Abstract
The potential negative impact of early blood oxygenation on development of specific cognitive and motor outcomes in children born at very low birth weight (VLBW; 1000 − 1500g) has not been examined even though these infants are exposed to varying durations and amounts of oxygen as part of their neonatal care. While this is the largest group of preterm infants, they receive much less research attention than extremely low birth weight infants (ELBW < 1000g).
Although neonatologists are questioning the routine use of oxygen therapy for all neonates, research has focused primarily on the more medically fragile ELBW infants. To date there are no systematic studies available to guide decision making for oxygen supplementation for a large segment of the preterm infant population. The aim of the present study was to determine if there is an association between blood oxygenation in the first four hours of life and specific cognitive and motor skills in preterm infants with acute respiratory disorders but no severe intracranial insult using a selected cohort from a longitudinal study children recruited in 1991 and 1992 designed to examine the role of biological immaturity as defined by gestational age and parenting in development. From this cohort, 55 children had acute respiratory disorders without severe intracranial insult. Of these, 35 children had at least one partial pressure of oxygen obtained from arterial blood (PaO2) during the first four hours of life as part of their clinical care. Higher early PaO2 values were associated with lower impulse control and attention skills in the elementary school age period. Models that examined for relations between PaO2 values that also included birth weight and parenting quality across the first year of life revealed that higher PaO2 remained associated with impulse control but not attention skills. Birth weight was not associated with any outcomes. These results suggest that hyperoxia may be a risk factor for developmental problems that are not expressed until school age.
Keywords: very low birth weight, acute respiratory disorders, child development
Introduction
The potential negative impact of early blood oxygenation on the development of specific cognitive and motor outcomes in children born preterm with gestational ages ≥ 28 weeks has not been examined even though they are exposed to varying durations and amounts of oxygen as part of their neonatal care. While this is the largest group of preterm infants, they receive much less research attention than infants born at lower gestational ages.
There is growing evidence from animal models that relatively brief exposure to combined hypoxia and hyperoxia is associated with DNA damage, programmed delayed cell death, and inflammation, all of which are risk factors for impaired neuronal development and function (Bredesen et al., 2006; Edinger and Thompson, 2004). Studies with animal models using the P7 neonatal rat in our laboratories have suggested that even relatively brief exposure to hyperoxia can result in damage (Qiu et al., 2004; Hu et al., 2003; Hu et al., 2005). This animal model is particularly relevant as, based on overall growth, cell production, neuronal migration and white matter myelination, it is thought to approximate the mid-third trimester human brain, (Jacobsen, 1963; Dobbing and Sands, 1979; Vannucci, et al., 1999). At this stage of neuronal development, oligodendrocytes and microglia is likely to play a role in the sensitivity of the brain to injury from hypoxic and hyperoxic exposure.
Preterm infants are the largest group of children who receive oxygen supplementation as part of their neonatal care. Recent studies in human neonates have implicated hyperoxia in pulmonary and ocular damage (e.g., Chow et al., 2003; Venot et al., 2001; Askie et al., 2003). These studies have changed the course of oxygen supplementation for the more medically fragile preterm neonates, often born at less than 27 wks gestation. As a result, the frequency of severe visual deficits has declined. Fortunately, this group of neonates only comprises 20% of preterm infants. The remaining 80% of preterm infants born closer to the expected delivery age of 40 wks gestation also receive supplemental oxygen that varies in concentration (e.g., 35−40% to 100%), delivery (e.g., face mask, vent), and duration (e.g., few to many days). The majority of preterm infants in this group are likely to be born at greater than 27 wks gestation but less than 36 wks and to have acute neonatal complications such as respiratory distress syndrome and transient tachypnea of the newborn without evidence of severe intracranial insult. This group of preterm infants often is considered “healthier” than more immature preterm infants and to be at lower risk for global developmental problems (Landry et al., 2001; Smith et al., 2006).
Deficits in specific cognitive and motor skills including attention, impulse control, and visual-motor coordination for preterm children have been documented (Brandt et al., 1992; Breslau et al., 2000; Hack et al., 1992; Vohr & Garcia-Coll, 1985). Aylward (2003) has termed this set of deficits “subtle dysfunctions”, as they occur despite average global cognitive abilities and are often not apparent until children enter a formal school setting. Although economic status (Bendersky & Lewis, 1994) and quality of parenting are known to predict development in children born at VLBW (Smith et al., 2006), these “subtle dysfunctions” have been difficult to explain by environmental factors. It is possible that factors related to neonatal treatments, such as oxygen supplementation, contribute to the presence of school age deficits, however there are few available studies.
The goal of the current project was to determine the extent to which blood oxygenation in the first four hours of life is associated with variability in child outcomes during three phases of development; infancy, early school age, and elementary school age for preterm children with a gestational of > 27 weeks. Because of the potential toxic effect of oxygen on neurodevelopment that may occur even with brief oxygen exposure found in our research, it was hypothesized that higher PaO2 values in the first four hours of life would be associated with lower visual-motor, attention, and impulse control skills. Because school age is the time when these skills are maturing, it was expected that associations with early oxygen would be more apparent across early school ages rather than in infancy.
Methods
Participants
A cohort of 185 families that had either a term (n = 97) or preterm (n = 63) infant born in 1991 through 1992 at the University of Texas Medical Branch were evaluated across infancy (i.e., 6, 12, 24 mos), early school age (3, 4, 6 yrs), and elementary school age (8, 10, 12 yrs) as part of a longitudinal study of the relation of biological risk and parenting behaviors in children's development. Of the 63 preterm children that had not been excluded due to other medical conditions that would affect their development, 55, or 87%, had gestational ages > 27 wks with no evidence of a chronic respiratory disorder or severe intracranial insult. A review of medical records revealed that 35 had at least one arterial PaO2 during the first four hours of life as part of the infant's clinical care, or 64%, of the available cohort. Twenty-three of the 35 had one arterial PaO2, nine had two and three had more than two during the first four hours of life. The following reasons were determined as to why the other 20 infants did not have an available arterial PaO2 : 1) six had blood oxygenation measured via capillary blood, 11%, 2) four were transferred from another hospital, 7%, and 3) 10 were unknown, 18%. Because the PaO2 values measured via arterial and capillary blood differ, only those with an arterial measure of PaO2 were included in the study.
Procedures
A neonatologist reviewed all medical records to ensure that infants met the study criteria and to exclude those that had other medical conditions that would impact development (e.g., intrauterine growth retardation, pulmonary hemorrhage; metabolic acidosis). The neonatal course also was reviewed in order to ensure that medical events occurring later in the hospitalization such as hyper- or hypotension, metabolic acidosis, etc would not compromise development and three children were excluded due to intrauterine growth retardation (n = 2) and cerebral hemorrhage (n = 1). Four infants were found to be small for gestational age as defined by a birth weight of < 10% on the Lubchenco growth chart (Lubchenco, Hansman, and Boyd, 1966). Medical information including birth weight, gestational age, small versus appropriate for gestational age, Apgar scores at 1 and 5 min, and all diagnoses were recorded. All PaO2 values obtained during the first 12 hrs of life were recorded.
Details regarding the neurological evaluations conducted by a developmental pediatrician, tests administered to evaluate child outcomes, and coding procedures for quantifying parenting quality have been published in detail (e.g., Anderson et al., 1999; Landry et al., 2001; Smith et al., 2006). Table 1 summarizes the tests used to measure each of the outcomes. Maternal responsiveness was coded “live” during a 60 -minute observation of daily activities and 10 min mother-child play in the home when children were 6 and 12 months of age. Three developmentally appropriate toys were provided by the research staff for the toy play interaction. Ratings of two aspects of maternal responsiveness, warm sensitivity and contingent responsiveness, were made every 20 minutes during the daily observation and at the end of toy play using a five point Likert type scale that ranged from “minimal” to “almost always”. All examiners were unaware of the child's medical background.
Table 1.
Summary of Tests Used to Measure Child Outcomes
| Infancy 6, 12, 24 months |
Early School Age 3, 4, 6 years |
Elementary School Age 8, 10, 12 years |
|
|---|---|---|---|
| Global IQ | Mental Subscale, Bayley | Stanford-Binet Intelligence | |
| Scales of Infant Development | Test, 4th Ed. | ||
| Motor Skills | Psychomotor Subscale, Bayley | Copying Subtest | |
| Scales of Infant Development | Stanford-Binet Intelligence | ||
| Test, 4th Ed. | |||
| Neurological Status | Presence of neurological abnormalities in motor tone, motor coordination, and reflexes. | ||
| Attention Impulse Control | Child Behavior Checklist — Parent report | Continuous Performance Task |
Blood oxygenation
Partial pressure of oxygen in arterial blood (PaO2) is a well accepted measure of blood oxygenation that has clinical relevance in medical management during the neonatal period. While the goal is to maintain “normal” levels (i.e., PaO2 < 100 mm Hg), the amount of oxygen provided differed in relation to the infant's needs for the first few hours after delivery and by clinical practice of the attending neonatologist. If multiple PaO2 values were recorded in the first four hours of life, the highest value was used in data analyses. Blood oxygenation was included in the analyses two ways: 1) a continuous variable and, 2) a categorical variable that reflected clinical practice (i.e., normal oxygenation, PaO2 40 − 105 mm Hg; moderate hyperoxygenation, PaO2 106 − 199 mm Hg; severe hyperoxygenation, PaO2 ≥ 200 mm Hg.
Neurological functioning
Children were examined by a developmental pediatrician who used a systematic approach based on the scales of Amiel-Tison (1976), Baird and Gordon (1983), and Swaiman (1989) with excellent reliability documented, generalizability coefficient = 0.93 (Mitchell, 1979). The examination consisted of seven subscales with 56 items that evaluated global and specific abnormalities (i.e., head circumference, general state, cranial nerves, motor tone, motor coordination, reflexes, and a neuro-sensory examination). The responses elicited for each item were scored from 0 to 2 (0= normal, 1= questionable, 2= clearly abnormal). Previous analyses of this measure revealed that the majority of variability in scores was found on three subscales: Motor Tone, Motor Coordination, and Reflexes (Anderson et al., 1996). The scores for items on these 3 subscales were average to obtain a composite score of Neurological abnormalities related to motor tone, coordination, and reflexes (TCR) that was used in data analyses . Higher TCR scores indicate greater abnormality.
Cognitive skills
Global intelligence was assessed using the Mental Subscale of the Bayley Infant Development Scales (Bayley, 1969) during infancy and the Stanford-Binet Intelligence Scale, 4th Ed.,(Thorndike at al., 1986) during early school ages. Both measures are frequently used in clinical and research studies and have good reliability and validity. To maintain the integrity of the study design and to avoid introducing bias by changing tests, a decision was made to use these versions of the two tests even though newer versions became available. In this report, the Global IQ score with a M = 100 (SD = 16) was used in data analyses. Higher scores indicated better general cognitive abilities.
Motor skills
Motor skills were assessed using the Psychomotor Subscale of the Bayley Infant Development Scales across infancy (Bayley 1969). During the early school ages, the Copying Subtest from the Stanford-Binet Intelligence Scale, 4th Ed. was used to measure visual -motor integration (Thorndike et al., 1986). In this test, children are first required to use blocks to reproduce a model and later items require copying of increasingly complex designs with paper/pencil. Standardized scores (M = 50, SD = 10) were used in data analyses. Higher scores on both tests indicate better motor skills.
Attention and impulse control skills
Across early school ages, the Child Behavior Checklist (CBCL) was used to measure the maternal report of the child's behavior including difficulty with attention and impulse control. The CBCL (Achenbach, 1991) is a well known behavioral rating scale for children aged 2 − 16 yr. Extensive reliability and factor analytic studies have led to the construction of an overall problem behavior score. For this project, the total raw score was used in data analyses. Higher scores indicate greater behavioral problems.
During the elementary school ages the Continuous Performance Task (CPT) (Halperin et al., 1991) was used to measure the ability to sustain attention and to inhibit behavior (impulse control). The CPT is a widely used research and clinical tool to document difficulty with attention and impulse control (Barkley, 1997; NICHD Early Child Care Research Network, 2005). For both measures, higher scores indicate poorer attention and impulse control.
Parenting quality
The rating criteria for warm sensitivity included degree of sensitivity to infant cues (i.e., acceptance of interests and needs, pacing of interaction to fit infant) amount of physical affection, and positive voice tone and verbalizations. The rating criteria for contingent responsiveness included degree of prompt and appropriate response to infant needs and interests (Landry et al., 2001). Interrater reliability for these two observational measures was assessed using generalizability coefficients through a second observer coding at least 20% of the interactions at each age. Generalizability coefficients are recommended for studies using continuous, behavioral observational data and coefficients > 50 indicate adequate reliability (Mitchell, 1979). Generalizability coefficients for the maternal responsiveness ratings ranged from 0.82 to 0.85.
For each child, the four scores from each of the two rating scales were averaged to obtain a single score at 6 and 12 months of age. Pearson product moment correlations revealed them to be significantly intercorrelated within each age point (range, r = 0.50 to 0.93). These scores were then averaged to obtain a measure of responsiveness during infancy. Using hierarchical clustering analyses (Ward, 1963), distinct groups of mothers were found. For the sample in this project these included: higher, n = 16 , moderate n = 4, minimal, n = 7. Because of the small sample size, the moderate and minimal groups were combined for analyses.
Data Analyses
A series of analytic approaches were used to evaluate the hypothesized relation between early PaO2 values and children's outcomes across three developmental periods. In the first step, single order correlations between the PaO2 values with a) neonatal medical factors and, b) the outcomes were obtained. In the second step, a mixed model analysis using growth curve modeling (Littell et al., 1996) was conducted for the outcomes for which there was at least one significant correlation with the PaO2 values. Just as one can examine growth in anthropomorphic measures such as height, examination of children's outcomes over time has begun to examine “growth” in development (Burchinal, Nelson, and Poe, 2006). With three data collection points it is possible to characterize “growth” in a specific area of development using an intercept that reflects the level of skill typically set within the mid-point of the age range studied), the rate of skill change, or slope parameter, and the extent to which change over time is not linear, or a quadratic parameter (Bryk and Raudenbush, 1992).
In our project, “growth” curve modeling allowed for determination of whether PaO2 values were associated with the intercept, slope, and quadratic parameters. Because of significant differences in the clinical groups in birth weight and the known influence of the environment on children's development, birth weight and parenting quality were included in the model analyses to determine if the association of PaO2 values remained in the presence of these biological and environmental influences.
Results
Associations between PaO2 values and child outcomes were obtained using the PaO2 value as a continuous variable. For information, a comparison of medical and demographic characteristics based on three clinically relevant blood oxygenation groups is presented in Table 2. In clinical practice, the goal is to maintain blood oxygenation between 40 and 100 mm Hg and severe hyperoxia is > 200 mm Hg. The three clinical groups differed in average PaO2 values and in birth weight but duration of mechanical ventilation and total days on oxygen supplementation were comparable. The three clinical PaO2 groups also were on demographic variables including socioeconomic status. In the total sample, 54% of the children were female and 63% experienced a higher quality of parenting across the first year of life. The largest ethnicity in the sample was African American (51%) and this reflects the demographics of infants born VLBW in the early 1990s (Merritt et al., 2003). Twenty-nine percent of the children were Caucasian and 20% were of Hispanic background but spoke English. This requirement was at the request of the primary project funding source as at the time the assessment tools for Spanish-speaking children were limited.
Table 2.
Medical Characteristics of Participants by Clinically Relevant Groups and Total Sample1
| Normal | Moderate | Severe | ||
|---|---|---|---|---|
| Oxygenation | Hyperoxia | Hyperoxia | Total | |
| n = 6 | n = 15 | n = 12 | n = 35 | |
| Highest arterial PaO2 (mm Hg)2 | 65 (14) a | 154 (30) b | 274 (52) c | 182 (86) |
| Gestational age — wks | 31.5 (1.9) | 31.1 (2.2) | 29.9 (1.3) | 30.9 (2.0) |
| Birthweight — gms2 | 1460 (95)a | 1286 (225) b | 1210 (130) b | 1290 (204) |
| Umbilical Cord pH | 7.28 (0.03) | 7.27 (0.08) | 7.29 (0.08) | 7.28 (0.07) |
| Days on Ventilator | 1.8 (1.7) | 1.1 (1.4) | 2.2 (2.6) | 1.5 (1.9) |
| Total Days on Oxygen | 1.5 (1.2) | 2.5 (4.6) | 4.7 (5.2) | 3.0 (4.4) |
| Apgar, 5 min % >8 | 50% | 73.2% | 50% | 52.6 |
| Socioeconomic status3 | 27.8 (8.5) | 25.4 (5.0) | 27.7 (10.4) | 27 (9.0) |
All data are Mean (S.D.) with exception of Apgar score.
PaO2 groups, Normal Oxygenation = 40 − 105 mm Hg; Moderate Hyperoxia = 106 − 199 mm Hg; Severe Hyperoxia = ≥ 200 mm Hg; 3Indicates significant group effect, p < .05, groups with different letters are significantly different from other groups.
Based on Hollingshead, Four Factor Scale (1994); range = 0 to 66.
Correlations of PaO2 with medical factors
Higher PaO2 values were significantly correlated with lower birth weight, r(35) = −0.36, p < 0.05 but not with any other medical factor (i.e., gestational age, days on a ventilator, days on oxygen). However, there was a significant correlation between birth weight and gestational age, r(35) = 0.66, p < 0.01.
Correlations of PaO2 with child outcomes (Table 3)
Table 3.
Correlation Coefficients for PaO2 and Child Outcomes
| Infancy (months) | Early School Age (years) | Elementary School Age (years) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 | 12 | 24 | 3 | 4 | 6 | 8 | 10 | 12 | |
| Global IQa | 0.18 | −0.05 | −0.06 | −0.13 | −0.01 | −0.11 | |||
| Neuro Abnormalitiesb | −0.06 | −0.18 | −0.33 | ||||||
| Motor/Visual — Motora | 0.16 | 0.00 | 0.11 | −0.04 | −0.26 | −0.20 | |||
| Behaivor - Parent Reportb | −0.07 | −0.11 | −0.04 | −0.14 | |||||
| Impulse Controlb | 0.54* | 0.12 | 0.35 | ||||||
| Inattentionb | 0.16 | 0.35* | −0.02 | ||||||
Higher scores = higher skill
Higher scores = lower skill
p < .05
During early development, no significant associations were found between PaO2 values and neurological abnormalities. In the early school age period, no significant correlations with early PaO2 values and global intelligence, visual-motor integration skills, or parent report of behavior problems based on the CBCL were found. In the elementary school age, higher PaO2 values were significantly associated with greater difficulty with impulse control at the 8 year age point. A trend for a similar association was found at 12 years of age. Higher PaO2 values also were significantly associated with greater difficulty in sustaining attention but only at 10 years of age.
Association of PaO2 values with change in skills over time
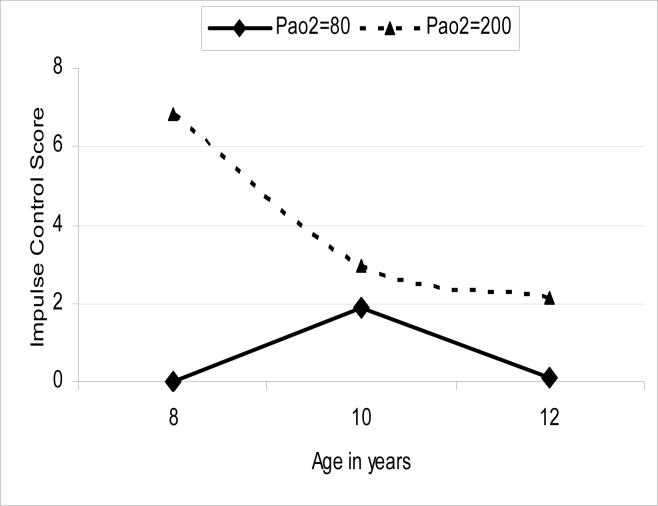
Early PaO2 values did not predict the intercept, or level of impulse control, at 10 years of age. However, it significantly predicted the rate of change, or slope, F(1, 16) = 4.61, p < 0.05. When added to the model, neither birth weight nor parenting quality was associated with impulse control. The model was used to obtain expected means at the 8, 10, and 12 year age points for two PaO2 values; normal, 80 mm Hg and severe hyperoxygenation, 200 mm Hg and these are illustrated in Figure 1.
Figure 1.
Estimated predicted impulse control scores at 8, 10, and 12 years of age for two PaO2 values (80, 200 mm Hg) based on the results of the mixed model analyses Lower impulse control scores equal better skill.
PaO2 values showed no association with either the level or slope parameters in the children's ability to sustain attention across the elementary school ages. Birth weight also was not associated with either parameter for this outcome. However, parenting quality significantly predicted level of attention skill across this developmental period, F(1, 18) = 6.94, p < 0.02. Children who experienced higher responsiveness across the first year of life had lower attention scores indicating better attention skills.
Discussion
The aim of the present study was to determine if associations exist between blood oxygenation in the first hours of life and neurodevelopmental outcomes across three developmental periods; infancy, early school age, and elementary school age, in a cohort of preterm children born at > 27 weeks gestation and without severe medical complications. Medically, this group of preterm children is considered relatively “healthy” because of greater gestational age at birth, heavier birth weights, > 1000 g, and acute medical complications rather than chronic disorders. Although general cognitive and motor skills appear to develop well in this group of preterm infants, they remain at risk for deficits in visual-motor, attention, and impulse control skills that may not be apparent until school age. Evidence for an association between early blood oxygenation and child outcomes was found in the present study but only in the elementary school age period, across 8, 10, and 12 years of age.
It is recognized that the study was not conducted prospectively but rather allowed for preliminary investigation of an important clinical question with a highly relevant group of children with longitudinal data. The present study could be even more of a challenge today given that invasive procedures such as blood drawings have virtually been eliminated from clinical practice for the “healthy” preterm infant unless clinically indicated. This is in contrast to practice during in the early 1990s when blood draws were more routinely obtained, often every 2 to 3 hrs on the majority of infants admitted to the Neonatal Intensive Care Unit. Today, pulse oximetry is the primary means of monitoring oxygen saturation for “healthy” preterm infants. While pulse oximetry measures were available for children in the present study, this measurement approach has a maximum of 100% saturation, and for preterm neonates born in the early 1990s, the maximum was frequently reached. Thus, in the present study we elected to study children with the most direct measure of blood oxygenation, PaO2. It also is recognized that while this study considered the influence of a single high blood oxygen level, in a prospective study the ability to examine the association between the duration of higher blood oxygenation and child outcomes will be important.
Development of Impulse Control and Attention Skills
Children's ability to gain control over impulses and learn to sustain their attention, particularly in the face of “boring” tasks, is an important foundational skill for development of later more complex executive functioning skills (Landry and Smith, in press). In this study, the finding that different processes supported development of these two skills was of note.
The importance of impulse control, or the ability to inhibit behavior, is recognized as a central characteristic in the development of attention deficit disorders (Barkley, 1997). The ability to control impulses requires neurological maturation and the young child's ability to inhibit a “prepotent response”, that is to inhibit a behavior that may be “neurologically wired”. For example, presenting young children with two cups, in which the child watches as a toy is hidden under one of the cups, requires the child to inhibit reaching for both of the cups simultaneously in order to find the reward. At older ages, impulse control is often measured with the computer task included in the present study where the child is presented with a sequence of letters of the alphabet on the screen that are presented at variable rates. The child is required to push a button every time a letter is seen, thereby establishing the “pre-potent” response. However, the child also is directed to “not push the button” or, inhibit the response, when the letter “X” is presented.
Impulse control appears to be a critical developmental skill that is likely to impact many areas of social and cognitive functioning and one that can be difficult to achieve for preterm children (Breslau et al., 2000). What the present study cannot address is whether the negative association between blood oxygenation and impulse control is apparent in the preschool age or if the negative association is only apparent at later school ages.
Children's ability to regulate attention in this study was associated with blood oxygenation but when examined in a model that included quality of parenting across the first year of life, it was no longer a unique predictor. This result was in direct contrast to that found for impulse control where blood oxygenation was a significant predictor in change in impulse control over time and parenting quality was not significant. The relation between parenting quality during infancy and cognitive outcomes is less frequently investigated than relations between parenting during infancy to infant attachment or other aspects of emotional development (e.g., Ainsworth et al., 1978). However, our results are consistent with that of the large NICHD Child care study where family quality was the primary predictor of attention skills during first grade even when considering multiple other child and environmental factors (NICHD Early Child Care Research Network, 2005).
Development of motor skills
The present study found no significant relation of early blood oxygenation and early school age development of visual-motor integration skills. Children born at VLBW irrespective of their families' economic circumstances are at increased risk for difficulty in coordinating eye-hand movements that are often evidenced in difficulty with coloring “inside the lines” and with early print skills (Cornhill and Case-Smith, 1996). It is possible that the lack of a significant association was due to our limited sample size. This may be particularly true given the relatively small group of children who had “normal” blood oxygenation. However, the lack of findings also may be due to the age at which these skills were studied and/or the task requirements. For example, the task in the present study did not have a time pressure and it may be that deficits are more apparent when time to complete the task is a critical feature.
Growth curves versus simple order correlations
In experimental studies, particularly using animal models, the ability to control potential confounding influences is a critical feature. Such control often can be assumed in intervention studies with humans where randomization results in no differences between potentially important factors that are beyond experimental control. However, preliminary data are necessary in order to focus clinical trials and in this regard, longitudinal studies can provide important information with which to guide experimental research. Within developmental psychology, the use of growth curve modeling has become a well-established and accepted means of better understanding the impact of variables of interest on development as opposed to a skill at one point in time. The realities of clinical research are such that the assumptions associated with the use of more traditional approaches, while well suited for highly controlled experiments, are difficult to meet. This becomes even more difficult as research attempts to determine the longer term impact on development. Given the importance of translating basic science research into the clinical arena, using a set of analytical approaches that cross basic science and clinical research appears to be important. In the present study, the combination of correlational approaches with more sophisticated statistical approaches that rely on similar methodologies (e.g., regression) provides a more complete understanding of the questions of interest; that is the association of early blood oxygenation on outcomes across multiple developmental periods.
Summary
In summary, the preterm infant frequently is faced with a double dose of oxidative stress: first anoxia/hypoxia during the birth process, then hyperoxia during resuscitation and early management of lung disease. One clinical response to these insults is evolving through implementation of gentle resuscitation therapies (e.g., room air, 40% oxygen) as opposed to 100% oxygen supplementation which should reduce the degree of oxidative stress experienced by preterm infants. The results presented here suggest that the risks of neonatal hyperoxia may not be expressed until late in childhood.
Acknowledgements
This study was funded, in part, by the National Institute of Child Health and Human Development, Health PO1 HD039833 to J. R. P-P and HD25128 to S. H. L. , Children's Learning Institute, University of Texas, Houston Health Science Center, Dr. K. E. S. Co-Investigator. Support also was provided by SHC 8540 from the Shriner's Hospital for Children to J. R. P-P.
The authors would like to thank Jennifer Caffey, M..D., and Donna Simmons, M.D. for their assistance in the review of medical records and Damir Janigro, Ph.D., for comments on earlier versions of the manuscript.
Abbreviations
- VLBW
Very low birth weight
- PaO2
partial pressure of oxygen obtained from arterial blood
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T. Manual for the Child Behavior Checklist/4−19 and the 1991 Profile. University of Vermont; Burlington: 1991. [Google Scholar]
- Ainsworth MDS, Blehard M, Walters E, Wall S. Patterns of Attachment: A Psychological Study of the Strange Situation. Erlbaum; Hillsdale: 1978. [Google Scholar]
- Amiel-Tison C. A method for neurologic evaluation within the first year of life. Current Problems in Pediatrics. 1976;7:1–50. [PubMed] [Google Scholar]
- Anderson A, Swank P, Wildin S, Landry S, Smith KE. Modeling analysis of change in neurologic abnormalities in children born prematurely: a novel approach. Journal of Child Neurology. 1999;14:502–8. doi: 10.1177/088307389901400804. [DOI] [PubMed] [Google Scholar]
- Askie SM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. New England Journal of Medicine. 2003;349:959–67. doi: 10.1056/NEJMoa023080. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Cognitive function in preterm infants: no simple answers. The Journal of the American Medical Association. 2003;289:705–11. doi: 10.1001/jama.289.6.752. [DOI] [PubMed] [Google Scholar]
- Baird HW, Gordon EC. Clinics in Neurodevelopmental Medicine. Lippincott; Philadelphia: 1983. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–74. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 1st Ed Psychological Corporation; NY: 1969. [Google Scholar]
- Bendersky M, Lewis M. Environmental risk, biological risk, and developmental outcome. Developmental Psychology. 1994;30:484–94. [Google Scholar]
- Brandt P, Magyary D, Hammond M, Barnard K. Learning and behavioral-emotional problems of children born preterm at second grade. Journal of Pediatric Psychology. 1992;17:291–311. doi: 10.1093/jpepsy/17.3.291. [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Johnson EO, Andreski P, Lucia VC. Neurologic soft signs and low birthweight: Their association and neuropsychiatric implications. Biological Psychiatry. 2000;47:1005–11. doi: 10.1016/s0006-3223(99)00131-6. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical Linear Models. Sage Publication; London: 1992. [Google Scholar]
- Burchinal M, Nelson L, Poe M. Growth curve analysis: An introduction to various methods for analyzing longitudinal data. Monographs of the Society for Research in Child Development. 2006;71:65–87. [Google Scholar]
- Chow LC, Wright KW, Sola A, the CSMC Oxygen Administration Study Group. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–45. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- Cornhill S, Case-Smith J. Factors that relate to good and poor handwriting. American Journal of Occupational Therapy. 1996;50:732–39. doi: 10.5014/ajot.50.9.732. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ, Gleser GC, Rajaratnam N. Theory of generalizability: A liberalization of reliability theory. British Journal of Mathematical and Statistical Psychology, 1963;16:137–73. [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Human Development. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Fleiss JL. The Design and Analysis of Clinical Experiments. Wiley; New York: 1986. [Google Scholar]
- Hack M, Breslau N, Aram D, Weissman B, Klein N, Borzwski-Clark E. The effect of very low brithweight and social risk on neurocognitive abilities at school age. Journal of Developmental and Behavioral Pediatrics. 1992;13:412–20. [PubMed] [Google Scholar]
- Halperin JM, Sharma V, Greenblatt E, Schwartz ST. Assessment of the Continuous Performance Test reliability and validity in a nonreferred sample. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3:603–8. [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Department of Sociology, Yale University; New Haven: 1975. [Google Scholar]
- Hu X, Qiu J, Grafe MR, Rea HC, Rassin DK, Perez-Polo JR. Bcl-2 family members make different contributions to cell death in hypoxia and/or hyperoxia in rat cerebral cortex. International Journal of Developmental Neuroscience. 2003;21:371–77. doi: 10.1016/s0736-5748(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Hu X, Nesic-Taylor O, Qiu J, Rea, Harriett C, Fabian R, Rassin DK, Perez-Polo JR. Activation of nuclear factor-κB signaling pathway by interleukin-1 after hypoxia/ischemia in neonatal rat hippocampus and cortex. J. Neurochemistry. 2005;93:26–37. doi: 10.1111/j.1471-4159.2004.02968.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen S. Sequence of mylenization in the brain of the albino rat. A. Cerebral cortex, thalamus and related structures. Journal of Comparative Neurology. 1963;121:5–29. doi: 10.1002/cne.901210103. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ. Rat model of perinatal hypoxic-ischemic brain damage. Journal of Neuroscience Research. 1999;55:158–163. doi: 10.1002/(SICI)1097-4547(19990115)55:2<158::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE. Early social and cognitive precursors and parental support for self-regulation and executive function: Relations from early childhood into adolecence. In press.
- Landry SH, Smith KE. Early social and cognitive precursors and parental support for self-regulation and executive function: Relations from early childhood into adolecence. In press.
- Landry SH, Smith KE, Swank PR, Assel MA, Vellet S. Does early responsive parenting have a special importance for children's development or is consistency across early childhood necessary? Developmental Psychology. 2001;37:387–403. doi: 10.1037//0012-1649.37.3.387. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS Systems for Mixed Models. SAS Institute, Inc.; Cary: 1996. [Google Scholar]
- Lubchenco LO, Hansman C, Boyd E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics. 1966;37:403–8. [PubMed] [Google Scholar]
- Merritt TA, Pillers D, Prows SL. Early NICU discharge of very low birth weight infants: A critical review and analysis. Seminars in Neonataology. 2003;8:95–115. doi: 10.1016/S1084-2756(02)00219-1. [DOI] [PubMed] [Google Scholar]
- Mitchell F. Interobserver agreement, reliability, and generalizability data collected in observational studies. Psychological Bulletin. 1979;86:376–390. [Google Scholar]
- NICHD Early Child Care Research Network Predicting individual differences in attention, memory, and planning in first graders from experiences at home, child, care and school. Developmental Psychology. 2005;41:99–114. doi: 10.1037/0012-1649.41.1.99. [DOI] [PubMed] [Google Scholar]
- Qiu J, Hu X, Nesic O, Grafe MR, Rassin DK, Wood TG, et al. Effects of NF-kB Oligonucleotide “Decoys” on Gene Expression in P7 Rat Hippocampus after Hypoxia/Ischemia. Journal of Neuroscience Research. 2004;77:108–118. doi: 10.1002/jnr.20156. [DOI] [PubMed] [Google Scholar]
- Smith KE, Landry SH, Swank PR. The role of early maternal responsiveness in supporting school-aged cognitive development for children who vary in birth status. Pediatrics. 2006;117:1608–17. doi: 10.1542/peds.2005-1284. [DOI] [PubMed] [Google Scholar]
- Swaiman KF. Neurologic examination after the newborn period until 2 years of age. In: Swaiman KF, editor. Pediatric neurology principles and practice. Mosby; St. Louis: 1989. pp. 35–44. [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. Guide for administering and scoring the Stanford-Binet Intelligence Scale. 4th ed Riverside Publishing; Chicago: 1986. [Google Scholar]
- Tin W, Walker S, Lacamp C. Oxygen monitoring in preterm babies: too high, too low? Pediatric Respiratory Reviews. 2003;4:9–14. doi: 10.1016/s1526-0542(02)00307-x. [DOI] [PubMed] [Google Scholar]
- Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001;107:642–647. doi: 10.1542/peds.107.4.642. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Garcia-Coll CT. Neurodevelopmental and school performance of very low-birth-weight infants: A seven-year longitudinal study. Pediatrics. 1985;76:345–350. [PubMed] [Google Scholar]
- Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Soc. 1963;77:841–847. [Google Scholar]