Abstract
Glutamate-coded signaling in corticostriatal circuits has been shown to be important in various forms of learning and memory. In the present study, the authors found that N-methyl-D-aspartate (NMDA) receptor antagonism in the central nucleus of the amygdala (CeA) and the posterior lateral striatum (PLS) impaired instrumental conditioning but had no effect in the anterior dorsal striatum. NMDA receptor antagonism in the CeA and PLS also affected spontaneous motor behavior and certain aspects of feeding. The present findings extend knowledge of the dynamic neurophysiological processes, instantiated in a complex neural network, required for instrumental learning in the mammalian brain.
Instrumental conditioning involves the consequences of certain responses influencing the likelihood of those responses occurring again in the future, under similar conditions. In a typical arrangement, a hungry rat presses a lever that produces sucrose pellets; lever presses (LPs) generally increase over time or sessions. Many experiments suggest a crucial role for the glutamate receptor known by the artificial ligand that stimulates it, the N-methyl-D-aspartate (NMDA) receptor, in instrumental learning. For example, blockade of NMDA receptors within the nucleus accumbens core, basolateral amygdala (BLA), and medial prefrontal cortex impairs instrumental learning, but not performance of instrumental behavior, once learned (Baldwin, Holahan, Sadeghian, & Kelley, 2000; Kelley, Smith-Roe, & Holahan, 1997). Baldwin et al. (2000) also showed that NMDA receptor inactivation in the dorsal or ventral subiculum does not impair this form of learning or performance, thereby suggesting that the neurophysiological events that accompany initial instrumental learning are distributed across a distinct network of physiological structures. The nucleus accumbens, part of the ventral striatal region of the basal ganglia, is known for its role in mediating the reinforcing effects of drugs of abuse (Kelley & Berridge, 2002; Koob & Le Moal, 2001; Volkow et al., 1999; Wise & Bozarth, 1987) and is thought to be involved in behaviors influenced by natural reinforcers such as food, drink, sex, exploration, and instrumental learning (Berridge & Robinson, 1998; Kelley et al., 2002; Robinson & Berridge, 2000; Salmon & Butters, 1995; White, 1997; Zhang & Kelley, 2000). NMDA receptors have received a great deal of attention with respect to learning because their activation is thought to underlie neural plasticity and long-term potentiation (Abel & Lattal, 2001). The role of NMDA receptors in other cortical, striatal, and limbic structures on instrumental learning, however, has yet to be investigated.
Interest in the role of the central nucleus of the amygdala (CeA) in learning has been sparked by studies showing a critical role for the CeA in emotional processing; aversive, or “fear,” conditioning; and attentional processes (Davis, 1992; Gallagher, Graham, & Holland, 1990; Gallagher & Holland, 1994; Holland & Gallagher, 1999; Holland, Han, & Gallagher, 2000; LeDoux, 1992). de Olmos and Heimer (1999) have argued that the CeA is part of a larger continuous structure in the basal forebrain referred to as the “extended amygdala,” whereas Cassell, Freedman, and Shi (1999) have argued that the CeA and the nucleus accumbens should be viewed as continuous, given that there is little change in architecture between the two structures. Swanson and Petrovich (Swanson & Petrovich, 1998) have suggested that the CeA is striatal in nature. Therefore, because of this strong connectivity with other regions of the ventral forebrain, the CeA likely plays an important role in the motivational and cognitive functions of the ventral forebrain (de Olmos, Alheid, & Beltramino, 1985; de Olmos & Heimer, 1999), and perhaps a role in instrumental learning as well.
The suggestion by Swanson and Petrovich (1998) that the CeA is striatal in nature raises important questions about the role of the striatum in instrumental learning per se. Typically, the striatum is thought to include the caudate nucleus, putamen, globus pallidus, nucleus accumbens, and olfactory tubercle. With retrograde and anterograde tracing methods, Kelley, Domesick, and Nauta (1982) found strong connectivity between the BLA and striatum, and McGeorge and Faull (1989) demonstrated connectivity between the cerebral cortex and striatum. Behaviorally, lesions of subregions of the striatum disrupt dissociable aspects of behavior (Brasted, Humby, Dunnett, & Robbins, 1997; Brown & Robbins, 1989a, 1989b, 1991). It appears that the dorsolateral striatum involves the selection of responses, whereas the dorsomedial striatum is involved in inhibitory control over responding. Therefore, the CeA and several dissociable subregions of the striatum may be involved in instrumental learning because of their strong interconnectivity with other ventral striatal regions and the BLA, regions already shown to be involved in learning and behavioral selection.
Using a cannula-mapping approach, we attempted here to determine the role of NMDA receptor activation in several sites throughout the basal ganglia and limbic system, specifically the CeA and two sites within the dorsal striatum. Little research has been conducted on the role of NMDA receptors in the dorsal striatum, an area some have identified as important for “stimulus–response or habit learning” (Mishkin, quoted in White, 1997, p. 165). However, the concept of the dorsal striatum (presumably the nonventral area of the striatum) encompasses a large and probably nonhomogeneous area, and references to the “dorsal striatum” are often ambiguous, at best. Another goal of the current research was to explore the microstructure of instrumental behavior over the course of learning, to possibly dissociate the role of the different sites in the complex pattern of behavior that emerges during instrumental learning. Lastly, when deficits were found in conditioning, we conducted control experiments to investigate spontaneous feeding and motor behavior patterns.
Method
Subjects
Male Sprague–Dawley rats (Harlan, Madison, WI) were housed in pairs in polyethylene cages in colony room with a 12:12-hr light–dark cycle. They were approximately 90 days old at the start of experimentation and weighed approximately 300 g each. They were weighed and handled daily and provided with food and water ad libitum before surgery. After recovery from surgery, each rat was reduced to 85% of its ad-lib (presurgical) weight. During food restriction, and before the start of testing, rats were given approximately 3 g of sucrose pellets in their home cages per day; the 85% weight was maintained for the remainder of the experiment. Care of the rats was in accordance with University of Wisconsin—Madison animal care committee guidelines.
Apparatus
Operant chambers
Operant learning sessions were conducted in eight identical commercially constructed operant chambers (Coulbourn Instruments, Allentown, PA) enclosed in sound-attenuating, ventilated chests. Fans provided some masking noise continuously throughout the session. Two retractable levers, approximately 6 cm apart, could be projected into the chamber through the right-side wall. Spaced equally between the two levers was a feeder trough, into which 45-mg Bio-Serv (Frenchtown, NJ) sucrose pellets could be delivered. The feeder trough was equipped with a photo sensor, such that the number and timing of nose pokes into the tray could be recorded. Above the feeder trough were a row of three stimulus lights (red, yellow, and green) and a 28-V houselight. Experimental events were arranged and recorded with a PC located in the same room as the chambers, running Graphic State Notation (Version 1.013-00), interfaced with L91-04S Habitest Universal Lincs (both from Coulbourn Instruments, Allentown, PA).
Locomotion and feeding cages
Clear polycarbonate cages (24 cm wide × 45 cm long × 21 cm high) with a wire mesh floor and wire lid with a water bottle served as test cages. Two small ceramic dishes filled with sucrose pellets were affixed to the mesh floor with pliable adhesive (Poster Tac; Pacer Technology, Rancho Cucamonga, CA). Data were obtained by means of an event recorder connected to a PC. Measures included the amount of sucrose eaten, latency to eat, amount of time spent feeding, number of feeding bouts, number of center crossings, and number of rears.
Surgery
Rats were anesthetized with a ketamine–xylazine mixture (1.0 mg/kg) and placed in a standard stereotaxic surgery device (incisor bar at −5.0 mm; flat skull). Indwelling stainless cannulas (23 gauge) were implanted bilaterally and secured to the skull with stainless steel screws and dental cement. Cannulas were aimed 2.5 mm above the injection targets. Stainless steel stylets prevented occlusion of the cannulas. Coordinates (flat skull, in millimeters) were as follows: CeA, −2.0 AP from bregma, ±4.0 ML from midline, and −5.7 DV from the skull surface; posterior lateral striatum (PLS), −1.0 AP, ±4.5 ML, and −4.0 DV; and anterior dorsal striatum (ADS), +1.5 AP, ±2.8 ML, and −3.0 DV.
Drugs and Microinfusions
2-amino-5-phosphonopentanoic acid (AP-5) was dissolved in isotonic sterile saline. Doses of 1 μg AP-5 (5 nmol) or vehicle (saline) were administered through bilateral intracerebral microinfusions in a volume of 0.5 μl per side. After the stylets were removed, injectors (30 gauge) were inserted 2.5 mm below the tips of the guide cannulas to the site of the infusion (−8.2 mm DV for CeA, −6.5 mm DV for PLS, and −5.5 mm DV for ADS). A Harvard Apparatus (South Natick, MA) pump, set at a rate of 0.32 μl/min, infused drug or vehicle for 1 min 33 s, followed by 1 min of diffusion time. The injectors were removed and the stylets replaced. After microinfusions, rats were immediately placed in the operant chambers or locomotor and feeding cages.
Experimental Design and Behavioral Testing
Operant learning
All sessions lasted 15 min. All rats were habituated to the chamber for three sessions on 3 consecutive days before testing. Before the first 2 habituation sessions, rats were given a mock infusion, in which an injector was lowered to the end of the cannula, but not into brain tissue, and the microdrive pump was turned on, but no drug was infused. Rats were then immediately placed into the chambers, with both levers retracted, the houselight turned on, and sucrose pellets in the food trough. The number and timing of nose pokes into the tray were recorded. Before the third session, rats were given a vehicle (saline) infusion as specified in the previous section. Again, they were placed in the chambers immediately after the infusion, with both levers retracted, the houselight on, and pellets in the trough. Subjects were matched on the basis of the frequency of nose poking and randomly assigned to one of two groups (drug or vehicle). The experiment proceeded in three phases. Before the next five sessions (Phase 1), rats were infused with drug or vehicle, depending on group assignment, and placed immediately into the operant chamber. Both levers were projected into the chamber, with one lever randomly designated as the correct lever and the other as the incorrect lever; lever assignments remained the same for each rat for the entirety of the experiment. Sucrose pellets were taped to the correct lever for the first two sessions. Lever presses on the correct lever were reinforced by turning off the houselight, turning on the red stimulus light for 1 s, and delivering one pellet. After 20 reinforcers were earned in any one session, the contingencies changed to a random ratio 2 (RR-2); each lever press was reinforced with a probability of 0.5. The behavioral contingencies remained the same regardless of infusion type throughout the entirety of the experiment. For the next five or seven sessions (Phase 2), no infusions were given, but before the next session, an infusion was given to test for performance effects. If differences in responding were seen during that session, a test for recovery with no infusion was run on the following day (Phase 3).
For Experiment 1, two groups of rats with bilateral indwelling cannulas aimed at the CeA received infusions of AP-5 (n = 8) or vehicle (n = 6). For Experiment 2, AP-5 (n = 8) or vehicle (n = 8) was infused into the PLS, and for Experiment 3, AP-5 (n = 7) or vehicle (n = 6) was infused into the ADS.
Locomotion and feeding
Locomotion and feeding control experiments were conducted to investigate the unconditional effects of drug infusions, if significant learning impairments were found in operant learning experiments. In a within-subjects design, rats were infused with either drug of vehicle before being placed in the locomotor and feeding cages. Surgery, handling, and deprivation conditions were identical to those of the operant learning experiments. Each rat was given an infusion of AP-5 and an infusion of vehicle on consecutive days, in a randomized order, by an experimenter who was blind to rats’ group assignments. As noted earlier, rats were placed immediately into the locomotion and feeding cages after infusions; latency to eat, amount of sucrose eaten, amount of time spent feeding, number of feeding bouts, number of center crossings, number of rears, number of drinking bouts, and length of drinking were recorded. For both infusion sites, 7 rats were used. Because we did not find any effects of AP-5 in the ADS on instrumental learning or nose poking, we did not conduct an experiment on the effect of AP-5 in the ADS on locomotion and feeding.
Histological Analysis
After the completion of the experiment, all rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with isotonic saline followed by 10% (wt/vol) formalin. Brains were stored in a 10% (wt/vol) sucrose–10% formalin mixture before being cut in 60-μm sections, which were then stained with Cresyl Violet and examined under a light microscope for location of infusion sites. Fifty-seven rats were found to have adequate placements in the targeted sites.
Statistical Analysis
Lever presses were first analyzed by multivariate analysis of variance (ANOVA), with treatment as the between-subjects variable and sessions and lever type (correct vs. incorrect) as within-subjects variables. Nose pokes were analyzed, with treatment as the between-subjects variable and sessions as the within-subjects variable. Lever presses and nose pokes were analyzed in three phases: Sessions 1–5 (Phase 1: infusion sessions); Sessions 6–10 or 6–12 (Phase 2: no infusion sessions); and performance test–recovery Sessions 11–12, 13–14, or 11 (Phase 3: infusion–no infusion). The lever-type variable was included in the design to control for the possible effects of infusions on motor behavior and displacement activity. Because there were very few incorrect lever presses across the entirety of the experiment, this variable was eliminated from subsequent analyses, yielding drug as a between-subjects variable and sessions as a within-subjects variable. Handling the data this way makes the results more easily interpretable; inclusion of lever type as a variable does not materially change any of the conclusions of the present results. Data from the locomotion and feeding control experiments were analyzed with one-way ANOVA or dependent-samples t tests, where appropriate.
Analysis of the Microstructure of Behavior
Statistical analyses were supplemented by detailed microstructural behavior analysis of operant learning experiments by exporting raw data files from the Coulbourn system into Microsoft Excel. Correct lever presses (LPs), incorrect lever presses (iLPs), nose pokes (NPs), and reinforcers (earned reinforcer [eRs]) that occurred during each rat’s session were time-stamped by Graphic State Notation. The order of events and their temporal relation were analyzed by counting all the dyads of events that occurred. For example, an NP could be followed by an LP, an iLP, an eR, or another NP, yielding four types of dyads (NP–LP, NP–iLP, NP–eR, or NP–NP). These dyads were used to compute conditional probabilities: For example, the probability of an LP given that an NP had just occurred was the number of NP–LP dyads divided by the total number of NPs, or NP–LP + NP–iLP + NP–eR + NP–NP. These conditional probabilities were averaged across rats per session and differentiated by group assignment. Interevent times and latencies—for example, the time between an eR–NP dyad—were also computed and averaged across rats per session by group. The amount of time that passed between consecutive LPs was termed an interresponse time, and the time between an eR and an NP was termed latency to remain consistent with general conventions in the literature. The results of the microstructural analysis yielded 66 separate measures across sessions; however, only a few of the most interesting behavioral patterns are presented.
Results
Experiment 1: Effects of NMDA Receptor Antagonism in CeA
Histology
Cannula placements for all rats in Experiment 1 are shown in Figure 1. Examples of Cresyl Violet-stained sections are also shown. Although several placements were located in the ventral–lateral portion of the CeA, all were clearly in the CeA.
Figure 1.
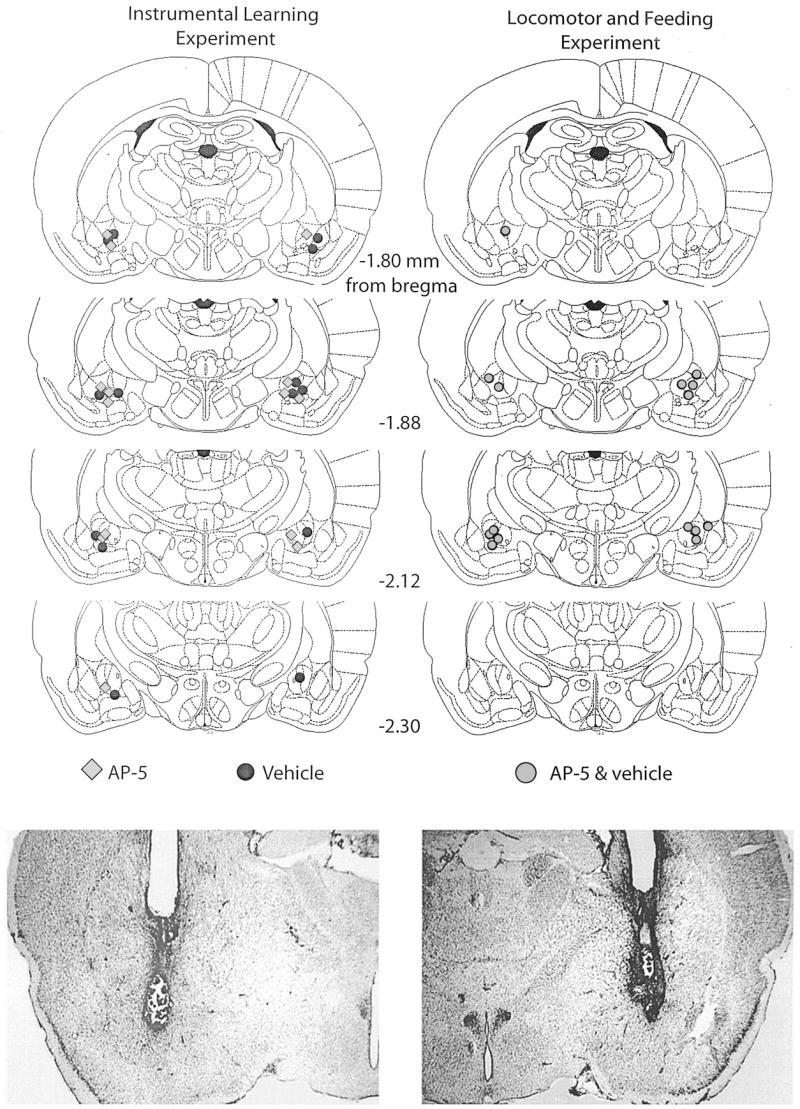
Top: Histological reconstructions of cannula placements in the central nucleus of the amygdala (Experiment 1) are represented in schematic form. Histological sections were examined under a light microscope, and the site of the infusion was estimated. AP-5, n = 8; vehicle, n = 6; AP-5 + vehicle, n = 7. AP-5 = 2-amino-5 phosphonopentanoic acid. Reprinted from The Rat Brain in Stereotaxic Coordinates, 4th ed., G. Paxinos and C. Watson, Figures 26–29, Copyright 1998, with permission from Elsevier. Bottom: Photomicrograph examples of sites represented in the schematic diagrams.
Instrumental learning
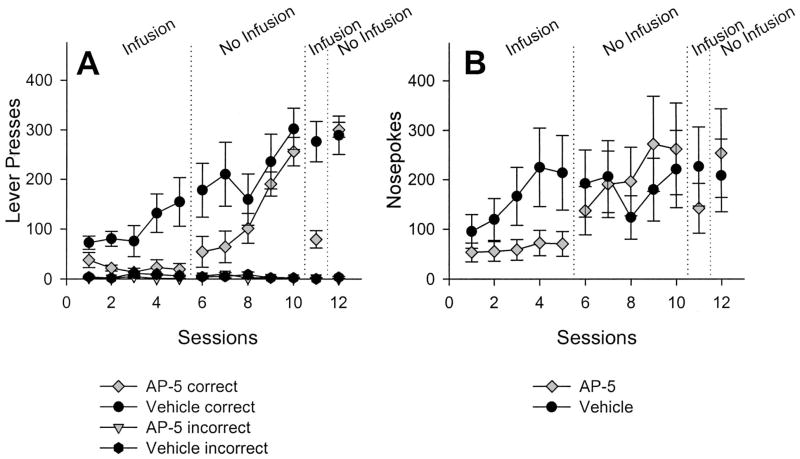
Figure 2A shows that AP-5 infusions into the CeA affected instrumental conditioning. For vehicle-treated rats, the mean number of LPs increased during the first five sessions (Phase 1). In contrast, the number of LPs in AP-5-treated rats decreased over the same sessions. Before Sessions 6–10 (Phase 2), no infusions were given, and the average number of LPs for previously drug-treated rats quickly increased, reaching roughly the same level as that of vehicle-treated rats. Infusions of AP-5 given before Session 11 decreased lever pressing, with a return to previous levels of lever pressing during Session 12 (Phase 3). The results of the statistical analyses performed on lever presses during the three phases on Experiment 1 are shown in Table 1.
Figure 2.
Mean (± SEM) number of correct and incorrect lever presses (A) and nose pokes (B) over sessions after 2-amino-5 phosphonopentanoic acid (AP-5) infusion (n = 8) or vehicle infusion (n = 6) into the central nucleus of the amygdala. See the Results section for details on the statistical analyses.
Table 1.
Results of Statistical Analyses for Experiment 1 (AP-5 in CeA)
| Source of variation | df | F | p |
|---|---|---|---|
| Phase 1: Sessions 1–5 | |||
| Drug | 1, 12 | 9.898 | .008 |
| Session | 4, 48 | 1.935 | .120 |
| Drug × Session | 4, 48 | 2.557 | .050 |
|
| |||
| Phase 2: Sessions 6–10 | |||
| Drug | 1, 12 | 2.174 | .166 |
| Session | 4, 48 | 30.674 | < .001 |
| Drug × Session | 4, 48 | 3.613 | .012 |
|
| |||
| Phase 3: Sessions 11–12 | |||
| Drug | 1, 12 | 6.476 | .026 |
| Session | 1, 12 | 44.713 | < .001 |
| Drug × Session | 1, 12 | 35.137 | < .001 |
Note. AP-5 = 2-amino-5 phosphonopentanoic acid; CeA = central nucleus of the amygdala. Boldface indicates a significant effect of AP-5.
Table 1 confirms what can be seen in Figure 2A, namely, an effect of drug and Drug × Session interaction. Typically, a reliable effect of sessions would be expected; however, this was not seen, possibly because of the small sample size in the vehicle-infused group and/or high variability within this group. During Phase 2, no main effect of drug was seen, but a reliable effect of sessions and Drug × Session interaction was noted. These statistical analyses indicate that lever pressing increased across sessions for both groups, but more rapidly for the drug-infused group than the vehicle-infused group. The performance test and recovery sessions (Sessions 11–12) were also analyzed with factorial ANOVA. The statistically significant Drug × Session interaction indicates an effect of infusions on lever pressing.
The number of nose pokes into the food trough is shown in Figure 2B. Analyses of nose pokes from Sessions 1–5 revealed a main effect of drug, F(1, 13) = 6.93, p = .02, and sessions, F(4, 52) = 4.46, p < .01, but no interaction. Analyses on nose poking from Sessions 6–10 found a significant effect of sessions, F(4, 44) = 7.10, p < .01; the effect of previous treatment, F(1, 12) = 1.30, p = .28, was not reliable. The Session × Previous Treatment interaction, F(4, 44) = 3.81, p = .05, was marginally reliable. Nose pokes during Session 11 approached statistical significance, t(12) = −2.15, p = .05, and were not statistically differentiated on Session 12, t(12) = 1.05, p = .31.
The facts that AP-5 infusions decreased lever pressing during Session 11 and that recovery appeared complete during Session 12 raised additional questions about the nature of infusions and warranted further experimentation. In other words, it is possible that AP-5 infusions decreased lever pressing because (a) the rats were sensitized to the drug in some way; (b) the presence of AP-5 cued the absence of sucrose through a discrimination learning process; or (c) there was an unconditional effect of the drug, irrespective of previous conditioning. To test these hypotheses, we split the drug and vehicle groups into two groups matched on the basis of lever pressing during Session 12. Before 13th session, 4 of the previously drug-treated rats and 3 of the previously vehicle-treated rats received an AP-5 infusion, and the remaining 4 previously drug-treated and 3 previously vehicle-treated rats received a vehicle infusion, yielding a 2 × 2 design, with previous drug history as one variable and current drug state as the second variable. The results of AP-5 infusions on lever pressing during Session 13 are presented in Figure 3, and the results of the statistical analysis are presented in Table 2.
Figure 3.
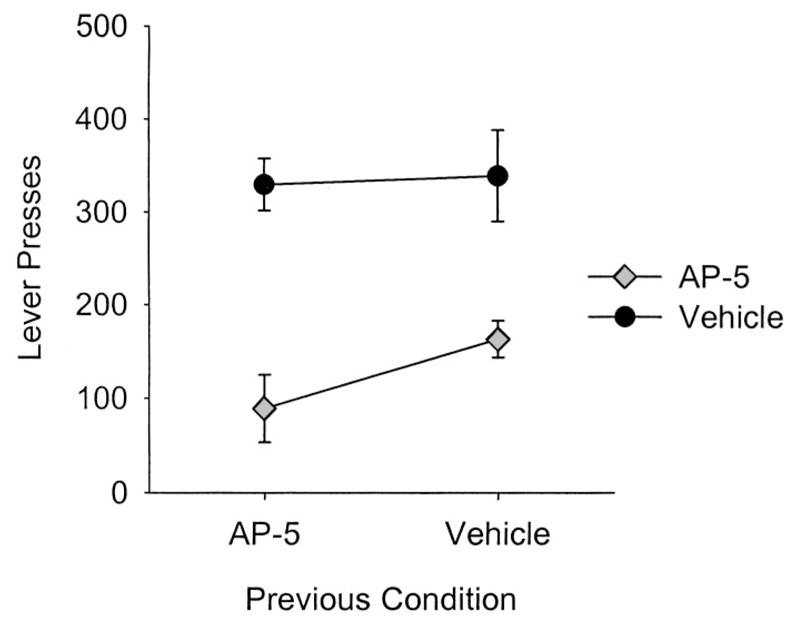
Mean (± SEM) number of lever presses during Session 13 after 2-amino-5 phosphonopentanoic acid (AP-5) or vehicle infusions into the central nucleus of the amygdala. Previously AP-5-treated rats (n = 8) and previously vehicle-treated rats (n = 6) were divided into two groups (n = 7 per group). One group received an AP-5 infusion before Session 13; the remaining group received a vehicle infusion. AP-5 infusions decreased the number of lever presses regardless of previous drug history.
Table 2.
Results of Statistical Analyses for Experiment 1, Session 13 (Phase 4; AP-5 in CeA)
| Source of variation | df | F | p |
|---|---|---|---|
| Previous drug history | 1, 10 | 1.440 | .258 |
| Current drug state | 1, 10 | 35.830 | < .001 |
| Previous × Current | 1, 10 | 0.859 | .376 |
Note. AP-5 = 2-amino-5 phosphonopentanoic acid; CeA = central nucleus of the amygdala. Boldface indicates a significant effect of AP-5.
As can be seen in Figure 3 and Table 2, the effect on AP-5 infusions was an unconditional one; AP-5 infusions reduced lever pressing regardless of drug history, thereby ruling out the hypotheses that sensitization or drug-discrimination learning processes influenced the current results.
Microstructural analyses
The results of three of the microstructural behavior analyses are presented in Figure 4. Figure 4A shows the probability of a nose poke given a nose poke [Pr(NP|NP)]. In vehicle-infused rats, this probability systematically decreased across sessions, whereas it remained near 0.90 for drug-infused rats until the infusions ceased. One of the measures complementary to Pr(NP|NP) is shown in Figure 4B: the probability of a correct lever press given a nose poke [Pr(LP|NP)]. Panel 4B parallels the learning curve shown in Figure 2A and shows the interdependency of various measures of behavior in the instrumental learning paradigm. Figure 4C shows the probability of a nose poke given a reinforcer [Pr(NP|eR)]. In both drug-treated and vehicle-treated rats, the Pr(NP|eR) increased over sessions to nearly 0.80. This may represent the associative strength of the events surrounding feeder operation (e.g., the magazine light, the red stimulus light, the sound of the feeder) and the sucrose pellets (a stimulus–outcome relation). In other words, Figure 4C shows that when a reinforcer was delivered, there was a high likelihood that the next measured event for all rats was a nose poke, regardless of treatment. The decrease in subsequent sessions is likely given the fact the contingencies changed to a RR-2 (from fixed ratio 1 after 20 lever presses); it becomes less advantageous to nose poke after every lever press, and more advantageous to lever press with increasing rapidity because of the positive feedback function of the RR-2 schedule (more presses = more reinforcers). Because vehicle-treated rats encountered the RR-2 earlier in the experiment than the drug-treated rats, the Pr(NP|eR) should decrease because the Pr(LP|eR) was increasing (which was the case, but not shown).
Figure 4.
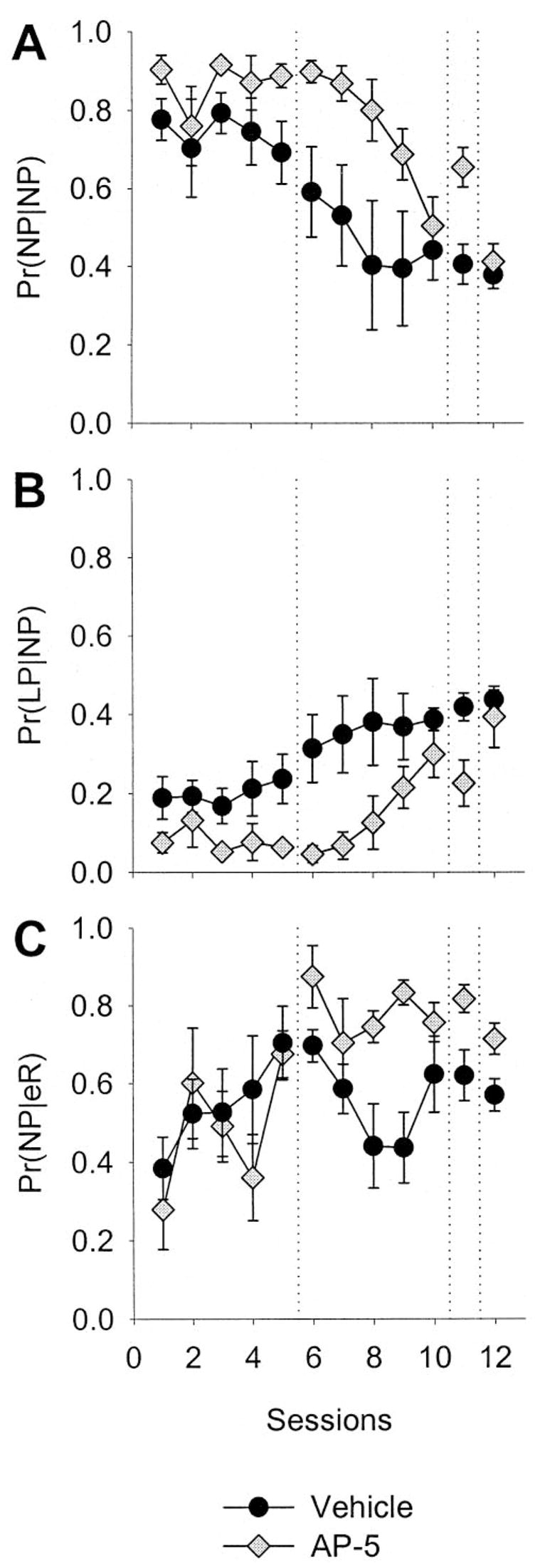
Microstructural behavioral analysis of N-methyl-D-aspartate antagonism in the central nucleus of the amygdala. Data are means (± SEM). A: Probability of a nose poke given a nose poke [Pr(NP|NP)], averaged across rats in each group over sessions. B: Probability of a lever press given a nose poke [Pr(LP|NP)]. C: Conditional probability of a nose poke given a reinforcer as the last recorded event [Pr(NP|eR)]. AP-5 = 2-amino-5 phosphonopentanoic acid.
Locomotion and feeding
The effects of AP-5 infusions into the CeA on measures of locomotion and feeding are presented in Figure 5.
Figure 5.
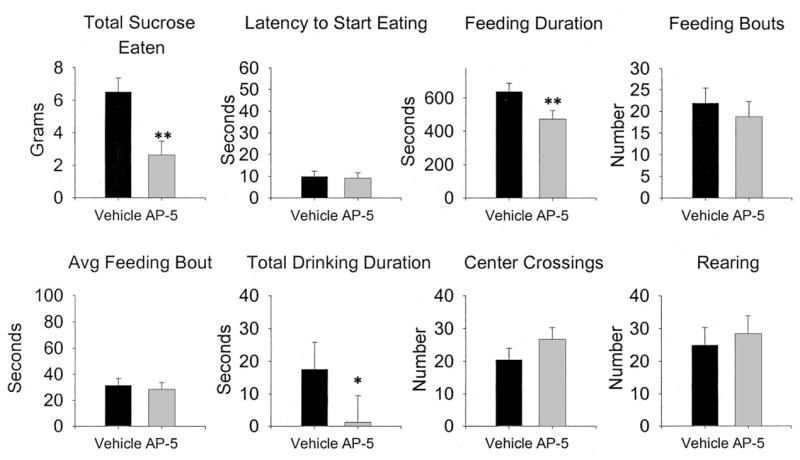
Effects of 2-amino-5 phosphonopentanoic acid (AP-5) infusions in the central nucleus of the amygdala on spontaneous feeding and locomotor behavior. Error bars are the standard error of the difference, which is the appropriate statistic for representing variability in a within-subjects experiment. n = 7. Avg = average. * p < .05, ** p < .01.
AP-5 infusions into the CeA affected feeding behavior of food-deprived rats in several ways. As can be seen, total sucrose intake (grams in 15 min), and total eating duration were reduced by AP-5 infusions. However, latency to begin eating and the number of feeding bouts were not affected, although differences in the average length of a feeding bout approached significance ( p = .076). Measures of motor behavior, although not statistically reliable, seem to indicate more activity following infusions of AP-5, especially the number of center crossings.
Experiment 2: Effects of NMDA Receptor Antagonism in the PLS
Histology
In Experiment 2, cannula placements (see Figure 6) were generally in the lateral portion, near the external capsule, of the posterior striatum.
Figure 6.
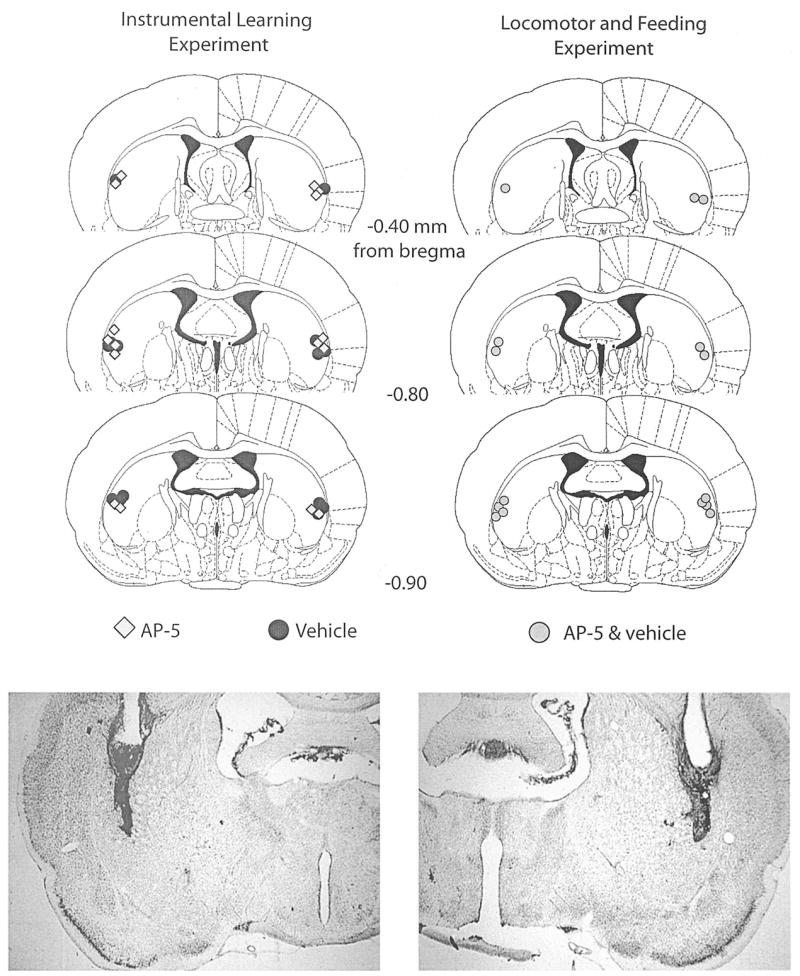
Top: Histological reconstructions of cannula placements in the posterior lateral striatum (Experiment 2) are represented in schematic form. AP-5, n = 8; vehicle, n = 7; AP-5 + vehicle, n = 7. AP-5 = 2-amino-5 phosphonopentanoic acid. Reprinted from The Rat Brain in Stereotaxic Coordinates, 4th ed., G. Paxinos and C. Watson, Figures 20–22, Copyright 1998, with permission from Elsevier. Bottom: Photomicrograph examples of sites represented in the schematic diagrams.
Instrumental learning
The effects of AP-5 infusions into the PLS on instrumental conditioning are presented in Figure 7, and the results of the accompanying statistical analyses are presented in Table 3.
Figure 7.
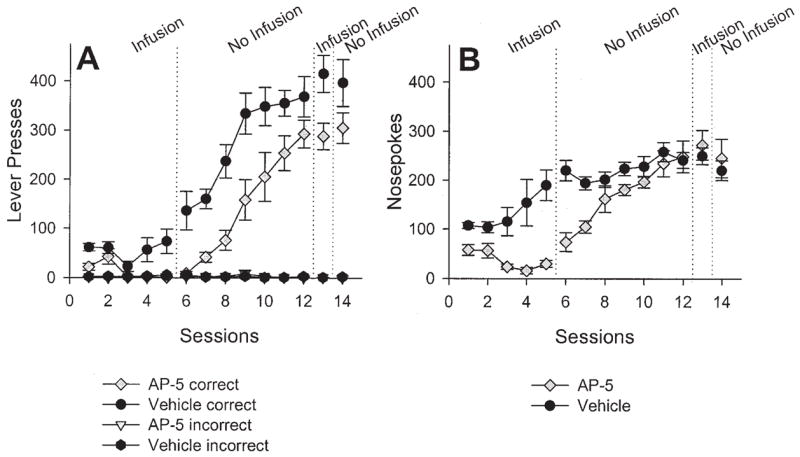
Mean (± SEM) number of correct and incorrect lever presses (A) and nose pokes (B) over sessions after 2-amino-5 phosphonopentanoic acid (AP-5) infusion (n = 8) or vehicle infusion (n = 7) into the posterior lateral striatum. See the Results section for results of the statistical analyses.
Table 3.
Results of Statistical Analyses for Experiment 2 (AP-5 in PLS)
| Source of variation | df | F | p |
|---|---|---|---|
| Phase 1: Sessions 1–5 | |||
| Drug | 1, 13 | 12.858 | .003 |
| Session | 4, 52 | 3.490 | .013 |
| Drug × Session | 4, 52 | 2.598 | .047 |
|
| |||
| Phase 2: Sessions 6–12 | |||
| Drug | 1, 13 | 10.605 | .006 |
| Session | 6, 78 | 41.636 | < .001 |
| Drug × Session | 6, 78 | 0.876 | .516 |
|
| |||
| Phase 3: Sessions 13–14 | |||
| Drug | 1, 13 | 7.598 | .016 |
| Session | 1, 13 | 0.014 | .907 |
| Drug × Session | 1, 13 | 0.353 | .563 |
Note. AP-5 = 2-amino-5 phosphonopentanoic acid; PLS = posterior lateral striatum. Boldface indicates a significant effect of AP-5.
Table 3 indicates reliable effects of drug, session, and a Drug × Session interaction. During Sessions 6 –12, the number of lever presses increased systematically for both groups, although the previously AP-5-treated group appeared to lag behind, which explains the main effect of drug. However, the fact that the Drug × Session interaction was not reliable suggests that the increase in lever pressing across sessions was roughly parallel. Before Session 13, rats received AP-5 or vehicle infusions to test effects on performance; no infusions were given before Session 14. From Figure 7A, it appears that the infusions had little effect on lever pressing; this was tested by analyzing Session 13–14 with a factorial ANOVA. A main effect of prior treatment was found; however, there was no effect of sessions or an interaction. The lack of effect of sessions, combined with the effect of prior treatment, indicates that the infusion of AP-5 before Session 13 had no effect on ongoing performance. The difference in the mean number of lever presses was a residual effect of previous drug treatment (i.e., Sessions 1–5), as seen in Figure 7A.
Figure 7B shows the average number of nose pokes per session across sessions. An ANOVA on nose pokes during the first five sessions revealed a significant main effect of treatment, F(1, 14) = 23.70, p <.001, and a significant Treatment × Session interaction, F(4, 56) = 3.81, p =.008. An ANOVA on nose pokes during Sessions 6–12 revealed main effects of prior treatment, F(1, 14) = 11.83, p =.004, and a Treatment × Session interaction, F(6, 84) = 3.98, p =.002. An ANOVA on nose pokes during Sessions 13–14 revealed no effect of treatment, sessions, or interaction (Fs < 1.2).
Microstructural analyses
As in Experiment 1, the results of three of the microstructural behavior analyses are shown in Figure 8, and are nearly identical to the results of Experiment 1, except that the Pr(NP|eR) decreases to zero.
Figure 8.
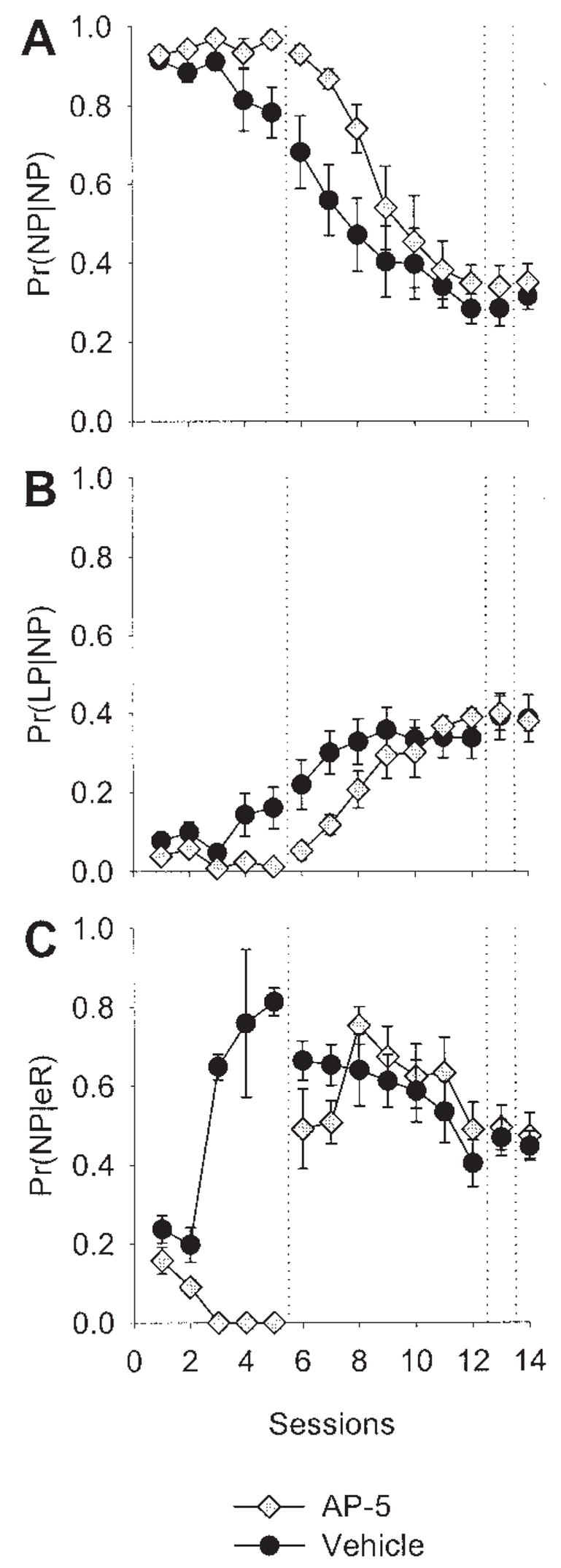
Microstructural behavioral analysis of N-methyl-D-aspartate antagonism in the posterior lateral striatum. Data are means (± SEM). A: Probability of a nose poke given a nose poke [Pr(NP|NP)], averaged across rats in each group over sessions. B: Probability of a lever press given a nose poke [Pr(LP|NP)]. C: Probability of a nose poke given an earned reward [Pr(NP|eR)]. AP-5 = 2-amino-5 phosphonopentanoic acid.
In Figure 7B, nose pokes in the drug-treated group decreased over the first five sessions, as well as the Pr(NP|eR), as seen in Figure 8C. Although the two measures are related, if a rat did not earn at least five reinforcers in a session, its Pr(NP|eR) was not used in calculating the group mean (which is displayed in Figure 8). Thus, the decrease in Pr(NP|eR) to zero is not because the drug-treated group’s average rate of nose poking decreased, but appears to reflect some impairment on learning the contingency between the events surrounding the feeder’s operation (sound, light, etc.) and the delivery of a pellet (e.g., a stimulus–stimulus contingency), for the rats in this group that did earn reinforcers. However, the Pr(NP|eR) in drug-treated rats increased to levels near those of vehicle-treated rats after infusions were discontinued, suggesting a performance, rather than learning, effect. Note also, the decrease in the Pr(NP|eR) for both groups in Session 6 –12, which is possibly due to the fact that the RR-2 engenders high rates of responding, with increased runs of lever presses.
Locomotion and feeding
The effects of AP-5 infusion into the PLS on measures of locomotion and feeding are shown in Figure 9.
Figure 9.
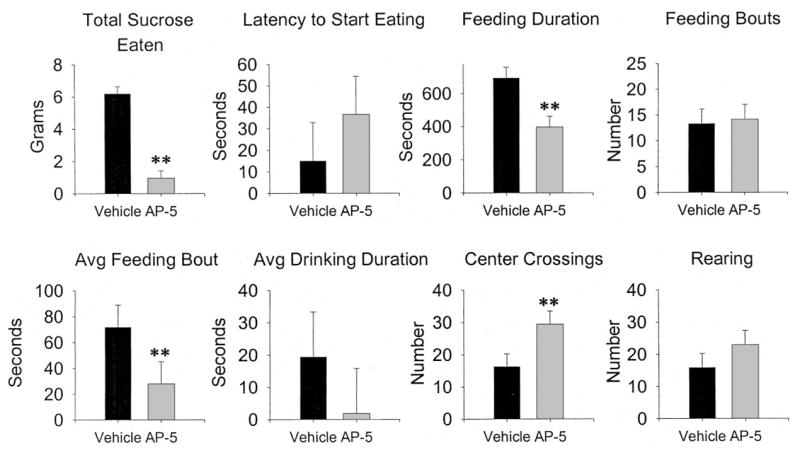
Effects of 2-amino-5 phosphonopentanoic acid (AP-5) infusions in the posterior lateral striatum on spontaneous feeding and locomotor behavior. Error bars are the standard error of the difference, which is the appropriate statistic for representing variability in a within-subjects design. n = 8. Avg = average. ** p < .01, significantly different from vehicle.
AP-5 infusions markedly decreased the amount of sucrose eaten, feeding duration, average feeding bout, and increased center crossings, but had no effect on latency to start eating or number of feeding bouts, a pattern similar to that found with AP-5 infusions into the CeA.
Experiment 3: Effects of NMDA Receptor Antagonism in the ADS
Histology
Cannula placements are shown in Figure 10. All placements were in the anterior and dorsal portion of the striatum.
Figure 10.
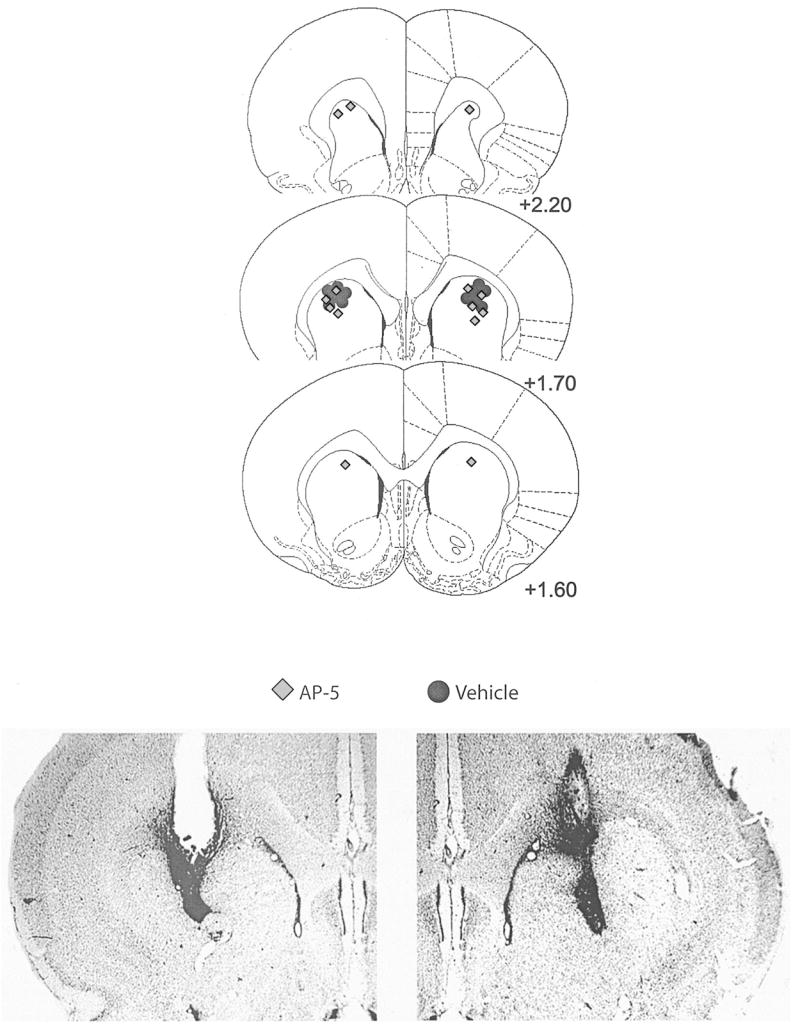
Top: Histological reconstructions of cannula placements in the anterior dorsal striatum (Experiment 3, instrumental learning). AP-5, n = 7; vehicle, n = 6. AP-5 = 2-amino-5 phosphonopentanoic acid. Numbers to the right indicate distance (in millimeters) from bregma. Reprinted from The Rat Brain in Stereotaxic Coordinates, 4th ed., G. Paxinos and C. Watson, Figures 10–12, Copyright 1998, with permission from Elsevier. Bottom: Photomicrograph examples of sites represented in the schematic diagrams.
Instrumental learning
The effects of AP-5 infusions into the ADS on instrumental conditioning are presented in Figure 11A. Separate statistical analyses on lever presses on Sessions 1–5, 6–10, and 11 revealed no reliable effects of treatment and no Treatment × Sessions interaction, and are shown in Table 4. The statistically reliable effect of sessions during Phases 1 and 2 reflects learning that is typical in situations with the contingencies used in the present experiments, without drug manipulations.
Figure 11.
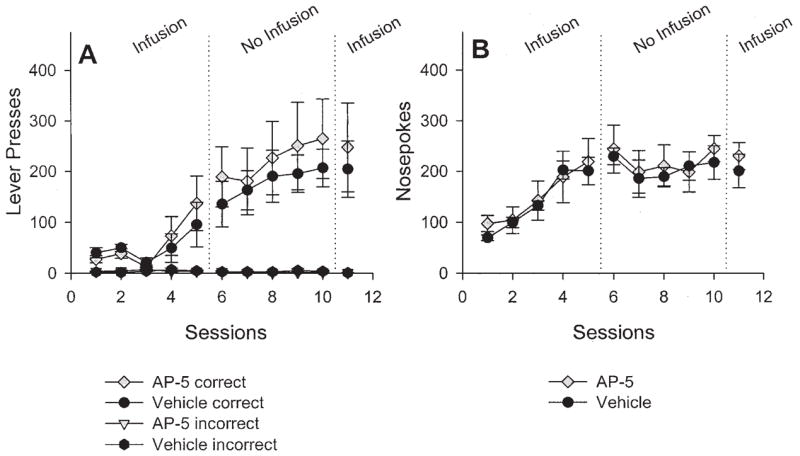
Mean (± SEM) number of correct and incorrect lever presses (A) and nose pokes (B) over sessions after 2-amino-5 phosphonopentanoic acid (AP-5) infusion (n = 7) or vehicle infusion (n = 6) into the anterior dorsal striatum. See the Results section for details on the statistical analyses.
Table 4.
Results of Statistical Analyses for Experiment 3 (AP-5 in ADS)
| Source of variation | df | F | p |
|---|---|---|---|
| Phase 1: Sessions 1–5 | |||
| Drug | 1, 11 | 0.058 | .814 |
| Session | 4, 44 | 5.569 | .001 |
| Drug × Session | 4, 44 | 0.590 | .672 |
|
| |||
| Phase 2: Sessions 6–10 | |||
| Drug | 1, 11 | 0.266 | .616 |
| Session | 4, 44 | 6.334 | < .001 |
| Drug × Session | 4, 44 | 0.446 | .775 |
|
| |||
| Phase 3: Session 11 | |||
| Drug | 1 | 0.398 | .699 |
Note. AP-5 = 2-amino-5 phosphonopentanoic acid; ADS = anterior dorsal striatum. Boldface indicates a significant effect of AP-5.
The effects of AP-5 infusions into the ADS on nose poking are presented in Figure 11B. Once again, separate statistical analyses on Sessions 1–5, 6–10, and 11 revealed no differences between groups and no interactions.
Microstructural analyses
Figure 12 shows the results of the same microstructural analyses conducted for the ADS data. In contrast to the results of Experiments 1 and 2, the panels of Figure 12 show a general overlap of measures for both vehicle-treated and drug-treated rats that is consistent with the results presented in Figure 11. Once again, a decrease in the Pr(NP|eR) over later sessions was observed (Panel C).
Figure 12.
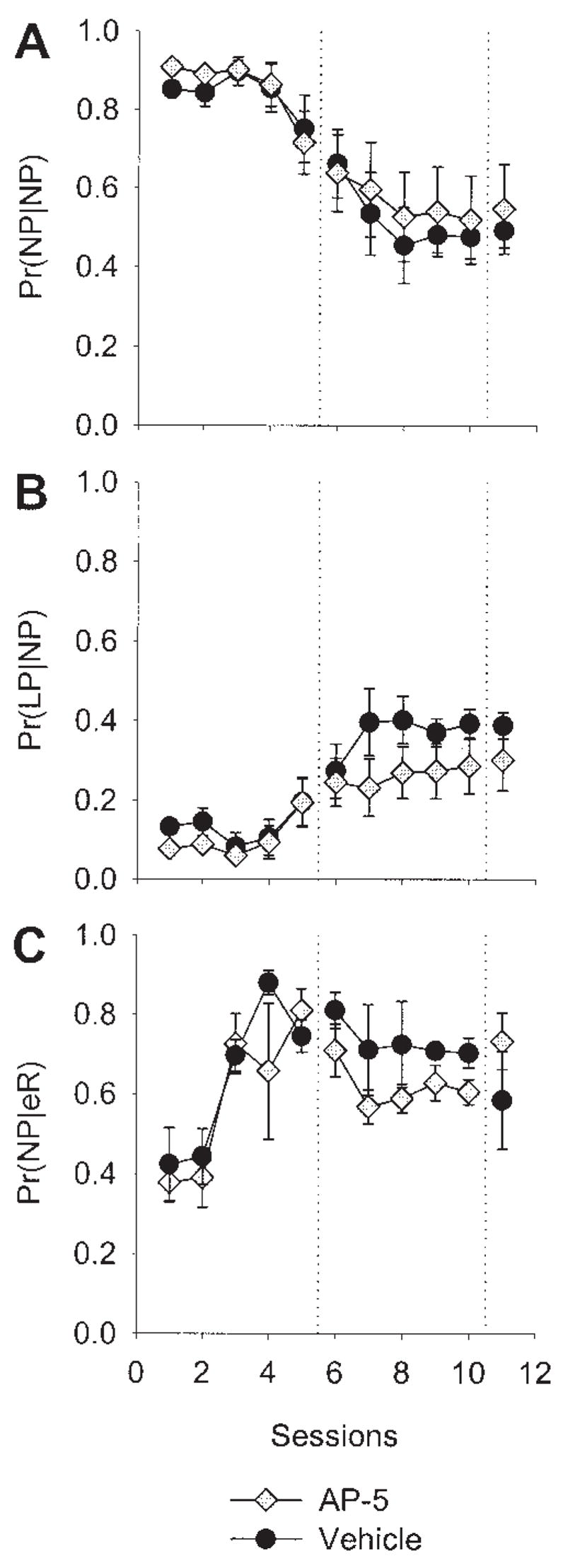
Microstructural behavioral analysis of N-methyl-D-aspartate antagonism in the anterior dorsal striatum. Data are means (± SEM). A: Probability of a nose poke given a nose poke [Pr(NP|NP)], averaged across rats in each group over sessions. B: Probability of a lever press given a nose poke [Pr(LP|NP)]. C: Probability of a nose poke given an earned reward [Pr(NP|eR)]. AP-5 = 2-amino-5 phosphonopentanoic acid.
Discussion
Interdependency of Instrumental and Pavlovian Contingencies
The instrumental conditioning preparation used here entails a response–stimulus–reinforcer contingency (lever press → red light → sucrose pellet). However, several other learned and unlearned relations could have affected the potency of this contingency. As some have suggested (Colwill & Rescorla, 1988; Mowrer, 1960; Rescorla, 1990, 1994; Rescorla & Solomon, 1967), sucrose pellets are not only the putative reinforcing stimulus in the instrumental case, but are also an unconditional stimulus (US) that elicits food-directed responses like salivation, arousal, approach, or the stereotyped feeding pattern seen in rats. In the case of Pavlovian responding, the sucrose pellets (USs) may also enter into stimulus–stimulus relations with the context, the lever, the proprioceptive feedback of lever pressing, or other discriminative stimuli, to name a few possibilities. Thus, the context, the lever, the proprioceptive feedback, and/or other features may become conditional stimuli (CSs) that elicit approach, salivation, or stereotyped feeding, or enhance ongoing behavior (e.g., Pavlovian-to-instrumental transfer [PIT]). Experiments designed to assess the role of Pavlovian binary relations in instrumental learning situations, however, have failed to demonstrate a complete reducibility of the instrumental to the Pavlovian (Rescorla, 1994). In addition, nose poking (i.e., conditioned approach), which is sometimes thought to reflect the value of the US, could also be the result of an instrumental process because nose pokes are occasionally reinforced with a sucrose pellet (nose poke → sucrose pellet; Rescorla, 1994). The nature of the instrumental contingency in the present experiments, with the illumination of the red stimulus light for 1 s followed by a pellet, also introduced the possibility that conditioned reinforcement played a role, although the red light was not conditioned before training, as would be the case in a traditional conditioned reinforcement preparation.
NMDA Receptor Antagonism in CeA
Infusions of AP-5 into the CeA impaired instrumental learning; however, infusions also affected performance of lever pressing after rats were conditioned (Figure 2, Session 11), affected several measures of food-directed behavior, and substantially reduced lever-pressing in drug-naive rats (before Session 13). Hence, NMDA receptor antagonism in the CeA during initial instrumental conditioning sessions appears to have altered one or more ancillary processes that in turn reduced or impaired lever-pressing, but not instrumental learning per se. One plausible explanation is that AP-5 affected the reinforcing value of sucrose pellets (the unconditional value) by rendering the rats less hungry, for example. As seen in Table 1, AP-5 infusions reduced total sucrose intake and total feeding duration, even though latency to begin eating and number of feeding bouts were not statistically differentiated. Basal rates of nose poking did not decrease during AP-5 infusions, a measure of the reinforcing value of sucrose pellets, even though there were between-group differences (Figure 2B) that were due primarily to an increase in nose poking in the vehicle-treated rats. Figure 4C shows that Pr(NP|eR) increased over the course of infusion sessions in both groups, although the data are somewhat variable in the drug-infused group. Thus, it appears that CeA AP-5 infusions do not impair a rat’s ability to approach the reinforcer after it was delivered (conditioned approach). Moreover, Pr(NP|NP) did not decrease during drug infusions (Figure 4A), in contrast to vehicle treatment, but when infusions were discontinued, the Pr(NP|NP) decreased, whereas Pr(LP|NP) increased. The Pr(NP|NP) increased once again after an infusion of AP-5, suggesting an unconditional effect of the infusion on food-directed behavior. Gallagher and Holland (1992, 1994) have demonstrated that rats with CeA lesions are not impaired in first-order or second-order conditioning, common tests for a stimulus’s motivational significance. There was no evidence of a general motoric impairment (e.g., less movement), but, in fact, there appeared to be a trend toward hyperactivity after AP-5 infusions, as evidenced by a greater number of center crossings and rears, although neither was statistically reliable. Together, these data suggest that AP-5 infusions did not reduce food-motivation because a process akin to first-order conditioned approach (nose poke) was unimpaired in the instrumental learning AP-5-infused group, and the latency to begin eating and total feeding bouts were not lessened with AP-5 infusions, even though several other food-directed responses were substantially affected. Although the role for conditioned reinforcement cannot be completely ruled out, it is unlikely to have been a significant factor given the brief time the red light was on (1 s) and the fact that albino rats are severely visually impaired (Prusky, Harker, Douglas, & Whishaw, 2002). In sum, it appears that AP-5 infusions in the CeA affected consummatory measures of feeding (e.g., total intake), but not appetitive measures (e.g., latency).
In apparent contrast to other research, Ahn and Phillips (2002) found that inactivation of the CeA with lidocaine increased overall food intake—a possible measure of consummatory behavior—but impaired normal anticipatory responses to food, increased latency to begin feeding, and produced inefficient eating—potential appetitive measures of feeding. We also observed inefficient eating in the locomotion and feeding portion of the first experiment (average amount of spillage: AP-5 treated, 13.29 g; vehicle-treated, 0.13 g); however, we recorded data only from the last 4 subjects on the last infusion day. However, several procedural variations between the present experiments and Ahn and Phillips (2002) cloud a clear comparison (e.g., 15-min feeding session vs. 50-min feeding session; microinfusion vs. reverse dialysis), and whereas we selectively inactivated NMDA receptors, Ahn and Phillips inactivated all neurons in the CeA, excitatory (e.g., glutamate) as well as inhibitory (e.g., GABA). Other studies support a role for the CeA in certain aspects of ingestive behavior. For example, the CeA receives massive input from cortical and subcortical taste areas (Cho, Li, & Smith, 2003), and inactivation of the CeA with muscimol disrupts nucleus accumbens–DAMGO-induced feeding (Will, Franzblau, & Kelley, 2004). These results suggest that infusions into the CeA unconditionally affected eating, an aspect of feeding behavior, itself a complex behavioral pattern. Nevertheless, it is clear that the CeA plays an important role in food-directed behavior, that NMDA receptors mediate some of those effects, and that these are important coincident activities to instrumental learning. It appears, though, that the effects of NMDA receptor antagonism in the CeA may not affect plasticity or learning per se, but are involved in instrumental learning through actions on food-directed behaviors.
Another possible explanation of the present results involves processes akin to PIT. A typical PIT procedure entails three phases. In Phase 1, a stimulus is conditioned (e.g., a light CS) to a US (e.g., sucrose pellets), and then in Phase 2, an instrumental response (e.g., lever press) is shaped by differential reinforcement with sucrose pellets. In the third, or test, phase, under conditions of extinction, the CS is presented in the presence of the operandum (e.g., lever) to which instrumental responding was shaped. In general, the presence of the CS increases rates of lever pressing and has been interpreted as evidence that CSs can modulate instrumental responding through motivational or discriminatory processes. However, rats with CeA lesions do not exhibit enhanced instrumental responding in the PIT preparation (Hall, Parkinson, Connor, Dickinson, & Everitt, 2001; Holland & Gallagher, 2003). Therefore, in the present experiments, the various contextual stimuli in the operant chamber may have become CSs that arouse and enhance instrumental responding, and AP-5 infusions may have impaired this process. The role of PIT on initial instrumental learning, in contrast to ongoing instrumental responding, remains an open question and warrants further research.
As the major output nucleus of the amygdala complex to the brainstem and hypothalamus, the convergence of information in the CeA or the generation of response output appears dependent on NMDA receptor activation (Pitkänen, Savander, & LeDoux, 1997). The peripheral nervous system, via the nucleus of the solitary tract and the parabrachial nuclei in the brainstem, project strongly to the CeA and send taste, gastrointestinal, and cardiovascular information (Alden, Besson, & Bernard, 1994; Bernard, Alden, & Besson, 1993; Norgren, 1976; Voshart & van der Kooy, 1981). The CeA also receives cortical and thalamic input (McDonald, Shammah-Lagnado, Shi, & Davis, 1999), and strong reciprocal connections between the CeA and lateral hypothalamus have been noted repeatedly (Allen, Saper, Hurley, & Cechetto, 1991; Ottersen, 1980; Turner & Herkenham, 1991; van der Kooy, Koda, McGinty, Gerfen, & Bloom, 1984). The CeA also seems to modulate aspects of dopamine efflux in the nucleus accumbens core, via GABAergic connections to the ventral tegmental area and the substantia nigra (Ahn & Phillips, 2003), regions important in modulating the effects of reinforcers and the control of motor behavior. Thus, the CeA’s importance becomes apparent when its relation to other structures is considered. Nevertheless, although the CeA was originally thought to mediate aversive learning or emotional processes (Davis, 1992; LeDoux, 1992), the present results contribute to a growing body of literature expanding the function of the CeA into appetitive domains (de Olmos et al., 1985; de Olmos & Heimer, 1999; Everitt, Cardinal, Parkinson, & Robbins, 2003; Hall et al., 2001; Holland & Gallagher, 2003).
NMDA Receptor Antagonism in the Striatum
Infusions of AP-5 into the PLS disrupted instrumental learning, nose poking, and several measures of feeding and motor behavior. Infusions did not, however, affect instrumental behavior or nose poking during the performance test. It is possible that after infusions, AP-5 diffused to adjacent cortical structures and affected learning or lever pressing. In Experiment 1, diffusion into adjacent areas was less problematic because infusions of AP-5 into the adjacent BLA do not affect the performance of previously learned instrumental responding (Baldwin et al., 2000). The possible role of cortical structures in instrumental learning is of particular interest to us; future research, however, is needed to ascertain the cortex’s function, and specifically NMDA receptor activation in the cortex, in instrumental learning.
In contrast to the results of Experiment 1, AP-5 infusions in the PLS decreased nose poking, decreased the Pr(NP|eR), increased locomotion, and decreased the average length of a feeding bout, whereas AP-5 infusions in the CeA had no discernible effect on basal rates of nose poking, the Pr(NP|eR), locomotion, or average length of a feeding bout. Infusions of AP-5 in the CeA did, however, decrease lever pressing during the performance test session, whereas infusions in the PLS had no effect.
Like the results of Experiment 1, many of the present results are somewhat inconsistent with the hypothesis that AP-5 infusions in the PLS made the rats less hungry, primarily because of the lack of an effect of the drug on performance during the test session, on latency to begin feeding, and on the number of feeding bouts. No evidence of a general motoric impairment was noted; in fact, NMDA receptor antagonism in this area of the striatum increased locomotion and rearing, although the latter was not statistically reliable. The significant increase in activity may have produced the learning impairment and decrement in nose poking, but it appears that the effects of infusions on nose poking attenuated after instrumental conditioning. It is possible that with an increase in the response strength of lever pressing, the effects of the drug are overcome or that there is a shift in the mechanisms that mediate this form of learning and behavior. In the plus-maze preparation, behavior is first controlled by extramaze cues (place learning) but shifts to control by interoceptive cues (response learning) with training. Packard and McGaugh (1996) demonstrated that inactivation of a similar area of the striatum (caudate nucleus) with lidocaine impaired response learning in the plus-maze, suggesting that the mechanisms that instantiate this shift in strategy are mediated in part by the striatum. Posttrial AP-5 infusions into this area of the striatum have no effect on escape latencies in a spatial Morris water maze task, but reduce latencies in the cued water maze preparation, thereby intimating the dorsal striatum in procedural or simple associative learning (Packard & Teather, 1997). In humans, it appears that the neostriatum (the caudate nucleus and putamen) is required for implicit memory tasks or simple conditioning (Knowlton, Mangels, & Squire, 1996). In any event, many researchers have noted systematic changes in the topography, force, direction, and/or rate of responding during the course of instrumental conditioning (Ferster & Skinner, 1957; Herrnstein, 1970), contradicting the notion that the form of instrumental learning merely involves the “stamping-in” of information, stimulus–response “habit” learning, or formation of simple associations. A more tenable position is that instrumental learning is a dynamic and temporally extended process involving multiple corticostriatal systems with complex time courses (Baldwin, Sadeghian, Holahan, & Kelley, 2002; Baldwin, Sadeghian, & Kelley, 2002; Hernandez, Sadeghian, & Kelley, 2002). A shift or change in the control of instrumental behavior during training may have occurred in the present experiments and likely involve NMDA receptors in the lateral striatum.
The rationale for assessing the role of the PLS in instrumental learning was based in part on its strong connectivity with other areas known to be involved in instrumental learning, most notably the BLA. In addition, striatal lesions have been shown to impair learning using other preparations (Brown & Robbins, 1989a, 1989b, 1991). However, the term dorsal striatum appears to refer to any region of the striatum that is not ventral, irrespective of the region’s position in the anterior–posterior plane. Experiment 3 demonstrated clear functional heterogeneity in the dorsal striatum with respect to instrumental learning because NMDA receptor antagonism in a more anterior site had no effect on learning, performance, or measures of food-directed behavior. Future research is needed to address the heterogeneity of the region referred to as the dorsal striatum.
Summary
NMDA receptor activation is thought to be critical to the cascade of neuronal events that underlie enduring neural plasticity associated with learning and memory. Instrumental learning is construed as behavioral adaptation to changing environmental contingencies instantiated in an environment–organism feedback system (Baum, 1973; Skinner, 1953) and is contrasted with other forms of learning that do not appear to have the same sort of feedback loops. However, the conceptualization of rats pressing levers as simple habit learning or stimulus–response learning belies the complexity of these actions, for instrumental actions must be viewed in the context of other possible actions. Herrnstein (Herrnstein, 1970) argued that even in the simple environment of a one-lever conditioning chamber, the lever press was interwoven with other possible actions (e.g., scratching, sniffing, exploring, grooming, etc.), which influence the instrumental behavior. Instrumental learning most likely requires the coordination of a diverse set of physiological processes: motivational, attentional, and motoric. Impairing instrumental learning can occur as a result of influencing those other actions. In Experiment 1, it appears that food-directed responses were affected by NMDA receptor inactivation within the CeA, whereas in Experiment 2, NMDA receptor antagonism seems to have produced hyperactivity; this hyperactivity interfered with learning, but not performance. Clearly, NMDA receptor activation in the CeA and distinct regions of the striatum is necessary for initial instrumental learning. However, although the present results extend knowledge of the distributed corticostriatal network that mediates instrumental learning, additional experimentation is needed to discern the multiple effects of NMDA antagonism on this complex form of learning and behavior.
Acknowledgments
This research was supported by National Institute on Drug Abuse Grants DA016465-01 to Matthew E. Andrzejewski and DA04788 to Ann E. Kelley.
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Current Opinion in Neurobiology. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Ahn S, Phillips AG. Modulation by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. Journal of Neuroscience. 2002;22:10958–10965. doi: 10.1523/JNEUROSCI.22-24-10958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Phillips AG. Independent modulation of basal and feeding-evoked dopamine efflux in the nucleus accumbens and medial prefrontal cortex by the central and basolateral amygdalar nuclei in the rat. Neuroscience. 2003;116:295–305. doi: 10.1016/s0306-4522(02)00551-1. [DOI] [PubMed] [Google Scholar]
- Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: A PHA-L study in the rat. Journal of Comparative Neurology. 1994;341:289–314. doi: 10.1002/cne.903410302. [DOI] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. Journal of Comparative Neurology. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behavioral Neuroscience. 2000;114:84–98. doi: 10.1037//0735-7044.114.1.84. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiology of Learning and Memory. 2002;77:44–62. doi: 10.1006/nlme.2000.4002. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Kelley AE. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. Journal of Neuroscience. 2002;22:1063–1071. doi: 10.1523/JNEUROSCI.22-03-01063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum WM. The correlation-based law of effect. Journal of the Experimental Analysis of Behavior. 1973;20:137–153. doi: 10.1901/jeab.1973.20-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: A Phaseolus valgaris leucoagglutinin (PHA-L) study in the rat. Journal of Comparative Neurology. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Review. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Humby T, Dunnett SB, Robbins TW. Unilateral lesions of the dorsal striatum in rats disrupt responding in egocentric space. Journal of Neuroscience. 1997;17:8919–8926. doi: 10.1523/JNEUROSCI.17-22-08919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, Robbins TW. Deficits in response space following unilateral striatal dopamine depletion in the rat. Journal of Neuroscience. 1989a;9:983–989. doi: 10.1523/JNEUROSCI.09-03-00983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, Robbins TW. Elementary processes of response selection mediated by distinct regions of the striatum. Journal of Neuroscience. 1989b;9:3760–3765. doi: 10.1523/JNEUROSCI.09-11-03760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, Robbins TW. Simple and choice reaction time performance following unilateral striatal dopamine depletion in the rat. Impaired motor readiness but preserved response preparation. Brain. 1991;114(Part 1B):513–525. doi: 10.1093/brain/114.1.513. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. In: McGinty JF, editor. Annals of the New York Academy of Sciences: Vol. 877. Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug abuse. New York: New York Academy of Sciences; 1999. pp. 217–241. [Google Scholar]
- Cho YK, Li CS, Smith DV. Descending influences from the lateral hypothalamus and amygdala converge onto medullary taste neurons. Chemical Senses. 2003;28:155–171. doi: 10.1093/chemse/28.2.155. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Rescorla RA. Associations between the discriminative stimulus and the reinforcer in instrumental learning. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:155–164. [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Alheid GF, Beltramino CA. Amygdala. In: Paxinos G, editor. The rat nervous system. Sydney, Australia: Academic Press; 1985. pp. 223–334. [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. In: McGinty JF, editor. Annals of the New York Academy of Sciences: Vol. 877. Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug abuse. New York: New York Academy of Sciences; 1999. pp. 1–32. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. In: Shinnick-Gallagher P, Pitkänen A, Shekhar A, Cahill L, editors. Annals of the New York Academy of Sciences: Vol. 985. Amygdala in brain function: Basic and clinical approaches. New York: New York Academy of Sciences; 2003. pp. 233–250. [PubMed] [Google Scholar]
- Ferster CB, Skinner BF. Schedules of reinforcement. Upper Saddle River, NJ: Prentice-Hall; 1957. [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned behavior. Journal of Neuroscience. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Holland PC. Understanding the function of the central nucleus: Is simple conditioning enough? In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 307–321. [Google Scholar]
- Gallagher M, Holland PC. The amygdala complex: Multiple roles in associative learning and attention. Proceedings of the National Academy of Sciences, USA. 1994;91:11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. European Journal of Neuroscience. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Hernandez PJ, Sadeghian K, Kelley AE. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nature Neuroscience. 2002;5:1327–1331. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. On the law of effect. Journal of the Experimental Analysis of Behavior. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends in Cognitive Science. 1999;3(2):65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. European Journal of Neuroscience. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Han JS, Gallagher M. Lesions of the amygdala central nucleus alter performance on a selective attention task. Journal of Neuroscience. 2000;20:6701–6706. doi: 10.1523/JNEUROSCI.20-17-06701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiology & Behavior. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. Journal of Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat—an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proceedings of the National Academy of Sciences, USA. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996 September 6;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Brain mechanisms of emotion and emotional learning. Current Opinion in Neurobiology. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. In: McGinty JF, editor. Annals of the New York Academy of Sciences: Vol. 877. Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug abuse. New York: New York Academy of Sciences; 1999. pp. 309–338. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Mowrer OH. Learning theory and behavior. Oxford, England: Wiley; 1960. [Google Scholar]
- Norgren R. Taste pathways to hypothalamus and amygdala. Journal of Comparative Neurology. 1976;166:17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat and cat: II. Afferents from the hypothalamus and the basal telencephalon. Journal of Comparative Neurology. 1980;194:267–289. doi: 10.1002/cne.901940113. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Double dissociation of hippocampal and dorsal-striatal memory systems by posttraining intracerebral injections of 2-amino-5-phosphonopentanoic acid. Behavioral Neuroscience. 1997;111:543–551. doi: 10.1037//0735-7044.111.3.543. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego, CA: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: An emerging framework for understanding functions of the amygdala. Trends in Neuroscience. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Harker KT, Douglas RM, Whishaw IQ. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behavioural Brain Research. 2002;136:339–348. doi: 10.1016/s0166-4328(02)00126-2. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. The role of information about the response-outcome relation in instrumental discrimination learning. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16:262–270. [PubMed] [Google Scholar]
- Rescorla RA. Control of instrumental performance by Pavlovian and instrumental stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:44–50. [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychological Review. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N. Neurobiology of skill and habit learning. Current Opinion in Neurobiology. 1995;5:184–190. doi: 10.1016/0959-4388(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Skinner BF. Science and human behavior. New York: The Free Press; 1953. [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in Neuroscience. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: A test of the amygdala’s role in sensory processing. Journal of Comparative Neurology. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. Journal of Comparative Neurology. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. Journal of Pharmacology and Experimental Therapeutics. 1999;291:409–415. [PubMed] [Google Scholar]
- Voshart K, van der Kooy D. The organization of the efferent projections of the parabrachial nucleus of the forebrain in the rat: A retrograde fluorescent double-labeling study. Brain Research. 1981;212:271–286. doi: 10.1016/0006-8993(81)90462-5. [DOI] [PubMed] [Google Scholar]
- White NM. Mnemonic functions of the basal ganglia. Current Opinion in Neurobiology. 1997;7:164–169. doi: 10.1016/s0959-4388(97)80004-9. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau E, Kelley AE. The amygdala is critical for opioid-mediated “binge” eating in rats. 2004. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94:469–492. [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: Microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]