Abstract
The crucial role of individual Notch receptors and the mechanism by which they maintain intestinal crypt progenitor cells were assessed by using a series of inducible gut-specific Notch mutant mice. We found that Notch1 and Notch2 receptors function redundantly in the gut, as only simultaneous loss of both receptors results in complete conversion of proliferating crypt progenitors into post-mitotic goblet cells. This conversion correlates with the loss of Hes1 expression and derepression of the cyclin-dependent kinase (CDK) inhibitors p27Kip1 and p57Kip2. We also found that the promoter of both CDK inhibitor genes is occupied by the Notch effector Hes1 in wild-type crypt progenitor cells. Thus, our results indicate that Notch-mediated Hes1 expression contributes to the maintenance of the proliferative crypt compartment of the small intestine by transcriptionally repressing two CDK inhibitors.
Keywords: Notch, intestine, CDKI, growth arrest, differentiation
Introduction
The intestinal epithelium is a self-renewing tissue with a high turnover rate and cellular processes such as proliferation, migration and cell death must be highly regulated to ensure homeostasis of the intestinal epithelium. Despite their diversity, these processes are controlled by a relatively small number of signalling pathways. Until recently, the Wnt pathway was thought to be the predominant signalling cascade active in regulating the pool of undifferentiated crypt progenitor cells, as inactivation of Tcf4 (transcription factor 4) or β-catenin (two downstream mediators of Wnt signals), or overexpression of the soluble Wnt inhibitor Dickkopf1, results in the loss of the proliferative crypt compartment (reviewed by Radtke & Clevers, 2005).
Another signalling pathway that has received attention in recent years because of its involvement in gut homeostasis is the Notch cascade. Notch proteins comprise a family of four single transmembrane-bound receptors that influence cell fate decisions and differentiation processes through cell-to-cell signalling in many different organisms (Artavanis-Tsakonas et al, 1999). Notch signalling is triggered by ligand binding, inducing a cascade of proteolytic cleavages that release the intracellular domain of Notch receptors (NICD). NICD then translocates to the nucleus, where it binds to the transcription factor Rbpj (recombination signal sequence binding protein for Jk genes, also known as CBF-1 or Csl) and activates target gene transcription. Some of the best-known targets belong to the family of hairy/enhancer of split (Hes) genes, which function as transcriptional repressors.
Expression of several components of the Notch cascade in the murine intestine suggested that Notch signalling might have important functions in the gut (Schroder & Gossler, 2002; Sander & Powell, 2004). Pharmacological studies using γ-secretase inhibitors (Milano et al, 2004; Wong et al, 2004) and genetic loss-of-function experiments in mouse for Hes1 (Jensen et al, 2000) and Math1 (Yang et al, 2001), or in zebrafish for DeltaD (a Notch ligand) and mindbomb (Crosnier et al, 2005), suggest that Notch signalling might regulate a binary cell fate decision of intestinal progenitors that must choose between the secretory and absorptive lineages.
Furthermore, inducible tissue-specific loss-of-function and gain-of-function studies of Notch signalling components in the murine intestine point to an additional role for Notch signalling in the maintenance of proliferative and undifferentiated crypt progenitor compartments. Conditional gut-specific inactivation of Rbpj, which mediates Notch signalling of all four Notch receptors, results in the complete loss of proliferating crypt progenitors followed by their conversion into post-mitotic goblet cells (van Es et al, 2005). In reciprocal gain-of-function studies, expression of NICD in the intestine inhibits differentiation of crypt progenitor cells, resulting in a large increase in undifferentiated transient amplifying cells (Fre et al, 2005). These complementary loss-of-function and gain-of-function studies indicate an essential role for Notch signalling as a gatekeeper of the gut progenitor compartment.
As the mechanism by which Notch exerts this function is currently unknown, we used inducible tissue-specific loss-of-function approaches to investigate the role of the Notch1 and Notch2 receptors in the intestine, and how they maintain the undifferentiated crypt compartment.
Results And Discussion
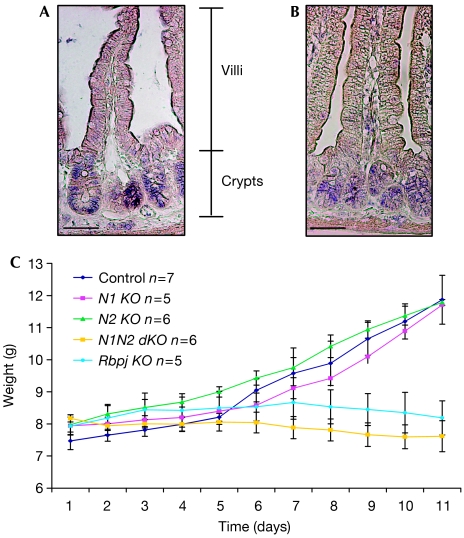
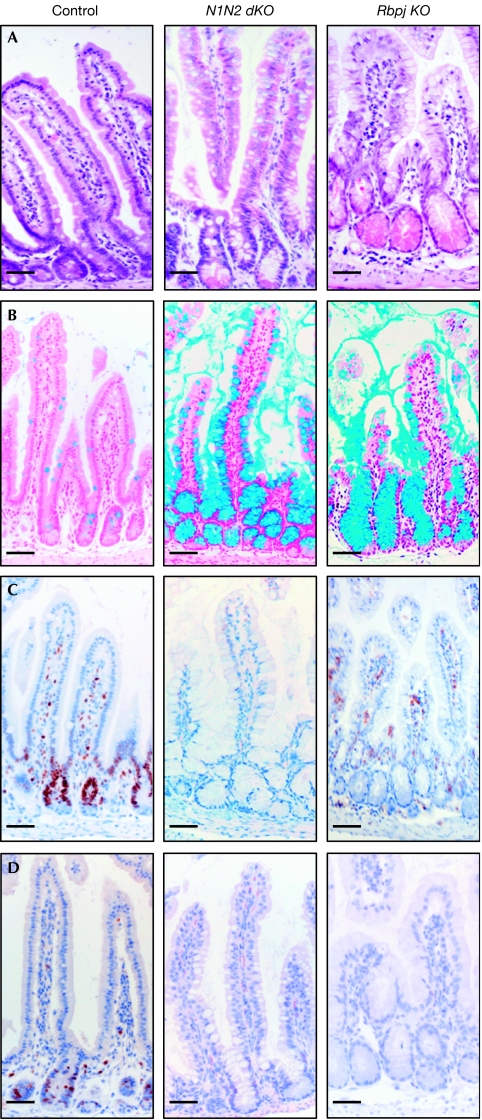
To investigate which of the Notch receptors mediates the putative gatekeeper function in the crypt progenitor compartment of the small intestine, the expression of Notch1 (N1) and Notch2 (N2) was analysed by using in situ hybridization. Both receptors are most prominently expressed in epithelial cells of the crypts (Fig 1A), whereas Notch3 and Notch4 are expressed in the villus mesenchyme and endothelial cells, as previously shown (Schroder & Gossler, 2002; Sander & Powell, 2004). These expression studies show that signalling through N1 and/or N2 is important for maintaining the undifferentiated crypt compartment. To investigate directly the roles of both Notch receptors in crypt homeostasis, mice with floxed (loxP sequence containing) alleles of N1 (Radtke et al, 1999), N2 (Besseyrias et al, 2007) or both genes were crossed with transgenic mice expressing Cre-ERT2 under the control of the villin promoter (el Marjou et al, 2004). Twelve-day-old Notch1lox/lox-vil-Cre-ERT2 (N1 KO), Notch2lox/lox-vil-Cre-ERT2 (N2 KO), Notch1/Notch2lox/lox-vil-Cre-ERT2 (N1N2 dKO), Rbpjlox/lox-vil-Cre-ERT2 (Rbpj KO) and corresponding littermate controls lacking the vil-Cre-ERT2 transgene were injected intraperitoneally with tamoxifen on 5 consecutive days and analysed 12 days after the last injection (the deletion efficiency of the corresponding genes is shown in supplementary Fig 1 online). The body weight of these mice was controlled daily. Littermate control animals and mice with gut-specific inactivation of N1 or N2 alone gained weight normally. By contrast, mice in which N1 and N2 were simultaneously inactivated did not gain weight, similar to mice with gut-specific inactivation of the Rbpj gene (van Es et al, 2005; Fig 1B). Histological analysis showed that inactivation of the N1 or N2 genes alone did not result in a gut phenotype, as the morphology of the intestine of either gene-targeted mice was comparable to that of littermate controls (supplementary Fig 2 online). However, simultaneous inactivation of both Notch receptors resulted in the same phenotype as loss of Rbpj function in the gut. The entire crypt compartment was replaced by post-mitotic goblet cells as shown by haematoxylin/eosin and Alcian blue staining (Fig 2A,B). Ki67 staining (Fig 2C) and 5-bromodeoxyuridine incorporation (Fig 2D) showed a complete lack of proliferating cells in these mice, whereas the proliferative crypt compartment of N1- or N2-deficient mice appeared to be normal. Paneth cells were present in the crypts of all gene-targeted mice, as shown by Ledrum's phloxine tartazine staining, and the number of synaptophysin-positive enteroendocrine cells was unchanged (data not shown). These results indicate that N1 and N2 function redundantly in the intestine, as signalling through only one of the two receptors is sufficient to maintain the crypt compartment. γ-Secretase inhibitors are under development for the treatment of Alzheimer patients, and recently also for treatment of T-cell leukaemia patients to block Notch signalling in cancer cells (Aster, 2005). However, most of these γ-secretase inhibitors show unwanted side effects such as goblet cell metaplasia within the crypt compartment (Milano et al, 2004; Wong et al, 2004), which is recapitulated in mice with gut-specific inactivation of both N1 and N2. These results indicate that drugs now being developed inhibit Notch signalling mediated by both N1 and N2 receptors, and thus most likely signalling through all Notch receptors. The development of γ-secretase inhibitors that can specifically block N1 but not N2 signalling would certainly attenuate or even fully extinguish the unwanted intestinal side effects, as signalling through N2 alone is sufficient to maintain gut homeostasis.
Figure 1.
Expression pattern of Notch receptors in the small intestine of wild-type mice. In situ hybridization for (A) N1 and (B) N2 shows expression of both receptors in the crypts of the small intestine. Scale bars, 50 μm. (C) Bodyweight curves of control (N1/N2lox/lox), N1 KO (Notch1lox/lox-vil-Cre-ERT2), N2 KO (Notch2lox/lox-vil-Cre-ERT2), N1N2 dKO (Notch1/Notch2lox/lox-vil-Cre-ERT2) and Rbpj KO (Rbpjlox/lox-vil-Cre-ERT2) post tamoxifen-induced Cre activation. The number of mice analysed is indicated.
Figure 2.
Redundant function of N1 and N2 signalling in the small intestine. Conversion of the proliferative crypt compartment into post-mitotic goblet cells occurs only after simultaneous inactivation of both N1 and N2. Representative sections of the small intestine from control, N1N2 dKO (Notch1/Notch2lox/lox-vil-Cre-ERT2) and Rbpj KO (Rbpjlox/lox-vil-Cre-ERT2) stained with (A) haematoxylin and eosin, (B) Alcian blue for goblet cell identification, and antibodies against (C) Ki67 and (D) 5-bromodeoxyuridine to identify proliferating crypt progenitors. Scale bars, 50 μm. N1, Notch1; N2, Notch2.
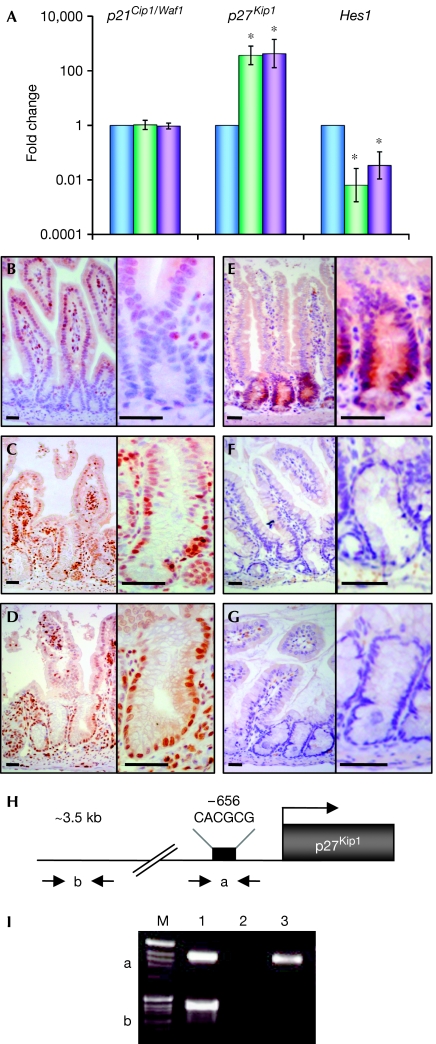
To gain further mechanistic insights into how Notch signalling maintains the proliferation of crypt progenitors, and with the knowledge that Wnt signalling is also involved in this function, we investigated a potential cross-talk between the two signalling cascades. Proliferating crypt cells show active Wnt signalling, which is characterized by the accumulation of nuclear β-catenin and expression of Wnt target genes such as CD44 (van de Wetering et al, 2002). Expression studies for CD44 and β-catenin performed in all Notch mutant mice indicate that the Wnt cascade is still active even though crypt progenitor cells no longer proliferate in N1N2 dKO and Rbpj-deficient intestines (supplementary Fig 3 online). Mechanistically, active Wnt signalling was shown to sustain the proliferation of cells in the crypt by β-catenin/Tcf4-dependent upregulation of c-Myc, which subsequently binds to Miz1 and represses the cell-cycle inhibitor p21Cip1/Waf1 (van de Wetering et al, 2002). As no differences were observed in β-catenin and CD44 expression in Notch-deficient intestines, we investigated whether Notch signalling controls the proliferation of crypt progenitor cells by influencing the expression of p21Cip1/Waf1 in a β-catenin-independent manner. Therefore, RNA from crypt sections derived from control, N1N2 dKO and Rbpj KO mice was isolated by laser capture microdissection to perform quantitative reverse transcription–PCR (RT–PCR) for p21Cip1/Waf1. The expression levels of p21Cip1/Waf1 for control, N1N2 dKO and Rbpj KO crypts were similar (Fig 3A), indicating that Notch signalling does not regulate proliferation of crypt progenitor cells through the cell-cycle regulator p21Cip1/Waf1. To confirm genetically that the cell-cycle arrest of Notch-deficient crypt cells is not mediated by p21Cip1/Waf1, p21Cip1/Waf1−/− mice were intercrossed with Rbpj KO mice. Intestines deficient for both Rbpj and p21Cip1/Waf1 or for p21Cip1/Waf1 alone were compared with those of littermate controls. The intestines of p21Cip1/Waf1-deficient and control animals were indistinguishable, as they had normal morphology, goblet cell content and proliferation, as shown by haematoxylin/eosin, periodic acid-Schiff (PAS) and Ki67 staining (supplementary Fig 4 online). Simultaneous loss of Rbpj and p21Cip1/Waf1 did not allow crypt progenitor cells to re-enter the cell cycle. The intestines of these mice are identical to Rbpj KO mice, as they show complete conversion of crypt progenitor cells into post-mitotic goblet cells, as shown by PAS and Ki67 staining (supplementary Fig 4 online), indicating that the cell-cycle arrest in Notch signalling-deficient crypt cells is not mediated by p21Cip1/Waf1. To investigate whether the cell-cycle arrest in Notch signalling-deficient intestines is mediated by another family member of the cyclin-dependent kinase inhibitors, quantitative RT–PCR for p27Kip1 was performed on RNA from laser capture microdissected crypt cells from control, N1N2 dKO and Rbpj KO mice. The expression levels of p27Kip1 increased 400- and 500-fold in N1N2 dKO and Rbpj KO animals, respectively (Fig 3A). To confirm these messenger RNA expression studies at the protein level, immunostaining for p27Kip1 was performed. In littermate controls, p27Kip1 protein expression was found exclusively in the nuclei of the villi, whereas crypt cells were negative (Fig 3B). However, nuclear p27Kip1 staining was found in crypt cells of N1N2- and Rbpj-deficient mice (Fig 3C,D). These results show that Notch signalling within the small intestine represses the cyclin-dependent kinase inhibitor p27Kip1 to maintain proliferating crypt progenitor cells.
Figure 3.
Loss of Notch signalling in the crypt compartment results in loss of Hes1 expression and derepression of the cyclin-dependent kinase inhibitor p27Kip1. (A) Crypt regions of control (blue), N1N2 dKO (Notch1/Notch2lox/lox-vil-Cre-ERT2; green) and Rbpj KO (Rbpjlox/lox-vil- Cre-ERT2; purple) mice were laser microdissected and RNA isolated for quantitative reverse transcription–PCR. Relative levels of messenger RNA of p21Cip1/Waf1, p27Kip1 and Hes1 genes normalized with glyceraldehyde phosphate dehydrogenase are shown (logarithmic scale, *P<0.05; results represent the average of four independent experiments). (B–D) Immunohistochemical analysis of sections of the small intestine from (B) control, (C) N1N2 dKO and (D) Rbpj KO stained with an antibody against p27Kip1 bars. (E–G) Immunostaining for Hes1 on sections of the small intestine derived from (E) control, (F) N1N2 dKO and (G) Rbpj KO. The right-hand panels show a higher magnification of crypts from the left-hand panels. Scale bars, 50 μm. (H) Schematic representation of the p27Kip1 promoter indicating the primers used (a and b) for the chromatin immunoprecipitation (ChIP) analysis. The black rectangle corresponds to a class C Hes1-binding site (CACGCG; Murata et al, 2005). Primers indicated by ‘b', 3 kb upstream of the ATG, were used for control PCR. (I) ChIP assay. Epithelial cells from crypt enrichment preparations were processed for ChIP with an antibody against Hes1 (lane 3) and purified rabbit IgGs as a control (lane 2). PCRs of two distinct regions indicated by ‘a', containing the class C Hes1-binding site, and by ‘b', an Hes1-binding-site-free region, which was used as a negative control, are shown. Lane 1 corresponds to the input DNA.
Hes gene family members are among the best-characterized target genes of Notch signalling (Kageyama et al, 2005). Furthermore, the fetal intestines of Hes1−/− mice show increased numbers of goblet cells at the expense of adsorptive enterocytes (Jensen et al, 2000), indicating that the phenotype observed in Notch signalling-deficient mice might be, at least in part, mediated by the loss of Hes1 expression. To investigate this possibility further, we performed quantitative RT–PCR followed by immunostaining for Hes1. Hes1 mRNA levels showed a 50- and 40-fold decrease in N1N2- and Rbpj-deficient animals, respectively, compared with controls (Fig 3A). Hes1 immunostaining was confined to the crypt compartment in control animals (Fig 3E), whereas it was not detected in N1N2- and Rbpj-deficient crypts (Fig 3F,G), confirming the role of Hes1 as a target gene of N1 and/or N2 in the small intestine.
Protein levels of the cell-cycle regulator p27Kip1 in cycling cells are regulated at the transcriptional and post-transcriptional levels by protein degradation through the ubiquitin–proteasome system (Hengst & Reed, 1996). Notch signalling has been linked to both of these regulatory mechanisms. One study indicates that p27Kip1 expression is repressed by Hes1 at the transcriptional level (Murata et al, 2005), whereas another report suggests that N1 controls expression of the S-phase kinase-associated protein 2 (Skp2)—the F-box subunit of the ubiquitin-ligase complex SCFSkp2 that targets proteins for degradation (Sarmento et al, 2005). We investigated the expression of Skp2 by quantitative RT–PCR and immunohistochemistry. However, we were unable to detect Skp2 expression at either mRNA or protein levels on gut sections derived from the different gene-targeted mice. Therefore, we focused our studies on the possibility that Hes1 might directly bind to, and thereby repress, the p27Kip1 promoter, as previously suggested (Murata et al, 2005). Promoter analysis identified one important Hes1-binding site (class C site) within the p27Kip1 promoter that conveys transcriptional repression (Murata et al, 2005). Therefore, we performed a chromatin immunoprecipitation (ChIP) assay on isolated crypts from wild-type mice to investigate whether Hes1 binds to the p27Kip1 promoter in crypt progenitor cells. DNA of the p27Kip1 promoter region including a proximal class C site, but not that of the p27Kip1 upstream sequence, was specifically detected in the Hes1 immunoprecipitated DNA complex from formaldehyde-treated crypt cells, indicating Hes1 occupancy of the p27Kip1 promoter in vivo. These results show that Notch-induced Hes1 expression contributes to the transcriptional repression of the p27Kip1 gene in vivo. Whether other Hes gene family members that are also expressed in the crypt compartment contribute to the repression of p27Kip1 remains to be investigated.
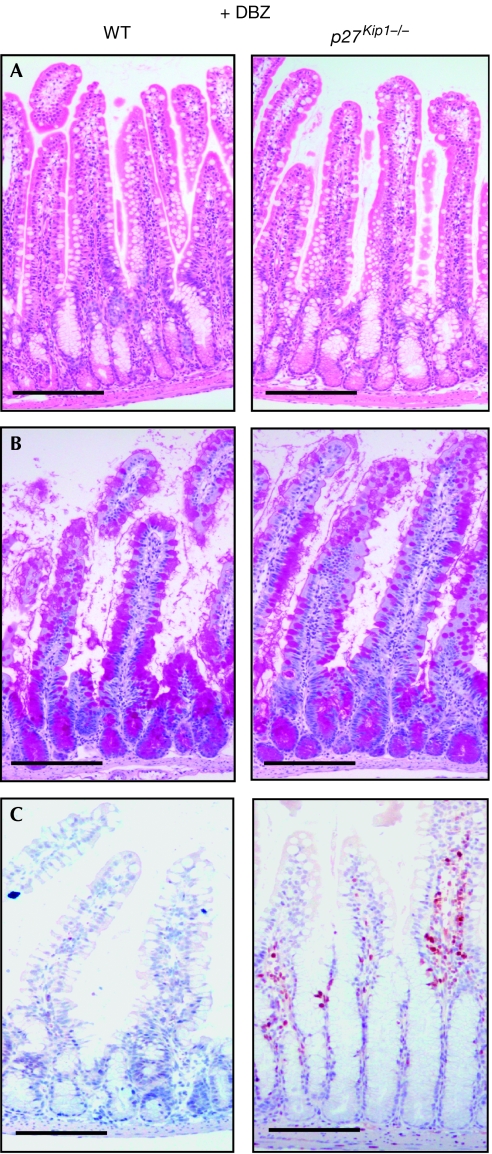
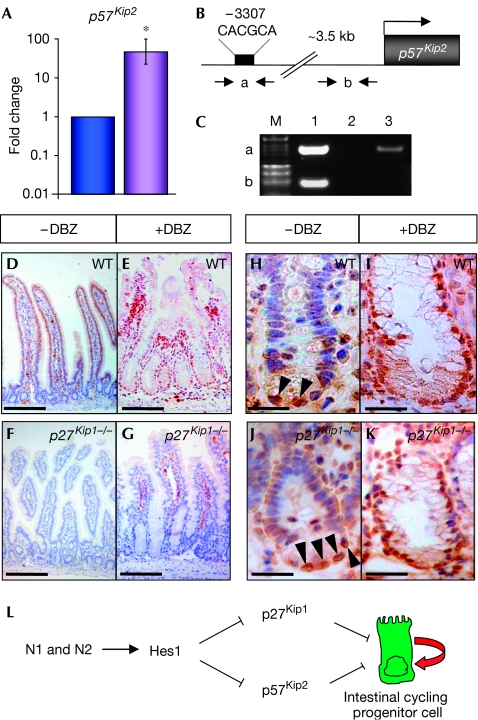
To confirm genetically that upregulation of p27Kip1 owing to loss of Notch signalling causes crypt progenitor cells to exit the cell cycle and differentiate into post-mitotic goblet cells, p27Kip1−/− and control mice were treated with the γ-secretase inhibitor dibenzazepine (DBZ; Milano et al, 2004). Surprisingly, the phenotypes of DBZ-treated p27Kip1−/− and control mice were indistinguishable and were characterized by the complete conversion of proliferating crypt progenitor cells into post-mitotic goblet cells, as shown by Ki67 and PAS staining (Fig 4A–C). Although the loss of Notch signalling clearly leads to upregulation of p27Kip1 in crypt progenitor cells (Figs 3C,D,5E), its loss is not sufficient to allow N1N2-deficient crypt progenitors to re-enter the cell cycle, indicating that additional inhibitory cell-cycle regulators are active. To investigate this further, quantitative RT–PCR for additional cyclin-dependent kinase inhibitors was performed on RNA from laser microdissected crypts from control and Rbpj-deficient mice. We chose to study Cdkn2a and p57Kip2, the latter having been shown to be transcriptionally regulated by Hes1 in the pancreas (Georgia et al, 2006). Expression of Cdkn2a was not detected, whereas the expression levels of p57Kip2 increased 47-fold in Rbpj KO mice (Fig 5A). These mRNA expression studies were confirmed further by immunostaining for p57Kip2 in vehicle- and DBZ-treated control and p27Kip1−/− mice, respectively. In the crypts of littermate controls and p27Kip1−/− mice, p57Kip2 staining was found only in Paneth cells (Fig 5H,J, arrowheads), whereas the nuclei of crypt progenitors were negative. By contrast, nuclear p57Kip2 staining was found in control and p27Kip1−/− animals in which Notch signalling was blocked by γ-secretase treatment (Fig 5I,K). This indicates that the loss of Notch signalling results in upregulation of two cyclin-dependent kinase inhibitors of the Kip family. Promoter analysis of p57Kip2 led to the identification of a potential class C Hes1-binding site (Fig 5B), which might convey transcriptional repression similar to that observed for the p27 promoter (Fig 3). ChIP experiments performed on isolated crypt cells from wild-type mice show that this particular Hes1-binding site, but not that of an unrelated p57Kip2 downstream sequence, is occupied by Hes1 in vivo (Fig 5C), indicating that Hes1 contributes to the transcriptional repression of p57Kip2.
Figure 4.
Loss of p27Kip1 in Notch signalling-inhibited progenitors is not sufficient to maintain their proliferative and undifferentiated phenotype. Treatment of wild type (WT; left) and p27Kip1−/− mice (right) with the γ-secretase inhibitor dibenzazepine (DBZ) followed by morphological and immunohistochemical analysis is shown. Representative small intestine sections stained with (A) haematoxylin and eosin, and (B) periodic acid-Schiff or (C) with an antibody against Ki67 are shown. Scale bars, 100 μm.
Figure 5.
Derepression of p57Kip2 expression in the Notch signalling-deficient crypt compartment of the small intestine. (A) Crypt regions of control (blue) and Rbpj KO (purple) mice were laser microdissected followed by RNA isolation and quantitative reverse transcription–PCR for p57Kip2. (B) Schematic representation of the p57Kip2 promoter indicating the primers used (a and b) for the chromatin immunoprecipitation (ChIP) analysis. The black rectangle corresponds to a class C Hes1-binding site. Primers indicated by ‘b', localizing about 1 kb upstream of the ATG, were used for the control PCR. (C) ChIP assay. Epithelial cells from crypt enrichment preparations were processed for ChIP with an antibody against Hes1 (lane 3) and purified rabbit IgGs as control (lane 2). PCRs of two distinct regions, indicated by ‘a', containing the class C Hes1-binding site, and by ‘b', an unrelated downstream sequence within the p57 promoter used as negative control, are shown. Lane 1 corresponds to input DNA. (D–K) Immunohistochemical analysis of small intestine sections from wild-type (WT) and p27Kip1−/− mice treated with either vehicle alone (−DBZ (dibenzazepine)) or with the γ-secretase inhibitor DBZ (+DBZ) using antibodies against p27Kip1 (D–G) or p57Kip2 (H–K). Note that p57Kip2 is expressed only in the nuclei of Paneth cells (arrowheads) when mice are treated with vehicle alone (H,J). By contrast, γ-secretase-mediated blockage of Notch signalling results in nuclear p57Kip2 staining of crypt progenitor cells (I,K). Scale bars in sections showing p27Kip1 immunostaining correspond to 100 μm, whereas in sections showing p57Kip2 staining, they correspond to 50 μm. (L) Model indicating a possible mechanism by which the Notch signalling pathway promotes the cycling of progenitor cells. Signalling through Notch1 (N1) and Notch2 (N2) in crypt progenitor cells represses the two cyclin-dependent kinase inhibitors p27Kip1 and p57Kip2.
The simultaneous upregulation and the redundant function of p27Kip1 and p57Kip2 in Notch signalling-deficient crypt progenitors might explain why the loss of p27Kip1 is not sufficient to maintain proliferation of crypt progenitors.
Together, our results show that N1 and N2 function redundantly in the small intestine. Unlike in the skin, in which Notch and Wnt signalling have opposing functions (Nicolas et al, 2003), both signalling cascades work together to preserve the proliferative crypt compartment of the small intestine. Wnt signalling results in repression of the cyclin-dependent kinase inhibitor p21Cip1/Waf1 (van de Wetering et al, 2002), whereas Notch signalling represses the cell-cycle regulators p27Kip1 and p57Kip2 at least in part through its target gene Hes1 (Fig 5L). Although the Wnt and the Notch pathways do not show any apparent cross-talk in crypt progenitor cells, both show similar functions by inhibiting cyclin-dependent kinases.
Methods
Animals. Gene-targeted and transgenic mice are described in the supplementary information online.
Immunohistochemistry, antibodies and in situ hybridization. Intestinal tissue preparation and immunohistochemistry were performed as described previously (van Es et al, 2005). Antibodies, laser capture microdissection and quantitative RT–PCR are described in the supplementary information online.
Crypt enrichment preparation and chromatin immunoprecipitation. Crypts from wild-type mice were prepared as described previously (Kobayashi et al, 2005). ChIP analysis was performed in three independent experiments by using the ChIP assay kit (Millipore/Upstate, Zug, Switzerland) following the manufacturer's instructions. The Hes1 antibody was incubated at 4°C for 24 h. An IgG antibody was used as a negative control. The mouse primer set for identification of the Hes1 C-box-binding site of the p27Kip1 gene was sense 5′-CGGCCGTTTGGCTAGTTTGTTTGT-3′ and antisense 5′-CAAGGTTTGGAGAGGGGCTGCGTT-3′ (Murata et al, 2005) and for control, sense 5′-CTTCGATTCTATCGCTAAACTC-3′ and antisense 5′-GTGAAGAAGGAGGCGGAGATCAG-3′. The primer set for the C-box of the p57Kip2 promoter was sense 5′-AGGGGAGACAAGTCTCAACTCTAC-3′ and antisense 5′-AAGTCAGTTTAGTTTCCCAGAACG-3′ and for control, sense 5′-CTCTGCAGGGCCTTTCAAGTATGT-3′ and antisense 5′-TTGGCTGGAAGTAGTTATGCTAGA-3′.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary information
Acknowledgments
We thank A. Wilson for critical reading of the manuscript, D. Louvard and S. Robine for the Villin-Cre-ERT2 mice, R. Kageyama for providing the Hes1 antibody, A. Gossler for providing the in situ probes, and G.-F. Mancini and G. Badic for technical assistance. This work was in part supported by, the Swiss cancer league, the Swiss National Science Foundation and the Deutsche Forschungsgemeinschaft.
Footnotes
The authors declare that they have no conflict of interest.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284: 770–776 [DOI] [PubMed] [Google Scholar]
- Aster JC (2005) Deregulated NOTCH signaling in acute T-cell lymphoblastic leukemia/lymphoma: new insights, questions, and opportunities. Int J Hematol 82: 295–301 [DOI] [PubMed] [Google Scholar]
- Besseyrias V, Fiorini E, Strobl LJ, Zimber-Strobl U, Dumortier A, Koch U, Arcangeli ML, Ezine S, Macdonald HR, Radtke F (2007) Hierarchy of Notch–Delta interactions promoting T cell lineage commitment and maturation. J Exp Med 204: 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J (2005) Delta–Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132: 1093–1104 [DOI] [PubMed] [Google Scholar]
- el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S (2004) Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39: 186–193 [DOI] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S (2005) Notch signals control the fate of immature progenitor cells in the intestine. Nature 435: 964–968 [DOI] [PubMed] [Google Scholar]
- Georgia S, Soliz R, Li M, Zhang P, Bhushan A (2006) p57 and Hes1 coordinate cell cycle exit with self-renewal of pancreatic progenitors. Dev Biol 298: 22–31 [DOI] [PubMed] [Google Scholar]
- Hengst L, Reed SI (1996) Translational control of p27Kip1 accumulation during the cell cycle. Science 271: 1861–1864 [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD (2000) Control of endodermal endocrine development by Hes-1. Nat Genet 24: 36–44 [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R (2005) Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res 306: 343–348 [DOI] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA (2005) Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307: 731–734 [DOI] [PubMed] [Google Scholar]
- Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, Jacobs RT, Zacco A, Greenberg B, Ciaccio PJ (2004) Modulation of notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci 82: 341–358 [DOI] [PubMed] [Google Scholar]
- Murata K, Hattori M, Hirai N, Shinozuka Y, Hirata H, Kageyama R, Sakai T, Minato N (2005) Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1. Mol Cell Biol 25: 4262–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F (2003) Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 33: 416–421 [DOI] [PubMed] [Google Scholar]
- Radtke F, Clevers H (2005) Self-renewal and cancer of the gut: two sides of a coin. Science 307: 1904–1909 [DOI] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M (1999) Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10: 547–558 [DOI] [PubMed] [Google Scholar]
- Sander GR, Powell BC (2004) Expression of notch receptors and ligands in the adult gut. J Histochem Cytochem 52: 509–516 [DOI] [PubMed] [Google Scholar]
- Sarmento LM, Huang H, Limon A, Gordon W, Fernandes J, Tavares MJ, Miele L, Cardoso AA, Classon M, Carlesso N (2005) Notch1 modulates timing of G1–S progression by inducing SKP2 transcription and p27 Kip1 degradation. J Exp Med 202: 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder N, Gossler A (2002) Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns 2: 247–250 [DOI] [PubMed] [Google Scholar]
- van de Wetering M et al. (2002) The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250 [DOI] [PubMed] [Google Scholar]
- van Es JH et al. (2005) Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963 [DOI] [PubMed] [Google Scholar]
- Wong GT et al. (2004) Chronic treatment with the γ-secretase inhibitor LY-411,575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem 279: 12876–12882 [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY (2001) Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294: 2155–2158 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary information