The third international symposium on the Epithelial–Mesenchymal Transition was an EMBO workshop organized by TEMTIA (The International EMT Organization) and the European network EpiPlastCarcinoma. It took place between 10 and 12 September 2007, at the Larisha Palace in Krakow, Poland, and was organized by P. Savagner, A. Moustakas, A. Garcia de Herreros and A. Cano.
Introduction
Epithelial–mesenchymal transitions (EMTs) have been described primarily during embryonic development when tissue remodelling and cell migration shape the future organism. In addition, they have also been described in pathological situations such as tumour progression. During EMT, epithelial cells lose the adherent and tight junctions that keep them in contact with their neighbours; they can also break through the basal membrane and migrate over long distances owing to profound changes in their cytoskeleton architecture.
Although EMT has been recognized as a crucial process during embryonic development, its potential role in the progression of carcinoma was neglected for many years. Recent data from embryos of various species and different human pathologies, including fibrosis and cancer, are helping us to understand the physiological and pathological aspects of EMTs. In addition to somatic mutations and the control of gene expression, crosstalk between signalling pathways and regulatory elements such as microRNAs (miRNAs), natural antisense transcripts, sophisticated transcription complexes and the control of protein transport and stability, have fuelled increasing interest in this exciting field.
This EMBO workshop in Krakow, Poland, brought together around 80 people working on EMTs, and was an excellent opportunity to discuss recent advances in a relaxed and friendly atmosphere. However, the meeting began on a sad note as we expressed our condolences on the recent loss of Elizabeth Hay, who was scheduled to open the workshop. Betty Hay was the founder of the epithelial-to-mesenchymal transformation concept (Fig 1), and, in her memory, R. Kalluri (Harvard, MA, USA) and J.P. Thiery (Proteos, Singapore) opened the meeting describing her outstanding contribution to the field. In 1967, Hay realized that the EMT was of crucial importance during gastrulation (Trelstad et al, 1967). This seminal study was followed by other papers, culminating in 1982 with the publication of a model to study EMT in three-dimensional collagen cultures (Greenburg & Hay, 1982). Throughout her scientific life, Hay remained an extremely rigorous and passionate investigator in her quest to understand how cells assemble into functional tissues to shape the embryo. Her last co-authored paper—showing that transforming growth factor β3 (TGFβ3) induces EMT during palatal fusion through the activation of the Wnt signalling target LEF1—was presented at the meeting by A. Nawshad (Lincoln, NE, USA; Nawshad et al, 2007).
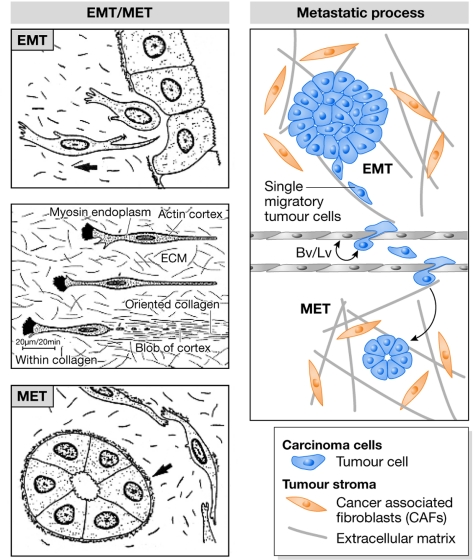
Figure 1.
The epithelial–mesenchymal transition in cancer: past, present and future perspectives. Betty Hay coined the term epithelial-to-mesenchymal transformation some 40 years ago and left us beautiful drawings that not only show the transformation to mesenchyme, but also the transient nature of the process and the reversion to the epithelial character (MET; Hay, 1968, 1995). This is why the preferred term is now epithelial-to-mesenchymal transition (EMT). The EMT, as an initial step in the metastatic cascade, has until recently remained a matter of debate; however, powerful imaging tools have convincingly shown that individual cells delaminate from primary tumours (Condeelis & Segall, 2003). Bv, blood vessels; ECM, extracellular matrix; Lv, lymphatic vessels.
E-cadherin repressors and EMT
E-cadherin is essential for the maintenance of epithelial integrity of many embryonic and adult tissues. Consequently, its repression is a crucial step of the EMT, both during embryonic development and in pathological situations in the adult. Snail genes were the first direct repressors of E-cadherin expression to be described (Batlle et al, 2000; Cano et al, 2000). However, additional E-cadherin repressors have since been identified, including basic helix–loop–helix transcription factors such as E47 and Twist, and the Zeb factors, ΔEF1/Zeb1 (Zeb for zinc finger E-box binding homeobox) and Sip1/Zeb2 (Sip for Smad interacting protein; Peinado et al, 2007). At the meeting, A. Cano (Madrid, Spain) presented E2-2, another basic helix–loop–helix transcription factor that is able to induce full EMT in Madine–Darby canine kidney cells by directly repressing E-cadherin promoter activity.
The activity of E-cadherin repressors is tightly regulated at various levels, as reflected by several recent studies that were presented in Krakow. Snail1 (Snail) was shown to control Zeb2 activity by the splicing of a 5′UTR fragment in the Zeb2 mRNA. This produces a natural antisense transcript that leads to an increase in the protein levels of Zeb2 (A. Garcia de Herreros, Barcelona, Spain). Another way to enhance the activity of E-cadherin repressors was presented by H. Peinado (Madrid, Spain), who showed that the lysine oxidase, LoxL2, increases the stability and activity of Snail1 (Peinado et al, 2005). Indeed, the co-expression of Snail and LoxL2 in tumour models and human squamous cell carcinomas correlates with malignancy or local recurrence. LoxL2 silencing augments E-cadherin expression and induces epithelial differentiation. With respect to the control of Snail subcellular localization, M.A. Nieto (Alicante, Spain) identified specific amino-acid residues in the Snail1 protein that are required for the binding of several importins to mediate its nuclear translocation and function as a transcription factor.
New EMT inducers
Various posters and talks at the meeting presented new proteins that induce full EMT. One example is Pez, a protein tyrosine phosphatase that induces TGFβ expression and EMT in Madine–Darby canine kidney cells (Wyatt et al, 2007). The interleukin-like EMT inducer (ILEI) is also able to induce tumour growth and metastasis in various cell lines (Waerner et al, 2006), whereas functional interference with ILEI reverts TGFβ-induced EMT. This work, from the laboratory of H. Beug (Vienna, Austria), was the worthy recipient of the first Elizabeth Hay poster prize awarded to A. Csiszar. Another interesting study showed that the high mobility group protein, HMGA2, is required for the induction of EMT by TGFβ (Thuault et al, 2006), which is mediated primarily by Snail induction through the Smad proteins (A. Moustakas, Uppsala, Sweden).
New insights into cell adhesion, polarity and migration
By measuring the strength of cadherin-mediated cell adhesion, Thiery showed that cells expressing Type I cadherins adhere more rapidly and more strongly than those expressing Type II cadherins, which are often present in migratory cells (Chu et al, 2006). In addition, he showed that the strength of E-cadherin adhesion also depends on the cortical actin cytoskeleton, p120 interaction and the presence of an activated integrin pathway, suggesting that cadherins and integrins reciprocally control adhesive forces.
The dynamic behaviour of desmosomal cadherins also involves two adhesion states. Desmosomes in normal epidermis or confluent cells are hyper-adhesive and calcium independent, whereas those that are active in the early embryo, in subconfluent cultures or during wound healing are less adhesive and calcium dependent (D. Garrod, Manchester, UK). These different adhesion states could have implications for EMT, as an initial step in mesenchymal transition might involve a desmosome switch owing to changes in protein kinase C activity (Kimura et al, 2007). During wound healing, cells maintain some desmosomal proteins and they establish an intermediate state between the epithelial and mesenchymal states, referred to as the metastable phenotype (P. Savagner, Montpellier, France). This phenotype corresponds to a partial EMT characterized by the maintenance of cell–cell adhesion structures that do not preclude active cell migration. In relation to this, Nieto provided evidence that the function of Snail genes in regulating adhesion might or might not be associated with the induction of a full EMT, depending on the cellular context. Snail2 (Slug) induces a full EMT in the mesoderm of the early chick embryo, whereas two Snail1 proteins—snail1a and snail1b—cooperate in the migration of the axial mesendoderm in the zebrafish embryo by decreasing adhesion without inducing EMT (Blanco et al, 2007). In both cases, E-cadherin is downregulated, but in the latter, Snail-positive cells move and push Snail-negative/E-cadherin-positive neighbouring cells, which act as pioneers. This movement might parallel that of the leader cells during the collective migration of the epithelial monolayers (Poujade et al, 2007). Indeed, collective cell migration of pioneer cells can occur in the absence of cadherin downregulation both in vitro and in vivo, as shown by G. Christofori (Basel, Switzerland). This process is regulated by a mucin-like molecule called podoplanin, which induces cytoskeletal changes and the formation of filopodia (Wicki et al, 2006). The combination of different cell movements has also been described in Drosophila embryos, in which the use of a photoactivatable green fluorescent protein has allowed M.J. Murray (Canberra, Australia) to resolve the behaviour of mesodermal cells in live embryos. In this case, some cells migrate as a group over the ectoderm, whereas others disperse independently (Murray & Saint, 2007).
Surprisingly, the changes in cell polarity concomitant with EMT had not been addressed until recently. The homologues of the Drosophila genes that establish cell polarity have now been analysed in vertebrates, and these studies have implicated the Scribble polarity complex in regulating polarity and migration during development and tumorigenesis (P.O. Humbert, Melbourne, Australia; Dow et al, 2007). Polarity genes are targeted by E-cadherin repressors in conjunction with the induction of EMT. Indeed, Snail and Zeb1 directly repress Crumbs3 transcription (E. Whiteman, Ann Arbor, MI, USA; A. Eger, Vienna, Austria), and ZEB1 also downregulates Lgl2 (lethal giant larvae 2) in colorectal and breast cancer human cells (T. Brabletz, Freiburg, Germany and A. Eger; Aigner et al, 2007; Spaderna et al, 2008). Interestingly, the Par complex establishes long-lasting apico–basal polarity in contacting keratinocytes in conjunction with Tiam 1 (T-cell lymphoma invasion and metastasis 1, an activator of Rac), whereas chemotactic migration is favoured in non-contacting cells by stabilizing front-to-rear polarization (J. Collard, Amsterdam, The Netherlands; Pegtel et al, 2007).
Signalling pathways in EMT
Although important questions linking TGFβ signalling and EMT were recently addressed at a meeting in Tucson, AZ, USA (Padgett & Reiss, 2007), new data were also presented in Krakow. To understand the dual role of TGFβ in growth inhibition and EMT, C. Hill (London, UK) has studied the function of Smad3 in two distinct cell systems: one undergoing EMT in response to TGFβ (EpH4 cells), and another resistant to TGFβ-induced growth arrest (EpRas cells). She showed that Smad3 is necessary for TGFβ to induce EMT in EpH4 cells, but that it is dispensable for the maintenance of the mesenchymal phenotype. Interestingly, Smad3 is more stable in quiescent cells than in cycling cells, and its stabilization in EpRas cells restores the inhibitory growth response to TGFβ without affecting the induction of the EMT. Together, these results indicate that the level of Smad3 protein modulates the cellular response to TGFβ. R.B. Runyan (Tucson, AZ, USA) focused on the functions of different TGFβ isoforms in promoting either EMT or cell invasion and migration (Mercado-Pimentel & Runyan, 2007). By using the atrio–ventricular canal as an experimental system, he showed that TGFβ2 and TGFβ3 act through the receptors TGFβRIII and RII, respectively, to activate Smad2/3 sequentially and induce EMT and cell spreading, and through Alk2 to promote cell invasion by Smad1/5 signalling.
Other groups focused on the crosstalk between TGFβ and other signalling pathways. A. Östman (Stockholm, Sweden) showed the importance of the platelet-derived growth factor (PDGF) pathway in the tumour microenvironment in inducing EMT. PDGF acts synergistically with TGFβRII and the vascular endothelial growth factor (Ostman & Heldin, 2007). Savagner focused on the importance of the epidermal growth factor (EGF) and Snail2 for wound healing, during which signalling through the EGF receptor preferentially activates extracellular signal-regulated kinase (ERK) pathways. He found that the Erk5 pathway specifically enhances Snail2 promoter activity and controls in vitro wound healing in keratinocyte-derived HaCaT cells.
The hepatocyte growth factor (HGF)/Met signalling pathway is also involved in inducing EMT and in growth invasion. P. Comoglio (Turin, Italy) presented a microarray screening of cells exposed to HGF and identified two coagulation genes—plasminogen activator inhibitor, type I (Pai-1) and cyclooxygenase 2 (Cox2)—which are induced by HGF/Met, and the overexpression of which induces thrombohaemorrhagic syndrome and precedes the onset of hepatocarcinomas in mice. These data confirm the observations of Trousseau in 1865 that thrombophlebitis migrans is a sign of occult malignancy (Boccaccio & Comoglio, 2006). This provoked an interesting discussion as to whether the HGF signalling pathway, similar to many other developmental pathways, is part of a genetic programme controlling physiological processes that is usurped by cancer cells. Indeed, many questions relating to this issue remain unresolved; for example, the observation that HGF seems to induce the inverse mesenchymal-to-epithelial transition in some cellular contexts (M. López-Cabrera, Madrid, Spain).
Much effort has been devoted to understanding NF-κB activation during EMT. The data available show that NF-κB activates Snail expression both in vitro and in vivo. M. Klymkowsky (Boulder, CO, USA) showed that the induction of Snail2 and Twist expression by NF-κB is necessary for mesoderm formation in Xenopus (Zhang et al, 2006). Snail1 expression is also activated by NF-κB in human squamous carcinoma cells through the insulin-like growth factor receptor and Akt pathways (L. Larue, Orsay, France; Julien et al, 2007), and during the induction of EMT in mesothelial cells (R. Strippoli, Madrid, Spain).
Regulating the β-catenin pool
β-Catenin interacts with E-cadherin at adherens and tight junctions to maintain the epithelial phenotype. In response to exogenous signals, β-catenin is translocated from the cell membrane to the cytoplasm where it is either ubiquitinated and degraded, or directed to the nucleus where it can regulate gene transcription and induce EMT. In Krakow, several studies were presented that highlighted the importance of nuclear β-catenin in controlling cell invasion and growth inhibition. It is worth noting that cell density modulates adhesion through the turnover of β-catenin and E-cadherin (Conacci-Sorrell et al, 2003). At low cell density, β-catenin translocates to the nucleus and activates the repression of E-cadherin transcription by Snail2. Indeed, this process also occurs in colon cancer cells and at the invasion front of tumours where β-catenin activates the L1 cell adhesion molecule, a disintegrin and metalloprotease 10 (Adam10) and Fascin, thereby promoting EMT and metastasis (A. Ben Ze'ev, Rehovot, Israel, in collaboration with Brabletz; Gavert et al, 2007).
Nuclear β-catenin induces EMT and growth arrest in hepatocytes by increasing the levels of the cell-cycle inhibitor p16ink4a (Fischer et al, 2007). Attenuating cell proliferation seems to be a conserved characteristic associated with EMT because SIP1/ZEB2 represses Cyclin D expression (G. Berx, Ghent, Belgium; Mejlvang et al, 2007), as previously shown for Snail1 (Vega et al, 2004).
The relationship between the pools of E-cadherin and β-catenin is crucial for the induction and maintenance of the mesenchymal phenotype. The overexpression of E-cadherin sequesters β-catenin and maintains its association with the cell membrane, preventing its role as a transcription factor important for EMT. E-cadherin also helps to maintain the epithelial phenotype by decreasing NF-κB activity, which impairs Snail1 activation and, consequently, the induction of ZEB1 genes and other mesenchymal markers (Garcia de Herreros).
EMT and organ fibrosis
Until recently, fibrosis and, in particular, renal fibrosis was thought to be mediated by the activation of interstitial fibroblasts that deposit an excess of collagen fibres. It now seems clear that renal tubular epithelial cells can also undergo EMT and become collagen-producing myofibroblasts (Iwano et al, 2002; Boutet et al, 2006). Kalluri has shown previously that bone morphogenetic protein 7 (Bmp7) can revert renal fibrosis by inhibiting TGFβ signalling in mice (Zeisberg et al, 2003). At the meeting, he discussed that Bmp7 can also revert the TGFβ-induced endothelial-to-mesenchymal transition during cardiac fibrosis (Zeisberg EM et al, 2007) and that EMT is also involved in liver fibrosis (Zeisberg M et al, 2007). Other examples of the pathological involvement of EMT were presented, including the participation of bronchial EMT in asthma (S. Letuve, Paris, France) and of mesothelial cells during peritoneal dialysis (López-Cabrera), indicating that EMT is a common process during tissue development and organ fibrosis.
A molecular signature for EMT in development and cancer
In the past few years, much effort has been devoted to identifying a molecular signature for cancer and for the metastatic potential of tumour cell lines and human tumours (Chin et al, 2006; Neve et al, 2006; Nguyen & Massague, 2007). At the meeting, E. Thompson (Melbourne, Australia) elaborated on the identification of a molecular signature for EMT that might be useful in prognosis and therapy. The characterization of a molecular signature for EMT during embryonic development could provide insight into the participation of EMT in physiological processes such as a wound healing, or in pathological circumstances such as cancer progression or organ fibrosis. Therefore, the use of genome-wide analyses and/or high-throughput screening to reveal EMT-inducing signals and their cellular response in embryos will be welcomed by the EMT community. Both M. Morkel (Berlin, Germany) and Hill presented screening strategies to identify new EMT regulators in vivo in mouse and Xenopus embryos, respectively. These animal models offer distinct opportunities to evaluate cell behaviour and molecular networks. An impressive study—as part of a long-term collaboration with E.H. Davidson (Pasadena, CA, USA)—allowed D. McClay (Durham, NC, USA) to present the pre-EMT gene regulatory network that controls endomesoderm specification in the sea urchin embryo (see Ben-Tabou de-Leon & Davidson, 2007). Conversely, a functional analysis in the sea urchin embryo of known inducers of EMT such as Snail and Twist, revealed new data indicating that Snail is involved in E-cadherin protein transport, as well as in its transcriptional repression (Wu & McClay, 2007).
MicroRNAs and EMT in cancer
MiRNAs are short non-coding RNAs involved in many developmental processes, as well as cell proliferation and differentiation. Although they have only recently been implicated in cancer, some intriguing relationships have been identified between miRNAs and tumours of diverse origins. Interesting data were presented to show that five members of the miR-200 family are repressed during TGFβ-induced EMT (G.J. Goodall, Adelaide, Australia). Indeed, it seems that their normal function is to downregulate the repressors of E-cadherin, Zeb1 and Sip1/Zeb2, and thus reinforce the epithelial phenotype. Conversely, J. Zavadil (Stony Brook, NY, USA) showed that miR-21—which is upregulated in human carcinomas—is induced during TGFβ-mediated EMT in a model of renal injury and fibrosis. Accordingly, miR-21 seems to target the tissue inhibitor of metalloproteinase-3 (TIMP-3), augmenting the degradation of the extracellular matrix. Together, these results suggest that the upregulation or downregulation of various miRNAs is fundamental for the regulation of the epithelial phenotype, as well as for EMT and tumour progression. This is in agreement with recent data obtained in breast cancer cells and tumours (Ma et al, 2007).
EMT and tumour progression
In deciphering the role of Snail genes during tumour progression, Cano showed that growth is impaired in tumours derived from keratinocyte and mammary tumour cell lines in which Snail1 and/or Snail2 were knocked down, and that they had a lower metastatic potential in nude mice (Olmeda et al, 2007). By contrast, the complementary gain-of-function approach adopted by Berx showed an increase in the sensitivity to chemical carcinogens when ectopic Snail1 expression was driven by the keratin14 promoter in the skin. These results highlight the importance of Snail in epidermal tumour progression.
Östman discussed cancer-associated fibroblasts (CAFs) and the interaction between malignant cells and the stroma. CAFs are activated myofibroblasts that can be of bone marrow origin—the so-called mesenchymal cancer stem cells (Karnoub et al, 2007)—or generated by the activation of local fibroblasts, or they might originate from cancer cells after EMT (Brabletz et al, 2005). These are not necessarily mutually exclusive scenarios, as different CAFs might co-exist (Fig 1). Therefore, it is crucial to distinguish the CAFs within and around the tumour to decipher the origin of the different mesenchymal cells. This will significantly advance our knowledge of tumour biology and the design of anti-invasive drugs.
Conclusions and future perspectives
The use of sophisticated animal models and high-throughput technologies are helping us to understand the EMT that occurs during the formation of many organs, and to reveal the associated cellular processes and molecular networks. Powerful imaging techniques are unveiling the behaviours of migratory cells in physiological conditions such as embryonic development and wound healing, and also confirm that EMT is important in the first step of the metastatic cascade in carcinomas. Indeed, single non-dividing migratory cells delaminate from primary tumours. The characterization of authentic markers will undoubtedly help to identify the nature and origin of all mesenchymal cells found in the stroma and in the vicinity of the primary tumour.

From left: Jean Paul Thiery, M. Angela Nieto & Hervé Acloque
Acknowledgments
We are grateful to the organizers for a wonderful meeting, and to the participants for helpful comments and suggestions. We apologize to those participants whose work has not been mentioned owing to space constraints. Work in our laboratories is supported by grants from the Spanish Ministry of Education and Science to M.A.N. (BFU2005-05772, NAN2004-09230-C04-04 and CONSOLIDER-INGENIO 2010 CSD2007-00017 and CSD2007-00023) and A*STAR Singapore to J.P.T.
References
- Aigner et al. (2007) The transcription factor ZEB1 (deltaEF1) promotes tumour cell differentiation by repressing master regulators of epithelial polarity. Oncogene 26: 6979–6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2: 84–89 [DOI] [PubMed] [Google Scholar]
- Ben-Tabou de-Leon S, Davidson EH (2007) Gene regulation: gene control network in development. Annu Rev Biophys Biomol Struct 36: 191–212 [DOI] [PubMed] [Google Scholar]
- Blanco MJ, Barrallo-Gimeno A, Acloque H, Reyes AE, Tada M, Allende ML, Mayor R, Nieto MA (2007) Snail 1a and 1b cooperate in the anterior migration of the axial mesendoderm in the zebrafish embryo. Development 134: 4073–4081 [DOI] [PubMed] [Google Scholar]
- Boccaccio C, Comoglio PM (2006) Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer 6: 637–645 [DOI] [PubMed] [Google Scholar]
- Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA (2006) Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 25: 5603–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T (2005) Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer 5: 744–749 [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83 [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze'ev A (2003) Autoregulation of E-cadherin expression by cadherin–cadherin interactions: the roles of β-catenin signaling, Slug, and MAPK. J Cell Biol 163: 847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Segall JE (2003) Intravital imaging of cell movement in tumours. Nat Rev Cancer 3: 921–930 [DOI] [PubMed] [Google Scholar]
- Chin K et al. (2006) Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell 10: 529–541 [DOI] [PubMed] [Google Scholar]
- Chu YS, Eder O, Thomas WA, Simcha I, Pincet F, Ben-Ze'ev A, Perez E, Thiery JP, Dufour S (2006) Prototypical type I E-cadherin and type II cadherin-7 mediate very distinct adhesiveness through their extracellular domains. J Biol Chem 281: 2901–2910 [DOI] [PubMed] [Google Scholar]
- Dow LE, Kauffman JS, Caddy J, Peterson AS, Jane SM, Russell SM, Humbert PO (2007) The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene 26: 2272–2282 [DOI] [PubMed] [Google Scholar]
- Fischer AN, Fuchs E, Mikula M, Huber H, Beug H, Mikulits W (2007) PDGF essentially links TGF-β signaling to nuclear β-catenin accumulation in hepatocellular carcinoma progression. Oncogene 26: 3395–405 [DOI] [PubMed] [Google Scholar]
- Gavert N et al. (2007) Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res 67: 7703–7712 [DOI] [PubMed] [Google Scholar]
- Greenburg G, Hay ED (1982) Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol 95: 333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED (1968) Organization and fine structure of epithelium and mesenchyme in the developing chick embryo. In Epithelial–Mesenchymal Interactions, R Fleischmajer and R.E. Billingham (eds), pp 31–55. Baltimore, MD, USA: Williams & Wilkins Co [Google Scholar]
- Hay ED (1995) An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 154: 8–20 [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG (2002) Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L (2007) Activation of NF-κB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene 26: 7445–7456 [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449: 557–563 [DOI] [PubMed] [Google Scholar]
- Kimura TE, Merritt AJ, Garrod DR (2007) Calcium-independent desmosomes of keratinocytes are hyper-adhesive. J Invest Dermatol 127: 775–781 [DOI] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Weinberg RA (2007) Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449: 682–688 [DOI] [PubMed] [Google Scholar]
- Mejlvang J, Kriajevska M, Vandewalle C, Chernova T, Sayan AE, Berx G, Mellon JK, Tulchinsky E (2007) Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell 18: 4615–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Runyan RB (2007) Multiple transforming growth factor-β isoforms and receptors function during epithelial–mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs 185: 146–156 [DOI] [PubMed] [Google Scholar]
- Murray MJ, Saint R (2007) Photoactivatable GFP resolves Drosophila mesoderm migration behaviour. Development 134: 3975–3983 [DOI] [PubMed] [Google Scholar]
- Nawshad A, Medici D, Liu CC, Hay ED (2007) TGFβ3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2–Smad4–LEF1 transcription complex. J Cell Sci 120: 1646–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RM et al. (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10: 515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Massague J (2007) Genetic determinants of cancer metastasis. Nat Rev Genet 8: 341–352 [DOI] [PubMed] [Google Scholar]
- Olmeda D, Moreno-Bueno G, Flores JM, Fabra A, Portillo F, Cano A (2007) SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res 67: 11721–11731 [DOI] [PubMed] [Google Scholar]
- Ostman A, Heldin CH (2007) PDGF receptors as targets in tumor treatment. Adv Cancer Res 97: 247–274 [DOI] [PubMed] [Google Scholar]
- Padgett RW, Reiss M (2007) TGFβ superfamily signaling: notes from the desert. Development 134: 3565–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG (2007) The par–tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol 17: 1623–1634 [DOI] [PubMed] [Google Scholar]
- Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F (2005) A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J 24: 3446–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7: 415–428 [DOI] [PubMed] [Google Scholar]
- Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, Buguin A, Silberzan P (2007) Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci USA 104: 15988–15993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaderna S et al. (2008) The transcriptional repressor ZEB1 promotes metastasis and a loss of cell polarity in cancer. Cancer Res 68: 537–544 [DOI] [PubMed] [Google Scholar]
- Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A (2006) Transforming growth factor-β employs HMGA2 to elicit epithelial–mesenchymal transition. J Cell Biol 174: 175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad RL, Hay ED, Revel JD (1967) Cell contact during early morphogenesis in the chick embryo. Dev Biol 16: 78–106 [DOI] [PubMed] [Google Scholar]
- Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA (2004) Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 18: 1131–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waerner T, Alacakaptan M, Tamir I, Oberauer R, Gal A, Brabletz T, Schreiber M, Jechlinger M, Beug H (2006) ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell 10: 227–239 [DOI] [PubMed] [Google Scholar]
- Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G (2006) Tumor invasion in the absence of epithelial–mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell 9: 261–272 [DOI] [PubMed] [Google Scholar]
- Wu SY, McClay DR (2007) The Snail repressor is required for PMC ingression in the sea urchin embryo. Development 134: 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt L, Wadham C, Crocker LA, Lardelli M, Khew-Goodall Y (2007) The protein tyrosine phosphatase Pez regulates TGFβ, epithelial–mesenchymal transition, and organ development. J Cell Biol 178: 1223–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM et al. (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961 [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R (2003) BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968 [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R (2007) Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 282: 23337–23347 [DOI] [PubMed] [Google Scholar]
- Zhang C, Carl TF, Trudeau ED, Simmet T, Klymkowsky MW (2006) An NF-κB and slug regulatory loop active in early vertebrate mesoderm. PLoS ONE 1: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]