Abstract
There are several endocytic pathways, which are either dependent on or independent of clathrin. This study focuses on a poorly characterized mechanism—clathrin- and caveolae-independent endocytosis—used by the interleukin-2 receptor β (IL-2Rβ). We address the question of its regulation in comparison with the clathrin-dependent pathway. First, we show that Ras-related C3 botulinum toxin substrate 1 (Rac1) is specifically required for IL-2Rβ entry, and we identify p21-activated kinases (Paks) as downstream targets. By RNA interference, we show that Pak1 and Pak2 are both necessary for IL-2Rβ uptake, in contrast to the clathrin-dependent route. We observe that cortactin, a partner of actin and dynamin—two essential endocytic factors—is required for IL-2Rβ uptake. Furthermore, we find that cortactin acts downstream from Paks, suggesting control of its function by these kinases. Thus, we describe a cascade composed of Rac1, Paks and cortactin specifically regulating IL-2Rβ internalization. This study indicates Paks as the first specific regulators of the clathrin-independent endocytosis pathway.
Keywords: cytokine receptors, cytoskeleton, kinase, Rho GTPase, cortactin
Introduction
Receptor-mediated endocytosis is crucial for eukaryotic cells. In mammalian cells, there are several pathways. However, the only route well characterized so far is the clathrin-dependent one used by many receptors, including the transferrin (Tf) receptor. Briefly, the formation of the vesicle involves a clathrin coat, adaptors and scaffolding proteins (Mousavi et al, 2004). The detachment of the clathrin vesicle from the plasma membrane requires dynamin, actin and their partners, such as intersectin, syndapin, neuronal Wiskott–Aldrich syndrome protein (N-WASP) and cortactin (Schafer, 2002).
At least three clathrin-independent internalization pathways exist, but they are all poorly defined (Kirkham & Parton, 2005). The point shared by the receptors in these pathways is their association at the plasma membrane with lipid microdomains enriched in cholesterol and glycosphingolipids and also the requirement of sphingolipids for their uptake (Cheng et al, 2006). Caveolae are a subset of these domains that contain caveolin (Nabi & Le, 2003). In two of these mechanisms, dynamin and actin polymerization are also essential. These proteins are involved in the caveolae-dependent uptake used by viruses and toxins (Pelkmans et al, 2002) as well as the caveolae-independent endocytosis of some immune cell receptors, including the interleukin-2 receptor β chain (IL-2Rβ; Lamaze et al, 2001) and the common cytokine receptor γc (Sauvonnet et al, 2005). However, dynamin is not required in the third clathrin-independent mechanism, which is used by the glycosylphosphatidylinositol-anchored proteins (Sabharanjak et al, 2002) and also ricin (Llorente et al, 1998). In addition, kinases were shown to be important in caveolae uptake, but their functions remain to be elucidated (Pelkmans et al, 2005). Apart from this limited information, the functioning of the three types of clathrin-independent vesicle is far from being understood (Gesbert et al, 2004; Kirkham & Parton, 2005).
Interestingly, dynamin and F-actin are crucial to most endocytic processes that coexist within the cell. These common factors must be tightly controlled and perhaps differentially regulated according to the endocytotic mechanisms. The upstream regulators include the Rho GTPases, because RhoA has opposite effects on the clathrin-dependent and -independent mechanisms (Lamaze et al, 1996, 2001; Sabharanjak et al, 2002). However, the targets of Rho GTPases in each pathway remain to be found. Rho GTPases are known to be involved in the control of actin dynamics (Jaffe & Hall, 2005). They act on actin nucleators and regulators. For instance, Ras-related C3 botulinum toxin substrate 1 (Rac1) can control actin polymerization by activating the serine/threonine p21-activated kinases (Paks; Ridley, 2006). The group I Paks (Pak1, Pak2 and Pak3) are powerful actin cytoskeleton regulators that phosphorylate many substrates (Vidal et al, 2002; Hofmann et al, 2004). Among these, cortactin is of particular interest, because it is a partner of actin and dynamin that promotes actin polymerization (Schafer, 2002).
In this study, we investigated the action and targets of Rac1, which our laboratory has previously suggested to be involved, similarly to RhoA, in the endocytosis of IL-2Rβ (Lamaze et al, 2001). Thus, we compared simultaneously two internalization routes that require both dynamin and actin: the clathrin-dependent endocytosis of Tf and the clathrin-independent uptake of IL-2Rβ.
Results And Discussion
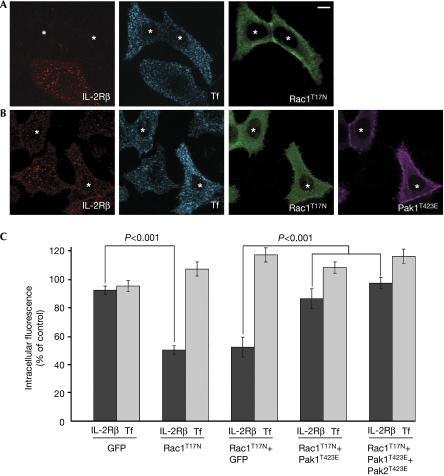
To investigate the role of Rac1 in endocytosis, we used Hep2 cells expressing stable IL-2Rβ (supplementary information online). These cells, designated Hep2β, produced a similar amount (1.4-fold greater) of IL-2Rβ as does a natural killer cell line (YT) that natively expresses the receptor, and we verified that IL-2Rβ endocytosis is dynamin-dependent (supplementary Fig 1 online). Hep2β cells were transfected with a dominant-negative mutant of Rac1 (Rac1T17N), and internalization of IL-2Rβ was initiated by the addition of a monoclonal antibody against it (anti-IL-2Rβ) coupled with Cy3 fluorochrome. Cells were incubated with the antibody for 15 min at 37°C, a sufficient time to observe the majority of receptors in the endosomes. For comparison, clathrin-dependent uptake was measured in same cells by the simultaneous addition of Tf coupled with Alexa Fluor 647 fluorochrome. Strikingly, the quantification of the data showed that the expression of Rac1T17N inhibited 50% of IL-2Rβ entry as compared with the non-transfected cells, but did not affect Tf uptake (Fig 1A,C). The same inhibitory effect of Rac1T17N was observed after 30 min of endocytosis (supplementary Fig 2 online). Therefore, these two pathways are affected differently by the prevention of Rac1 action. Indeed, Rac1 activity is required only for the clathrin-independent endocytosis of IL-2Rβ.
Figure 1.
A Rac1 dominant-negative mutant inhibits specifically IL-2Rβ endocytosis and can be counteracted by an active form of Pak1. (A) Endocytosis of IL-2Rβ (red) and Tf (blue) were examined by immunofluorescence in Hep2β cells transfected with a dominant-negative mutant of Rac1, myc-Rac1T17N (green). The cells were incubated for 15 min at 37°C in the presence of Cy3-coupled IL-2Rβ antibody and Alexa Fluor 647-coupled Tf. Cells were then fixed, permeabilized and reacted with myc antibody and the corresponding secondary antibody. A medial section is shown; asterisks indicate the cells expressing myc-Rac1T17N. (B) Hep2β cells were transfected with myc-Rac1T17N and a constitutively active form of Pak1, HA-Pak1T423E. Endocytosis of IL-2Rβ and Tf were followed as described in (A). (C) Quantification of (A), (B) and Hep2β cells transfected with myc-Rac1T17N and GFP, as well as Hep2β cells transfected with myc-Rac1T17N, HA-Pak1T423E and a constitutively active form of Pak2, GFP-Pak2T423E. To quantify endocytosis, the intracellular fluorescence intensity was measured with Metamorph software (mean±s.e.; n≈100 cells from three independent experiments). The results are expressed as a percentage of the intracellular fluorescence intensity of non-transfected cells. GFP, green fluorescent protein; HA, haemagglutinin; IL-2Rβ, interleukin-2 receptor β; Pak, p21-activated kinase; Rac1, Ras-related C3 botulinum toxin substrate 1; Tf, transferrin.
Paks are targets of Rac1 of particular interest because they have been shown to be involved in many cellular processes including cytoskeleton reorganization (Jaffer & Chernoff, 2002). In addition, Pak1 was shown to be required for macropinocytosis (Dharmawardhane et al, 2000). This prompted us to test whether this kinase could act downstream from Rac1 in receptor-mediated uptake. To do so, we tried to rescue the inhibitory effect of the mutant Rac1T17N by coexpressing a constitutively active form of Pak1 (Pak1T423E) in Hep2β cells. This form of Pak1 has a substitution in its autophosphorylation site (Thr 423) that prevents the inactive folding of the kinase (Zenke et al, 1999). Cells expressing both Rac1T17N and Pak1T423E showed 86% of IL-2Rβ endocytosis (Fig 1B,C). This rescue of uptake is statistically significant (P<0.001). Rescue was slightly, but not significantly, increased (97% of IL-2Rβ entry) when Pak1T423E and an active form of Pak2 (Pak2T423E) were expressed with Rac1T17N (Fig 1C). As a control, cells expressing Rac1T17N and green fluorescent protein (GFP) showed an inhibition of IL-2Rβ internalization of about 50% (Fig 1C). As expected, Tf internalization was unaffected by Pak expression. These results indicate that the inhibitory effect of the dominant-negative mutant of Rac1 can be overcome by the expression of a constitutively active form of Pak1. Therefore, our data indicate that Pak1 is a downstream target of Rac1 that is involved in clathrin-independent endocytosis.
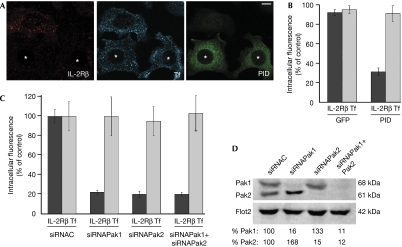
To further investigate the role of Pak1 in IL-2Rβ internalization, Hep2β cells were transfected with a gene encoding the Pak inhibitory domain (PID), a Pak1 domain (83–149 aa) that interacts with the kinase region and inhibits the activity of Paks (Zhao et al, 1998). Cells overexpressing the PID had about 70% inhibition of IL-2Rβ endocytosis. By contrast, Tf uptake was not affected (Fig 2A,B). As the three members of group I Paks are similar, they can be inactivated by the same dominant-negative mutant of Pak1. Hep2β cells express both Pak1 and Pak2, but not Pak3. Thus, our results indicate that at least one of these two kinases is involved in IL-2Rβ internalization. To determine which one, or both, is required for this endocytic pathway, we used small interfering RNAs (siRNA) to knock down Pak1 and/or Pak2 in Hep2β cells, and then assayed for Tf and IL-2Rβ endocytosis. We observed by western blot that, in transfected cells, each siRNA specifically knocked down its target and did not knock down the other one (84–89% of depletion; Fig 2D). The quantification of the endocytosis data indicates that cells depleted in either Pak1 or Pak2 had a roughly 78% reduction in IL-2Rβ internalization as compared with control cells (Fig 2C). The depletion of both kinases led to an inhibition of 80% of endocytosis (Fig 2C). By contrast, Tf entry was not affected (Fig 2C). As a control, we checked that two other pairs of siRNAs specific to Pak1 and Pak2 led to the same results (data not shown). Therefore, both Pak1 and Pak2 are necessary for IL-2Rβ entry but not for Tf endocytosis. These two kinases might have distinct functions in internalization. Taken together, these results show that group I Paks are downstream targets of Rac1 that are specifically required for clathrin-independent endocytosis.
Figure 2.
Pak1 and Pak2 are necessary for IL-2Rβ entry but not for transferrin uptake. (A) Endocytosis of IL-2Rβ (red) and Tf (blue) in cells transfected with the Pak1 inhibitory domain, myc-PID (green). Endocytosis and immunofluorescence were carried out as described in Fig 1A (asterisks indicate the cells expressing myc-PID). (B) Quantification of (A) and of Hep2β cells transfected with GFP as a control was carried out as described in Fig 1C. (C) Quantification of intracellular IL-2Rβ and Tf in Pak1- and/or Pak2-knockdown cells. Hep2β cells were transfected with small interfering RNA (siRNA) against Pak1 (siRNAPak1), Pak2 (siRNAPak2) or against an irrelevant protein (siRNAC). Endocytosis and quantification were carried out for 200 cells. The results are expressed as a percentage of the intracellular fluorescence intensity of control cells (siRNAC). (D) Western blots of siRNA-transfected cells were probed with antibodies against Pak1, Pak2 or against flotillin 2 (Flot2) as a control; quantification by Storm FluoroImager. GFP, green fluorescent protein; IL-2Rβ, interleukin-2 receptor β; Pak, p21-activated kinase; PID, Pak inhibitory domain; Rac1, Ras-related C3 botulinum toxin substrate 1; Tf, transferrin.
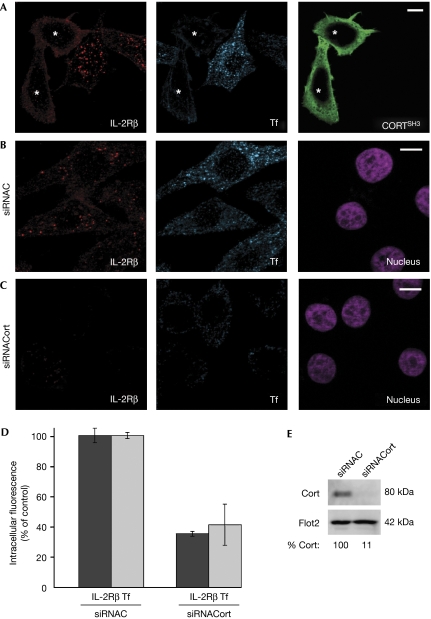
We next addressed the action of Paks on receptor-mediated uptake by searching for a downstream target. Paks have been shown to be important regulators of cytoskeletal dynamics. The requirement for the actin cytoskeleton in endocytosis has indicated a new role for dynamin, which interacts with several proteins associated with F-actin (Schafer, 2002). Among the dynamin–actin partners, cortactin was shown to be necessary for two examples of endocytic pathways, dependent on and independent of clathrin (Cao et al, 2003; Sauvonnet et al, 2005). Cortactin is an activator of actin polymerization that contains several domains enabling its direct interaction with actin-related protein 3 (Arp3) and F-actin, and, through its Src-homology 3 (SH3) domain, with dynamin, N-WASP and probably other factors (Daly, 2004). Moreover, cortactin was shown to be the target of several kinases, and it has many phosphorylated sites on tyrosine, serine and threonine residues (Martin et al, 2006). Interestingly, in several studies, cortactin was shown to be a substrate of group I Paks (Vidal et al, 2002; Webb et al, 2006). Therefore, we investigated whether IL-2Rβ endocytosis requires cortactin, as seen in the internalization of the common cytokine receptor γc (Sauvonnet et al, 2005). To this end, Hep2β cells were transfected with a dominant-negative mutant of cortactin, CortSH3, a truncated form of the protein keeping its SH3 domain (Du et al, 1998). Cells overexpressing CortSH3 had a reduction of 60% of IL-2Rβ endocytosis, indicating that cortactin was necessary for this entry route (Fig 3A). As expected, Tf internalization was also inhibited by about 55% in the CortSH3-expressing cells (Fig 3A). To further test our hypothesis on the role of cortactin, we used siRNA to specifically deplete this protein, as verified by western blot (89% of depletion, Fig 3E). In cortactin-depleted cells, IL-2Rβ endocytosis was reduced by about 65% compared with control cells (Fig 3B–D). In addition, Tf uptake was also inhibited to about 60% when compared with control cells (Fig 3B–D). Thus, cortactin is an essential factor for IL-2Rβ and for Tf endocytosis, confirming that it belongs to a family of proteins necessary for both clathrin-dependent and -independent internalizations.
Figure 3.
Cortactin is necessary for IL-2Rβ and for transferrin endocytosis. (A) Hep2β cells transfected with a dominant-negative mutant of cortactin, Flag-CortSH3 (green). (B) Cells treated with control small interfering RNA (siRNAC). (C) Cells treated with siRNA against cortactin (siRNACort). (A–C) Endocytosis and immunofluorescence were carried out as described in Fig 1A (asterisks indicate the cells expressing CortSH3). Nuclei were stained with Hoechst (purple). (D) Quantification of IL-2Rβ and Tf endocytosis was carried out as described in Fig 2C. (E) Western blots of siRNA-transfected cells were probed with antibodies against cortactin or flotillin 2 (Flot2) as a control; quantification by Storm FluoroImager. IL-2Rβ, interleukin-2 receptor β; Pak, p21-activated kinase; Rac1, Ras-related C3 botulinum toxin substrate 1; Tf, transferrin.
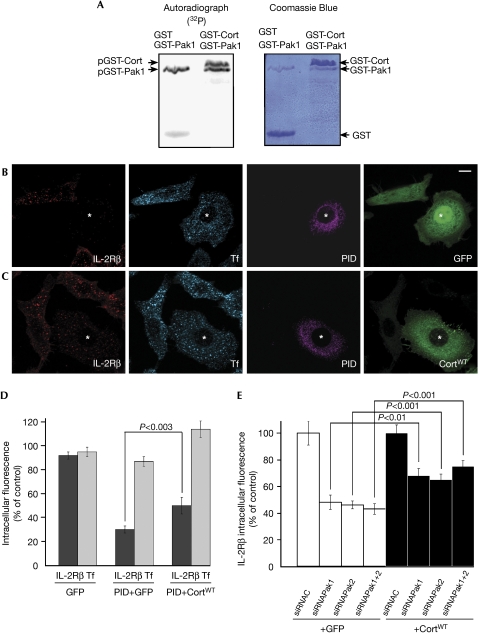
We then investigated whether cortactin acts downstream from Paks in receptor-mediated endocytosis. First, we tested whether Pak1 and Pak2 can phosphorylate glutathione-S-transferase–cortactin by using an in vitro kinase assay and this was confirmed (Fig 4A; supplementary Fig 3 online). Then, we tried to rescue the effect of the PID by co-overexpressing a wild-type form of cortactin (CortWT) in Hep2β cells. The inhibitory effect of PID could be partly overcome by coexpression of CortWT: cells coexpressing PID and CortWT had about 50% of IL-2Rβ endocytosis, whereas only 30% of uptake was measured in cells coexpressing PID and GFP (Fig 4B–D, P<0.003). Tf entry was not affected by CortWT (Fig 4B–D). We also tried to rescue IL-2Rβ endocytosis in Pak1-, Pak2- or Pak1–Pak2-depleted cells by overexpressing cortactin. Between 65 and 75% of IL-2Rβ internalization was seen in Pak1- and/or Pak2-depleted cells coexpressing CortWT, whereas 43% of uptake was observed when GFP was coexpressed as a control (Fig 4E, P<0.001). Therefore, overproduction of cortactin can partly counteract the inhibition of endocytosis observed in Pak1–Pak2-depleted cells. The fact that the recovery of IL-2Rβ uptake was only partial can be explained in two ways. Either the level of cortactin expression is not sufficient or cortactin is not the only target of Pak1 and/or Pak2 involved in IL-2Rβ internalization.
Figure 4.
Cortactin acts downstream of Paks in IL-2Rβ endocytosis. (A) In vitro phosphorylation of cortactin by Pak1. Purified GST-Pak1 (1 μg) was incubated with 5 μg of purified GST-cortactin (GST-Cort) or GST and 5 μCi γ[32P]ATP. Autoradiography (left), Coomassie blue gel (right). (B) Hep2β cells were transfected with myc-PID and GFP. (C) Hep2β cells were transfected with myc-PID and GFP-cortactin wild-type (CortWT). Cells were treated and analysed as described in Fig 1 (asterisks indicate the cells coexpressing the constructs). (D) Quantification of the endocytosis results was carried out as described in Fig 1C. (E) Hep2β cells were transfected with small interfering RNA (siRNA) against Pak1 (siRNAPak1) and/or Pak2 (siRNAPak2) or against an irrelevant protein (siRNAC) and transfected either with GFP-cortactin or GFP. Quantification of the endocytosis results was carried out as described in Fig 1C (mean±s.e.; n=50 cells in two independent experiments). GFP, green fluorescent protein; GST, glutathione-S-transferase; IL-2Rβ, interleukin-2 receptor β; Pak, p21-activated kinase; PID, Pak inhibitory domain; Rac1, Ras-related C3 botulinum toxin substrate 1; Tf, transferrin.
Our results indicate that the role of cortactin in clathrin-independent endocytosis is linked to Paks that are themselves controlled by Rac1. Next, we tested whether Rac1 or Pak1 stimulation might affect cortactin recruitment to the plasma membrane. We found that cells expressing a constitutively active form of Rac1 (Rac1G12V) or Pak1 (Pak1T423E) showed an enrichment of cortactin at the plasma membrane (supplementary Figs 4,5 online). Control cells expressing the dominant-negative mutant Rac1T17N or the PID showed no such enrichment, as expected. The phosphorylation of cortactin by Paks could enhance its function as a regulator of actin dynamics (Daly, 2004). Previous studies showed that the rate of actin polymerization promoted by cortactin is dependent on its binding to F-actin, Arp3 and N-WASP (Weaver et al, 2002). Interestingly, a recent report proposed that the affinity of cortactin to N-WASP would be regulated by its phosphorylation (Martinez-Quiles et al, 2004). Thus, we tested whether Rac1 or Pak1 stimulation might affect the localization of cortactin with N-WASP. We found that cells expressing Rac1G12V or Pak1T423E showed localization of cortactin with N-WASP at the plasma membrane, in contrast to cells expressing Rac1T17N or PID (supplementary Figs 4,5 online). Therefore, our results suggest that Rac1 and Paks enable a better interaction of cortactin with N-WASP and could enhance actin polymerization during clathrin-independent entry. Because Paks are not involved in clathrin-dependent internalization, the mechanism of regulation of cortactin might be different. For example, syndapin and intersectin, which are required for clathrin-dependent uptake, bind to dynamin and N-WASP (Qualmann & Kelly, 2000; Hussain et al, 2001) and could therefore allow the recruitment of N-WASP to cortactin, thereby enhancing the rate of actin polymerization. The fact that syndapin and intersectin are not required for clathrin-independent endocytosis (Sauvonnet et al, 2005) reinforces the hypothesis of a differential regulation of actin polymerization according to the endocytic route taken.
In conclusion, our data, together with the earlier results from our laboratory (Lamaze et al, 2001), show that the clathrin- and caveolae-independent endocytosis pathway (reviewed by Mayor & Pagano, 2007) requires both RhoA and Rac1. Interestingly, and consistently, clathrin-dependent endocytosis is inhibited by the constitutively active forms of RhoA and Rac1 (Lamaze et al, 1996). The similar effects of RhoA and Rac1 on clathrin-independent endocytosis contrasts with their generally antagonistic roles in controlling cell migration and adhesion (Burridge & Doughman, 2006).
In addition, we have identified what we believe to be the first factors specifically involved in the clathrin-independent endocytosis of IL-2Rβ, that is, the serine/threonine kinases Pak1 and Pak2, which are stimulated by Rac1. Moreover, we found that cortactin is a downstream target of the Paks. Finally, this study sheds light on the differential regulation of two endocytic pathways that share important factors such as dynamin, actin and cortactin, indicating that Rac1–Pak1–Pak2 act as upstream regulators that specifically switch on the clathrin-independent pathway.
Methods
Endocytosis, immunofluorescence and microscopy. Endocytosis of IL-2Rβ and Tf at 37°C were measured at 15 min as described previously (Lamaze et al, 2001), with 0.7 μg per coverslip of anti-IL-2Rβ (mouse antibody 561; Lamaze et al, 2001) conjugated to Cy3 fluorochrome (GE Healthcare, Amersham, Buckinghamshire, UK) and 50 nM human iron-loaded Tf conjugated to Alexa Fluor 647 (Amersham). Hep2β cells were fixed and permeabilized as described previously (Lamaze et al, 2001) and reacted with anti-haemagglutinin (rat antibody (Invitrogen, Cergy-Pontoise, France), 1/100), anti-myc (mouse antibody 9E10, ascites, 1/400) or anti-FlagM2 (mouse antibody (Sigma-Aldrich, Saint Quentin Fallavier, France), 1/2000). The following antibodies were used as secondary antibodies: Cy5-coupled anti-rat IgG (Chemicon, Billerica, MA, USA, 1/100), FITC-coupled anti-mouse IgG (Southern Biotechnology, 1/100) or Alexa Fluor 350-coupled anti-mouse IgG1 (Molecular Probes, Birmingham, AL, USA, 1/200). Fluorescence images were obtained with an Apotome microscope (Zeiss) equipped with a × 63 objective and a Roper Scientific Coolsnap HQ camera. A z-series of 1 μm optical sections was photographed, and a medial section is shown in each figure. For quantification of the data, we obtained images with an epifluorescence microscope equipped with a × 25 objective under the same acquisition settings. Images collected from three independent experiments were analysed with Metamorph software. To quantitate the fluorescence intensity, the area of at least 100 cells was traced, and the mean fluorescence intensity per unit area was determined for each channel (Cy3 and Alexa Fluor 647). Following a background subtraction, each value was divided by the mean of intensity of non-transfected cells and expressed as a percentage. For the siRNA experiment, the intensities of at least 200 cells were quantified for each channel and normalized by using the mean of siRNAC and expressed as a percentage of control cells. Student's t-test was used for statistical analysis. For further methods, see supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Dr Subtil for Hep2β cells. Dr Chernoff, Dr Hall, Dr van Nhieu, Dr Bougnères, Dr Qualmann and Dr Parsons are gratefully acknowledged for the generous gift of reagents. We thank Dr Alcover and Dr Thoulouze for discussion. We thank the Plateforme d'Imagerie Dynamique of the Institut Pasteur for technical help. This work was supported by Action Concertée, Programme Dynamique et Réactivité des Assemblages Biologiques and by Action Concertée Biologie Cellulaire, Moléculaire et Structurale.
Footnotes
The authors declare that they have no conflict of interest.
References
- Burridge K, Doughman R (2006) Front and back by Rho and Rac. Nat Cell Biol 8: 781–782 [DOI] [PubMed] [Google Scholar]
- Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA (2003) Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol 23: 2162–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ZJ, Singh RD, Sharma DK, Holicky EL, Hanada K, Marks DL, Pagano RE (2006) Distinct mechanisms of clathrin-independent endocytosis have unique sphingolipid requirements. Mol Biol Cell 17: 3197–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly RJ (2004) Cortactin signalling and dynamic actin networks. Biochem J 382: 13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM (2000) Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell 11: 3341–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Weed SA, Xiong WC, Marshall TD, Parsons JT (1998) Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol Cell Biol 18: 5838–5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesbert F, Sauvonnet N, Dautry-Varsat A (2004) Clathrin-independent endocytosis and signalling of interleukin 2 receptors. In Signalling from Internalised Growth Factor Receptors (Current Topics in Microbiology and Immunology), Madshus HI (ed), Vol. 286, pp 119–148. Berlin, Germany: Springer [PubMed] [Google Scholar]
- Hofmann C, Shepelev M, Chernoff J (2004) The genetics of Pak. J Cell Sci 117: 4343–4354 [DOI] [PubMed] [Google Scholar]
- Hussain NK et al. (2001) Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol 3: 927–932 [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A (2005) Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21: 247–269 [DOI] [PubMed] [Google Scholar]
- Jaffer ZM, Chernoff J (2002) p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol 34: 713–717 [DOI] [PubMed] [Google Scholar]
- Kirkham M, Parton RG (2005) Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta 1745: 273–286 [DOI] [PubMed] [Google Scholar]
- Lamaze C, Chuang T-H, Terlecky LJ, Bokoch GM, Schmid SL (1996) Regulation of receptor-mediated endocytosis by Rho and Rac. Nature 382: 177–179 [DOI] [PubMed] [Google Scholar]
- Lamaze C, Dujeancourt A, Baba T, Lo C, Benmerah A, Dautry-Varsat A (2001) Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell 7: 661–671 [DOI] [PubMed] [Google Scholar]
- Llorente A, Rapak A, Schmid SL, van Deurs B, Sandvig K (1998) Expression of mutant dynamin inhibits toxicity and transport of endocytosed ricin to the Golgi apparatus. J Cell Biol 140: 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KH, Jeffery ED, Grigera PR, Shabanowitz J, Hunt DF, Parsons JT (2006) Cortactin phosphorylation sites mapped by mass spectrometry. J Cell Sci 119: 2851–2853 [DOI] [PubMed] [Google Scholar]
- Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS (2004) Erk/Src phosphorylation of cortactin acts as a switch on–switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol 24: 5269–5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Pagano RE (2007) Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 8: 603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SA, Malerod L, Berg T, Kjeken R (2004) Clathrin-dependent endocytosis. Biochem J 377: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR, Le PU (2003) Caveolae/raft-dependent endocytosis. J Cell Biol 161: 673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Puntener D, Helenius A (2002) Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296: 535–539 [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M (2005) Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436: 78–86 [DOI] [PubMed] [Google Scholar]
- Qualmann Q, Kelly RB (2000) Syndapin isoforms participate in receptor mediated endocytosis and actin organisation. J Cell Biol 148: 1047–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16: 522–529 [DOI] [PubMed] [Google Scholar]
- Sabharanjak S, Sharma P, Parton RG, Mayor S (2002) GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell 2: 411–423 [DOI] [PubMed] [Google Scholar]
- Sauvonnet N, Dujeancourt A, Dautry-Varsat A (2005) Cortactin and dynamin are required for the clatrin-independent endocytosis of γc cytokine receptor. J Cell Biol 168: 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA (2002) Regulating actin dynamics at membranes: a focus on dynamin. Traffic 5: 463–469 [DOI] [PubMed] [Google Scholar]
- Vidal C, Geny B, Melle J, Jandrot-Perrus M, Fontenay-Roupie M (2002) Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the cortical-actin binding protein cortactin. Blood 100: 4462–4469 [DOI] [PubMed] [Google Scholar]
- Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA (2002) Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol 12: 1270–1278 [DOI] [PubMed] [Google Scholar]
- Webb BA, Zhou S, Eves R, Shen L, Jia L, Mak AS (2006) Phosphorylation of cortactin by p21-activated kinase. Arch Biochem Biophys 456: 183–193 [DOI] [PubMed] [Google Scholar]
- Zenke FT, King CC, Bohl BP, Bokoch GM (1999) Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J Biol Chem 274: 32565–32573 [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Chen XQ, Chong C, Leung T, Lim L (1998) A conserved negative regulatory region in alphaPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol 18: 2153–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information