new gene targeting experiment (for details, see supplementary Fig 3 online), which resulted in a second recombinant line (Citnull/null), characterized by the complete loss of both Cit-K and Cit-N proteins (Fig 5A). As Citnull/null mice show a neuroblast cytokinesis phenotype that is indistinguishable from that observed in Cit-K−/− mice, leading to a similar histological structure and survival phenotype (data not shown), we thought that the specific role of Cit-N could be investigated by comparing the two lines. We first addressed this biochemically, by measuring the levels of Cit-N-interacting proteins (Fig 1E) in total brain lysates and in crude synaptosomes. We observed no differences between total lysates of Cit-K−/− and Citnull/null, although both lines showed some differences with respect to the wild-type control (Fig 5B). Interestingly, compared with Cit-K−/−, crude synaptosomes of Citnull/null showed a significant decrease in TGN38, Psd95 and RhoA, and a slight decrease in RockII and actin (Fig 5B,C), which was in good agreement with the in vitro experiments.
Figure 5.
The loss of Citron-N affects dendritic spine morphology in vivo. (A) SDS lysates (30 μg) of P4 cerebellum from mice of the indicated genotypes were analysed by western blotting with Citron antibodies (upper panel). Antibodies against the related kinase RockII were used as a positive control (lower panel). (B) Levels of the indicated proteins were analysed by western blotting in total lysates (left) and crude synaptosomes (right) of P15 brains of the indicated genotypes. Data are representative of four independent experiments. (C) Quantitative analysis of data shown in (B). The signals were normalized to the internal loading control (Tubulin) and the results of the mutant mice are referred to the wild-type control. Error bars, standard deviation; **P<0.01, *P<0.05 (t-test). (D) High-magnification field, showing comparable and representative dendritic segments of the indicated genotypes. The arrows indicate stubby spines. Error bars, standard error; scale bar, 10 μm. (E) Quantitative analysis of spine density in P17 cortical pyramidal neurons of the indicated genotypes. (F) Quantitative assessment of spine morphology in dendrites of P17 cortical pyramidal neurons of the indicated genotypes. Cit-K, Citron kinase; Cit-N, Citron-N; WT, wild type.
Next, we studied whether these mice show qualitative and quantitative differences on synapse and dendritic spines. In particular, we focused on cortical pyramidal neurons at P17. Compared with control mice, there was a marked reduction in the spine density in both Cit-K−/− and Citnull/null mice (Fig 5D,E). However, the spines of Cit-K−/− mice showed a morphology comparable with that of wild-type mice, with most having a mushroom shape, whereas the spines of Citnull/null mice showed an immature appearance, with a strong prevalence of stubby and filopodia types (Fig 5D,F). Confocal microscopy showed that the colocalization of Psd95, N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors with pre-synaptic markers, such as the vesicular glutamate transporter 1 (Vglut1), was not different among genotypes (supplementary Fig 4 online), indicating that the observed phenotype is not primarily due to defective synaptogenesis. These results indicate that Cit-N is specifically required for the production of morphologically mature dendritic spines in vivo.
Discussion
Here, we have shown that Cit-N is a crucial mediator of maturation and maintenance of dendritic spines, both in vivo and in vitro. Previously, we had established that Cit-N is essential for maintaining Golgi architecture in young neurons, by RhoA- and actin-dependent mechanisms involving a RockII-dependent actin-polymerizing complex (Camera et al, 2003). Here, we have shown that Cit-N exerts a similar activity on the peripheral Golgi membranes associated with dendritic spines. Interestingly, knockdown of Cit-N in mature neurons had a much weaker effect on somatic TGN38-positive compartments and on GM130-positive membranes (Fig 2C; data not shown), when compared with younger cells (Camera et al, 2003). This indicates that the effect of Cit-N RNAi on spines is rather specific and not due to a general disturbance of Golgi architecture, and suggests that the functional requirement of this protein shifts towards dendritic compartments during neuronal maturation. Furthermore, the knockdown of Cit-N prevented the accumulation of active RhoA and F-actin at spines, providing strong support for the idea that local organization of membranes and the cytoskeleton at spines are tightly intertwined processes. However, at the same time, they raise the question of what could be the primary event. In this regard, it is important to note that RhoA has recently been shown to interact directly with ionotropic and methabotropic glutamate receptors, and to mediate spine actin polymerization through RockII (Schubert et al, 2006). On the basis of this, it would be tempting to speculate that the first determinant of spine organization could be the recruitment of Cit-N–RockII complex at synaptic sites by active RhoA associated to glutamate receptors, followed by actin polymerization and Golgi membranes tethering through the CMG domain of Cit-N. However, the results obtained by overexpressing the Cit-ΔCMG mutant are not in agreement with this scenario. Besides being unable to associate with TGN38-positive membranes, this mutant was not localized to excitatory synapses, although it has a functional RhoA-binding domain. Therefore, a more likely possibility is that the Cit-N–RockII complex becomes first associated with TGN38-positive membranes, by the CMG domain of Cit-N, and regulates their organization by RhoA-dependent actin polymerization. In the second phase, the entire complex, with its associated membranes, would be transferred to synapses, where it could contribute to maintaining the characteristic organization of spine actin and membranes. The findings that Cit-N overexpression prevents the activity-elicited relocalization of active RhoA and F-actin from spines to dendritic shafts (Fig 3B), and that the proteins normally associated with Cit-N in synaptosomes are specifically decreased in Citnull/null mice (Fig 5B), add further support to this view.
As glutamate receptors show high levels of associated RhoA activity under basal conditions (Schubert et al, 2006), the requirement for Cit-N function could be largely activity independent. However, in the light of the recent discovery that the activity-dependent growth of spines depends on exocytic membrane trafficking from early endosomes (Park et al, 2006), it will be of interest to investigate the dynamic behaviour and functional involvement of the Cit-N complex during activity-dependent processes.
Methods
Cell culture and transfection. Primary cultures of rat embryonic hippocampal neurons were prepared as described previously (Goslin & Banker, 1991). For morphological analysis, 100,000 cells were plated per 60 mm dish, each with six poly-L-lysine-coated glass coverslips. Plasmid DNA was transfected by using the Effectene reagent (Qiagen, Dusseldorf, Germany). For short-interfering RNA inhibition experiments, Cit-N and deletion mutant overexpression experiments, the neurons were transfected at 21 DIV and fixed at 23 DIV.
Immunoblotting and immunoprecipitations. Brain proteins were extracted with lysis buffer (1% Triton X-100, 150 mM sodium chloride, 50 mM Tris–HCl pH 7.5, protease inhibitors (Roche, Basel, Switzerland) and 1 mM phenylethylsulphonyl fluoride). Equal amounts of proteins were resolved by reducing SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose filters, which were incubated with the indicated antibodies and developed by using the ECL system (Amersham Biosciences, Piscataway, NJ, USA). Fractionation experiments and immunoprecipitation from crude synaptosomes were performed essentially as described previously by Schubert et al (2006). See the supplementary information online for details.
Immunofluorescence microscopy. Cells were fixed in paraformaldehyde/SEM buffer (4% paraformaldehyde, 0.12 M sucrose, 3 mM EGTA and 2 mM MgCl2 in PBS), quenched with 50 mM ammonium chloride and extracted with 0.1% Triton X-100. To reveal active RhoA, cells were pre-extracted with 0.1% Triton X-100 for 10 s before fixation (Schubert et al, 2006). Neuronal stimulation with KCl was performed as according to Schubert et al (2006) (for details, see the supplementary information online). Specific protein detection was performed by using previously mentioned antibodies, followed by incubation with appropriate Alexa Fluor-conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA). F-actin was labelled with Texas red isothiocyanate-conjugated phalloidin (Sigma-Aldrich, St Louis, MO, USA) or with Alexa Fluor 350-conjugated phalloidin (Molecular Probes). Cells were observed using an Axiovert 200M Zeiss microscope, with a × 63 oil immersion lens, and images were acquired using the MetaMorph Software (Molecular Devices, Toronto, Canada).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to B. Hellias, F. Bianchi, F. Cristofani and E. Cassin for the excellent preparation of primary cultures, and to O. Azzolino for her assistance with gene targeting. The financial support of Cassa di Risparmio di Torino foundation to A.V., and of Telethon-Italy and of the Jérôme Léjeune foundation to F.D.C. is gratefully acknowledged.
References
- Berto G, Camera P, Fusco C, Imarisio S, Ambrogio C, Chiarle R, Silengo L, Di Cunto F (2007) The Down syndrome critical region protein TTC3 inhibits neuronal differentiation via RhoA and Citron kinase. J Cell Sci 120: 1859–1867 [DOI] [PubMed] [Google Scholar]
- Camera P, da Silva JS, Griffiths G, Giuffrida MG, Ferrara L, Schubert V, Imarisio S, Silengo L, Dotti CG, Di Cunto F (2003) Citron-N is a neuronal Rho-associated protein involved in Golgi organization through actin cytoskeleton regulation. Nat Cell Biol 5: 1071–1078 [DOI] [PubMed] [Google Scholar]
- Di Cunto F et al. (2000) Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron 28: 115–127 [DOI] [PubMed] [Google Scholar]
- Dillon C, Goda Y (2005) The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci 28: 25–55 [DOI] [PubMed] [Google Scholar]
- Gardiol A, Racca C, Triller A (1999) Dendritic and postsynaptic protein synthetic machinery. J Neurosci 19: 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Banker GA (1991) Culturing Nerve Cells, pp 251–281. Cambridge, MA, USA: MIT Press [Google Scholar]
- Kennedy MJ, Ehlers MD (2006) Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci 29: 325–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD (2006) Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 52: 817–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert V, Da Silva JS, Dotti CG (2006) Localized recruitment and activation of RhoA underlies dendritic spine morphology in a glutamate receptor-dependent manner. J Cell Biol 172: 453–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Sheng M (2006) Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol 16: 95–101 [DOI] [PubMed] [Google Scholar]
- Zhang W, Benson DL (2006) Targeting and clustering citron to synapses. Mol Cell Neurosci 31: 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Vazquez L, Apperson M, Kennedy MB (1999) Citron binds to PSD-95 at glutamatergic synapses on inhibitory neurons in the hippocampus. J Neurosci 19: 96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information