Abstract
Pneumococcal surface protein (PspA) is a virulence factor expressed by all clinical isolates of Streptococcus pneumoniae. PspAs are variable in structure and have been grouped into clades and cross-reacting families based on sequence similarities and immunologic cross-reactivity. At least 98 percent of PspAs are found in PspA families 1 or 2. PspA has been shown to interfere with complement deposition on pneumococci, thus reducing opsonization and clearance of bacteria by the host immune system. Prior studies using pooled human sera have shown that PspA interferes with C3 deposition on a single strain of S. pneumoniae, WU2, and that mouse antibody to PspA can enhance the deposition of C3 on WU2. The present studies have demonstrated that these previous findings are representative of most normal human sera and each of 7 different strains of S. pneumoniae. It was observed that PspAs of PspA families 1 and 2 could inhibit C3 deposition in the presence of immunoglobulin present in all but 3 of 22 normal human sera. These studies have also demonstrated that rabbit and human antibody to PspA can enhance the deposition of C3 on pneumococci expressing either family 1 or 2 PspAs and either capsular types 2, 3, or 11. A vaccine candidate that can elicit immunity that neutralizes or compensates for S. pneumoniae’s ability to thwart host immunity would be of value.
Keywords: Streptococcus pneumoniae, antibody, Complement, C3, Pneumococcal surface protein A, PspA
1. Introduction
Streptococcus pneumoniae is a major health concern worldwide, causing pneumonia, bacteremia, meningitis, and otitis media. Its major disease burden is in children, the elderly, and patients with HIV and other immunosuppressive conditions [1–3]. Vaccines containing polysaccharide antigens are commercially available but have some limitations. For example, a 23-valent polysaccharide-based vaccine was 75% efficacious against invasive disease in immunocompetent individuals over 65 years of age [1]. However, children younger than 2 years do not generate good immune responses to these T-cell independent pneumococcal polysaccharides [2, 4]. A 7-valent polysaccharide-protein conjugate vaccine licensed in 1999 has been shown to be immunogenic in young children and highly efficacious against invasive disease caused by the serotypes covered by the vaccine [4, 5]. The fact that protection elicited by the vaccine is restricted to the serotypes included in these vaccines could be an issue as there is a large diversity among the capsular polysaccharides produced by S. pneumoniae [6] and in some countries as many as 66 percent of the childhood strains would not be covered [7, 8]. Additionally, a shift towards prevalence of serotypes not included in the current commercially available vaccines is a concern [9, 10].
Several pneumococcal proteins have been under investigation as potential vaccine candidates including pneumococcal surface protein A (PspA), which is a virulence factor expressed by all clinical S. pneumoniae isolates [8, 11–13]. It consists of five domains: i) a signal peptide, ii) an alpha-helical domain, iii) a proline-rich domain believed to span the cell wall, iv) a choline-binding domain that anchors the protein to the cell surface and v) a short C-terminal tail [14–16]. The alpha-helical region is variable in length and amino acid sequence, but is very cross-reactive [17, 18]. PspA proteins have been grouped into three families encompassing 6 different clades based on the C-terminal 100 amino acids of the sequence of this region. Family 1 is comprised of clade 1 and 2, and family 2 is comprised of clade 3, 4, and 5, and family 3 only has clade 6. Families were discriminated by having less than 55% sequence identity and PspA proteins within the same clade have more than 90% sequence identity [14]. PspA families can also be distinguished with adsorbed polyclonal antisera [12] or by genotyping using family specific primer pairs [13, 14]. S. pneumoniae strains expressing family 1 or 2 PspA proteins constitute 98 % of clinical isolates whereas strains with family 3 PspA proteins are rarely identified [8, 12, 14].
The importance of the innate immune system and in particular the role of the complement system in host defense against S. pneumoniae infection has been extensively studied in animal models [19–21]. Activation of the complement system leads to deposition of complement component C3 fragments on the activating surface [22]. PspA has been shown to interfere with complement deposition on the pneumococcal surface [23–25]. Sera from mice infected with a S. pneumoniae capsular type 3 strain expressing family 1 clade 2 PspA protein had higher levels of circulating C3, than mice infected with a PspA− strain [26]. The role of PspA in protecting pneumococci from C3-dependent host immunity was shown in the same study where C3-deficient mice were observed to be as susceptible to infection with the PspA-negative mutant strain as to infection with its PspA-expressing parent strain.
Active immunization with family 1 PspA proteins, family 2 PspA proteins, or truncated fragments thereof, elicited protection against lethal pneumococcal sepsis in mice [27–31]. Passive immunization with antibodies to PspA also conferred protection against lethal sepsis in mice. Using PspA-specific monoclonal antibodies, protective epitopes have been mapped to the alpha helical region of PspA [30, 32]. These findings suggest that the alpha-helical region could elicit immune responses protective against S. pneumoniae infection independent of capsule type. Furthermore, PspA was found to be well tolerated and immunogenic in a phase 1 clinical trial where human volunteers were injected with a family 1 clade 2 recombinant PspA from strain Rx1 [33]. The ability of sera from the PspA/Rx1 immunized subjects to protect mice from otherwise fatal S. pneumoniae challenge was significantly increased post-immunization [34].
Prior studies have shown that mouse antibody to clade 2 Rx1 PspA can enhance complement deposition on WU2 pneumococci expressing clade 2 PspA [21]. This result has provided evidence for an attractive explanation of how antibodies to PspA are able to protect against pneumococcal infection. Although virtually all strains of pneumococci express PspA, it is of variable structure, and it is not clear that PspA is adequately exposed on the surface of all strains to permit antibody to PspA to enhance complement deposition.
Except for the passive protection of mice with pre- and post-immune human sera [18], there is no laboratory surrogate of protection elicited in man by immunization with PspA. In the passive protection study with human sera, the protective potency of antibody to PspA (as determined by ELISA) varied more than 4-fold among sera from 5 immunized humans [18]. Other studies with minimally immunized mice have shown that the mice that are protected are not necessarily those making the highest amounts of antibody [35]. These findings suggest that all antibodies to PspA are not equally protective, making an ELISA a poor surrogate of protection. The need for an in vitro surrogate for immunity to PspA is further emphasized by the fact that efforts to mediate killing of pneumococci by phagocytes in vitro have not been successful. Using the heparanized whole blood assay used to study antibody-mediated phagocytic killng of group A streptococci [36], it was possible to show antibody mediated killing with Ab to capsule, but not with Ab to PspA [37]. Moreover the opsonophagocytosis and killing assay commonly used to measure functionality of antibody to capsule, does not detect opsonophagocytosis and killing mediated by antibody to PspA (J Russell and DE Briles, unpublished).
To determine whether significant complement deposition by antibodies to pneumococci is a general property of antibody to PspA the present studies have examined 1) antibodies to PspA produced in rabbits and humans, 2) antibodies to three different PspA clades, and 3) the effects of antibodies to PspA on the levels of C3 deposited on strains of three capsular types, collectively expressing 5 PspA clades. These studies have also examined the degree to which inhibition of complement deposition by PspA on pneumococci can be observed in the presence of normal immunoglobulins in the serum from 22 different individuals. The information gained suggests that in vitro complement deposition may be able to be used as an in vitro surrogate of protective immunity elicited in man by immunization with PspA.
2. Results
2.1. PspA decreased deposition of complement on pneumococci in the presence of immunoglobulin from most normal human sera
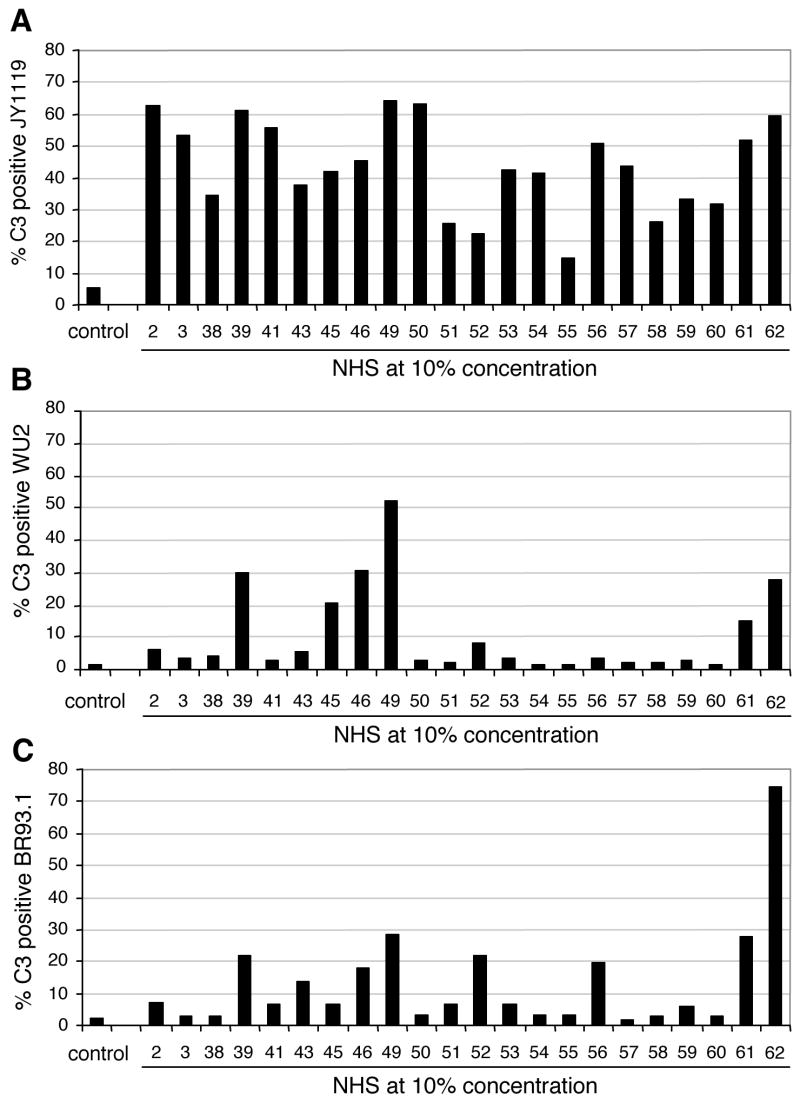
Sera from 22 healthy adults were collected, heat-inactivated, and examined for their ability to mediate C3 deposition on pneumococci. To reduce sample variation due to potential differences in human complement proteins in different individuals, baby rabbit complement was used as an exogenous complement source in this study. S. pneumoniae strains were incubated with heat-inactivated normal human serum (NHS) followed by incubation with baby rabbit complement. C3 deposition on the bacteria was quantified by flow cytometric analyses [34]. A capsular type 3 strain WU2 expressing family 1 clade 2 PspA, strain BR93.1, an isogenic derivative of WU2 expressing family 2 clade 3 PspA, and strain JY1119, a PspA-negative mutant of WU2, were used (Table 1). In the absence of heat-inactivated NHS the background levels of C3 deposition were low on all three strains (left-most column of Fig. 1A, 1B and 1C). By contrast, when pneumococci were incubated with heat-inactivated NHS, the fraction of JY1119 bacteria positive for C3 varied from sample to sample, ranging from 15% to over 60% (Fig. 1A). For the majority of serum samples, the complement deposition on strains WU2 (Fig. 1B) and BR93.1 (Fig. 1C) was lower than on the PspA− strain JY1119 (Fig 1A). PspA expressed on the pneumococcal surface has previously been shown to inhibit complement deposition [21]. The results in Figures 1A, 1B, and 1C confirmed that observation and demonstrated that PspA’s inhibitory effect on complement deposition could be seen in the presence of many different normal human sera and what ever pre-existing antibody they contain.
Table 1.
Strains of Streptococcus pneumoniae used in these studies
| Strain | Genetic Background | Capsular type | PspA Clade | PspA family | Reference |
|---|---|---|---|---|---|
| WU2 | Wild type | 3 | 2 | 1 | [54] |
| BR93.1a | WU2 | 3 | 3 | 2 | [25] |
| JY1119a | WU2 | 3 | nulla | nulla | [55] |
| 3JYP2670 | Wild type | 3 | 4 | 2 | [52] |
| ATCC6303 | Wild type | 3 | 5 | 2 | [46] |
| 3JY4182-95 | Wild type | 3 | 1 | 1 | [46] |
| D39 | Wild type | 2 | 2 | 1 | [52, 56] |
| BG7941 | Wild type | 11 | 5 | 2 | [57]b |
Strains BR93.1 and JY1119 are isogenic mutants of strain WU2. JY1119 does not express any PspA [55].
BG7941, from patient blood, was provided by Barry M. Gray from his collection of childhood strains of S. pneumoniae isolated in Birmingham, AL. The strain was used strain in 2002 [57], but it was not listed by name.
Figure 1.
Wide-ranging levels of C3 deposition on S. pneumoniae with normal human sera from healthy adult donors. Sera samples from 22 donors were heat-inactivated and incubated at 10% concentration with strains (A) JY1119 (PspA-negative), (B) WU2 (PspA clade 2) and (C) BR93.1 (PspA clade 3) followed by incubation with baby rabbit complement as exogenous complement source. The control samples were incubated with buffer instead of heat-inactivated NHS followed by incubation with baby rabbit complement. Quantification of C3 deposition was measured by flow cytometry.
Complement deposition in the presence of three of the heat-inactivated NHS samples did not follow the pattern of less C3 on PspA-positive strains WU2 and BR93.1 than on PspA-negative strain JY1119. The sera from donors 52 and 62 led to similar levels of C3 deposition on BR93.1 as on JY1119 (Fig 1A and 1C), while C3 deposition on WU2 was lower (Fig 1B). Serum from donor 49 resulted in comparable levels of C3 on both JY1119 and WU2, while less C3 was on BR93.1 (Fig 1A and 1B). The fact that some sera facilitated much more complement deposition on PspA expressing pneumococci than did other sera could be explained if the sera differed in their levels of antibody to PspA or other pneumococcal antigens. The fact that different amounts of C3 were deposited by some sera on BR93.1 the isogenic mutant of WU2, as on wild type strain WU2 is probably an indication of differences in the specificity of antibodies to different PspA proteins in different sera.
To determine whether the amount of C3 deposited on the pneumococcal surface was correlated to the level of specific antibody to the PspA protein binding its surface, the amount of antibodies to Rx1 or EF3296 PspA protein in human sera samples were plotted against C3 deposition on strains WU2 or BR93.1 (Fig 2A and 2B). The results showed that there is positive correlation between the level of specific antibody to PspA and the amount of C3 deposited on the bacterial surface, ie. the more antibodies to PspA present in the human serum, the more C3 generated on the pneumococcal surface.
Figure 2.
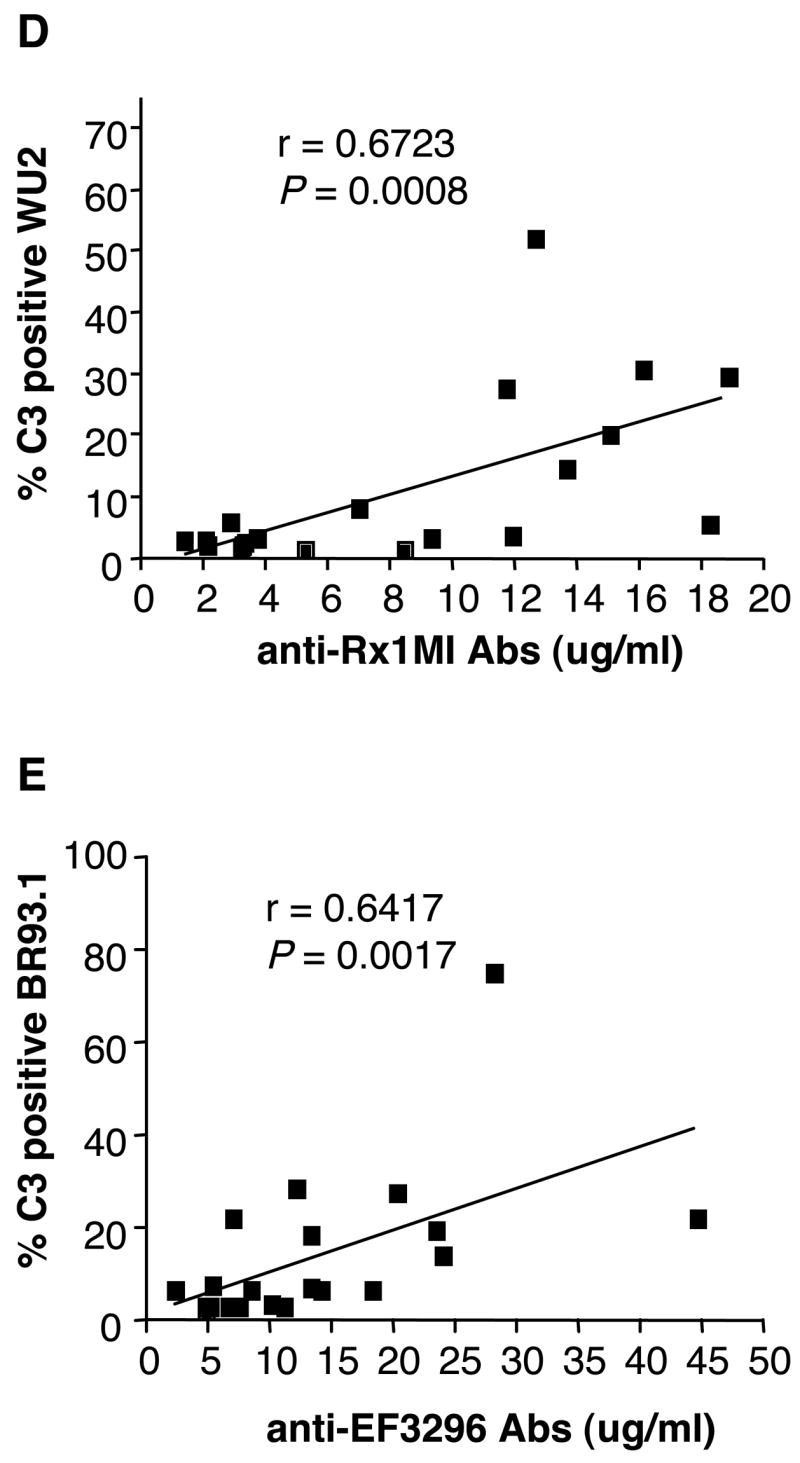
Correlation of antibody level and C3 deposition. Correlations between the level of anti-Rx1 and C3 deposition on WU2 (A), or anti-EF3296 and C3 deposition on BR93.1 (B) were shown. The nonparametric (Spearman) correlation r-value and two-tailed P value were indicated on the graphs.
2.2. Rabbit immune sera against family 1 or 2 PspA facilitated high levels of C3 deposition
To directly investigate the effects that antibodies to PspA might have on complement deposition, S. pneumoniae strains were incubated with heat-inactivated rabbit sera raised against the truncated recombinant PspA proteins Rx1, EF3296 and EF5668, representing PspA clades 2, 3 and 4, respectively, prior to incubation of the bacteria with baby rabbit complement. Antisera to these three PspA clades were chosen because earlier studies of PspA diversity and cross-reactivity had indicated that antibodies to these three clades should collectively have a good chance of reacting with all family 1 and family 2 PspAs [17, 38].
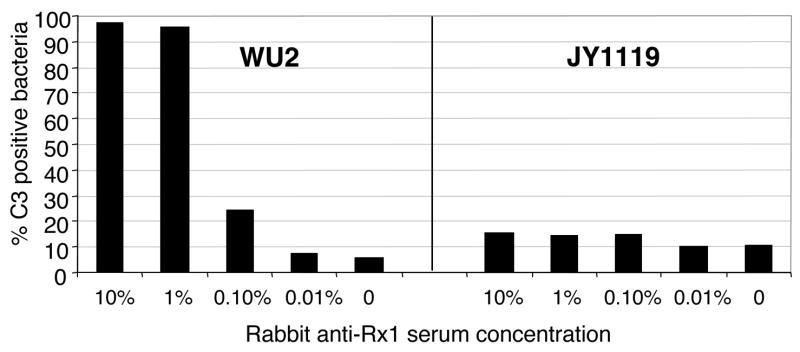
When strains WU2 and JY1119 were incubated with baby rabbit complement in the absence of rabbit anti-sera, minimal background levels of C3 deposition were detected (Fig 3). Incubation with increasing concentrations of anti-Rx1 serum did not lead to increases in C3 deposition on the PspA-negative strain JY1119. In contrast, incubation of strain WU2, expressing clade 2 PspA, with the clade 2-specific anti-Rx1 serum led to a strong serum concentration-dependent increase in surface-bound C3 (Fig 3). With 1% serum, more than 90% of WU2 were C3 positive. These findings demonstrated that the generation of anti-Rx1 antibodies by immunization with recombinant Rx1 PspA protein was sufficient to mediate high levels of C3 deposition on WU2.
Figure 3.
C3 deposition on S. pneumoniae was facilitated by rabbit antibodies to PspA in a dose-dependent manner. Strains WU2 (PspA clade 2) and JY1119 (PspA-negative) were incubated with different concentrations of heat-inactivated rabbit anti-Rx1 immune serum followed by incubation with 10% baby rabbit complement. The proportion of bacteria with C3 deposited was determined by flow cytometry.
Studies were also performed to test whether rabbit antibodies elicited to PspAs of clades 2, 3 or 4 mediated complement deposition on a panel of capsule serotype 3 S. pneumoniae strains expressing PspA proteins of clades 1 through 5 (Table 2). Incubation with each of the antisera to PspA was able to enhance C3 deposition on pneumococci expressing PspA of the same family as the immunizing PspA. These data also illustrated within family cross-reactivity of the antibodies to PspA since the anti-Rx1 (clade 2 PspA) serum facilitated high C3 deposition on the clade 1 expressing strain 3JY4185-95; and the anti-EF5668 (clade 4 PspA) serum facilitated high C3 deposition on the clade 5 expressing strain ATCC6303 (Table 2).
Table 2.
C3 deposition on S. pneumoniae strains expressing PspA proteins of clades 1 through 5 and capsular types 2, 3, and 11
| % C3 positive bacteria
|
||||||
|---|---|---|---|---|---|---|
| Strain | Capsule type | PspA family | PspA Clade | Antiserum | 1% anti-PspA + baby rabbit C′ | baby rabbit C′ alone |
| JY1119 | 3 | None | None | Anti-clade 2 | 14.1 | 10.5 |
| 3JY4182-95 | 3 | 1 | 1 | Anti-clade 2 | 85.2 | 0.6 |
| WU2 | 3 | 1 | 2 | Anti-clade 2 | 95.7 | 5.7 |
| BR93.1 | 3 | 2 | 3 | Anti-clade 3 | 96.4 | 8.6 |
| 3JYP2670 | 3 | 2 | 4 | Anti-clade 4 | 54.2 | 2.3 |
| ATCC6303 | 3 | 2 | 5 | Anti-clade 4 | 88.9 | 3.7 |
| D39 | 2 | 1 | 2 | Anti-clade 2 | 61.6 | 3.2 |
| BG7941 | 11 | 2 | 5 | Anti-clade 4 | 59.5 | 1.9 |
Note: S. pneumoniae strains were incubated with 1% heat-inactivated rabbit anti-PspA sera followed by baby rabbit complement (C′). The proportion of C3-positive bacteria was determined by flow cytometric analysis. Anti-PspA sera were generated in rabbits after immunization with the PspA proteins Rx1, EF3296 and EF5668 representing clades 2, 3 and 4 in the presence of Freund’s adjuvant.
Like the antiserum to Rx1, neither the anti-EF3296 PspA nor the anti-EF5668 PspA serum increased C3 deposition on PspA negative strain JY1119 (data not shown), confirming that the deposition of C3 seen on the PspA positive strains was the result of antibody specific for PspA. The fact that baby rabbit complement alone resulted in very little complement deposition even on strain JY1119 as compared to immune rabbit serum (Table 2, Fig 3) strongly suggests that the complement deposition observed in this assay is largely antibody-dependent.
The failure of the immune sera to enhance complement deposition on JY1119 suggests that these rabbit sera lack naturally occurring antibody that can efficiently activate C3 deposition onto pneumococci. To further address this question, all strains examined above were incubated with heat-inactivated 10% pre-immune normal rabbit serum followed by baby rabbit complement. Less than 10% of bacteria were positive for C3 (data not shown) demonstrating that non-immune normal rabbit serum does not mediate high levels of complement deposition on pneumococci.
The S. pneumoniae strains described above were all capsular type 3 strains. To see if antibodies to PspA could also facilitate C3 deposition on other capsular types, a capsular type 11 strain BG7941 and a capsular type 2 strain D39 were included in these studies. D39, expressing a clade 2 PspA, was incubated with rabbit anti-Rx1 serum, while BG7941, expressing a clade 5 PspA, was incubated with anti-EF5668 serum. The presence of antibodies to PspA strongly increased C3 deposition on both strains demonstrated that heat-inactivated sera contained antibodies to PspA that could enhance C3 deposition on strains of capsular types other than type 3 (Table 2). About 60% of the bacteria of the capsular type 11 and 2 strains were positive for C3 after treatment with 1% dilutions of the indicated antisera to PspA (Table 2).
2.3. Immunization of human subjects with PspA generates antibodies that can increase C3 deposition on S. pneumoniae
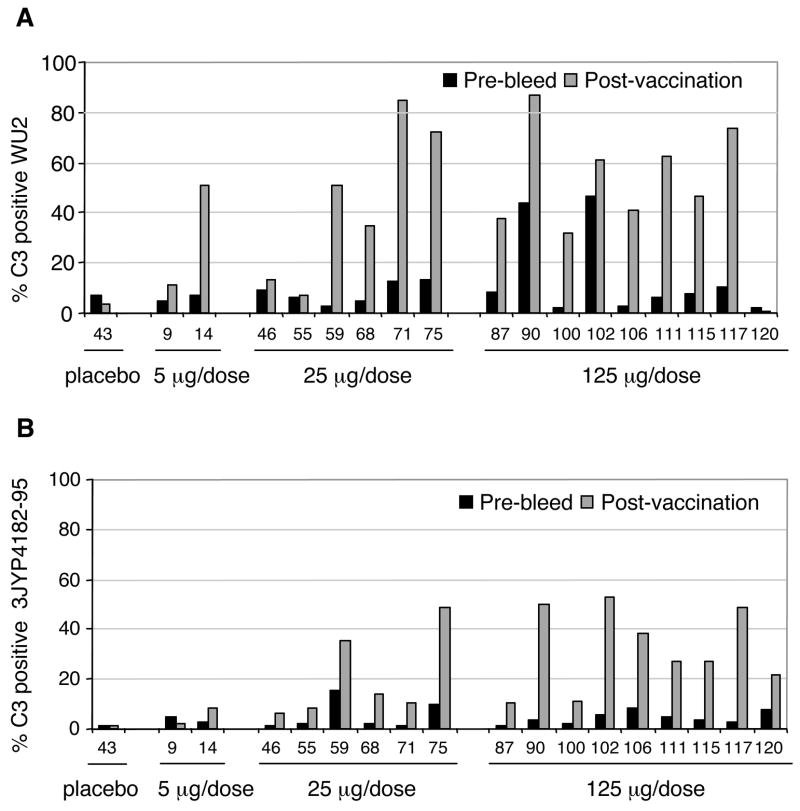
In a Phase I clinical trial, human subjects were immunized with recombinant Rx1 PspA protein [33]. Pre-bleed and post-vaccination sera from 17 Rx1-recipients were examined for their ability to increase C3 deposition on two S. pneumoniae strains, 3JY4182-95(capsular type 3, clade 1 PspA) and WU2 (capsular type 3, clade 2 PspA), respectively. C3 deposition on WU2 was substantially higher when the post-immune sera of 13 subjects were compared to the paired pre-immune sera (Fig. 4A). For subjects 59, 100 and 106 this difference was most pronounced; very little C3 deposition was detected with the pre-immune sera, but C3 deposition with post-vaccination sera showed more than a 10-fold increase.
Figure 4. Comparison of C3 deposition on pneumococcal surface with pre- and post-immune human sera from subjects vaccinated with Rx1 protein.
Sera were collected from subjects who received the placebo control or Rx1 protein at immunization doses of 5 μg/dose, 25 μg/dose and 125 μg/dose. Pneumococcal strains WU2 (PspA clade 2, panel A) and 3JY4182-95 (PspA clade 1, panel B) were incubated with 10% heat-inactivated pre- or post-vaccination human sera followed by incubation with baby rabbit complement. The bars showing percent of bacteria labeled with C3 were each based on 10,000 bacteria observed by flow cytometry. Thus, the bars are highly precise and the differences between pre- and post-sera were statistically significant in every case except for those few where neither the pre-or post-immune sera mediated complement deposition on more than 15% of bacteria. Using a Wilcoxon matched-pairs signed-ranks test we did pair-wise comparisons on complement deposition mediated by pre- and post-immune sera from immunizations with 25 μg and 125 μg recombinant Rx1 PspA. For both panels A and B, the greater complement deposition by the post-immune sera was significant at P < 0.0001 by the Wilcoxon matched-pairs signed-rank test.
C3 deposition on the clade 1 PspA-expressing strain 3JY4182-95 showed the same trend as observed for WU2 of higher C3 deposition with sera from subjects vaccinated with recombinant Rx1 clade 2 PspA protein than with pre-immune sera (Fig 3). Although the total C3 deposition was a little less than was observed with WU2, the fold increase comparing the post immune with the pre-immune sera was at least as great as was seen with WU2. .
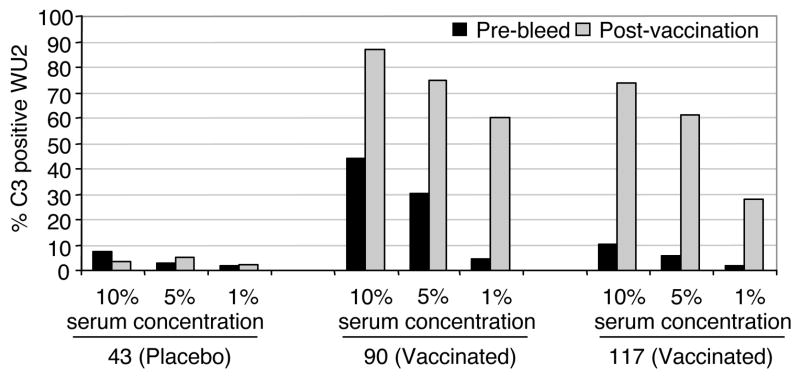
To investigate the effect of serum concentration on the amount of C3 deposition, pre- and post-immune sera from three trial participants were used at concentrations of 1%, 5% and 10% (Fig 5). Subjects 90 and 117 were from the group of vaccinated patients and subject 43 was from the patients that received the alum-only placebo. As the post-immune sera from the vaccinated participants increased in concentration from 1% to 5%, there was an increase in the proportion of C3 positive bacteria. Increasing the sera concentration again to 10% further increased in C3 deposition. At each of these three concentrations examined there were more pneumococci with C3 deposited as compared to the pre-immune sera from the same individuals using the equivalent concentrations. Sera from placebo subject 43 had background levels of C3 deposition at the 1%, 5% and 10% concentrations from each of the paired sera samples (less than 8% of the bacteria were C3 positive). These results (Fig. 5) showed that C3 deposition on pneumococci was serum dose-dependent and also PspA vaccine-dependent.
Figure 5.
C3 deposition increased with increasing the post-vaccination serum concentration. Different amounts of pre-bleed and post-vaccination sera from clinical trial subjects 43, 90 and 117 were incubated with strain WU2. Subject 43 had received a placebo vaccination and subjects 90 and 117 had been vaccinated with 125 μg/dose of Rx1 protein. The proportion of bacteria with C3 deposition was determined by flow cytometry.
3. Discussion
The essential contribution of complement in protection against pneumococcal disease is well established [39–42]. Opsonization by complement is known to enhance the clearance of pneumococci from infected animals. Mice treated with cobra venom factor or C3-deficient mice are more susceptible to pneumococcal infection than mice with an intact immune system [23, 41]. Antibodies against S. pneumoniae play an important role in clearance by enhancing opsonization [43]. Studies reported here investigated PspA-specific antibody-dependent deposition of the complement component C3 on S. pneumoniae in vitro. The S. pneumoniae strains used were all highly resistant to C3 deposition in the absence of antibodies directed against S. pneumoniae.
These studies used complement from baby rabbits as the complement source since it has very little if any antibody reactive with pneumococci [44, 45]. Prior incubation of bacteria with heat-inactivated PspA-specific sera generated by immunizing adult rabbits, resulted in an increase in C3 deposition with increasing concentrations of immune rabbit sera. C3 deposition was attributed to the presence of PspA-specific antibodies as strain JY1119, the PspA-negative mutant of strain WU2, had very little rabbit C3 deposited on its surface by pre-treatment with heat-inactivated immune rabbit sera. There was also a positive correlation between the level of the clade-specific antibodies to PspA in human serum and the amount of C3 deposited on pneumococcal strains expressing PspA of the same clade.
One of the strongest findings of the paper was the observation that immunization of human volunteers with PspA enhanced not only the antibody levels in their sera [11, 12, 33] and their ability to protect mice from pneumococcal infection [46], but also their ability to mediate complement deposition on pneumococci. Antibodies to PspA are cross-reactive [11, 12, 33] and cross-protective [29, 46, 47] within and sometimes between PspA families. In this paper it was demonstrated that this cross-reactivity within PspA families extends to the biologically important mediation of C3 deposition on intact pneumococci. Of the 7 different strains used only two, D39 and BR93.1, expressed a PspA identical to one of the PspAs used to immunize the patients or rabbits.
The ability of antibody to PspAto mediate deposition of C3 has highlighted a biological function of antibodies to PspA, which seems likely to be more relevant than the data gained from ELISA measurements of concentrations of antibodies to PspA. Prior studies with the immune sera from patients from the same phase I trial demonstrated that the immune sera could protect mice from otherwise fatal pneumococcal infection [46]. It seems likely that the protection observed may have been largely through the ability of the elicited antibodies to enhance complement deposition on pneumococci. The potential importance of C3 deposition mediated by antibodies to PspA was further emphasized by the observation that the effect was not restricted to capsular type 3 strains as antibodies to PspA also enhanced C3 deposition on capsular type 2 and type 11 strains. These were encouraging findings since for PspA to have utility as a vaccine component it is vital that immunization with PspA proteins elicit protection against strains expressing non-identical PspA proteins on multiple capsular types. It seems highly likely that protection elicited by immunization with PspA is dependent primarily on complement dependent opsonization of pneumococci.
By examining the sera of 22 healthy non-PspA-immunized adults, a better understanding of the ability of pre-existing anti-pneumococcal antibodies to result in C3 deposition on pneumococci was gained. The human sera examined were heat-inactivated to destroy their own complement activity; and baby rabbit complement was used as a uniform complement source for all sera in all experiments. As very little C3 was deposited in the absence of human sera, the C3 deposited in these studies was attributable to antibodies against S. pneumoniae present in the normal human sera. The variation in the levels of C3 deposition among the 22 normal sera suggested that the sera differed in their amounts of anti-pneumococcal antibody and confirmed the expectation that the general adult population has been naturally, and variably, primed by pneumococci [48–51].
Much higher levels of C3 were deposited by most sera on the PspA− strain JY1119 than on its isogenic PspA+ strains WU2 or BR93.1. This finding is consistent with earlier studies demonstrating that PspA can reduce complement deposition on pneumococci [21, 23, 25]. The accelerated deposition of baby rabbit C3 on the PspA− pneumococci was only seen in the presence of normal human serum. This result indicated that this deposition was antibody-dependent and most likely was occurring through the classical pathway. Our observation is consistent with our previous study demonstrating that PspA inhibits classical pathway complement activation [21], and with an earlier report that complement deposited on pneumococci is via the classical pathway [20]. It is likely that the antibody to PspA acts by two mechanisms. One is to interfere with PspA’s abilty to block C3 activation. The other mechanism is to actually bind C1q and activate complement through the classical pathway.
Some exceptions were observed, however. Sera from donors 52 and 62 led to deposition of C3 on strain BR93.1 as efficiently as on strain JY1119, but not on strain WU2. It is probable that these individuals had antibodies specific to a clade 3 PspA like that expressed by strain BR93.1. Similarly, donor 49 could have had antibodies that recognized a clade 2 PspA protein like on strain WU2. The fact that there is positive correlation between the amount of clade-specific antibodies to PspA and the amount of C3 deposited on pneumococcal strains supported the above speculations. Unfortunately, insufficient amounts of these sera were available for detailed studies of the differences in the anti-pneumococcal specificity of the antibodies in the different sera.
It has been shown that even though protection against pneumococci can clearly be transferred by antibody to PspA, the levels of antibody to PspA as determined by ELISA do not always correlate well with the level of protection in immunized animals [18, 31, 52]. Thus, the determination of antibody levels in sera by ELISA assay does not appear to necessarily distinguish the functional antibodies with biological relevance from total level of antibodies to PspA. To develop vaccines that can protect against pneumococci, it is highly desirable to have a surrogate in vitro assay for assessing protection that can be used to test for efficacy against a diversity of pneumococcal strains. To date, the only assay available for antibodies to PspA, is passive transfer of sera to mice prior to lethal challenge [18]. Considering the strong evidence for the importance of complement in protection against pneumococcal disease [39–41], it seems likely that in vitro complement deposition mediated by antibody may be able to be developed into a surrogate assay for the prediction of protection induced by surface antigens of pneumococci.
4. Materials and methods
4.1. Bacterial strains
The S. pneumoniae strains that were used in this study are shown in table 1. Strains were grown, and stored as described previously [25] except for BR93.1 and JY1119, which carry erythromycin resistance genes and stocks were maintained by growth in broth containing 300ng/ml of erythromycin (Calbiochem, La Jolla, CA).
4.2. C3 deposition assay
S. pneumoniae strains were grown in Todd-Hewitt broth (Becton Dickinson, Sparks, MD) with 0.5% yeast extract (Becton Dickinson, Sparks, MD) to mid logarithmic growth phase at 37°C with 5% CO2. Bacteria were harvested by centrifugation for two minutes at 10000 rpm in a microfuge, washed twice with phosphate buffered saline (PBS) (Invitrogen, Grand Island, NY), and the bacterial pellet was suspended in PBS to approximately 5 × 109 cfu/ml. 20 μl or 1 × 108 colony forming units (CFU) of the bacterial suspension were incubated with 80 μl of heat-inactivated serum for 30 min at 37°C in the presence of 5% CO2. The sera were heat-inactivated by incubation at 56°C for 30 min to destroy the activity of serum complement and diluted to pre-determined concentrations. The bacteria were then washed in PBS, the pellet suspended in 10% baby rabbit complement (Cedarlane, Hornby, Canada) in gelatin-veronal-buffer with magnesium and calcium (Sigma, St. Louis, MO) and incubated at 37°C in the presence of 5% CO2 for 30 min. The bacteria were then washed twice with PBS and incubated with fluorescein-isothiocyanate (FITC) conjugated goat anti-rabbit C3 antibody (1:20 dilution in PBS; ICN, Costa Mesa, CA) at room temperature for 30 min. To remove unbound fluorescent antibody, bacteria were washed twice with PBS and finally suspended in 500 μl of 1% paraformaldehyde (Electron Microscopy Sciences, Washington, PA)). The percentage of FITC-positive bacteria was determined by flow cytometry (EPICS® XL-MCL, Beckman coulter). Bacteria were considered positive if the fluorescence was above background, which included 98–100% of the bacteria examined in absence of fluorescent antibody to complement following a procedure developed in previous studies [34, 53].
4.3. Immune rabbit sera
New Zealand White rabbits were injected intramuscularly with 5 μg/dose of recombinant PspA-proteins (Sanofi Pasteur) adjuvanted with Complete Freund’s adjuvant. Two subsequent immunizations were administered with Incomplete Freund’s adjuvant at two weeks intervals. Each immunization was in a volume of 500 μl. Blood was collected two weeks after the final immunization. The following truncated PspA proteins were used: Rx1 ( family 1, clade 2), EF3296 (family 2, clade 3), and EF5668 (family 2, clade 4). Expression and purification of these proteins was described previously [33].
4.4. Human sera
Human sera were collected for these studies from healthy adult volunteers with unknown clinical history. The serum IgG levels against Rx1 ( family 1, clade 2) and EF3296 (family 2, clade 3) PspA proteins were measured by ELISA as described previously [33]. Other human sera used in these studies were from healthy adult volunteers who were participants of a phase I clinical trial that examined recombinant truncated Rx1 PspA protein as a potential vaccine against S. pneumoniae. These sera were all obtained with informed consent. Trial participants had been immunized on days 0 and 30 with recombinant truncated Rx1 protein adjuvanted with alum or a placebo [33]. Blood samples were taken at various times after immunization. The sera used in this study had been collected on day 90 or day 190 after the second immunization. The immune and pre-immune sera used here were selected from sera from an original population of 90 immunized and 30 placebo volunteers. As these sera had already been used for several studies, the sera selection was based solely on whether a sample was still available.
4.5. Statistical significance
Each FACS run was performed more than three times with essentially the same results. Thus, only a single run is shown for each experiment to illustrate the findings. In each run at least 10,000 bacteria were observed thus the 95% confidence interval is about 2% of the observed value. Thus, differences in percent bacteria stained larger than a few percent were highly significant.
Acknowledgments
We thank Grace Bittorf for her excellent technical assistance and our colleague Shaper Mirza for assistance with the electronic submission of the manuscript. We also acknowledge NIH grant AI21548 for partial support of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitney CG, Schaffner W, Butler JC. Rethinking recommendations for use of pneumococcal vaccines in adults. Clin Infect Dis. 2001 Sep 1;33(5):662–75. doi: 10.1086/322676. [DOI] [PubMed] [Google Scholar]
- 2.Obaro SK. The new pneumococcal vaccine. Clin Microbiol Infect. 2002 Oct;8(10):623–33. doi: 10.1046/j.1469-0691.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 3.Dowell SF. A new imprative of global pneumonia control: a commentary. Pediatr Infect Dis J. 2007;26:441–2. doi: 10.1097/01.inf.0000261197.70855.1e. [DOI] [PubMed] [Google Scholar]
- 4.Whitney CG, Pickering LK. The potential of pneumococcal conjugate vaccines for children. Pediatr Infect Dis J. 2002 Oct;21(10):961–70. doi: 10.1097/00006454-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002 Sep;21(9):810–5. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995 Oct;33(10):2759–62. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castaneda E, Leal AL, Castillo O, De La Hoz F, Vela MC, Arango M, et al. Distribution of capsular types and antimicrobial susceptibility of invasive isolates of Streptococcus pneumoniae in Colombian children. Pneumococcal Study Group in Colombia. Microb Drug Resist. 1997 Summer;3(2):147–52. doi: 10.1089/mdr.1997.3.147. [DOI] [PubMed] [Google Scholar]
- 8.Mollerach M, Regueira M, Bonofiglio L, Callejo R, Pace J, Di Fabio JL, et al. Invasive Streptococcus pneumoniae isolates from Argentinian children: serotypes, families of pneumococcal surface protein A (PspA) and genetic diversity. Epidemiol Infect. 2004 Apr;132(2):177–84. doi: 10.1017/s0950268803001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 10.Feikin DR, Klugman KP. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin Infect Dis. 2002 Sep 1;35(5):547–55. doi: 10.1086/341896. [DOI] [PubMed] [Google Scholar]
- 11.Crain MJ, Waltman WD, 2nd, Turner JS, Yother J, Talkington DF, McDaniel LS, et al. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990 Oct;58(10):3293–9. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vela Coral MC, Fonseca N, Castaneda E, Di Fabio JL, Hollingshead SK, Briles DE. Pneumococcal surface protein A of invasive Streptococcus pneumoniae isolates from Colombian children. Emerg Infect Dis. 2001 Sep–Oct;7(5):832–6. doi: 10.3201/eid0705.017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollingshead SK, Baril L, Ferro S, King J, Coan P, Briles DE. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J Med Microbiol. 2006 Feb;55(Pt 2):215–21. doi: 10.1099/jmm.0.46268-0. [DOI] [PubMed] [Google Scholar]
- 14.Hollingshead SK, Becker R, Briles DE. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect Immun. 2000 Oct;68(10):5889–900. doi: 10.1128/iai.68.10.5889-5900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yother J, Briles DE. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992 Jan;174(2):601–9. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDaniel LS, McDaniel DO, Hollingshead SK, Briles DE. Comparison of the PspA sequence from Streptococcus pneumoniae EF5668 to the previously identified PspA sequence from strain Rx1 and ability of PspA from EF5668 to elicit protection against pneumococci of different capsular types. Infect Immun. 1998 Oct;66(10):4748–54. doi: 10.1128/iai.66.10.4748-4754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabors GS, Braun PA, Herrmann DJ, Heise ML, Pyle DJ, Gravenstein S, et al. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies. Vaccine. 2000;18:1743–54. doi: 10.1016/s0264-410x(99)00530-7. [DOI] [PubMed] [Google Scholar]
- 18.Briles DE, Hollingshead SK, King J, Swift A, Braun PA, Park MK, et al. Immunization of humans with rPspA elicits antibodies, which passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis. 2000;182:1694–701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- 19.Hosea SW, Brown EJ, Frank MM. The critical role of complement in experimental pneumococcal sepsis. J Infect Dis. 1980 Dec;142(6):903–9. doi: 10.1093/infdis/142.6.903. [DOI] [PubMed] [Google Scholar]
- 20.Brown JS, Hussell T, Gilliland SM, Holden DW, Paton JC, Ehrenstein MR, et al. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):16969–74. doi: 10.1073/pnas.012669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren B, Szalai AJ, Hollingshead SK, Briles DE. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect Immun. 2004 Jan;72(1):114–22. doi: 10.1128/IAI.72.1.114-122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walport MJ. Complement. First of two parts. N Engl J Med. 2001 Apr 5;344(14):1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 23.Tu AH, Fulgham RL, McCrory MA, Briles DE, Szalai AJ. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immun. 1999 Sep;67(9):4720–4. doi: 10.1128/iai.67.9.4720-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neeleman C, Geelen SP, Aerts PC, Daha MR, Mollnes TE, Roord JJ, et al. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect Immun. 1999 Sep;67(9):4517–24. doi: 10.1128/iai.67.9.4517-4524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren B, Szalai AJ, Thomas O, Hollingshead SK, Briles DE. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect Immun. 2003 Jan;71(1):75–85. doi: 10.1128/IAI.71.1.75-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu A-HT, Fulgham RL, McCory MA, Briles DE, Szalai AJ. Pneumococcal surface protein A (PspA) inhibits complement activation by Streptococcus pneumoniae. Infect Immun. 1999;67:4720–4. doi: 10.1128/iai.67.9.4720-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tart RC, McDaniel LS, Ralph BA, Briles DE. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J Infect Dis. 1996 Feb;173(2):380–6. doi: 10.1093/infdis/173.2.380. [DOI] [PubMed] [Google Scholar]
- 28.Briles DE, King JD, Gray MA, McDaniel LS, Swiatlo E, Benton KA. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine. 1996 Jun;14(9):858–67. doi: 10.1016/0264-410x(96)82948-3. [DOI] [PubMed] [Google Scholar]
- 29.Briles DE, Hollingshead SK, Nabors GS, Paton JC, Brooks-Walter A. The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine. 2000 Dec 8;19( Suppl 1):S87–95. doi: 10.1016/s0264-410x(00)00285-1. [DOI] [PubMed] [Google Scholar]
- 30.Roche H, Hakansson A, Hollingshead SK, Briles DE. Regions of PspA/EF3296 best able to elicit protection against Streptococcus pneumoniae in a murine infection model. Infect Immun. 2003 Mar;71(3):1033–41. doi: 10.1128/IAI.71.3.1033-1041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roche H, Ren B, McDaniel LS, Hakansson A, Briles DE. Relative roles of genetic background and variation in PspA in the ability of antibodies to PspA to protect against capsular type 3 and 4 strains of Streptococcus pneumoniae. Infect Immun. 2003 Aug;71(8):4498–505. doi: 10.1128/IAI.71.8.4498-4505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDaniel LS, Ralph BA, McDaniel DO, Briles DE. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb Pathog. 1994 Nov;17(5):323–37. doi: 10.1006/mpat.1994.1078. [DOI] [PubMed] [Google Scholar]
- 33.Nabors GS, Braun PA, Herrmann DJ, Heise ML, Pyle DJ, Gravenstein S, et al. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine. 2000 Mar 6;18(17):1743–54. doi: 10.1016/s0264-410x(99)00530-7. [DOI] [PubMed] [Google Scholar]
- 34.Ren B, Szalai AJ, Hollingshead SK, Briles DE. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect Immun. 2004;72:114–22. doi: 10.1128/IAI.72.1.114-122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDaniel LS, Loechel F, Benedict C, Greenway T, Briles DE, Conry RM, et al. Immunization with a plasmid expressing pneumococcal surface protein A (PspA) can elicit protection against fatal infection with Streptococcus pneumoniae. Gene. 1997;188:279–84. doi: 10.1038/sj.gt.3300401. [DOI] [PubMed] [Google Scholar]
- 36.Lancefield RC. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J Exp Med. 1957;106:525–44. doi: 10.1084/jem.106.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briles DE, Forman C, Horowitz JC, Volanakis JE, Benjamin WH, Jr, McDaniel LS, et al. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989;57:1457 – 64. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollingshead SK, Becker RS, Briles DE. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect Immun. 2000;68:5889–900. doi: 10.1128/iai.68.10.5889-5900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown EJ, Hosea SW, Frank MM. The role of complement in the localization of pneumococci in the splanchnic reticuloendothelial system during experimental bacteremia. J Immunol. 1981 Jun;126(6):2230–5. [PubMed] [Google Scholar]
- 40.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991 Jul;4(3):359–95. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szalai AJ, Briles DE, Volanakis JE. Role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect Immun. 1996 Nov;64(11):4850–3. doi: 10.1128/iai.64.11.4850-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuste J, Ali S, Sriskandan S, Hyams C, Botto M, Brown JS. Roles of the alternative complement pathway and C1q during innate immunity to Streptococcus pyogenes. J Immunol. 2006 May 15;176(10):6112–20. doi: 10.4049/jimmunol.176.10.6112. [DOI] [PubMed] [Google Scholar]
- 43.Nahm MH, Apicella MA, Briles DE. Immunity to extracellular bacteria. In: Paul WE, editor. Fundamental Immunology. Philadelphia: Lippincott-Raven; 1999. pp. 1373–86. [Google Scholar]
- 44.Yu X, Gray B, Chang S, Ward JI, Edwards KM, Nahm MH. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J Infect Dis. 1999 Nov;180(5):1569–76. doi: 10.1086/315096. [DOI] [PubMed] [Google Scholar]
- 45.Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, et al. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997 Jul;4(4):415–22. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briles DE, Hollingshead SK, King J, Swift A, Braun PA, Park MK, et al. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis. 2000 Dec;182(6):1694–701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- 47.Roche H, Ren B, McDaniel LS, Hakansson A, Briles DE. Relative roles of genetic background and variation in PspA in the ability of antibodies to PspA to protect against capsular type 3 and 4 strains of Streptococcus pneumoniae. Infect Immiun. 2003;71:4498–505. doi: 10.1128/IAI.71.8.4498-4505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lifshitz S, Dagan R, Shani-Sekler M, Grossman N, Fleminger G, Friger M, et al. Age-dependent preference in human antibody responses to Streptococcus pneumoniae polypeptide antigens. Clin Exp Immunol. 2002 Feb;127(2):344–53. doi: 10.1046/j.1365-2249.2002.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briles DE, Scott G, Gray B, Crain MJ, Blaese M, Nahm M, et al. Naturally occurring antibodies to phosphocholine as a potential index of antibody responsiveness to polysaccharides. J Infect Dis. 1987 Jun;155(6):1307–14. doi: 10.1093/infdis/155.6.1307. [DOI] [PubMed] [Google Scholar]
- 50.Rapola S, Jantti V, Haikala R, Syrjanen R, Carlone GM, Sampson JS, et al. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J Infect Dis. 2000 Oct;182(4):1146–52. doi: 10.1086/315822. [DOI] [PubMed] [Google Scholar]
- 51.Baril L, Briles DE, Crozier P, King J, Punar M, Hollingshead SK, et al. Characterization of antibodies to PspA and PsaA in adults over 50 years of age with invasive pneumococcal disease. Vaccine. 2004 Dec 21;23(6):789–93. doi: 10.1016/j.vaccine.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 52.McDaniel LS, Yother J, Vijayakumar M, McGarry L, Guild WR, Briles DE. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA) J Exp Med. 1987 Feb 1;165(2):381–94. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren B, Szalai AJ, Thomas O, Hollingshead SK, Briles DE. Both family 1 and family 2 PspAs can inhibit complement deposition and confer virulence to a capsular 3 serotype Streptococcus pneumoniae. Infect Immun. 2003;71:75–85. doi: 10.1128/IAI.71.1.75-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yother J, Handsome GL, Briles DE. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J Bact. 1992;174:610–8. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDaniel LS, Scott G, Kearney JF, Briles DE. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J Exp Med. 1984 Aug 1;160(2):386–97. doi: 10.1084/jem.160.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992 Jan;60(1):111–6. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]