Abstract
The interleukin-12 (IL-12) family of cytokines which includes IL-12, IL-23, and IL-27 play critical roles in T cell differentiation and are important modulators of multiple sclerosis and experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis. Previously, we demonstrated that peroxisome proliferator-activated receptor (PPAR) -α agonists suppress the development of EAE. The present studies demonstrated that the PPAR-α agonist fenofibrate inhibited the secretion of IL-12p40, IL-12p70 (p35/p40), IL-23 (p19/p40), and IL-27p28 by lipopolysaccharide-stimulated microglia. The cytokines interferon-γ and tumor necrosis factor-α also stimulated IL-12 p40 and IL-27 p28 expression by microglia, which was suppressed by fenofibrate. Furthermore, fenofibrate inhibited microglial expression of CD14 which plays a critical role in TLR signaling, suggesting a mechanism by which this PPAR-α agonist regulates the production of these pro-inflammatory molecules. In addition, fenofibrate suppressed the secretion of IL-12p40, IL-23, and IL-27p28 by lipopolysaccharide-stimulated astrocytes. Importantly, fenofibrate suppression of EAE was associated with decreased expression of IL-12 family cytokine mRNAs as well as mRNAs encoding TLR4, CD14, and MyD88 known to play critical roles in MyD88-dependent TLR signaling. These novel observations suggest that PPAR-α agonists including fenofibrate may modulate the development of EAE, at least in part, by suppressing the production of IL-12 family cytokines and MyD88-dependent signaling.
Keywords: experimental autoimmune encephalomyelitis, fenofibrate, glia, interleukin-12, interleukin-23, interleukin-27
Multiple sclerosis (MS) is a chronic inflammatory disease that is characterized by demyelination and neuronal injury resulting from an inflammatory response directed at host CNS antigens (Martin and McFarland 1995; Sobel 1995; Coyle 1996). Self-reactive T cells are believed to initiate the disease (Stinissen et al. 1998; Xiao and Link 1999). The activation of CD4+ autoreactive T cells and their differentiation into a T-helper 1 (Th1) phenotype are believed to be crucial events in the development of a variety of autoimmune diseases including MS (Sospedra and Martin 2005).
The interleukin-12 (IL-12) family of proteins includes the prototypic member IL-12, as well as the more recently identified IL-23 and IL-27. Each of these are heterodimeric proteins with IL-12 composed of p40 and p35 subunits, and IL-23 composed of the same p40 subunit together with an unique p19 subunit. IL-27 is composed of Epstein-Barr virus-induced molecule 3 (EBI3) which is structurally related to p40 and p28 which is related to p35. IL-12 family cytokines have been demonstrated to play an important role in the generation of Th1 cells (Murphy and Reiner 2002; Robinson and O’Garra 2002; Trinchieri et al. 2003). These cytokines are produced by a variety of cells, including antigen presenting cells such as dendritic cells, and macrophages. In the CNS, microglia produce IL-12-family cytokines, whereas astrocytes production of these molecules remains controversial.
The innate immune system recognizes pathogen-associated molecular patterns present on viruses, bacteria, and fungi through a family of pattern recognition receptors termed Toll-like receptors (TLRs). CD14 is also a pattern recognition receptor critical to recognition of bacterial surface markers including lipopolysaccharide (LPS). Ligand binding to TLRs (with the exception of TLR3) can trigger recruitment of the adaptor molecule MyD88 resulting in the activation of MyD88-dependent signaling which results in the activation of the transcription factor nuclear factor-kappa B (NF-κB) which is known to stimulate the expression of a variety of genes that encode pro-inflammatory molecules (Kielian 2006). Exacerbations of MS can be triggered by pathogens and TLRs play a critical role in the development of experimental autoimmune encephalomyelitis (EAE) (Zekki et al. 2002; Racke et al. 2005; Hansen et al. 2006; Prinz et al. 2006). This suggests that agents capable of suppressing TLR signaling may be effective in the treatment of MS.
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor family of proteins. Nuclear receptors which include estrogen, progesterone, and androgen receptors, as well as thyroid and glucocorticoid receptors can function as transcription factors to regulate the expression of genes important in reproduction and metabolism. Three PPAR subtypes exist (α, β, and γ), which exhibit distinct tissue distribution and ligand specificities (Desvergne and Wahli 1999). The role of PPAR-α in lipid metabolism is well established (Lee et al. 2003). Furthermore, PPAR-α agonists are commonly prescribed for the treatment of hyperlipidemia and hypertriglyceridemia. However, the role of PPAR-α in regulating inflammatory response has not been thoroughly investigated. It has been demonstrated that administration of the PPAR-α agonists gemfibrozil and fenofibrate, inhibit the clinical signs of EAE, suggesting that PPAR-α agonists may be effective in the treatment of MS.
The aim of the present study was to investigate the effects of the PPAR-α agonist fenofibrate on the release of IL-12-family cytokines by activated glia as well as during the course of EAE, and to explore the possible mechanisms of the anti-inflammatory effects of fenofibrate. Our studies demonstrate that fenofibrate suppresses the production of IL-12 family cytokines by glia in vitro and in EAE mice in vivo, perhaps by inhibiting MyD88-dependent signaling. This suggests that fenofibrate may be effective in the treatment of MS.
Materials and methods
Reagents
The PPAR-α agonist fenofibrate, carboxymethylcellulose, LPS, the lectin Griffonia simplicifolia, oxaloacetate pyruvate insulin medium supplement, and l-leucine methyl ester hydrochloride were obtained from Sigma (St Louis, MO, USA). Dulbecco’s modified Eagle’s medium media, glutamine, trypsin, and antibiotics used for tissue culture were obtained from BioWhittaker (Walkersville, MD, USA). Fetal bovine serum was obtained from Hyclone (Logan, UT, USA). GM-CSF was obtained from BD Pharmingen (San Diego, CA, USA). Glial fibrillary acidic protein was obtained from Dako (Carpinteria, CA, USA). C57BL/6 mice were obtained from Harlan (Indianapolis, IN, USA) and bred in house.
Cell culture
Primary mouse microglia cultures were obtained through a modification of the McCarthy and deVellis protocol (McCarthy and de Vellis 1980). Briefly, cerebral cortices from 1- to 3-day-old C57BL/6 mice were excised, meninges removed, and cortices minced into small pieces. Cells were separated by trypsinization followed by trituration of cortical tissue. The cell suspension was filtered through a 70-µm cell strainer to remove debris. Cells were centrifuged at 153 g for 5 min at 4°C, resuspended in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 1.4 mmol/L l-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, oxaloacetate pyruvate insulin medium supplement, and 0.5 ng/mL recombinant mouse GM-CSF, and plated into tissue culture flasks. Cells were allowed to grow to confluence (7–10 days) at 37°C/5% CO2. Flasks were then shaken overnight (200 rpm at 37°C) in a temperature-controlled shaker to loosen microglia and oligodendrocytes from the more adherent astrocytes. These less adherent cells were plated for 2–3 h and then lightly shaken to separate oligodendrocytes from the more adherent microglia. Microglia were seeded in 24-well plates or six-well plates and incubated overnight at 37°C/5% CO2. Astrocyte medium contained all the substances described above for microglia medium, except GM-CSF. Astrocyte medium was also supplemented with 0.1 mmol/L l-leucine methyl ester hydrochloride to eliminate any remaining microglia. After shaking to remove microglia and oligodendrocytes, astrocytes were recovered by trypsinization, seeded in 24-well plates or six-well plates and incubated overnight at 37°C/5% CO2. After overnight incubation, microglia and astrocytes were treated with fenofibrate for 1 h, and then stimulated with LPS or interferon-γ (IFN-γ) plus tumor necrosis factor-α (TNF-α) for 24 h. Finally, tissue culture supernatants and cells were collected for ELISA and cell viability assay. The purity of microglia and astrocyte cultures was greater than 95% determined by immunohistochemical staining with the lectin Griffonia simplicifolia and glial fibrillary acidic protein, respectively.
Cell viability assays
Cell viability was determined by MTT reduction assay as described previously (Drew and Chavis 2001). Optical densities were determined using a Spectromax 190 microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 570 nm. Results were reported as percentage viability relative to untreated cultures.
Enzyme-linked immunosorbent assay
Cytokine (IL-12p40 and IL-12p70) levels in tissue culture media were determined by ELISA as described by the manufacturer (OptEIA Sets; BD Pharmingen). Cytokine IL-23 (p19/p40) levels in tissue culture media were determined by ELISA as described by the manufacturer (eBioscience, San Diego, CA, USA). Cytokine IL-27p28 levels in tissue culture media were determined by ELISA as described by the manufacturer (R&D Systems Inc., Minneapolis, MN, USA). Optical densities were determined using a Spectromax 190 microplate reader (Molecular Devices) at 450 nm. Cytokine concentrations in media were determined from standards containing known concentrations of the proteins.
RNA isolation and cDNA synthesis
Total RNA was isolated from microglia or spinal cord using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA samples were treated with DNaseI (Invitrogen) to remove any traces of contaminating DNA. The RT reactions were carried out using an iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions.
Real-time quantitative RT-PCR assay
Interleukin-12p40, IL-12p35, IL-23p19, IL-27EBI3, IL-27p28, CD14, MyD88, and TLR4 mRNA were quantified by real-time PCR using an iCycler IQ™ multicolor real-time PCR detection system (Bio-Rad). All primers and TaqMan minor groove binder probes (FAM™ dye-labeled) were designed and synthesized by Applied Biosystems (Foster City, CA, USA). The real-time PCR reactions were performed in a total volume of 25 µL using an iCycler™ kit (Bio-Rad). The levels IL-12p40, IL-12p35, IL-23p19, IL-27EBI3, IL-27p28, CD14, MyD88, and TLR4 mRNA expression in primary microglia or spinal cord were calculated after normalizing cycle thresholds against the ‘housekeeping’ gene glyceraldehyde-3-phosphate dehydrogenase and are presented as the fold change value (2−ΔΔCt) relative to LPS stimulated microglia or relative to vehicle treated mice in EAE studies.
Protein extraction and western blotting
Protein extracts were prepared from primary microglia cultured in six-well plates by lysing cells in Radio-Immuno Precipitation Assay buffer [1% Triton-X 100, 0.1% sodium dodecyl sulfate in phosphate-buffered saline (PBS), pH 7.4] supplemented with a Complete™ protease inhibitor cocktail tablet (Roche, Indianapolis, IN, USA). Lysates were incubated on ice for 30 min followed by centrifugation at 21 000 g for 20 min at 4°C to pellet debris. The resulting supernatants represent total cell protein lysates. The amount of total protein was quantitated by using a standard protein assay (bicinch-oninic acid protein assay reagent; Pierce, Rockford, IL, USA). Cellular proteins (25 µg) were separated electrophoretically on 4–15% Tris–HCl Ready Gels (Bio-Rad). Proteins were transferred electrophoretically to nitrocellulose membranes (NitroBind, MSI, Westborough, MA, USA) and then incubated with rat anti-mouse CD14 antibody (BD Pharmingen™) or rabbit anti-human/mouse MyD88 antibody (eBioscience) overnight at 4°C. Blots were then incubated with horseradish peroxidase-conjugated goat anti-rat IgG for CD14 or goat anti-rabbit IgG for MyD88 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) secondary antibodies for 1 h at room temperature. CD14 or MyD88 protein was detected by ChemoGlow West substrate as described by the manufacturer (Alpha Innotech, San Leandro, CA, USA) and visualized using a ChemiDoc XRS (Bio-Rad) image analysis system. Actin protein was detected in a similar manner using a rabbit anti-actin polyclonal antibody (Sigma; catalogue # A-5060) to verify uniformity in gel loading.
EAE induction and evaluation of disease
Experimental autoimmune encephalomyelitis was induced in C57BL/6 wild type mice by s.c. injection over four sites in the flank with 200 µg/mouse of myelin oligodendrocyte glycoprotein peptide 35–55 (C S Bio Co. Menlo Park, CA, USA) in an emulsion with Complete Freund’s Adjuvant (Difco, Detroit, MI, USA). Pertussis toxin (List Biological Laboratories Inc., Campbell, CA, USA) dissolved in PBS was injected i.p. at 200 ng/mouse at the time of immunization and 48 h later. Mice were scored on a scale of 0–6 as previously described (Racke et al. 1994): 0, no clinical disease; 1, limp/flaccid tail; 2, moderate hind limb weakness; 3, severe hind limb weakness; 4, complete hind limb paralysis; 5, quadriplegia or pre-moribund state; 6, death. Mice were monitored daily for disease. All mice were 7–10 week of age when experiments were performed. Fenofibrate suspended in 0.5% carboxymethylcellulose was fed by gavage once daily. Mice were given fenofibrate daily at 25 mg/kg beginning 3 days before immunization until 9 days after immunization. At days 10–18 post-immunization mice were given fenofibrate at 50 mg/kg each day by gavage. Vehicle-treated mice were given water containing 0.5% carboxymethylcellulose by gavage. At 18 days after immunization, mice were deeply anesthetized with halothane and transcardially perfused with PBS. The spinal cord was removed, and placed in TRIzol reagent for RNA isolation.
Statistics
Data derived from in vitro studies were analyzed by one-way anova followed by a Bonferroni post hoc test and data derived from in vivo studies were analyzed by Student’s t-test to determine the significance of difference.
Results
Effects of fenofibrate on production of IL-12 family cytokines by primary microglia stimulated with LPS
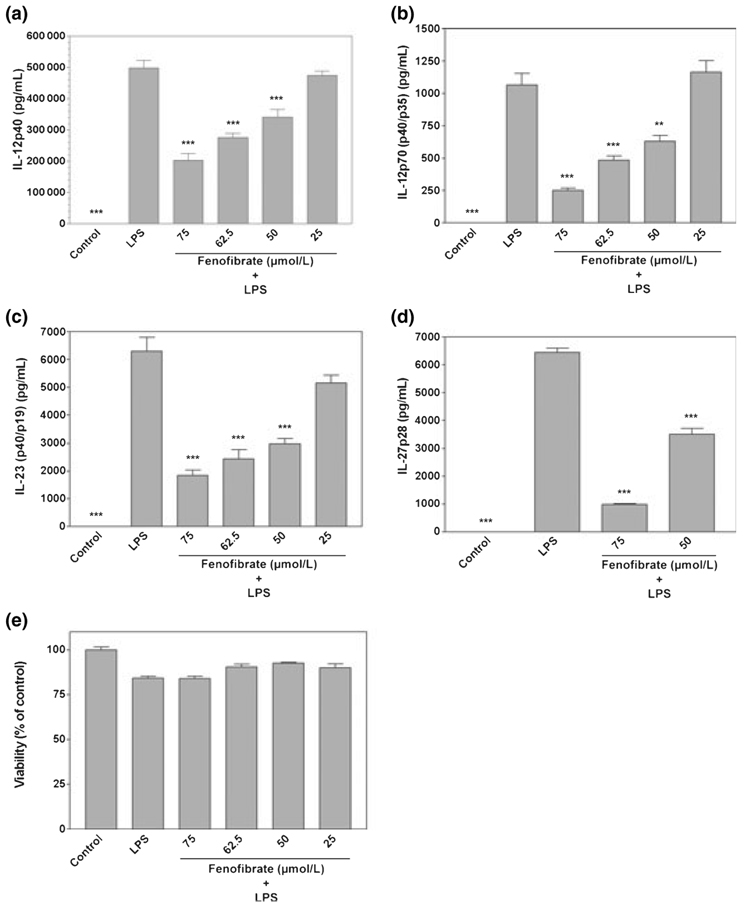
Relatively little is known concerning the expression of IL-12 family cytokines by resident CNS microglia. Furthermore, a variety of studies indicate that IL-12 family cytokines modulate the development of EAE. Therefore, in the present studies, we examined the expression of IL-12 family cytokines by microglia and determined the effects of fenofibrate on production of these cytokines. Fenofibrate was used in these studies because this PPAR-α agonist more potently inhibited LPS-induction of microglial production of pro-inflammatory molecules than other PPAR-α agonists examined in our previous studies (Xu et al. 2005) and strongly suppressed the development of EAE (Lovett-Racke et al. 2004). Primary microglia constitutively did not produce detectable levels of IL-12 family cytokines including IL-12 p40, IL-12 p70 (p35/p40), IL-23 (p19/p40), and IL-27 p28 as determined by ELISA analysis. However, LPS potently induced microglial production of these cytokines (Fig. 1). Furthermore, fenofibrate repressed LPS-induction of IL-12p40 (Fig. 1a), IL-12p70 (Fig. 1b), IL-23 (Fig. 1c), and IL-27p28 (Fig. 1d) production by microglia in a dose-dependent manner without effects on cell viability (Fig. 1e).
Fig. 1.
Fenofibrate inhibits lipopolysaccharide (LPS) induction of IL-12 family proteins in primary mouse microglia. Cells were pre-treated for 1 h with the indicated concentrations (µmol/L) of fenofibrate. LPS (0.1 µg/mL) was then added as indicated, and 24 h later, the concentration of IL-12p40 (a), IL-12p70 (p40/p35) (b), IL-23 (p40/p19) (c), and IL-27p28 (d) in the culture medium was determined. Cell viability was determined by MTT assay (e). Values represent the mean ± SEM for a representative experiment run in triplicate. Three independent experiments were conducted. **p < 0.01 and ***p < 0.001 versus LPS-treated cultures.
Effects of fenofibrate on production of IL-12 family cytokines by primary microglia stimulated with IFN-γ plus TNF-α
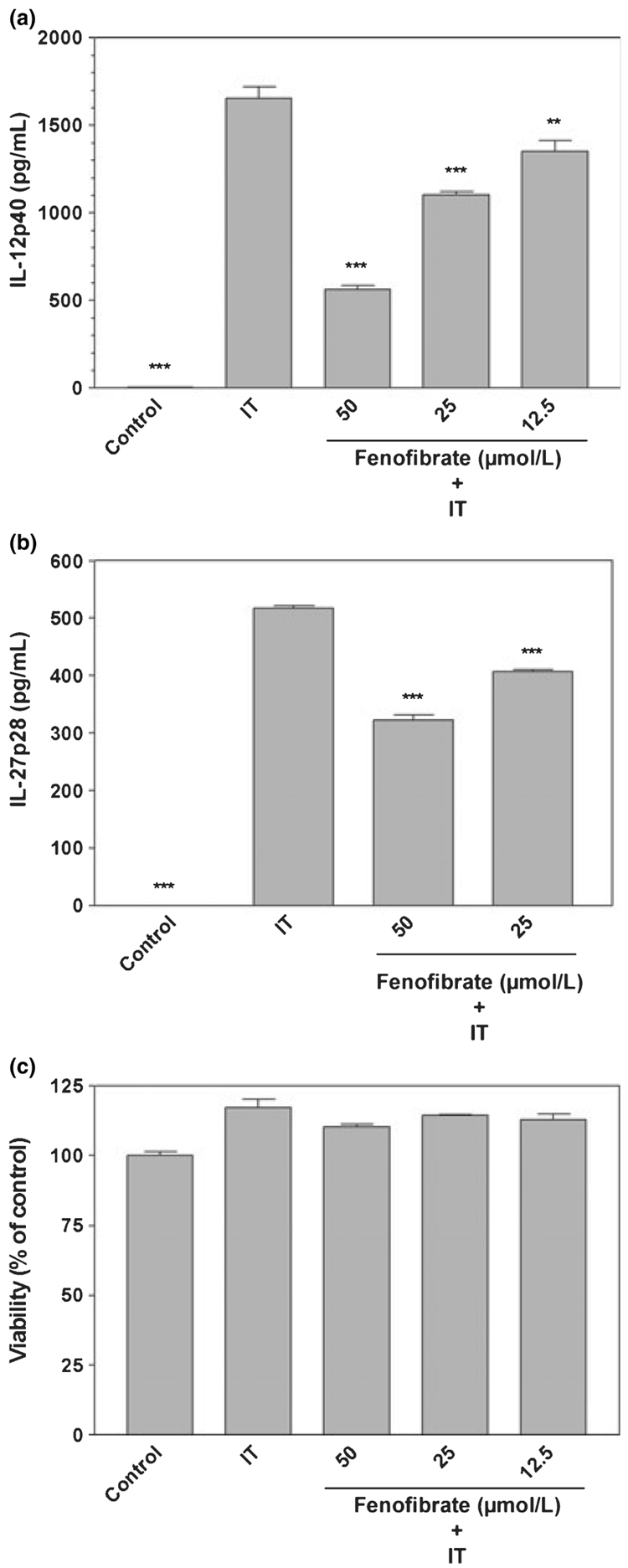
The pro-inflammatory cytokines IFN-γ and TNF-α activate microglia and are believed to contribute to the pathogenesis associated with MS. A combination of these cytokines potently induced the expression of IL-12 p40 (Fig. 2a) and IL-27 p28 (Fig. 2b), but not that of IL-12 p70 and IL-23 (data not shown). Fenofibrate suppressed the production of IL-12 p40 and IL-27 p28 protein expression in microglia stimulated with IFN-γ plus TNF-α, but did not affect cell viability (Fig. 2c). These studies demonstrate that fenofibrate is capable of suppressing IL-12 family cytokine production by microglia stimulated with LPS or a combination of IFN-γ plus TNF-α.
Fig. 2.
Fenofibrate inhibits interferon-γ (IFN-γ) plus TNF-α (IT) induction of IL-12p40 and IL-27p28 proteins in primary mouse microglia. Cells were pre-treated for 1 h with the indicated concentrations (µmol/L) of fenofibrate. IFN-γ (250 U/mL) and TNF-α (500 U/mL) were added as indicated, and 24 h later, the concentration of IL-12p40 (a) and IL-27p28 (b) in the culture medium was determined. Cell viability was determined by MTT assay (c). Values represent the mean ± SEM for a representative experiment run in triplicate. Three independent experiments were conducted. **p < 0.01 and ***p < 0.001 versus IFN-γ plus TNF-α-treated cultures.
Effects of fenofibrate on TLR signaling by primary microglia
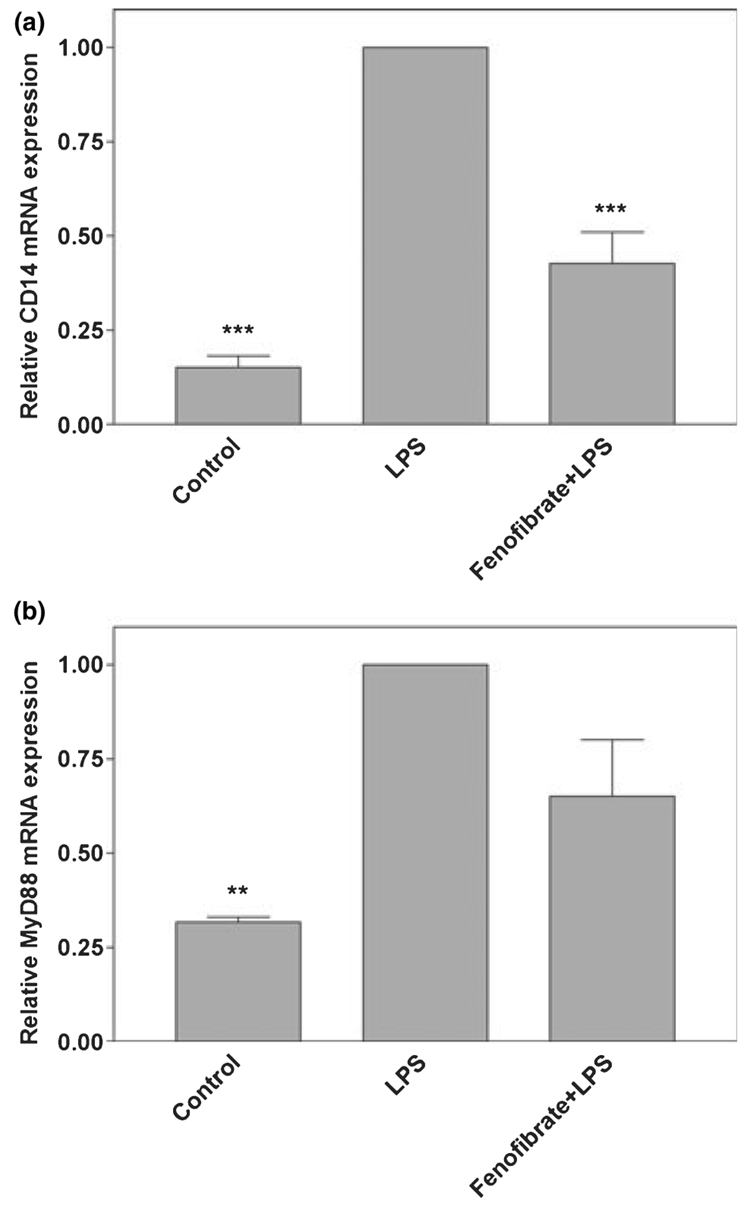
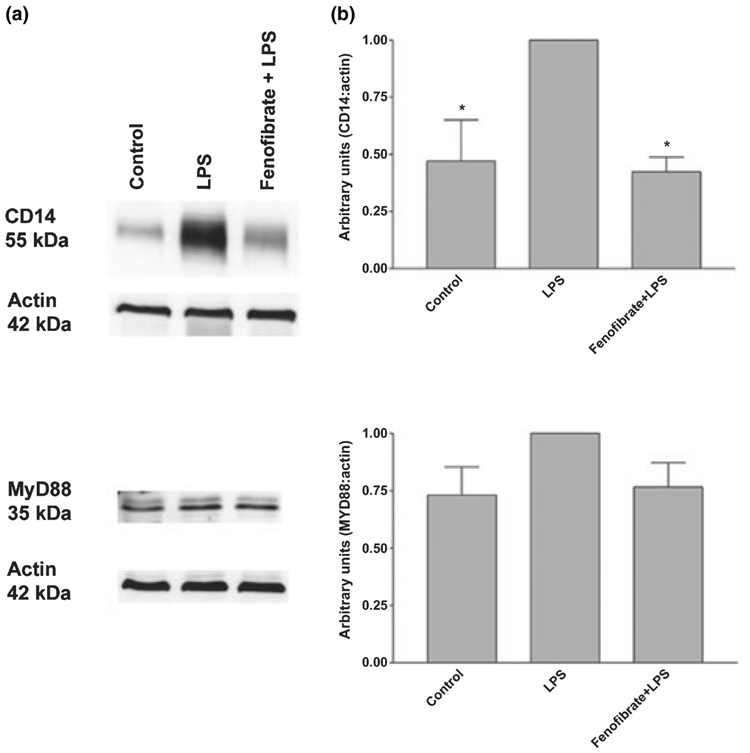
NF-κB plays an important role in the activation of a variety of genes which encode pro-inflammatory proteins. Fenofibrate inhibits LPS induction of NF-κB DNA binding activity in astrocytes (Xu et al. 2006) and microglia (data not shown). MyD88-dependent TLR signaling results in the activation of NF-κB. Therefore, we wished to determine the effects of fenofibrate on molecules critical in MyD88-dependent TLR signaling. Previously, we and others demonstrated that LPS does not induce the expression of the LPS receptor TLR4 (Kielian 2006; Xu and Drew 2007). LPS stimulated the production of the LPS co-receptor CD14 mRNA as well as the mRNA encoding the adaptor molecule MyD88, which is critical in MyD88-dependent TLR signaling. Interestingly, fenofibrate significantly inhibited CD14 mRNA expression by primary microglia (Fig. 3a). Fenofibrate also slightly inhibited MyD88 mRNA expression in these cells, although this inhibition was not statistically significant (Fig. 3b). Similarly, LPS induced the expression of CD14 and MyD88 protein expression as determined by western blot analysis. Fenofibrate inhibited the expression of CD14 and weakly inhibited the expression of MyD88 protein expression in these cells, although this inhibition again did not reach statistical significance (Fig. 4).
Fig. 3.
Effects of fenofibrate on lipopolysaccharide (LPS) induction of CD14 and MyD88 mRNA expression in primary mouse microglia. Cells were pre-treated for 1 h with fenofibrate (100 µmol/L). LPS (0.1 µg/mL) was added as indicated, and 6 h later, total RNA was isolated. CD14 (a) and MyD88 (b) mRNA levels were quantified by real-time quantitative RT-PCR. Results are expressed as fold changes relative to LPS-treated cells and all values were normalized against GAPDH. Values are mean ± SEM from four independent experiments, and duplicate reactions were performed on each sample in each experiment. **p < 0.01 and ***p < 0.001 versus LPS-treated cultures.(c)
Fig. 4.
Effects of fenofibrate on lipopoly-saccharide (LPS) induction of CD14 and MyD88 protein expression in mouse primary microglia. Cells were pre-treated for 1 h with fenofibrate (100 µmol/L). LPS (0.1 µg/mL) was added as indicated to cultures, and 24 h later, protein extracts from whole cell lysates were prepared. Protein expression for CD14 and MyD88 was evaluated by western blotting as described in Materials and methods. Western blot analyses are representative of three independent experiments (a). Each CD14, MyD88 and actin band was quantified by densitometry. Results are expressed in arbitrary units as the ratio of CD14 or MyD88 to actin, and LPS was used as reference and assigned the arbitrary value of 1. Values represent the mean ± SEM from three independent experiments. *p < 0.05 versus LPS-treated cultures (b).
Effects of fenofibrate on production of IL-12 family cytokines by primary astrocytes
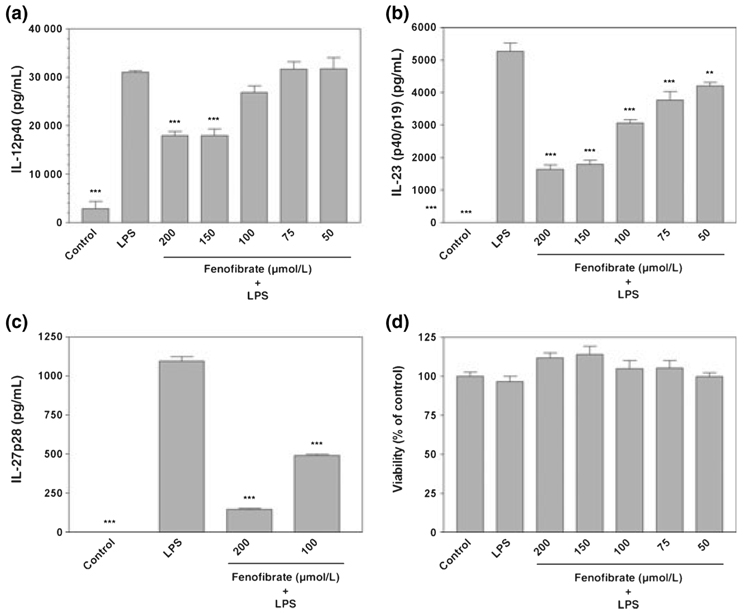
Interleukin-12 family cytokines are expressed by professional antigen presenting cells. However, expression of these cytokines by astrocytes remains controversial. Our current studies show that LPS can induce the secretion of IL-12p40 by activated astrocytes (Fig. 5a), but IL-12p70 (p35/p40) is undetectable in the same culture medium. Our studies also demonstrate that IL-23 (p19/p40) protein is expressed by LPS-treated astrocytes (Fig. 5b). Furthermore, LPS-stimu-lated astrocytes also express IL-27p28 as determined by ELISA (Fig. 5c). In addition, we demonstrate that the PPAR-α agonist fenofibrate inhibited LPS-induced IL-12p40 (Fig. 5a), IL-23 (Fig. 5b), and IL-27p28 (Fig. 5c) production in a dose-dependent manner without effects on cell viability (Fig. 5d). Collectively, the current studies demonstrate that the PPAR-α agonist fenofibrate inhibits the production of IL-12 family cytokines by CNS glia.
Fig. 5.
Fenofibrate inhibits lipopolysaccha-ride (LPS) induction of IL-12p40, IL-23 and IL-27p28 in primary mouse astrocytes. Cells were pre-treated for 1 h with the indicated concentrations (µmol/L) of fenofibrate. LPS (2.5 µg/mL) was then added as indicated, and 24 h later, the concentration of IL-12p40 (a), IL-23 (p40/p19) (b) and IL-27p28 (c) in the culture medium was determined. Cell viability was determined by MTT assay (d). Values represent the mean ± SEM for a representative experiment run in triplicate. Three independent experiments were conducted. **p < 0.01 and ***p < 0.001 versus LPS-treated cultures.
Effects of fenofibrate on production of IL-12 family cytokines in EAE model
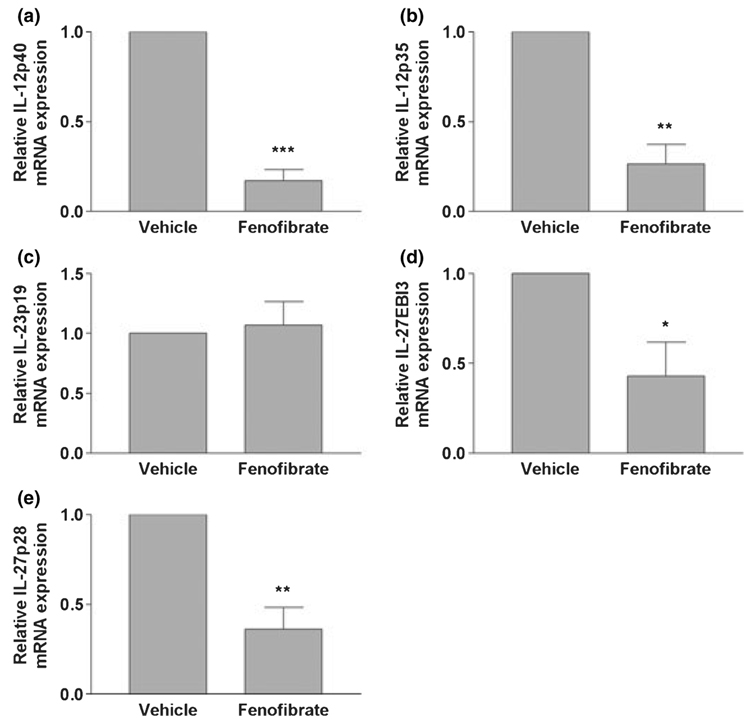
Previously, we demonstrated that fenofibrate suppresses the development of EAE (Lovett-Racke et al. 2004). We wished to determine if fenofibrate suppression of EAE was associated with altered expression of IL-12 family cytokines. Our current studies demonstrated that fenofibrate suppression of EAE was correlated with a decreased expression of IL-12 p40 (Fig. 6a), IL-12 p35 (Fig. 6b), IL-27 EBI3 (Fig. 6d), and IL-27 p28 (Fig. 6e), but not decreased expression of IL-23 p19 (Fig. 6c) mRNA. This suggests that fenofibrate suppresses the production of IL-12, IL-27, and possibly IL-23 in vivo.
Fig. 6.
Oral administration of fenofibrate inhibits IL-12 family subunit mRNA expression in experimental autoimmune encephalomyelitis mice. C57BL/6 mice were fed fenofibrate daily by gavage. Vehicle-treated mice were fed water containing 0.5% carboxymethylcellulose by gavage daily. Spinal cords were harvested on day 18 post-immunization, and total RNA was isolated. IL-12p40 (a), IL-12p35 (b), IL-23p19 (c), IL-27EBI3 (d), and IL-27p28 (e) mRNA levels were quantified by real-time quantitative RT-PCR. Results are expressed as fold-changes relative to experimental autoimmune encephalomyelitis mice in vehicle group and all values are normalized against GAPDH. Values are mean ± SEM from five independent experiments, and duplicate reactions were performed on each sample in each experiment. *p < 0.05, **p < 0.01, and ***p < 0.001 versus vehicle-treated mice.
Effects of fenofibrate on production of TLR signaling molecules in EAE model
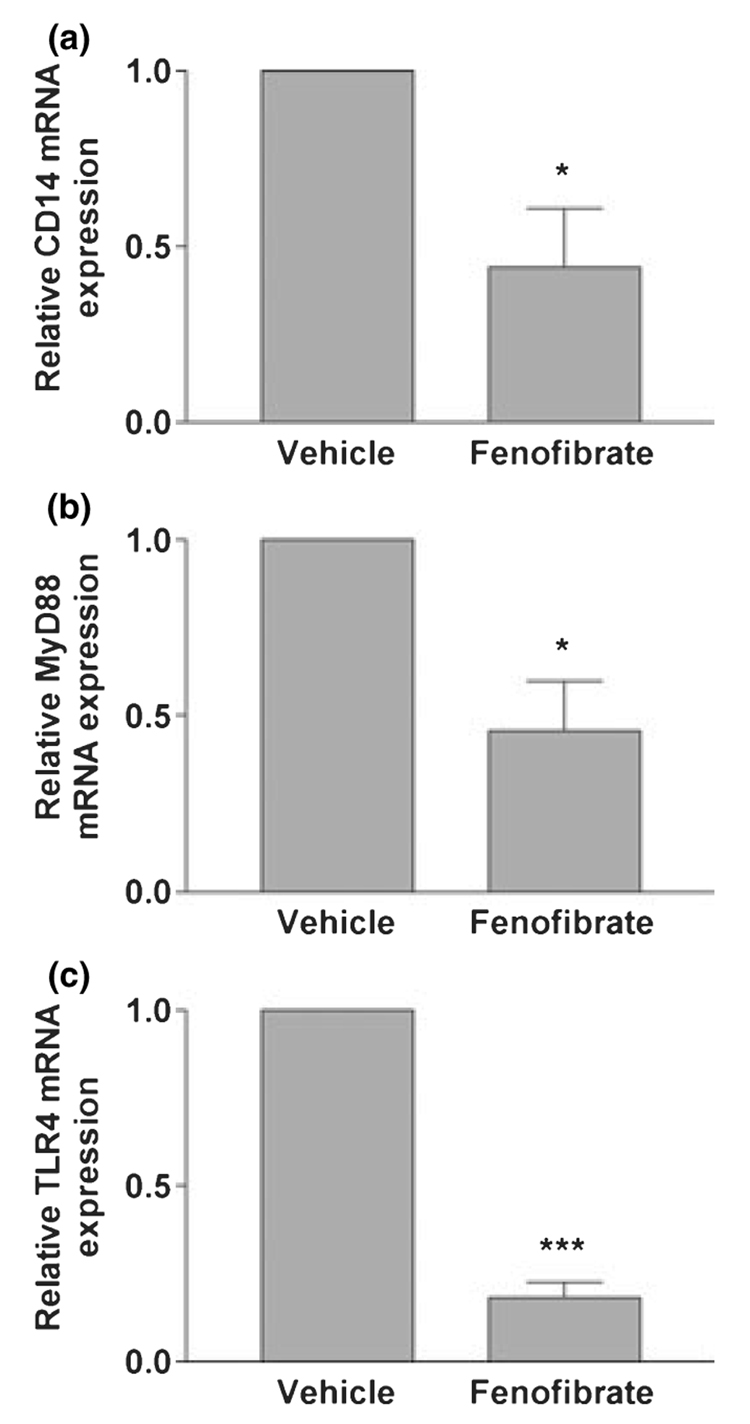
Fenofibrate suppression of EAE was also associated with suppression of CD14 (Fig. 7a), MyD88 (Fig. 7b), and TLR4 (Fig. 7c) mRNA. This suggests the possibility that fenofibrate inhibits EAE at least in part by inhibiting MyD88-dependent TLR signaling in vivo.
Fig. 7.
Oral administration of fenofibrate inhibits CD14, MyD88, and TLR4 mRNA expression in experimental autoimmune encephalomyelitis mice. C57BL/6 mice were fed fenofibrate daily by gavage. Vehicle-treated mice were fed water containing 0.5% carboxymethyl-cellulose by gavage daily. Spinal cords were harvested on day 18 post-immunization, and total RNA was isolated. CD14 (a), MyD88 (b), and TLR4 (c) mRNA levels were quantified by real-time quantitative RT-PCR. Results are expressed as fold-changes relative to experimental autoimmune encephalomyelitis mice in vehicle group and all values are normalized against GAPDH. Values are mean ± SEM from five independent experiments, and duplicate reactions were performed on each sample in each experiment. *p < 0.05 and ***p < 0.001 versus vehicle-treated mice.
Discussion
Our previous studies have shown that the PPAR-α agonists fenofibrate and gemfibrozil suppress the development of EAE (Lovett-Racke et al. 2004). These studies further demonstrated that these PPAR-α agonists increased the production of IL-4, a cytokine critical in Th2 cell differentiation, and suppressed the proliferation of T cell receptor transgenic T cells specific for MBPAc1–11. We also recently demonstrated that PPAR-α agonists block the activation of CNS microglia by repressing the production of nitric oxide and specific pro-inflammatory cytokines and chemokines (Xu et al. 2005). The current studies demonstrated that fenofibrate suppressed the LPS-induction of IL-12p40, IL-12p70 (p35/p40), IL-23 (p19/p40), and IL-27p28 by primary microglia. Fenofibrate also inhibited the induction of IL-12p40 and IL-27p28 by IFN-γ plus TNF-α stimulated microglia. In vivo, suppression of EAE correlated with suppression of IL-12 family cytokine mRNAs supporting a role for these cytokines in modulating disease. Furthermore, this PPAR-α agonist inhibited LPS-induction of IL-12p40, IL-23, and IL-27p28 by primary astrocytes. As IL-12 family cytokines are believed to contribute to the development of Th1 cells which likely exacerbate EAE, this suggests that PPAR-α agonists may suppress the development of EAE, at least in part, by suppressing glial production of IL-12 family cytokines. These studies further support the possibility that PPAR-α may be effective in the treatment of inflammatory diseases including MS.
Interleukin-12 plays a pivotal role for the differentiation of naive T cells into Th1 CD4+ lymphocytes (Hsieh et al. 1993; Manetti et al. 1993). Th1 cells produce IFN-γ and promote cell-mediated immunity which is essential for the response against intracellular pathogens, viruses, and bacteria (Dong and Flavell 2001; O’Shea and Paul 2002). The ability of IL-12 to strongly promote the development of Th1 cells makes it an ideal target for the treatment of Th1 cell-mediated diseases, such as MS. IL-12 can be detected in active lesions in MS patients, and increased IL-12 levels have been reported to correlate with disease activity (Windhagen et al. 1995; Nicoletti et al. 1996; Comabella et al. 1998). Antibodies prepared against IL-12 p40 have been shown to suppress the development and progression of EAE (Heremans et al. 1999). Further studies demonstrated that IL-12p40 knockout mice were resistant to EAE induction. In contrast, IL-12p35 knockout mice showed similar or even more severe clinical and pathological EAE compared with wild-type mice (Becher et al. 2002; Gran et al. 2002). These studies indicated a redundancy within the IL-12 system and suggested that other p40-containing cytokines, such as the more recently discovered IL-23, may be required for establishment of EAE.
Interleukin-23 p19 knockout mice were completely resistant to EAE (Cua et al. 2003). Furthermore, IL-23 p19 expression is increased in active lesions derived from MS patients (Li et al. 2007). These studies suggested that IL-23 rather than IL-12 is a critical factor in autoimmune demy-elination. Distinct from IL-12, IL-23 stimulates a unique subset of T cells to produce the pro-inflammatory cytokine IL-17, but only low levels of IFN-γ (Langrish et al. 2004). These novel T cell population was denoted ThIL-17. Recent studies showed that IL-23-dependent ThIL-17 cells drive autoimmune inflammation in the brain and that neutralization of soluble IL-17 by using antibodies partially protected mice from EAE (Hofstetter et al. 2005; Langrish et al. 2005). Here, our studies showed for the first time that fenofibrate potently inhibited the production of IL-12, IL-23, and their common subunit p40 by activated microglia, which suggest an additional mechanism by which fenofibrate may have beneficial effects on EAE.
Interleukin-27 is the newest member of the IL-12 family of heterodimeric cytokines (Pflanz et al. 2002). The functional role of IL-27 in MS is not clear. However, recent findings show that IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during EAE (Li et al. 2005), and neutralizing the function of IL-27p28 suppresses EAE (Goldberg et al. 2004). IL-27 is critical to the activation of T-bet which we recently demonstrated to be important not only in Th1 cell differentiation, but also in the function of pathogenic ThIL-17 cells in EAE (Gocke et al. 2007). Our present studies showed for the first time that fenofibrate inhibited the production of IL-27p28 by activated microglia. This suggests an additional mechanism by which PPAR-α agonists may modulate EAE, and possibly MS.
Unlike microglia, the ability of astrocytes to secrete IL-12p70 heterodimer is quite controversial. One early study showed that mouse astrocytes did not express IL-12p35 mRNA and did not secrete IL-12p70 protein upon stimulation with cytokines, LPS, or a neurotropic virus (Aloisi et al. 1997). Another study revealed that secretion of the IL-12 p70 heterodimer was detectable by ELISA from astrocytes treated with LPS plus IFN-γ, but not with LPS alone (Stalder et al. 1997). Furthermore, our present studies indicate that LPS induces the production of IL-12 p40, IL-23, and IL-27 p28 molecules, but not IL-12 p70 by primary astrocytes. However, a recent report showed that LPS-stimulated astrocytes are able to produce IL-12p40 and IL-12p70 protein, and express p19 and p35 mRNA (Constantinescu et al. 2005). These conflicting results cannot be presently explained. However, the different cell culture and assay systems used in the studies may be responsible for the disparate results. Importantly, fenofibrate inhibited LPS-induced IL-12p40, IL-23, and IL-27p28 production by primary astrocytes. This suggests that PPAR-α agonists may suppress EAE in part by inhibiting astrocyte expression of IL-12 family cytokines.
Exacerbations of MS can be triggered by pathogens and TLRs play a critical role in the development of EAE (Zekki et al. 2002; Racke et al. 2005; Hansen et al. 2006; Prinz et al. 2006). In addition, TLR signaling results in the production of NF-κB which is known to stimulate the production of a variety of pro-inflammatory molecules. Our results indicate that fenofibrate inhibits the production of molecules important in MyD88-dependent TLR signaling as well as NF-κB DNA-binding activity, suggesting a mechanism by which fenofibrate may modulate EAE.
In summary, we have demonstrated that PPAR-α agonist fenofibrate suppresses the production of IL-12-family cytokines including IL-12, IL-23, and IL-27p28 by activated glia in vitro as well as the mRNAs encoding these cytokines in vivo in EAE, possibly by suppressing MyD88-dependent TLR signaling. IL-12-family cytokines are key molecules involved in the development of MS. Therefore, these studies suggest that fenofibrate may be beneficial in the treatment of MS.
Acknowledgements
We thank Janet Chavis and Gail Wagoner for excellent technical assistance. This work was supported by grants from the National Institutes of Health (NS42860, NS44250, and NS047546), the National Multiple Sclerosis Society (RG 3427-B10/1T), and from the Arkansas Biosciences Institute.
Abbreviations used
- EAE
experimental autoimmune encephalomyelitis
- EBI3
EBV-induced molecule 3
- IFN-γ
interferon-γ
- IL
interleukin
- LPS
lipopolysaccharide
- MS
multiple sclerosis
- NF-κB
nuclear factor-kappa B
- PBS
phosphate-buffered saline
- PPAR
peroxisome proliferator-activated receptor
- Th1
T-helper 1
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
References
- Aloisi F, Penna G, Cerase J, Menendez Iglesias B, Adorini L. IL-12 production by central nervous system microglia is inhibited by astrocytes. J. Immunol. 1997;159:1604–1612. [PubMed] [Google Scholar]
- Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comabella M, Balashov K, Issazadeh S, Smith D, Weiner HL, Khoury SJ. Elevated interleukin-12 in progressive multiple sclerosis correlates with disease activity and is normalized by pulse cyclophosphamide therapy. J. Clin. Invest. 1998;102:671–678. doi: 10.1172/JCI3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu CS, Tani M, Ransohoff RM, et al. Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J. Neurochem. 2005;95:331–340. doi: 10.1111/j.1471-4159.2005.03368.x. [DOI] [PubMed] [Google Scholar]
- Coyle PK. The neuroimmunology of multiple sclerosis. Adv. Neuroimmunol. 1996;6:143–154. doi: 10.1016/0960-5428(96)00013-7. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [see comment] [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Dong C, Flavell RA. Th1 and Th2 cells. Curr. Opin. Hematol. 2001;8:47–51. doi: 10.1097/00062752-200101000-00009. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. The cyclopentone prostaglandin 15-deoxy-Delta(12,14) prostaglandin J2 represses nitric oxide, TNF-alpha, and IL-12 production by microglial cells. J. Neuroimmunol. 2001;115:28–35. doi: 10.1016/s0165-5728(01)00267-3. [DOI] [PubMed] [Google Scholar]
- Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J. Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- Goldberg R, Zohar Y, Wildbaum G, Geron Y, Maor G, Karin N. Suppression of ongoing experimental autoimmune encephalomyelitis by neutralizing the function of the p28 subunit of IL-27. J. Immunol. 2004;173:6465–6471. doi: 10.4049/jimmunol.173.10.6465. [DOI] [PubMed] [Google Scholar]
- Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J. Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- Hansen BS, Hussain RZ, Lovett-Racke AE, Thomas JA, Racke MK. Multiple toll-like receptor agonists act as potent adjuvants in the induction of autoimmunity. J. Neuroimmunol. 172;2006:94–103. doi: 10.1016/j.jneuroim.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Heremans H, Dillen C, Groenen M, Matthys P, Billiau A. Role of endogenous interleukin-12 (IL-12) in induced and spontaneous relapses of experimental autoimmune encephalomyelitis in mice. Eur. Cytokine Netw. 1999;10:171–180. [PubMed] [Google Scholar]
- Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [see comment] [DOI] [PubMed] [Google Scholar]
- Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J. Neurosci. Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- Li J, Gran B, Zhang GX, Rostami A, Kamoun M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J. Neurol. Sci. 2005;232:3–9. doi: 10.1016/j.jns.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Li Y, Chu N, Hu A, Gran B, Rostami A, Zhang GX. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain. 2007;130:490–501. doi: 10.1093/brain/awl273. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Hussain RZ, Northrop S, Choy J, Rocchini A, Matthes L, Chavis JA, Diab A, Drew PD, Racke MK. Peroxisome proliferator-activated receptor alpha agonists as therapy for autoimmune disease. J. Immunol. 2004;172:5790–5798. doi: 10.4049/jimmunol.172.9.5790. [DOI] [PubMed] [Google Scholar]
- Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit. Rev. Clin. Lab. Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Patti F, Cocuzza C, Zaccone P, Nicoletti A, Di Marco R, Reggio A. Elevated serum levels of interleukin-12 in chronic progressive multiple sclerosis. J. Neuroimmunol. 1996;70:87–90. doi: 10.1016/s0165-5728(96)00101-4. [DOI] [PubMed] [Google Scholar]
- O’Shea JJ, Paul WE. Regulation of T(H)1 differentiation–controlling the controllers. Nat. Immunol. 2002;3:506–508. doi: 10.1038/ni0602-506. [comment] [DOI] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, et al. IL-27, a hetero-dimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [see comment] [DOI] [PubMed] [Google Scholar]
- Prinz M, Garbe F, Schmidt H, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Rocken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J. Exp. Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke MK, Hu W, Lovett-Racke AE. PTX cruiser: driving autoimmunity via TLR4. Trends Immunol. 2005;26:289–291. doi: 10.1016/j.it.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Robinson DS, O’Garra A. Further checkpoints in Th1 development. Immunity. 2002;16:755–758. doi: 10.1016/s1074-7613(02)00331-x. [comment] [DOI] [PubMed] [Google Scholar]
- Sobel RA. The pathology of multiple sclerosis. Neurol. Clin. 1995;13:1–21. [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Stalder AK, Pagenstecher A, Yu NC, Kincaid C, Chiang CS, Hobbs MV, Bloom FE, Campbell IL. Lipopoly-saccharide-induced IL-12 expression in the central nervous system and cultured astrocytes and microglia. J. Immunol. 1997;159:1344–1351. [PubMed] [Google Scholar]
- Stinissen P, Medaer R, Raus J. Myelin reactive T cells in the autoimmune pathogenesis of multiple sclerosis. Mult. Scler. 1998;4:203–211. doi: 10.1177/135245859800400322. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [comment] [DOI] [PubMed] [Google Scholar]
- Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe MN, Cuzner ML, Hafler DA. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J. Exp. Med. 1995;182:1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao BG, Link H. Antigen-specific T cells in autoimmune diseases with a focus on multiple sclerosis and experimental allergic encephalomyelitis. Cell. Mol. Life Sci. 1999;56:5–21. doi: 10.1007/s000180050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists suppress the production of IL-12 family cytokines by activated glia. J. Immunol. 2007;178:1904–1913. doi: 10.4049/jimmunol.178.3.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Storer PD, Chavis JA, Racke MK, Drew PD. Agonists for the peroxisome proliferator-activated receptor-alpha and the retinoid X receptor inhibit inflammatory responses of microglia. J. Neurosci. Res. 2005;81:403–411. doi: 10.1002/jnr.20518. [DOI] [PubMed] [Google Scholar]
- Xu J, Chavis JA, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha and retinoid X receptor agonists inhibit inflammatory responses of astrocytes. J. Neuroimmunol. 2006;176:95–105. doi: 10.1016/j.jneuroim.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Zekki H, Feinstein DL, Rivest S. The clinical course of experimental autoimmune encephalomyelitis is associated with a profound and sustained transcriptional activation of the genes encoding toll-like receptor 2 and CD14 in the mouse CNS. Brain Pathol. 2002;12:308–319. doi: 10.1111/j.1750-3639.2002.tb00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]