Abstract
T helper responses have now grown to include three T cell subsets: Th1, Th2 and Th17. Th17 cells have recently emerged as a third independent T cell subset which may play an essential role in protection against certain extracellular pathogens. However, Th17 cells with specificity for self antigens are highly pathogenic and lead to the development of inflammation and severe autoimmunity. A combination of TGF-β plus IL-6 and the transcription factors STAT3 and RORγt were recently described to be essential for initial differentiation of Th17 cells, and IL-23 for the later stabilization of the Th17 cell subset. Here we introduce another player IL-21, which is produced by Th17 themselves, plays an important role in the amplification of Th17 cells. Thus Th17 cells may undergo three distinct steps of development: differentiation, amplification and stabilization in which distinct cytokine play a role.
Introduction
CD4+ T cells play an important role in the initiation of immune responses by providing help to other cells and by taking on a variety of effector functions during immune reactions. Upon antigenic stimulation, naive CD4+ T cells activate, expand and differentiate into different effector subsets termed T helper (Th) 1, Th2 and Th17 and characterized by the production of distinct cytokines and effector functions. Th cells produce interferon-γ (IFN-γ) and lymphotoxin (LT), and can mobilize the cellular arm of the immune system to combat intracellular pathogens. Th2 cells secrete IL-4, IL-13, and IL-25, which are essential for the generation of appropriate class of antibodies and for the elimination of extracellular pathogens [1]. The identification of IL-17 family of cytokine as well as IL-23-mediated expansion of IL-17-producing T cells uncovered a new subset of Th cells designated Th17 cells. Similar to Th1 and Th2 cells, Th17 cells require specific cytokines and transcription factors for their differentiation. While the function of these cell subtypes is not completely elucidated, emerging data suggest that Th17 cells may play an important role in host defense against extracellular pathogens, which are not efficiently cleared by Th1 and Th2-type immunity. While these subsets have specific effector functions in clearing infections, dysregulated expansion of CD4+ Th effector T cells causes immunopathology. Excessive Th1 responses are associated with various autoimmune and inflammatory disorders, whereas enhanced Th2 cytokine production is involved in atopic diseases, including allergies and asthma. Accumulating data suggest that Th17 are highly pro-inflammatory and that Th17 cells with specificity for self-antigens lead to severe autoimmunity in various animal models. Here we review the factors that are required for the differentiation, amplification and stabilization of Th17 cells including transcription factors that are important for their generation. Furthermore, the functions of Th17 in normal immune responses as well as their role in inducing autoimmunity will be discussed.
The IL-23/Th17 axis
The IL-17 cytokine family is a recently discovered group of cytokines, which includes 6 members; IL-17A, B, C, D, IL-17E (or IL-25) and IL-17F [2,3]. IL-17, (IL-17A) the original member of this family, was first identified in 1995 [4]. While IL-25 is mainly produced by Th2 cells [3], different cell types including T cells, gd T cells, NK cells and neutrophils produce IL-17A and IL-17F [2]. In CD4+ T cells, IL-17A expression is specifically expressed in a subset of T cells called Th17 for which it became the hallmark cytokine [5]. However, it is now becoming clear that Th17 cells also produce IL-17F, IL-21 and IL-22 [6-9]. In this subset, as well as other cell types, IL-17F is coexpressed with IL-17A suggesting that IL-17A and IL-17F might coordinately mediate their effector functions. This is further strengthened by the fact that IL-17A and IL-17F can also form heterodimers [10,11] and thus may work together to induce effector immune responses. However, subtle discordant expression of IL-17A and IL-17F in certain cell types also points to potentially different functions of these two cytokines. Therefore IL-17A and F may act both independently and synergistically.
Activation of T cells in the presence of IL-23, a member of IL-12 cytokine family, led to the expansion of IL-17 producing T cells [12] and resulted in the identification of the Th17 cell subset [5]. IL-23 is a heterodimeric cytokine formed by a p40 chain, which is shared with IL-12 and a unique p19 chain. It signals through a receptor complex made of the IL-12Rβ1 chain and the IL-23 specific subunit IL-23R [13]. Despite the similarities between their protein structures and their receptors, it quickly became apparent that IL-23 effects were different from those mediated by IL-12. Upon immunization, p19-deficient mice mount normal Th1 responses, but exhibit profound defects in the generation of IL-17–producing cells [14,15], suggesting that IL-23 induces the development of Th17 cells, which was confirmed by expanding Th17 cells in the presence of IL-23. However, later studies showed that IL-23 is not the differentiation factor for Th17 cells [16-18] but probably acts on previously differentiated Th17 cells to induce expansion and/or stabilization of the Th17 phenotype [16].
Functions of IL-17 and Th17 responses during infection
To date, the majority of reports have focused on three cytokines of the IL-17 family: IL-17A, IL-17E and IL-17F. The major function of these 3 members is to chemoattract different cell types through the induction of other cytokines and chemokines [2]. IL-25 (IL-17E) induces the expression of Th2 type cytokines and chemokines such as RANTES and Eotaxin-1 and plays a role in Th2-type allergic responses [3]. Both IL-17A and IL-17F have pro-inflammatory properties and act on a broad range of cell types to induce the expression of cytokines (IL-6, IL-8, GM-CSF, G-CSF), chemokines (CXCL1, CXCL10), and metalloproteinases. IL-17A and IL-17F are also key cytokines for the recruitment, activation and migration of neutrophils [2]. The functional analysis of IL-17 has suggested an important and unique role for this cytokine in host protection against specific pathogens. The production of IL-17 and the recruitment of neutrophils seem important in host protection against gram-negative bacteria and fungal infections. IL-17R deficient mice are highly susceptible to infection with the extracellular pathogen Klebbsiella and the fungus Candida [19,20].
In addition, the preferential production of IL-17 by T cells during infection with Klebsiella pneumonia [19,21], Bacteroides fragilis [22], Borrelia burgdoferi, mycobacterium tuberculosis [23] and fungal speciesspecies [24] suggest that Th17 responses are triggered by specific pathogens and are required for their clearance. In addition to neutrophils, IL-17 might also dictate the migration of other important effector cell types during infection. In support to this hypothesis, pathogen specific Th17 cells generated during mycobacterial infection induce the expression of CXCL9, CXCL10 and CXCL11, which attract IFN-γ-producing CD4+ Th1 cells to the lung in order to control the infection [25].
Role of Th17 subset in autoimmunity
In addition to infections, IL-17 and Th17 cells play a very important role in the induction and propagation of autoimmunity in animal models and potentially also in human autoimmune diseases. IL-17 expression has also been detected in the target tissue during the progression of various human autoimmune diseases such as multiple sclerosis, rheumatoid arthritis and psoriasis [26]. Consistent with these observations, IL-17–deficient mice [27] or mice treated with an IL-17R antagonist [28] are resistant to the development of adjuvant-induced arthritis. Similarly, IL-17–deficient animals develop experimental autoimmune encephalomyelitis (EAE) with delayed onset and reduced severity [29]. Furthermore, administration of an IL-17-blocking antibody in mice immunized with a myelin antigen prevents chemokine expression in the brain and the subsequent development of EAE [5,30]. These data support the idea that IL-17 is involved in the pathogenesis of several autoimmune diseases in mice and possibly also in humans.
The importance of the Th17 subset in autoimmne diseases was first demonstrated in mice deficient for the p19 chain of the IL-23. These mice had similar numbers of IFN-γ producing T cells than wildtype mice but showed a dramatic decrease in IL-17-producing T cells and were resistant to the development of EAE and collagen-induced arthritis [14,15]. Furthermore, the transfer of myelin specific Th17 cells could induce very potent EAE in SJL mice [5]. While many animal models of autoimmune diseases were previously thought to be mediated exclusively by Th1 type responses [31], these experiments highlighted the importance of the IL-23/Th17 axis in the pathogenicity of these diseases. Therapeutic neutralization of IL-23 can prevent EAE relapses and decrease CNS expression of IL-17 and IFN-γ [32]. In addition, the transfer of encephalitogenic T cells in p19 deficient animals or wildtype mice induced similar disease showing that disease can progress in the absence of IL-23 [33]. However Th1 cells from p19 deficient mice were not as encephalitogenic as those from the wildtype animals [33]. These results are consistent with the role of IL-23 in the maintenance of the Th17 subset but also suggest that elimination of the Th17 response might also impair function or migration of antigen specific Th1 cells.
Th17 lineage specific program
The differentiation of effector T cell is initiated by signals from TCR, costimulatory molecules and cytokine receptors. Th1 and Th2 differentiation are initiated by activation of T cells in the presence of IFN-γ or IL-4, respectively. These integrated signals then induce the expression of lineage-specific transcription factors that drive Th cell differentiation. Transcription factors T-bet, STAT1 and STAT4 specify Th1 cell fate, whereas GATA-3 and STAT6 govern Th2 cell differentiation [34]. These transcription factors are however not required for the differentiation of Th17 subsets [16,35] and their ablation has so far shown little effect on the differentiation of Th17 cells in vitro or in vivo [32,35-38], firmly establishing that Th17 cells are a unique lineage different from Th1 and Th2 cells. On the other hand, consistent with the role of IL-6 in the differentiation of Th17 cells (see below), STAT3 is required for the development of these cells [39,40]. In addition, Th17 cells specifically express and require the presence of the transcription factor RORγt [41].
Developing Th subsets cross regulate expansion and functions of each other. While IL-4 antagonizes differentiation of Th1 cells and IFN-γ inhibits the development of Th2 cells, both cytokines antagonize the development of Th17 cells [35,36]. More recently, IL-25, an IL-17 family member produced by Th2 cells, was also shown to antagonize the differentiation of Th17 cells [42]. Another cytokine, IL-27, important for the differentiation of Th1 cells acts as a potent inhibitor of Th17 differentiation [43,44]. IL-2, which is a growth factor for most T cells and T cell subsets appears however to have inhibitory effects on the expansion of Th17 cells [45].
Reciprocal relationship between Th17 and induced T-reg (iT-reg)
Although, IL-23 was described as the differentiation factor for Th17 cells, we were unable to differentiate naïve T cells in the presence of IL-23 [17], raising the possibility that another cytokine(s) was responsible for differentiating naïve T cells into Th17 cells. Several groups including ours' recognized that a combination of two cytokines namely IL-6 and transforming growth factor-β (TGF-β), was necessary for the differentiation of naïve T cells into Th17 cells [16-18]. This was surprising since TGF-β was considered a potent regulatory cytokine with inhibitory effects on T cell differentiation [46]. Indeed, TGF-β, which is produced by regulatory T cells (T-reg) [47,48] and cells of the innate immune system [46], induces Foxp3, a transcription factor that has been associated with the generation of T-reg cells [49]. We and other have shown that addition of TGF-β to Foxp3− T cells can lead to Foxp3+ T cells with regulatory activities [17,50]. Similarly, overexpression of TGF-β under the IL-2 promoter in TGF-β transgenic mice leads to the generation of T cell populations with regulatory function supporting an important role of TGF-β in regulating immune responses [51]. However, since autoimmune responses develop even in the face of regulatory T cells, this led us to hypothesize that pro-inflammatory cytokines produced in the inflammed tissue might affect the generation of induced (i) T-regs and also inhibit the function of already existing T-regs. This prompted us to investigate the effect of a panel of proinflammatory cytokines on the generation of Foxp3+ T-reg cells induced by TGF-β. Of all the cytokines tested, IL-6, an acute phase protein produced during inflammation, inhibited the induction of Foxp3 and generation of T-reg cells. Interestingly and against all our expectations, activation of T cells in the presence of TGF-β plus IL-6 resulted in the induction of Th17 cells suggesting that TGF-β plus IL-6 must be the differentiation factors for Th17 cells. The importance of TGF-β plus IL-6 in differentiation of Th17 cells was further confirmed in vivo in that the immunization of TGF-β transgenic mice with MOG in CFA resulted in an enhanced Th17 response and exacerbated EAE [17] while mice expressing a dominant negative form of the TGF-β receptor were deficient in Th17 cells and resistant to the development of EAE [52].
Based on this data, we proposed that there is a reciprocal relationship between Foxp3+ T-reg and Th17 cells in which IL-6 plays a pivotal role in dictating whether the immune response is dominated by pathogenic Th17 cells or protective T-reg cells. Consistent with this hypothesis, IL-6 deficient mice immunized with MOG35−55 fail to develop a Th17 response, have overwhelming numbers of T-reg cells in the peripheral repertoire and are resistant to the development of EAE [8,17]. Similarly, T cells isolated from the lamina propria of IL-6 deficient animals do not produce IL-17 and do not express RORγt [41]. Together, these data show that IL-6 is also a key player in the differentiation of Th17 cells.
Since IL-6 deficient mice have a very high frequency of T-reg cells in the peripheral repertoire, we tested the development of T cell responses and EAE in IL-6-deficient mice following depletion of T-reg cells. IL-6 deficient mice depleted of regulatory T cells and immunized with MOG, developed a significant T cell response and also became susceptible to EAE. Surprisingly, however, in T-reg depleted IL-6−/− mice, there was a reappearance of Th17 cells suggesting that there is alternate pathway by which Th17 cells are induced in IL-6 deficient mice [8].
IL-6, TGF-β, and IL-21: “ménage à trois?”
In our search for an alternate pathway that initiates a Th17 response, using the logic we had used to identify IL-6 as one of the differentiating factor for Th17 cells, we discovered that IL-21 could inhibit the generation of iT-regs but also, in combination with TGF-β, induced Th17 cells. IL-21 is a cytokine with homologies to IL-2, IL-4, and IL-15 proteins. IL-21 binds to a receptor complex composed of a unique IL-21Rα chain and the shared common γ-chain, which activates the STAT1/STAT3 pathway. IL-21, which is mainly produced by T-cells, acts on a variety of different immune and non-immune cells to control immune responses [53].
The combination of TGF-β plus IL-21 was able to differentiate naïve T cells into Th17 cells in the absence of IL-6 suggesting that IL-21 (together with TGF-β) is the cytokine combination responsible for the generation of pathogenic cells in IL-6 deficient mice depleted of T-reg cells. Although IL-21 is known to be produced by activated T cells and NK cells [53], our comparison of cytokine expression in the three subsets of Th cells showed that IL-21 is only present at a very low level in Th1 and Th2 cells but is highly expressed by Th17 cells. Furthermore, IL-21 is not only induced by IL-6 plus TGF-β but also by IL-6 alone. This suggests that IL-21 can be produced in vivo under inflammatory conditions and must develop a positive feedback loop to further amplify the Th17 response.
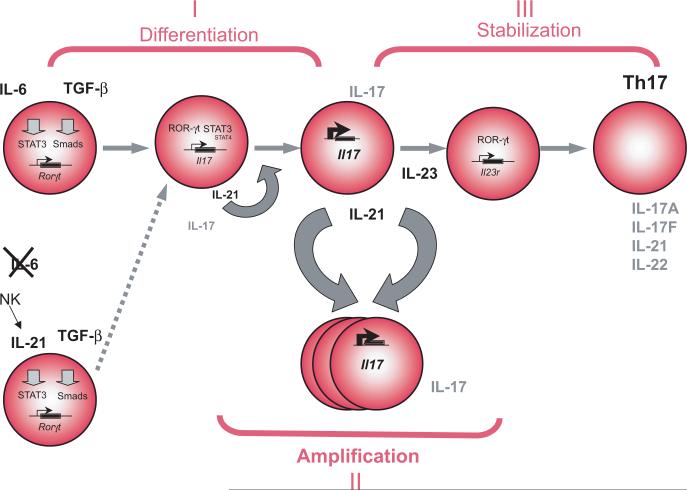
With another player added to the duo TGF-β-IL-6 in Th17 differentiation, this raises the issue of the role of IL-21 in normal Th17 differentiation and whether IL-21 is upstream or downstream of IL-6 during Th17 development. The fact that IL-6 plus TGF-β induced Th17-differentiation is partially impaired in IL-21 and IL-21R deficient mice [9] [8] and that the fraction of CD4+ CD44+ IL-17+ T cells is significantly reduced in IL-21R KO mice, suggest that even under classical TGF-β plus IL-6 mediated differentiation, IL-21 may be required for full commitment of Th17 cells. We propose that IL-21 produced by the newly generated Th17 cells must establish a positive feedback loop to further amplify Th17 differentiation increasing the numbers of Th17 cells in the milieu. This requires the expression of the transcription factor RORγt and the upregulation of the IL-23R. IL-23 is then necessary for the maintenance of the Th17 response (Figure 1). This also suggests that IL-21 is an integral part of the Th17 response which is further supported by the observation that the Th17 defect in the IL-21R deficient mice can not be overcome by addition of exogenous IL-23 [8].
Figure 1 legend. Model of Th17 lineage development.
The differentiation of Th17 cells is initiated by the activation of naïve T cells in the presence of IL-6 plus TGF-β. This leads to the expression of ROR-γt and production of IL-17. IL-6, produced by the innate immune system, is critical during this phase but when this cytokine is not present and T-reg cells are eliminated, IL-21 produced by NK cells together with TGF-β can initiate an alternate pathway of Th17 differentiation. Upon differentiation, IL-21 is also massively induced by the developing Th17 cells and act in autocrine fashion on Th17 cells to amplify this population. Then, IL-23 stabilizes previously differentiated Th17 cells and enables further expansion of the Th17 lineage with sustained production of its hallmark cytokines.
Conclusions
During the past 5 years, the Th1/Th2 paradigm has been updated to include a third subset called Th17. Through the induction of chemokines and the recruitment of other effector T cell populations, Th17 responses dominate in response to certain defined pathogens. It is therefore likely that the purpose of Th17 cells is to clear pathogens, which are not efficiently handled by Th1 and Th2 type of immunity. On the other hand, because of their cytokine profile and through the recruitment of other cell types, Th17 cells are highly proinflammatory and can mediate autoimmune diseases. Although TGF-β plus IL-6 are crucial factors for their differentiation and IL-23 is essential for maintaining the differentiated Th17 cells, we have added a new player, IL-21, in this pathway which plays a role in amplification of Th17 responses. Thus we propose that induction of Th17 responses must go through three distinct steps: Induction, amplification and stabilization where TGF-β plus IL-6 induce differentiation of Th17 cells, IL-21 amplifies the frequency of Th17 cells and IL-23 stabilizes the phenotype of previously differentiated Th17 cells. Loss of any of one of the members in the pathway (IL-6, IL-21 or IL-23) severely limits the Th17 response.
Acknowledgements
This work was supported by grants from the National Multiple Sclerosis Society, the National Institutes of Health, the Juvenile Diabetes Research Foundation Center for Immunological Tolerance at Harvard, and the Deutsche Forschungsgemeinschaft. VKK is the recipient of Javits Neuroscience Investigator Award from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Paper of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 4.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- •5.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [Of special interest.This paper was the first to establish the pathogenic role of Th17 cells in organ specific autoimmunity. Transfer of IL-23 driven Th17 cells were more efficient in inducing EAE in recipient SJL mice than Th1 cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2006 doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- ••8.Korn T, Bettelli E, Gao W, Awasthi A, Jaeger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternate pathway to induce proinflammatory Th17 cells. Nature. 2007 doi: 10.1038/nature05970. [Of outstanding interest.This paper show that depletion of regulatory T cells leads to the re-appearance of Th17 cells in IL-6 deficient mice suggesting an additional pathway by which Th17 can be generated in vivo. It further shows that IL-21, cooperates with TGF-β to induce Th17 cells in naive Il6−/− T cells and that IL-21 receptor deficient T cells are partially defective in generating a Th17 response.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••9.Nurieva R, O Yang X, Martinez G, Zhang Y, Athanasia D, Ma L, Schluns K, Tian Q, Watowich SS, Jetten M, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007 doi: 10.1038/nature05969. [Of outstanding interest.This paper shows that IL-21 is another effector cytokine produced by Th17 cells. IL-21 is proposed to be necessary and sufficient for the development of Th17 cells since IL-21 deficient animals have impaired Th17 responses and are protected from EAE.] [DOI] [PubMed] [Google Scholar]
- 10.Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, Qiu Y, Whitters MJ, Tomkinson KN, Dunussi-Joannopoulos K, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 11.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 13.Kastelein RA, Hunter CA, Cua DJ. Discovery and Biology of IL-23 and IL-27: Related but Functionally Distinct Regulators of Inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- ••14.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [Of outstanding interest.In this paper, p19 (IL-23 specific subunit) deficient animals were shown to be resistant to the development of EAE. IL-23 was further suggested to have important effects on macrophages. The authors suggested for the first time that IL-12 (and possible Th1 cells) but not IL-23 are dispensable for the development autoimmune diseases such as EAE.] [DOI] [PubMed] [Google Scholar]
- 15.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••16.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [Of outstanding interest.This publication is the first of three independent papers showing that a combination of TGF-β plus IL-6 induces the differentiation of naïve T cells into Th17. IL-23, although not required for the differentiation, was shown to play a role in the survival /expansion of Th17 cells.] [DOI] [PubMed] [Google Scholar]
- ••17.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [Of outstanding interest.This paper shows that the combination of TGF-β plus IL-6 plays an important role in vitro and in vivo for the differentiation of Th17 cells. A reciprocal relationship between the generation of pathogenic Th17 and induced regulatory T cells was further shown in vitro and suggested in vivo.] [DOI] [PubMed] [Google Scholar]
- •18.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [Of special interest.This paper shows that TGF-β is a cytokine critical for commitment to Th17 development and for the upregulation of the IL-23R. This paper clearly shows that IL-23 is required for host protection against a bacterial pathogen, Citrobacter rodentium.] [DOI] [PubMed] [Google Scholar]
- 19.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 21.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung DR, Kasper DL, Panzo RJ, Chitnis T, Grusby MJ, Sayegh MH, Tzianabos AO. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003;170:1958–1963. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 23.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 24.Leibundgut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007 doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 25.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 26.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 27.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 28.Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802–805. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- 29.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 30.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 31.O'Garra A, Steinman L, Gijbels K. CD4+ T-cell subsets in autoimmunity. Curr Opin Immunol. 1997;9:872–883. doi: 10.1016/s0952-7915(97)80192-6. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thakker P, Leach MW, Kuang W, Benoit SE, Leonard JP, Marusic S. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2589–2598. doi: 10.4049/jimmunol.178.4.2589. [DOI] [PubMed] [Google Scholar]
- 34.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 35.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 36.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, Kaplan MH. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108:1595–1601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 40.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- ••41.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The Orphan Nuclear Receptor RORgammat Directs the Differentiation Program of Proinflammatory IL-17(+) T Helper Cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [Of outstanding interest.In this report, ROR-γt was identified as a transcription factor specifically expressed in Th17 cells and a regulator of Th17 differentiation. The authors further show that Th17 cells are constitutively present throughout the intestinal lamina propria, express RORgammat, and are absent in mice deficient for RORgammat or IL-6. In addition, mice with RORgammat-deficient T cells have attenuated autoimmune disease and lack tissue-infiltrating Th17 cells.] [DOI] [PubMed] [Google Scholar]
- 42.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 44.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 45.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 47.Li MO, Wan YY, Flavell RA. T Cell-Produced Transforming Growth Factor-beta1 Controls T Cell Tolerance and Regulates Th1- and Th17-Cell Differentiation. Immunity. 2007 doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Faria AM, Weiner HL. Oral tolerance and TGF-beta-producing cells. Inflamm Allergy Drug Targets. 2006;5:179–190. doi: 10.2174/187152806778256034. [DOI] [PubMed] [Google Scholar]
- 49.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 50.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007;178:179–185. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 52.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 53.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]