Abstract
Using primary cultures of immature rat granulosa cells and adenoviral infections we expressed two mutants of the human lutropin receptor (hLHR) that do not activate the phosphoinositide cascade. One mutant (hLFF) has the extracellular domain of the hLHR and the transmembrane and intracellular domains of the hFSHR. The other (hLHR-L457D) has a leucine to aspartate mutation in residue 457 of transmembrane helix 3.
When expressed in immature rat granulosa cells the hLHR stimulates cAMP and inositol phosphate accumulation, transactivates the epidermal growth factor receptor (EGFR), elicits a transient increase in Akt phosphorylation, and a sustained increase in ERK1/2 phosphorylation but aromatase expression is not enhanced. When expressed at comparable densities, hLFF and hLHR-L457D support cAMP accumulation and transient Akt phosphorylation but do not support inositol phosphate accumulation, EGFR transactivation or a sustained phosphorylation of ERK1/2. Cells expressing either of these two mutants respond to hCG with increased aromatase expression.
We also show that addition of hCG to cells expressing the hLHR antagonizes the effects of hFSH on aromatase expression whereas addition of hCG to cells expressing the hLHR-L457D mutant does not.
These results show that activation of the phosphoinositide cascade is upstream of EGFR transactivation and ERK1/2 phosphorylation and that this pathway is a negative regulator of aromatase expression in granulosa cells.
Introduction
One of the outstanding questions in the development of the ovarian follicle is why two highly homologous hormone receptor pairs (FSH/FSHR and LH/LHR) elicit divergent effects on ovarian gene expression. Recent studies on the functional properties of the recombinant LHR expressed in immature rat granulosa cells show that these divergent effects of the FSH/FSHR and LH/LHR pairs are likely due to the level of receptor expression and signaling cascades that are activated and not due to the expression of the LHR only in differentiated granulosa cells (Andric and Ascoli, 2006; Bebia et al., 2001; Donadeu and Ascoli, 2005; Zeleznik et al., 2003).
By comparing the actions of hFSH and hCG in primary cultures of immature rat granulosa cells expressing different densities of the recombinant hFSHR or hLHR we have shown that both hormones induce aromatase expression when the density of their cognate receptor is low and neither hormone induces aromatase expression when the density of their cognate receptor is high (Donadeu and Ascoli, 2005). We have also shown that when acting on low or high receptor densities, hFSH and hCG activate the cAMP signaling pathway and induce a rapid and transient phosphorylation of ERK1/2 and Akt (Andric and Ascoli, 2006; Donadeu and Ascoli, 2005) whereas at high receptor densities hFSH and hCG also promote the hydrolysis of phosphatidylinositols and provoke a sustained increase in the phosphorylation of ERK1/2 (Andric and Ascoli, 2006; Donadeu and Ascoli, 2005).
Several investigators, including us, have already shown that the ERK1/2 pathway is a negative regulator of aromatase expression (Andric and Ascoli, 2006; McDonald et al., 2006; Su et al., 2006; Zeleznik et al., 2003). We have also shown that pharmacological inhibitors of the EGF network and ERK1/2 phosphorylation restore the ability of gonadotropins to induce aromatase expression in immature granulosa cells expressing a high density of receptors (Andric and Ascoli, 2006). The lack of useful experimental manipulations that can be used to inhibit the phosphoinositide signaling cascade has made it difficult to test for its involvement as the first step of the proposed pathway leading to the repression of aromatase induction (Andric and Ascoli, 2006). Here we use two mutants of the hLHR that do not activate the phosphoinositide cascade to directly and conclusively document the importance of this signaling cascade on the transactivatiion of the EGFR, the sustained phosphorylation of ERK1/2, and the repression of aromatase expression.
MATERIALS AND METHODS
Materials
Purified hCG (CR-127) and purified hFSH (AFP-5720D) were purchased from the National Hormone and Pituitary Agency (Torrance, CA). Purified recombinant hCG and hFSH were kindly provided by Ares Serono (Randolph, MA). Cell culture medium was obtained from Invitrogen Corp. (www.invitrogen.com). Other supplies and reagents used for granulosa cell extraction and culture were obtained from Sigma-Aldrich Corp. (www.sigmaaldrich.com), BD Biosciences (www.bdbiosciences.com) and Fisher (www.fishersci.com). Molecular biology reagents were obtained from Invitrogen Corp. (www.invitrogen.com) and Roche Diagnostics Corp. (www.roche-applied-science.com). All other chemicals were obtained from commonly used suppliers.
Viruses, plasmids and cells
The mutant named hLFF was constructed using standard PCR strategies and it contains the extracellular domain of the hLHR (amino acid residues 1−363) spliced into the transmembrane, and intracellular domains (amino acid residues 373−695) of the hFSHR. The boundaries of the spliced regions of this mutant correspond to the exact boundaries of a chimera of the rat FSHR and LHR (named LFF) previously characterized in this laboratory (Nakamura et al., 1999). An expression vector for hLHR-L457D was kindly provided by D. Segaloff (Shinozaki et al., 2001). These two constructs were subcloned into the RAPAd adenoviral vector and the recombinant adenoviral particles (Ad-hLFF and Ad-hLHR-LD) were prepared by the Gene Transfer Vector Core at The University of Iowa. The preparation of recombinant adenoviral particles coding for the hLHR (Ad-hLHR), hFSHR (Ad-hFSHR) and β–galactosidase (Ad-βgal) have been described (Andric and Ascoli, 2006; Donadeu and Ascoli, 2005).
The methods used to isolate, maintain and infect primary cultures of immature rat granulosa cells have also been previously described (Andric and Ascoli, 2006; Donadeu and Ascoli, 2005). These procedures were approved by the Institutional Animal Care and Use Committee at the University of Iowa. Infections with the different receptor-coding viral constructs were done as indicated in the figure legends but the total amount of virus used for each infection was always adjusted with Ad-βgal to give a constant MOI (multiplicity of infection) of 300.
An expression vector for a C-terminally tagged EGFR-GFP was donated to us by Dr. John Koland of this institution. I-10 cells a clonal strain of Leydig tumor cells that lack LHR (Ascoli, 2007; Shin, 1967), were purchased from the American Type Culture Collection (CCL-83), and maintained in DMEM/F12 medium supplemented with 15% horse serum, 20 mM Hepes and 50 μg/ml gentamicin, pH 7.4.
Binding, second-messenger and mRNA assays
The binding of 125I-hCG was measured during a 4 h incubation with 100 ng/ml of 125I-hCG (Donadeu and Ascoli, 2005). Cyclic AMP accumulation was measured during a 30 min incubation in cells incubated without or with hCG (100 ng/ml) but in the presence of 1 mM isobutylmethylxanthine, a phosphodiesterase inhibitor (Donadeu and Ascoli, 2005; Hipkin et al., 1995a; Hipkin et al., 1995b; Steiner et al., 1972). Inositol phosphate accumulation was measured during a 1 hour incubation in cells incubated without or with hCG (500 ng/ml) but in the presence of 20 mM LiCl, an inhibitor of the phosphatases that dephosporylate inositol phosphates (Ascoli et al., 1989; Donadeu and Ascoli, 2005; Hipkin et al., 1995a; Hipkin et al., 1995b; Hirakawa et al., 2002). Epiregulin and aromatase mRNA were quantitated using real time PCR at the end of a 9 or 48 hour incubation, respectively, without or with 100 ng/ml hCG as described elsewhere (Andric and Ascoli, 2006; Donadeu and Ascoli, 2005)
The incubation times and hCG concentrations chosen for binding and the second messenger and mRNA assays were previously shown to result in maximal effects (Andric and Ascoli, 2006; Donadeu and Ascoli, 2005; Hirakawa et al., 2002).
ERK and Akt phosphorylation assay
The methods for measuring ERK1/2 and Akt phosphorylation were as previously described (Andric and Ascoli, 2006; Donadeu and Ascoli, 2005) with a few modifications as follows. The membranes were first probed during an overnight incubation with a 1:5,000 dilution of dual phospho-specific p44/p42 (Thr202/Tyr204) ERK1/2 antibody (cat # 9101) or a 1:3,000 dilution of a phospho(Ser473) AKt antibody (cat # 9271) from Cell Signaling Technology (www.cellsignal.com). This was followed by a second 1-h incubation with a 1:10,000 dilution of a secondary antibody (cat # 170−6515) covalently coupled to horseradish peroxidase from BioRad (www.biorad.com). The immune complexes were visualized and quantified using Super Signal West femto maximum sensitivity detection system (www.piercenet.com) and a digital imaging system (Eastman Kodak Co.). After detection of phospho ERK1/2 or phospho Akt the membranes were stripped with 50 mM Tris-HCl pH 6.2, 100 mM β-mercaptoethanol, 2% SDS for 20−30 min at 70°C-80°C and incubated overnight with a 1:5,000 dilution of ERK-2 antibody (cat # sc-154) from Santa Cruz Biotechnology (www.sbct.com). This was followed by a 1h incubation with secondary antibody and detection of the immune complexes as described above. All the ERK1/2 and Akt phosphorylation data presented were corrected for the amount of total ERK-2 detected.
Bioassay for EGF-like growth factors
This assay was carried out by co-culturing primary granulosa cells expressing the hLHR or mutants thereof and I-10 Leydig cells expressing a GFP-tagged form of the human EGFR (henceforth referred to as test cells)1. When added to these co-cultures hCG acts only on granulosa cells because the test cells do not express the LHR (Ascoli, 2007; Shin, 1967; Shiraishi and Ascoli, 2007). Thus any hCG-dependent increase in the phosphorylation of the EGFR-GFP has to arise from extracellular, EGF-like factors that are produced by granulosa cells and act on the test cells. In addition, other potential direct effects of the LHR on the phosphorylation of the endogenous EGFR in granulosa cells would not be detected in this assay because the EGFR-GFP has a higher molecular weight than the endogenous EGFR and they can be readily resolved on SDS gels (Shiraishi and Ascoli, 2007).
I-10 cells were transfected with the EGFR-GFP using Lipofectamine® as described elsewhere (Hirakawa et al., 2002; Shiraishi and Ascoli, 2007). The transfected I-10 cells were trypsinized and plated (4 × 105 cells) on 6 well plates already containing 2 × 105 granulosa cells that had been infected one day earlier with Ad-hLHR, Ad-hLFF or Ad-hLHR-LD as described above. After plating the co-cultures were maintained in 2 ml of growth medium (DMEM/F12 medium supplemented with 1 mg/ml bovine serum albumin, 10 mM Hepes, insulin (1 μg/ml), transferrin (1 μg/ml), selenium (1 ng/ml), pH 7.4) for 8 hours. The medium was then replaced with assay medium (DMEM/F12 medium supplemented with 1 mg/ml bovine serum albumin, 20 mM Hepes, pH 7.4) and the cells were incubated in this medium for another 12 hours. The medium was then replaced with 1 ml of fresh assay medium and the co-cultures were incubated with buffer or hCG (100 ng/ml) for 9 hours as indicated. This incubation time was chosen because other experiments (not presented) measuring the phosphorylation of the EGFR-GFP as a function of time after hormone addition showed that a 9 hour incubation of the co-cultures was optimal to induce phosphorylation of the EGFR-GFP in the test cells.
At the end of the stimulation period the medium was aspirated and the cells were lysed with 100 μl of RIPA buffer (150 mM NaCl, 50 mM Tris, 1 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, pH 7.4) supplemented with an EDTA-free protease inhibitor cocktail from Roche Applied Science, 1 mM NaF and 1 mM sodium orthovanadate. The resulting lysates were clarified by centrifugation and assayed for protein content using the BCA protein assay kit from Bio-Rad Laboratories. Equal amounts of protein from each lysate (15 μg) were then resolved on 7.5% SDS-polyacrylamide gels and transferred electrophoretically to polyvinylidene difluoride membranes (Hirakawa et al., 2002). The blots were first incubated overnight at 4°C with a 1:1,000 dilution of a rabbit antibody to phosphotyrosine 1068 of the EGFR (Cell Signaling, catalog # 2234). After washing the blots were incubated for 1 h with a 1:3,000 dilution of a secondary antibody covalently coupled to horseradish peroxidase (from Bio-Rad Laboratories). After detection of the phospho-EGFR signal the blots were stripped and incubated with a 1:2,000 dilution of a goat anti GFP antibody coupled to horseradish peroxidase (from Abcam, cat # ab6663−1000) during an overnight incubation at 4°C.
All immune complexes were visualized and quantitated as described above and the phospho-EGFR signal was corrected for the amount of EGFR-GFP present in the blots.
Statistical analysis
Results were analyzed using ANOVA followed by Tukeys post hoc test or paired t-test as described in the figure legends using the Prism software package (www.graphpad.com). In all cases statistical significance was taken at P < 0.05.
Results
Initial characterization of hLHR mutants that do not activate the phosphoinositide cascade
To directly explore the impact of gonadotropin-induced activation of the phosphoinositide cascade on aromatase expression we examined the functional properties of two mutants of the hLHR. One mutant (designated hLFF) was constructed by grafting the extracellular domain of the hLHR on to the transmembrane and intracellular domains of the hFSHR. Since the binding specificity of the gonadotropin receptors is entirely dependent on the origin of the extracellular domain (Hirsch et al., 1996; Nakamura et al., 1999) and since the hLHR is more efficacious than the hFSHR in stimulating the phosphoinositide cascade when expressed in heterologous cells (Hirsch et al., 1996) we predicted that granulosa cells expressing hLFF would bind hCG and respond to it with cAMP accumulation, but not with inositol phosphate accumulation. The second hLHR mutant tested bears a leucine to aspartate mutation in codon 457 in transmembrane helix 3 (L457D, see Shinozaki et al., 2001). When expressed in 293 cells the hLHR-L457D mutant supports stimulation of the cAMP pathway but does not support the activation of the phosphoinositide cascade (Shinozaki et al., 2001).
To rule out the possibility that differences in the responses of granulosa cells expressing the hLHR, hLFF or hLHR-LD were due to differences in receptor expression, we equalized their expression levels as shown in Fig. 1. Cells infected with 200 MOI of Ad-hLHR, 300 MOI of Ad-hLFF or 300 MOI of Ad-hLHR-LD bound ∼2.5 ng of 125I-hCG/106 cells.
Figure 1. Expression of the hLHR and mutants thereof in immature granulosa cells.

Primary cultures of immature granulosa cells were infected with a mixture of Ad-hLHR at 200 MOI plus Ad-βgal at 100 MOI, Ad-hLFF at 300 MOI or Ad-hLHR-LD at 300 MOI as shown. 125I-hCG binding was measured 2 days after infection as described in Materials and Methods.
Each bar is the mean±SEM of 4−8 independent experiments
The level of 125I-hCG binding under the infection conditions used is what we consider a high LHR density (Andric and Ascoli, 2006; Donadeu and Ascoli, 2005), but is comparable to that attained when the expression of the endogenous LHR is induced by FSH. Immature rat granulosa cells maintained for 3 days in the presence of maximally effective concentrations of FSH bind ∼4 ng of 125I-hCG/106 cells (Shi and Segaloff, 1995). We also note that immature granulosa cells that are not infected with the hLHR or mutants thereof do not bind or respond to hCG and that expression of the hLHR, hLFF and hLHR-LD did not affect the ability of these cells to bind 125I-hFSH through the endogenus FSHR (data not shown and ref. Donadeu and Ascoli, 2005).
Figure 2A shows that hCG is effective in inducing a cAMP response in cells expressing the hLHR or mutants thereof. Human CG induced a similar increase (∼150-fold) in cAMP accumulation in cells expressing either the hLHR or hLFF. Cells expressing hLHR-LD also responded to hCG with an increase in cAMP accumulation but this increase (∼10-fold) was significantly lower than that observed in cells expressing hLHR or hLFF. The decreased cAMP responsiveness of granulosa cells expressing hLHR-LD was not expected from the results obtained in heterologous cells (Shinozaki et al., 2001) but we did not seek an explanation for this difference.
Figure 2. Second messenger responses in immature granulosa cells expressing the hLHR or mutant thereof.
Cells were infected with the indicated adenoviral constructs as described in Figure 1. Cyclic AMP and inositol phosphate accumulation were measured 2 days after infection in cells incubated with or without hCG as described in Materials and Methods.
Each bar is the mean±SEM of 4−8 independent experiments. Means within each panel with different letters are significantly different from each other (P<0.05, ANOVA).
Figure 2B shows that hCG stimulates inositol phosphate accumulation in cells expressing a high density of hLHR (also see Donadeu and Ascoli, 2005). In agreement with the results obtained in heterologous cell types (Hirsch et al., 1996; Shinozaki et al., 2001), we found that granulosa cells expressing a comparable density of hLFF or hLHR-LD do not respond to hCG with an increase in inositol phosphate accumulation (Figure 2B).
Human LHR mutants that do not activate the phosphoinositide cascade do not induce ERK1/2 phosphorylation but induce Akt phosphorylation
Addition of hCG to granulosa cells expressing a high density of hLHR results in a biphasic increase in ERK1/2 phosphorylation (Andric and Ascoli, 2006). A robust increase is detected within 5 min and it reaches a maximum in 15−30 min followed by a decline after 2h and a second robust increase after 6−9 hours of incubation (Andric and Ascoli, 2006).
Human CG stimulates ERK1/2 phosphorylation in cells expressing the hLHR at early (15 min, Figure 3A) and late time points (9 h, Figure 3B) but is unable to do so at either time point in granulosa cells expressing a comparable density of hLFF or hLHR-LD. The hCG-induced phospho ERK1/2 responses mediated by cells expressing hLFF or hLHR-LD during a 9 h incubation varied between ∼70% (Figure 3B) and ∼130% (see Table 1 below) of the phospho-ERK1/2 levels detected in cells incubated with buffer only. In the experiments presented in Figure 3B the level of phospho-ERK1/2 detected in hCG-stimulated cells are slightly lower than controls for both mutants but this difference was statistically significant only with the hLHR-LD mutant (also see legend to Table 1).
Figure 3. Transient and sustained hCG-induced ERK1/2 phosphorylation in immature granulosa cells expressing the hLHR or mutants thereof.
Primary cultures of immature granulosa cells were infected with the indicated adenoviral constructs as described in the legend to Fig 1. Two days after infection the cells were incubated with or without hCG as indicated and the levels of phospho-ERK1/2 were measured 15 min (panel A) or 9 hours later (panel B) as described in Materials and Methods. Total ERK2 was also measured in the blots as a loading control.
The blots shown are results of a representative experiment whereas the graphs show the average ± SEM of five independent experiments. In these graphs the asterisks indicate significantly different values (P<0.05, paired t-test) between the control and hCG-stimulated cells.
Table 1.
Effects of gonadotropins on second messengers, ERK1/2 phosphorylation and aromatase expression in immature granulosa cells expressing the endogenous FSHR only or the endogenous FSHR and the recombinant hLHR or hLHR-LD
| Cells infected with | Additions | cAMP (pmol/106 cells) | Inositol phosphates (cpm/106 cells) | Delayed P-ERK1/2 (fold over basal) | Aromatase mRNA (arbitrary units) |
|---|---|---|---|---|---|
| βgal | Buffer | 20 ± 1 | 2627 ± 408 | 1 | 0.004 ± 0 |
| hFSH | 5663 ± 451a | 2265 ± 416a | 0.6 ± 0.2a | 1.5 ± 0.1a | |
| hLHR | Buffer | 22 ± 2 | 2301 ± 447 | 1 | 0.003 ± 0 |
| hFSH, hCG | 6101 ± 324a | 13527 ± 3473b | 2.7 ± 0.5b | 0.2 ± 0.1b | |
| LD | Buffer | 24 ± 1 | 2551 ± 487 | 1 | 0.001 ± 0 |
| hFSH, hCG | 6909 ± 309a | 2307 ± 398a | 1.3 ± 0.1a | 1.0 ± 0.3a |
Primary cultures of immature granulosa cells were infected with Ad-βgal at 300 MOI, a mixture of Ad-hLHR at 200 MOI plus Ad-βgal at 100 MOI (hLHR), or Ad-hLHR-LD at 300 MOI (LD). Two days after infection the cells were incubated with buffer only, hFSH only (100 ng/ml) or hFSH and hCG (each at 100 ng/ml) as indicated. Cyclic AMP, inositol phosphates, delayed phospho-ERK1/2 (i.e, measured at 9h) and aromatase mRNA were measured as described in Materials and Methods.
Results are the mean ± SEM of 3-5 independent experiments. For stimulated cells, means within each column with different letters are significantly different from each other (P<0.05, ANOVA). Note that the delayed phospho-ERK1/2 response was somewhat variable when using hLHR-LD. In Figure 3 (see above) there is a small decrease in the response compared to cells incubated with buffer only and in Table 1 there is a small increase compared to cells incubated with buffer only. The important comparison in our analysis and conclusions is between the magnitude of the effects of hCG on the delayed phospho-ERK1/2 in cells expressing the hLHR or the hLHR-LD, however. The difference between these two groups is always statistically significant as indicated by the different letters in Table 1.
Since the Akt pathway has been implicated as a positive regulator of aromatase expression (Zeleznik et al., 2003) we also tested the effects of hCG on Akt phosphorylation in primary cultures of immature granulosa cells expressing the two hLHR mutants. In agreement with our previous data (Donadeu and Ascoli, 2005) we found that activation of the hLHR results in a rapid and robust increase in Akt phosphorylation (Figure 4). Akt phosphorylation was also readily enhanced by adding hCG to immature granulosa cells expressing hLFF or hLHR-LD (Figure 4).
Figure 4. Human CG-induced Akt phosphorylation in immature granulosa cells expressing the hLHR or mutants thereof.
Primary cultures of immature granulosa cells were infected with the indicated adenoviral constructs as described in the legend to Fig 1. Two days after infection the cells were incubated with or without hCG as indicated and the levels of phospho-AKt measured 15 min later as described in Materials and Methods. Total ERK2 was also measured in the blots as a loading control.
The blots shown are results of a representative experiment whereas the graphs shows the average ± SEM of three independent experiments. In these graphs the asterisks indicate significantly different values (P<0.05, paired t-test) between the control and hCG-stimulated cells.
Human LHR mutants that do not activate the phosphoinositide cascade do not increase the levels of bioactive EGF-like growth factors
To further test for the involvement of EGF-like growth factors as mediators of the gonadotropin-provoked ERK1/2 phosphorylation and aromatase repression we measured the production of bioactive EGF-like growth factors in primary cultures of immature granulosa cells. To accomplish this goal we co-cultured granulosa expressing the hLHR or mutants thereof with a test cell line that does not express the LHR but expresses a GFP-tagged form of the human EGFR (see Materials and Methods and Shiraishi and Ascoli, 2007). Since the test cells are unresponsive to hCG (Ascoli, 2007; Shin, 1967; Shiraishi and Ascoli, 2007) but they express the EGFR-GFP any hCG-induced phosphorylation of the EGFR-GFP in the test cells must originate from extracellular EGF-like growth factors that are produced by granulosa cells. We chose to use this co-culture system instead of measuring the phosphorylation of the endogenous EGFR in granulosa cells because this is the only way we can ensure that a gonadotropin-mediated increase in the phosphorylation of the EGFR is mediated by extracellular EGF-like growth factors. Since both gonadotropins can activate the Src family of kinases (Hunzicker-Dunn and Maizels, 2006; Mizutani et al., 2006; Shiraishi and Ascoli, 2006; Wayne et al., 2007) and this family of kinases can directly phosphorylate and activate the EGFR (Bromann et al., 2004) gonadotropin-mediated increases in the phosphorylation of the endogenous EGFR in granulosa cells are not necessarily mediated by the actions of extracellular EGF-like growth factors. In contrast, the hCG-induced phosphorylation of the EGFR-GFP in the test cells cannot occur by these intracellular signaling patwhays.
The results presented in Figure 5A show that addition of hCG to co-cultures containing granulosa cells expressing the hLHR results in a 2- to 3-fold increase in the phosphorylation of the EGFR-GFP in the test cells whereas addition of hCG to the co-cultures containing granulosa cells expressing hLFF or hLHR-LD does not stimulate the phosphorylation of the EGFR-GFP in the test cells.
Figure 5. Bioactive EGF-like growth factors and epiregulin expression in immature granulosa cells expressing the hLHR but not in those expressing mutants thereof.
Primary cultures of immature granulosa cells were infected with the indicated adenoviral constructs as described in the legend to Fig 1.
Panel A. Granulosa cells expressing the hLHR or mutants thereof were co-cultured with test cells expressing the EGFR-GFP and the co-cultures were incubated with or without hCG as indicated. Phosphorylated EGFR-GFP and total EGFR-GFP were measured at the end of a 9 hour incubation as described in Material and Methods. The blots shown are results of a representative experiment whereas the graph shows the average ± SEM of three independent experiments. In these graphs the asterisks indicate significantly different values (P<0.05, paired t-test) between the control and hCG-stimulated cells.
Panel B. Granulosa cells expressing the hLHR or mutants thereof were incubated with or without hCG for 9 hours as indicated. Total RNA was then collected and used to quantify epiregulin mRNA using RT followed by real-time PCR as described in Materials and Methods. Each bar is the mean ± SEM of 4 independent experiments. The asterisks indicate significantly different values (P<0.05, paired t-test) between the control and hCG-stimulated cells.
We next measured the expression of EGF-like growth factors in the granulosa cell cultures expressing the hLHR or mutants thereof. For these experiments we limited our analysis to epiregulin expression because it is the most abundant EGF-like growth factor detected in rat granulosa cells before or after hCG stimulation (Andric and Ascoli, 2006; Ashkenazi et al., 2005). In agreement with our previous data (Andric and Ascoli, 2006) we found that activation of the hLHR results in a robust increase in the expression of epiregulin (Figure 5B)2. Epiregulin expression was also readily enhanced by adding hCG to immature granulosa cells expressing hLFF or hLHR-LD (Figure 5B). The extent of epiregulin expression after hCG-stimulation was somewhat lower in cells expressing hLFF or hLHR-LD than in cells expressing the hLHR, however (Figure 5B).
These results show that the hLHR, hLFF and hLHR-LD are able to mediate an effect of hCG on epiregulin expression but the levels of EGF-like growth factor activity are enhanced only in cells expressing the hLHR.
Human LHR mutants that do not activate the phosphoinositide cascade increase aromatase expression
Human CG has only a small stimulatory effect on aromatase expression when added to cells expressing a high density of hLHR (Figure 6 and Andric and Ascoli, 2006; Donadeu and Ascoli, 2005). In contrast, hCG was able to induce robust aromatase expression in cells expressing a similar density of hLFF or hLHR-LD (Figure 6). The magnitude of the hCG-dependent induction of aromatase expression in cells expressing hLFF or hLHR-LD was in fact comparable to that elicited by hFSH acting on the endogenous FSHR (shown by the dashed line in Figure 6).
Figure 6. hCG-induced aromatase expression in immature granulosa cells expressing the hLHR or mutants thereof.
Primary cultures of immature granulosa cells were infected with the indicated adenoviral constructs as described in the legend to Fig 1. Two days after infection the cells were incubated with or without hCG for 48 hours as indicated. Total RNA was then collected and used to quantify or aromatase mRNA using RT followed by real-time PCR as described in Materials and Methods. Each bar is the mean ± SEM of 7−8 independent experiments. Means within each panel with different letters are significantly different from each other (P<0.05, ANOVA).
The dashed line shows the levels of aromatase mRNA in immature granulosa cells infected with 300 MOI of β-gal and stimulated with hFSH.
These data show that hCG can induce aromatase expression when acting through two distinct LHR mutants (hLFF and hLHR-LD) that do not activate the phosphoinositide cascade and ERK1/2 phosphorylation when expressed at high density. Since the hLHR-LD is also less efficacious in mediating the accumulation of cAMP (see Fig 2A) we sought to further characterize the actions of this mutant under conditions where cAMP accumulation is high. To accomplish this we used three groups of cells. One group was infected with β-gal and incubated with hFSH to activate the endogenous FSHR. The second group was infected with the hLHR and the third group was infected with the hLHR-LD. In these last two groups the endogenous FSHR and the recombinant hLHR or hLHR-LD were activated by addition of hFSH and hCG, respectively.
We have previously shown that addition of hFSH to cells expressing only the endogenous FSHR (i.e., βgal infected cells) results in maximal cAMP accumulation and increasing FSHR levels by infection with adenovirus coding for the hFSHR does not enhance the magnitude of the hFSH-induced cAMP response (Donadeu and Ascoli, 2005). In agreement with this finding the data presented in Table 1 show that addition of hCG and hFSH to cells expressing a high density of hLHR or hLHR-LD did not enhance cAMP accumulation over that induced by activating only the endogenous FSHR3. Using these experimental conditions we can compensate for the lower levels of cAMP accumulation induced by the LD mutant (Figure 2) and thus we can more readily compare the consequences of the unique activation of other signaling pathways by the hLHR or hLHR-LD in cells with constant levels of cAMP. As shown in Table 1 cells expressing the hLHR or hLHR-LD and stimulated with hFSH and hCG had the same levels of cAMP, but the accumulation of inositol phosphates and the delayed increase in ERK1/2 phosphorylation were still higher in cells expressing hLHR than in cells expressing hLHR-LD. In contrast, aromatase expression was much higher in cells expressing hLHR-LD than in cells expressing the hLHR as predicted. Lastly, the expression of aromatase in cells expressing the hLHR-LD was the same as that detected in cells expressing only the endogenous FSHR and stimulated with hFSH (Table 1).
Discussion
Studies using adenoviral-mediated expression of the FSHR or LHR in primary cultures of immature granulosa cells have shown that the signaling properties and aromatase induction profiles of these two receptors are similar but dependent on receptor density (Andric and Ascoli, 2006; Bebia et al., 2001; Donadeu and Ascoli, 2005; Zeleznik et al., 2003). At low receptor densities the LHR and FSHR stimulate cAMP accumulation, and elicit a transient increase in Akt and ERK1/2 phosphorylation but they do not stimulate inositol phosphate accumulation (this paper and Alam et al., 2004; Andric and Ascoli, 2006; Bebia et al., 2001; Donadeu and Ascoli, 2005; Gonzalez-Robayna et al., 1999; Gonzalez-Robayna et al., 2000; Hunzicker-Dunn and Maizels, 2006; Salvador et al., 2002; Zeleznik et al., 2003). At these low receptor densities the LHR and FSHR also stimulate epiregulin and aromatase expression (this paper and Andric and Ascoli, 2006; Bebia et al., 2001; Donadeu and Ascoli, 2005; Fitzpatrick et al., 1997; Fitzpatrick and Richards, 1993; Zeleznik et al., 2003). At higher receptor densities the LHR and FSHR increase cAMP accumulation, Akt phosphorylation and epiregulin expression but they also stimulate the accumulation of inositol phosphates, the phoshorylation of ERK1/2 becomes biphasic and aromatase expression is no longer supported (this paper and Andric and Ascoli, 2006; Donadeu and Ascoli, 2005).
These results lead us to postulate (Andric and Ascoli, 2006; Donadeu and Ascoli, 2005) that the gonadotropin-induced expression of aromatase in granulosa cells is dependent on the profile of signaling pathways that are activated which is in turn dictated by the density of receptors. We hypothesized that low receptor densities support aromatase expression because only the cAMP and Akt pathways are activated. Higher receptor densities do not support aromatase expression because they activate the phosphoinositide cascade, which in turn promotes the transactivation of the EGFR. The transactivation of the EGFR mediates the sustained phosphorylation of ERK1/2 that prevents aromatase expression.
Aromatase expression in granulosa cells can be stimulated by cAMP analogs or forskolin, but the magnitude of this effect (compared to the magnitude of the FSH effect) is rather variable (Donadeu and Ascoli, 2005; Fitzpatrick and Richards, 1993; 1991; Gonzalez-Robayna et al., 1999; Hickey et al., 1990; Kwintkiewicz et al., 2007; Richards, 1994; 2001a; Richards, 2001b; Richards et al., 2002; Simpson et al., 2002; Wayne et al., 2007; Zeleznik et al., 2003). Although most investigators agree that the cAMP/PKA pathway is an important component of the gonadotropin-induced aromatase expression (Conti, 2002; Fitzpatrick and Richards, 1993; 1991; Gonzalez-Robayna et al., 1999; Hickey et al., 1990; Kwintkiewicz et al., 2007; Richards, 1994; 2001a; Richards, 2001b; Richards et al., 2002; Simpson et al., 2002; Wayne et al., 2007) the involvement of additional pathways has also been proposed (Kwintkiewicz et al., 2007; Wayne et al., 2007). It is also known that transcriptional regulation of aromatase expression in the ovary is not uniquely effected by CREB. Other transcription factors such as SF1, LRH-1 and GATA4 also stimulate aromatase expression (Carlone and Richards, 1997; Dodson et al., 1997; Fitzpatrick and Richards, 1993; Kwintkiewicz et al., 2007).
The phosphorylation of Akt has been recently implicated as an additional gonadotropin-sensitive pathway involved in the induction of aromatase expression (Zeleznik et al., 2003). Many investigators have already shown that FSH activates Akt in granulosa cells (reviewed by Hunzicker-Dunn and Maizels, 2006) and we have previously shown that hFSH and hCG stimulate Akt phosphorylation in immature granulosa cells expressing a low or high density of the hFSHR or hLHR, respectively (Donadeu and Ascoli, 2005). Akt phosphorylation in granulosa cells is also stimulated by injection of hCG in female rats (Carvalho et al., 2003). We have shown here that the two mutants of the hLHR can readily enhance Akt phosphorylation as well. It is not currently known if hFSH and hCG stimulate Akt phosphorylation by similar or different pathways and if Akt phosphorylation is dependent or independent of cAMP (Hunzicker-Dunn and Maizels, 2006; Wayne et al., 2007). Since both LHR mutants are capable of stimulating Akt phosphorylation our data suggests that the hCG-induced Akt phosphorylation is independent of the activation of the phosphoinositide cascade. Moreover, the results obtained with the hLHR-LD also suggest that the hCG-induced Akt phosphorylation is not dependent on the cAMP pathway or that it requires only low levels of cAMP accumulation. Lastly, the finding that both mutants can readily mediate an increase in aromatase expression is also consistent with the notion that co-activation of the cAMP and Akt cascades may be needed for optimal aromatase induction by gonadotropins.
The experiments presented here with the two LHR mutants show that activation of the phosphoinositide cascade is the first step in gonadotropin-induced pathways that antagonize aromatase expression (Figure 7). When expressed in immature granulosa cells the hCG-induced cAMP response, the phosphorylation of Akt and the expression or epiregulin mediated by a high density of hLFF are similar to that mediated by the hLHR but the hCG-induced inositol phosphate response, the increase in EGF-like growth factor activity and the phosphorylation of ERK1/2 are all abolished. In agreement with our hypothesis a high density of hLFF allows hCG to support aromatase induction. The hCG-induced cAMP response in granulosa cells expressing a high density of hLHR-LD is substantially blunted but the phosphorylation of Akt and the expression of epiregulin are normal or only slightly reduced. Cells expressing a high density of hLHR-LD do not respond to hCG with an increase in inositol phosphates, EGF-like growth factor activity or ERK1/2 phosphorylation and hCG supports aromatase expression.
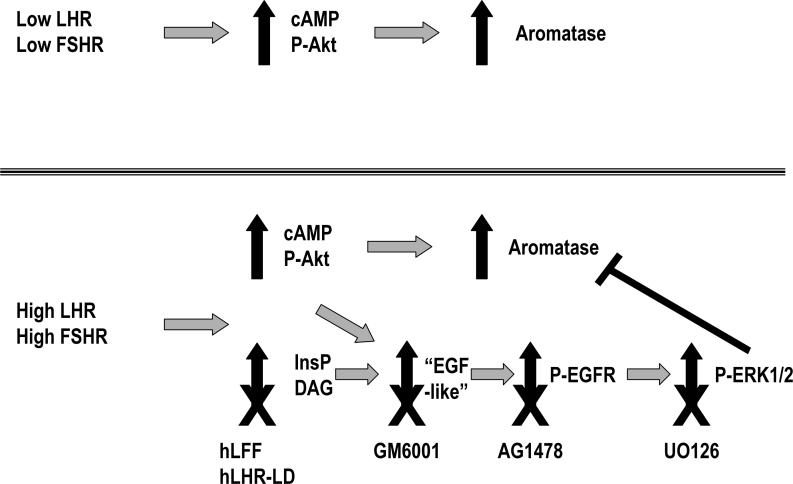
Figure 7. A model to explain the effects of low and high densities of gonadotropin receptors on aromatase expression in primary cultures of immature rat granulose cells.
When acting on their cognate receptors expressed at a low densities the gonadotropins stimulate the cAMP and Akt pathways and these stimulate aromatase expression. When acting on their cognate receptors expressed at a high densities, both gonadotropins continue to stimulate the cAMP and Akt pathways. When acting on these high receptor densities, however, the gonadotropins also activate the phosphoinositide cascade ultimately leading to a delayed increase in ERK1/2 phosphorylation, which is inhibitory to aromatase expression as indicated (Andric and Ascoli, 2006; Donadeu and Ascoli, 2005).
The location of action of the different pharmacological inhibitors used previously (Andric and Ascoli, 2006) or the receptor mutants used herein is indicated by the X's below the inhibited step. Here we show that when expressed at high densities two mutants of the hLHR (hLFF and hLHR-LD) do not support activation of the phosphoinositide cascade, an increase in bioactive EGF-like growth factors or the phosphorylation of ERK1/2 but allow hCG to increase aromatase expression. The involvement the EGF-like growth factors and the EGFR kinase as intermediates in this inhibitory pathway was also previously documented by the finding that an inhibitor of the metalloproteases that process precursors of the EGF-like growth factors (GM6001) and an inhibitor of the EGFR kinase (AG1478) prevent the gonadotropin-induced increase in ERK1/2 phosphorylation and allow gonadotropins to enhance aromatase expression even when acting on high receptor densities (Andric and Ascoli, 2006). The EGF-like growth factor(s) that mediate these actions of gonadotropins are not known and is thus indicated as “EGF-like” in the figure. Epiregulin has been shown to be a mediator of the actions of hCG in the mouse ovary (Hsieh et al., 2007; Park et al., 2004) and is the most abundant EGF-like growth factor in the rat ovary (Andric and Ascoli, 2006; Ashkenazi et al., 2005). We have previously shown that hFSH and hCG can increase epiregulin mRNA when acting on cells expressing a low or high density of their cognate receptors, but epiregulin mRNA is much higher in cells expressing a high density of gonadotropin receptors (Andric and Ascoli, 2006). Here we show that at high receptor densities the hLHR and the two mutants enhance the expression of epiregulin but only the hLHR supports an increase in bioactive EGF-like growth factors. The involvement the ERK1/2 cascade as an inhibitor of aromatase expression was previously documented by the finding that UO126 (an inhibitor of ERK1/2 phosphorylation) prevents the gonadotropin-induced increase in ERK1/2 phosphorylation but allows gonadotropins to enhance aromatase expression even when acting on high receptor densities (Andric and Ascoli, 2006).
Other compounds (not shown) such as H89, an inhibitor of PKA, and Ro-318220, an inhibitor of PKC, have also been previously shown to impair the effects of gonadotropins on the delayed phosphorylation of ERK1/2 (Andric and Ascoli, 2006). H89 cannot be used to test the involvement of this inhibitory pathway on aromatase expression because cAMP is also a stimulator of aromatase expression. Ro-318220 could not be used to study aromatase expression because is too toxic to the cells during the long incubation times needed for aromatase induction to occur (Andric and Ascoli, 2006).
InsP = inositol phosphates, DAG = diacylglycerol, P-Akt = phospho Akt, P-EGFR= phospho EGFR.
Maximal induction of aromatase by gonadotropins in immature granulosa cells expressing increasing densities of the hFSHR or hLHR occurs when the stimulated levels of cAMP are well below maximal (Donadeu and Ascoli, 2005). This finding is highly reminiscent of the low levels of cAMP needed to fully stimulate steroidogenesis in Leydig cells (Mendelson et al., 1975) and is highlighted here by the results obtained with hLHR-LD. This mutant mediates a robust induction of aromatase in spite of mediating a weak cAMP response. Because the levels of cAMP accumulation in hCG-stimulated cells expressing hLHR-LD are lower than those in cells expressing the hLHR we also measured aromatase induction in cells expressing hLHR or hLHR-LD but incubated with hFSH (to activate the endogenous FSHR) and with hCG to activate the recombinant hLHR or hLHR-LD. Under these conditions we equalized cAMP accumulation and the hLHR still mediated an hCG-induced increase in inositol phosphates and ERK1/2 phosphorylation and antagonized the actions of hFSH on aromatase induction. In contrast, hLHR-LD was still not able to mediate an hCG-induced increase in inositol phosphates, ERK1/2 phosphorylation, or to antagonize the actions of hFSH on aromatase induction.
We have previously shown that UO126 (an inhibitor of MEK, the kinase that catalyzes ERK1/2 phosphorylation) allows gonadotropins to enhance aromatase expression in cells expressing a high density of receptors (Andric and Ascoli, 2006) and the data presented here with hLFF and hLHR-LD show that when the phosphoinositide cascade is not activated, hCG fails to stimulate ERK1/2 phosphorylation but induces aromatase expression. Therefore these new results show for the first time that the gonadotropin-activated phosphoinositide cascade is upstream of ERK1/2 phosphorylation and of the inhibition of aromatase expression as shown in Figure 7.
The G protein-coupled receptor provoked activation of the ERK1/2 cascade is rather complex (reviewed by Dorsam and Gutkind, 2007) and the effects of gonadotropins on this pathway in the ovary are no exception. At low gonadotropin receptor densities only a rapid and transient increase in ERK1/2 phosphorylation is observed (Andric and Ascoli, 2006; Cottom et al., 2003; Salvador et al., 2002; Seger et al., 2001), whereas at high receptor densities an additional, delayed increase in phosphorylation becomes obvious (Andric and Ascoli, 2006). Here we show that the two LHR mutants that do not activate the phosphoinositide cascade also do not support a robust increase in the early or late increase in ERK1/2 phosphorylation.
The involvement of EGF-like growth factors and the phospho-EGFR as intermediates in the gonadotropin-induced phosphorylation of ERK1/2 and the inhibition of aromatase expression illustrated in Figure 7 is supported by several findings. First, EGF-like growth factors such as EGF, TGF-α and amphiregulin have been shown to inhibit the gonadotropin-induced expression or aromatase mRNA or enzymatic activity (Misajon et al., 1999; Wayne et al., 2007). Second, a pharmacological inhibitor of the proteases that degrade precursors of the EGF-like growth factors (GM6001) or an inhibitor of the EGFR kinase (AG1478) block the effects of gonadotropins on ERK1/2 phosphorylation and allow gonadotropins to induce aromatase expression in cells expressing a high density of receptors (Andric and Ascoli, 2006). These two inhibitors also block other ovarian actions of hCG that are mediated by EGF-like growth factors (Ashkenazi et al., 2005; Jamnongjit et al., 2005; Park et al., 2004). The effects of GM6001 suggest that, in addition to an increase in expression, an increase in the processing and release of EGF-like growth factors is an important component of this pathway (Andric and Ascoli, 2006). The results presented here show that an increase in epiregulin expression is not enough to support ERK1/2 phosphorylation and to prevent aromatase expression. Human CG increases epiregulin expression, the levels of bioactive EGF-like growth factors and ERK1/2 phosphorylation only in granulosa cells expressing the hLHR. In cells expressing hLFF or hLHR-LD, hCG increases epiregulin expression but does not increase bioactive EGF-like growth factor levels or ERK1/2 phosphorylation.
The gonadotropin-induced expression of EGF-like growth factors in the ovary is known to be a cAMP-dependent process (Park et al., 2004) but it is not known if cAMP is the only mediator of this response (Wayne et al., 2007). In primary cultures of rat granulosa cells the gonadotropin-induced expression of epiregulin increases as the density of gonadotropin receptors increases (Andric and Ascoli, 2006) but this additional increase in expression detected at higher receptor densities does not appear to be due the activation of the phosphoinositide cascade because hLFF and hLHR-LD still mediate a robust increase in epiregulin expression. Since only the hLHR mediates an increase in the levels of bioactive EGF-like growth factors we suggest that gonadotropin-induced activation of the phosphoinositide cascade is necessary to process the enhanced levels of and EGF-like growth factor precursor into its diffusible, bioactive form. This suggestion is also supported by the ability of GM6001 to prevent the gonadotropin-induced increase in ERK1/2 phosphorylation (Andric and Ascoli, 2006). In addition, the phosphoinositde cascade appears to regulate the processing of the EGF family of growth factor precursors in other cells (Blobel, 2005; Hackel et al., 1999; Yarden and Sliwkowski, 2001).
The identity of the EGF-like growth factor that mediate the effects described here is not yet known. Although changes in the expression of epiregulin mRNA are not always associated with an increase in EGF-like growth factor activity we cannot exclude the possibility that epiregulin is the EGF-like growth factor involved. The co-culture system used here to measure EGF-like growth factor activity is certainly capable of detecting epiregulin because it relies on measuring the phosphorylation of the transfected human EGFR (see Materials and Methods) and this receptor is readily phosphorylated by epiregulin (Linggi and Carpenter, 2006). Therefore, as discussed above activation of the phosphoinositide cascade may simply promote increased processing of the preformed epiregulin. Alternative explanations include the possibility that the phosphoinositide cascade promotes a more efficient translation of the epiregulin mRNA, or prevents the degradation of epiregulin. Regardless of the mechanism involved, the main conclusion drawn from these experiments is that only the hLHR and not hLFF or hLHR-LD allow hCG to increase the levels of bioactive EGF-like growth factors.
In summary the data presented here with the two hLHR mutants support the involvement of a pathway that is inhibitory to aromatase expression as shown in Figure 7. This pathway is activated only at high gonadotropin receptor densities, starts with the hydrolysis of phosphoinositides and, using the EGF network as an intermediary, ends with the phosphorylation of ERK1/2 which then acts as the most proximal mediator of the inhibition of aromatase expression. Ultimately then these results suggest that FSH stimulates aromatase expression during follicular development because the FSHR is expressed at a low density and allows FSH to activate only the stimulatory pathway whereas LH inhibits aromatase expression in the periovulatory and luteal phases because the LHR is expressed at a high density and it allows LH to activate the pathway that antagonizes aromatase expression (Figure 7).
Acknowledgments
We thank Marisa Zallocchi for construction of the hLFF chimera and Drs. Deborah Segaloff and John Koland for providing us with the hLHR-L457D mutant and the hEGFR-GFP construct. We also acknowledge the University of Iowa Gene Transfer Vector Core (supported by the NIH and by the Roy J. Carver Foundation) for the preparation of the all the recombinant adenoviral particles used in this project
*Supported by a grant from the National Institute of Child Health and Human Development (HD-28962).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
I-10 cells were chosen as test cells because they do not express the LHR, they can be maintained in the same medium as granulosa cells, they are routinely available in our laboratory, and they can be easily cultured and transfected. The fact that they are Leydig cells is irrelevant to this assay. In theory any other cell line that does not express the LHR and can be transfected with the EGFR-GFP could be used as test cells.
Addition of hFSH to cells granulosa cells expressing only the endogenous FSHR (i.e., cells infected with Ad-βgal) also enhances epiregulin expression (Andric and Ascoli, 2006) but does not lead to an increase in the phosphorylation of the EGFR-GFP in the co-culture system used here (data not shown).
These results are not surprising because G protein-mediated responses are saturable as dictated by the levels of receptors, G proteins and by their affinity for each other (Zhu et.al., 1994). It is thus possible that the endogenous FSHR has a high affinity for Gs and that all the available Gs can be activated by the endogenous FSHR. Alternatively, Gs may not be saturated but additional G proteins that antagonize the actions of Gs on adenylyl cyclase may be engaged by increasing levels of the recombinant FSHR or LHR and these may prevent a further increase in cAMP accumulation.
References
- Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M. J. Biol. Chem. 2004;279:19431–19440. doi: 10.1074/jbc.M401235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andric N, Ascoli M. Mol. Endocrinol. 2006;20:3308–3320. doi: 10.1210/me.2006-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli M. Immortalized Leydig cell lines as models for studying Leydig cell physiology. In: Payne AH, Hardy MP, editors. The Leydig Cell in Health and Disease. Humana Press; New York: 2007. pp. 373–392. [Google Scholar]
- Ascoli M, Pignataro OP, Segaloff DL. J. Biol. Chem. 1989;264:6674–6681. [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- Bebia Z, Somers JP, Liu G, Ihrig L, Shenker A, Zeleznik AJ. Endocrinology. 2001;142:2252–2259. doi: 10.1210/endo.142.6.8017. [DOI] [PubMed] [Google Scholar]
- Blobel CP. Nat. Rev. Mol. Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Bromann PA, Korkaya H, Courtneidge SA. Oncogene. 2004;23:7957–68. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- Carlone DL, Richards JS. Mol. Endocrinol. 1997;11:292–304. doi: 10.1210/mend.11.3.9900. [DOI] [PubMed] [Google Scholar]
- Carvalho CRO, Carvalheira JBC, Lima MHM, Zimmerman SF, Caperuto LC, Amanso A, Gasparetti AL, Meneghetti V, Zimmerman LF, Velloso LA, Saad MJA. Endocrinology. 2003;144:638–647. doi: 10.1210/en.2002-220706. [DOI] [PubMed] [Google Scholar]
- Conti M. Biol. Reprod. 2002;67:1653–1661. doi: 10.1095/biolreprod.102.004952. [DOI] [PubMed] [Google Scholar]
- Cottom J, Salvador LM, Maizels ET, Reierstad S, Park Y, Carr DW, Davare MA, Hell JW, Palmer SS, Dent P, Kawakatsu H, Ogata M, Hunzicker-Dunn M. J. Biol. Chem. 2003;278:7167–7179. doi: 10.1074/jbc.M203901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson MM, Michael LF, Simpson ER. Mol. Cell. Endocrinol. 1997;134:147–156. doi: 10.1016/s0303-7207(97)00178-0. [DOI] [PubMed] [Google Scholar]
- Donadeu FX, Ascoli M. Endocrinology. 2005;146:3907–3916. doi: 10.1210/en.2005-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS. Nature Reviews in Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Carlone DL, Robker RL, Richards JS. Steroids. 1997;62:197–206. doi: 10.1016/s0039-128x(96)00181-x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Richards JS. Mol. Endocrinol. 1993;7:341–354. doi: 10.1210/mend.7.3.8387157. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Richards JS. Endocrinology. 1991;129:1452–1462. doi: 10.1210/endo-129-3-1452. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Alliston TN, Buse P, Firestone GL, Richards JS. Mol. Endocrinol. 1999;13:1318–1337. doi: 10.1210/mend.13.8.0334. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Mol. Endocrinol. 2000;14:1283–1300. doi: 10.1210/mend.14.8.0500. [DOI] [PubMed] [Google Scholar]
- Hackel PO, Zwick E, Prenzel N, Ullrich A. Curr. Opin. Cell Biol. 1999;11:184–9. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- Hickey GJ, Krasnow JS, Beattie WG, Richards JS. Mol. Endocrinol. 1990;4:3–12. doi: 10.1210/mend-4-1-3. [DOI] [PubMed] [Google Scholar]
- Hipkin RW, Liu X, Ascoli M. J. Biol. Chem. 1995a;270:26683–26689. doi: 10.1074/jbc.270.44.26683. [DOI] [PubMed] [Google Scholar]
- Hipkin RW, Wang Z, Ascoli M. Mol. Endocrinol. 1995b;9:151–158. doi: 10.1210/mend.9.2.7776965. [DOI] [PubMed] [Google Scholar]
- Hirakawa T, Galet C, Ascoli M. Endocrinology. 2002;143:1026–1035. doi: 10.1210/endo.143.3.8702. [DOI] [PubMed] [Google Scholar]
- Hirsch B, Kudo M, Naro F, Conti M, Hsueh AJW. Mol. Endocrinol. 1996;10:1127–1137. doi: 10.1210/mend.10.9.8885247. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Mol. Cell. Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Maizels ET. Cell. Signal. 2006;18:1351–1359. doi: 10.1016/j.cellsig.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamnongjit M, Gill A, Hammes SR. Proc. Natl. Acad. Sci. USA. 2005;102:16257–16262. doi: 10.1073/pnas.0508521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwintkiewicz J, Cai Z, Stocco C. Mol. Endocrinol. 2007;21:933–947. doi: 10.1210/me.2006-0446. [DOI] [PubMed] [Google Scholar]
- Linggi B, Carpenter G. Trends Cell Biol. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- McDonald CA, Millena AC, Reddy S, Finlay S, Vizcarra J, Khan SA, Davis JS. Mol. Endocrinol. 2006;20:608–618. doi: 10.1210/me.2005-0245. [DOI] [PubMed] [Google Scholar]
- Mendelson C, Dufau M, Catt K. J. Biol. Chem. 1975;250:8812–8823. [PubMed] [Google Scholar]
- Misajon A, Hutchinson P, Lolatgis N, Trounson AO, Almahbobi G. Mol. Human Reprod. 1999;5:96–103. doi: 10.1093/molehr/5.2.96. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Shiraishi K, Welsh T, Ascoli M. Mol. Endocrinol. 2006;20:619–630. doi: 10.1210/me.2005-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Liu X, Ascoli M. J. Biol. Chem. 1999;274:25426–25432. doi: 10.1074/jbc.274.36.25426. [DOI] [PubMed] [Google Scholar]
- Park J-Y, Su Y-Q, Ariga M, Law E, Jin SLC, Conti M. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Richards JS. Endocr. Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- Richards JS. Mol. Endocrinol. 2001a;15:209–218. doi: 10.1210/mend.15.2.0606. [DOI] [PubMed] [Google Scholar]
- Richards JS. Endocrinology. 2001b;142:2184–2193. doi: 10.1210/endo.142.6.8223. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC. Rec. Progr. Horm. Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M. Endocrinology. 2002;143:2986–2994. doi: 10.1210/endo.143.8.8976. [DOI] [PubMed] [Google Scholar]
- Seger R, Hanoch T, Rosenberg R, Dantes A, Merz WE, Strauss JF, III, Amsterdam A. J. Biol. Chem. 2001;276:13957–13964. doi: 10.1074/jbc.M006852200. [DOI] [PubMed] [Google Scholar]
- Shi H, Segaloff DL. Mol. Endocrinol. 1995;9:734–744. doi: 10.1210/mend.9.6.8592519. [DOI] [PubMed] [Google Scholar]
- Shin S-I. Endocrinology. 1967;81:440–448. doi: 10.1210/endo-81-3-440. [DOI] [PubMed] [Google Scholar]
- Shinozaki H, Fanelli F, Liu X, Butterbrodt J, Nakamura K, Segaloff DL. Mol. Endocrinol. 2001;15:972–984. doi: 10.1210/mend.15.6.0661. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Ascoli M. Exp. Cell Res. 2007;314:25–37. doi: 10.1016/j.yexcr.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi K, Ascoli M. Endocrinology. 2006;147:3419–3427. doi: 10.1210/en.2005-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Annu. Rev. Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- Steiner AL, Parker CW, Kipnis DM. J. Biol. Chem. 1972;247:1106–1113. [PubMed] [Google Scholar]
- Su Y-Q, Nyegaard M, Overgaard MT, Qiao J, Giudice LC. Biol. Reprod. 2006;75:859–867. doi: 10.1095/biolreprod.106.052613. [DOI] [PubMed] [Google Scholar]
- Wayne CM, Fan H-Y, Cheng X, Richards JS. Mol. Endocrinol. 2007;21:1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Saxena D, Little-Ihrig L. Endocrinology. 2003;144:3985–3994. doi: 10.1210/en.2003-0293. [DOI] [PubMed] [Google Scholar]
- Zhu X, Gilbert S, Birnbaumer M, Birnbaumer L. Mol. Pharmacol. 1994;46:460–469. [PubMed] [Google Scholar]