Abstract
The binding of antibodies to the CD4-binding site (CD4bs) of the HIV-1 envelope glycoprotein gp120 has been shown to induce gp120 to undergo conformational changes that can expose and/or shield specific epitopes on gp120. Here, we study alterations in the antigenicity and immunogenicity of gp120 when complexed with human monoclonal antibodies (mAbs) specific for the CD4bs of gp120. The data showed that gp120 bound by anti-CD4bs mAbs had enhanced reactivity with mAbs to the V3 and N-terminal regions, but not with mAb to the C terminus. Moreover, mice immunized with the gp120/anti-CD4bs mAb complexes produced higher titers of gp120-specific serum IgG and IgA than mice immunized with uncomplexed gp120 or other gp120/mAb complexes. Notably, the enhanced antibody production was directed against V3 and correlated with better exposure of V3 on the gp120/anti-CD4bs mAb complexes. The higher antibody reactivity was evident against the homologous V3LAI peptide, but not against heterologous V3 peptides. Potent neutralization activity against HIV-1LAI was also observed in the sera from mice immunized with gp120/anti-CD4bs mAb complexes, although the sera exhibited poor neutralizing activities against other viruses tested. These results indicate that the anti-CD4bs antibodies alter the antigenicity and immunogenicity of gp120, leading to enhanced production of anti-gp120 antibodies directed particularly against the V3 region.
Keywords: HIV, immune complex, antibody, HIV envelope gp120
INTRODUCTION
The capacity of immune complexes to augment antibody (Ab) responses in an antigen-specific manner is well documented. Immune complexes are deposited and retained on follicular dendritic cells in the lymphoid follicles, inducing B cell activation and Ab production (Heyman, 2000). Indeed, immune complexes have been tested as vaccines to augment Ab responses to hepatitis B surface antigen (McCluskie et al., 1998; Wen, Qu, and Zhou, 1999), infectious bursal disease virus (Ivan et al., 2005; Kumar, 2001) as well as to equine herpesvirus 1 and porcine parvovirus (Alber, Killington, and Stokes, 2000; Roic et al., 2006). Additionally, Abs can affect the stability and conformation of antigens, while shielding or exposing specific antigenic sites (Jemmerson and Paterson, 1986). Previous studies with model antigens such as dinitrophenylated keyhole limpet hemocyanin, gamma globulin, and albumin have shown that immunization with antigen-antibody complexes results in qualitatively and quantitatively different Ab responses than with antigens alone (Heyman, 2000). Subsequently, immunization with hepatitis B surface antigen and Streptococcus mutans adhesin P1 bound by different mAbs was found to elicit distinct specificities of serum Abs, indicating the capacity of mAbs to redirect Ab responses (Bouige et al., 1996; Brady et al., 2000; Oli et al., 2004). The use of immune complexes has also been considered as a strategy to augment Ab responses to the HIV-1 envelope glycoprotein gp120 and especially to direct Abs toward critical neutralizing epitopes on this viral surface antigen. However, an earlier effort to immunize animals using HIV-1 envelope glycoprotein gp120 antigen complexed with mAb A32, which specifically induces the mAb binding to the chemokine-receptor binding site, did not enhance the production of cross-reactive neutralizing Abs against this conserved region on gp120 (Liao et al., 2004).
In this study, we investigated the capacity of Abs binding to the CD4-binding site (CD4bs) of gp120 to alter the antigenicity and immunogenicity of gp120. Anti-CD4bs Abs have broad binding activity for gp120 of various HIV-1 subtypes due to the highly conserved structure and function of the receptor-binding site (Moore et al., 1994). Extensive mutagenesis analyses have mapped the binding sites of different anti-CD4bs monoclonal antibodies (mAbs) and revealed that the footprint of these mAbs encompasses both the inner and outer domains of gp120, indicating that similar to CD4, anti-CD4bs mAbs bring together these two domains (Pantophlet et al., 2003; Thali et al., 1992; Xiang et al., 2002). Thermodynamic studies have also shown that the interaction of gp120 with anti-CD4bs mAbs induces significant conformational changes involving the rearrangement of the inner and outer domains (Kwong et al., 2002). The crystallographic structure of the gp120/anti-CD4bs mAb b12 complex and the structural modeling of anti-CD4bs mAb F105 further confirm the construction of the mAb binding sites from the two gp120 domains (Wilkinson et al., 2005; Zhou et al., 2007). Moreover, the binding of anti-CD4bs mAbs to gp120 has been shown to enhance the reactivity of anti-V3 mAbs (Pinter, Honnen, and Tilley, 1993), while at the same time, these mAbs block gp120 binding to the chemokine receptor (Raja et al., 2003), indicating that these mAbs trigger specific structural changes, albeit not necessary the same as the CD4-induced bridging sheet re-arrangement required for the chemokine receptor-binding site (Zhou et al., 2007). Hence, we postulate that because the binding of anti-CD4bs mAbs to gp120 changes antigenic properties of gp120, such that some regions of gp120 are better exposed while others are hidden or modified, the use of immune complexes made of gp120 and mAbs as immunogens should alter and direct the Ab responses to specific regions of the gp120 antigen.
To test this hypothesis, we probed the reactivity of gp120/anti-CD4bs mAb complexes using mAbs specific for different regions of gp120. Subsequently, we evaluated the levels and specificities of anti-gp120 Ab responses induced in mice immunized with gp120/anti-CD4bs mAb complexes as compared to immunization with uncomplexed gp120 or other gp120/mAb complexes. Our data indicate that the binding of anti-CD4bs mAb to gp120 enhances the exposure of the N terminal C1 region and neutralizing epitopes in the V3 loop. Importantly, immunization with gp120/anti-CD4bs mAb complexes consistently elicits higher levels of serum anti-gp120 Abs directed especially to the V3 region. Sera from the immunized mice also exhibit much more potent virus-neutralizing activity than sera of mice receiving uncomplexed gp120, although the neutralizing activity was observed primarily against the homologous HIV-1 strain.
MATERIALS AND METHODS
Antigens and mAbs
The recombinant HIV-1 gp120LAI used in the study was purchased from Progenics (Tarrytown, NY), while gp120YU-2 core protein was kindly provided by Richard Wyatt (Vaccine Research Center, NIH). Human mAbs were gifts from Susan Zolla-Pazner (New York University [NYU] School of Medicine, New York, NY), Abraham Pinter (Public Health Research Institute, UMDNJ, Newark, NJ), and James Robinson (Tulane University, New Orleans, LA). Except for the recombinant IgG1 b12, anti-gp120 mAbs were all generated from B cells of chronically HIV-1 infected subjects following Epstein Barr virus transformation and fusion with heteromyeloma. MAbs 654D, 559/68D, 1027/30D, IgG1b12, 5145A, and F105 are directed to the CD4bs (Jeffs et al., 2001; Karwowska et al., 1992; Kessler et al., 1997; Posner et al., 1991; Tuen et al., 2005), mAbs 694/98-D (Gorny et al., 1993) and 447/52D (Gorny et al., 1992) recognize V3, mAb 1006-30D is directed to C2 (Nyambi et al., 2000), mAb 1331A (Bandres et al., 1998; Hochleitner et al., 2000) is specific for C5, and mAb EH21 recognizes the N-terminus of gp120 (C1) (Haynes et al., 2005). MAbs 860-55D and 1418, which recognize the parvovirus B19 VP2 antigen (Gigler et al., 1999), were used as negative controls. Biotinylated mAbs were prepared using EZ-link sulfo-NHS-biotin reagents (Pierce, Rockford, IL). Gp120 peptides were obtained from NIBSC Centre for AIDS Reagents (EU Programme EVA/AVIP) or purchased from Macromolecular Resources (Colorado State University, Boulder, CO), Intracel (Cambridge,MA), or Sigma Genosys (Woodlands,TX). The following peptides were used: V3LAI/HXB2 (aa 272-291), V3SF162 (aa 272-291), V3JR-CSF (aa 272-296), V3MN (aa 272-296), six overlapping 20-mers representing C1LAI/HXB2 (aa 1-71) and three overlapping 20-mers representing C5LAI/HXB2 (aa 442-481). The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: soluble human CD4 (sCD4) from Dr. Sai Iyer, HIV-1 gp120 monoclonal antibody (F105) from Drs. Marshall Posner and Lisa Cavacini, IgG1 b12 from Drs. Dennis Burton and Carlos Barbas.
Ab reactivity to gp120 complexed with the anti-CD4bs mAb
Gp120 alone or gp120/mAb complexes were serially diluted and then adsorbed directly onto ELISA plate or captured with sheep-anti C5 Ab (Cliniqa, Fallbrook, CA). After washing, biotinylated anti-gp120 mAbs (1 μg/mL) were added, and detected by alkaline phosphatase-conjugated streptavidin (Invitrogen, Grand Island, NY) and p-nitrophenyl phosphate (Sigma-Aldrich, Saint Louis, MO).
Immunization with gp120/mAb combinations
Balb/c mice (female, >6 weeks old from Jackson Lab, 8 mice per group), were inoculated intraperitoneally with gp120LAI (3 μg gp120 per animal in 100 μL at two sites) alone or with mAb. Each immune complex was prepared at molar gp120/mAb ratios of approximately 1:3 or 1:10 by incubating gp120 with mAbs for 2 hrs at 37°C. Gp120 or the immune complexes were mixed according to the manufacturer’s directions with MPL+TDM adjuvant (Sigma-Aldrich, St Louis, MO). Animals were immunized 4 times at the designated intervals. Blood was collected one week after each immunization; at each time point, the sera from two animals in each group were pooled, and then stored at -80°C. The animal study was carried out according to the protocol approved by the VA and NYU IACUC.
ELISA for detection of serum Abs
The levels of gp120 specific Ab in the sera were determined using ELISA. Briefly, gp120 proteins (1 μg/ml) or peptides (1 μg/ml) were adsorbed onto ELISA plates (Immunoblot 2HB or Microfluor 2 Black; Thermo, Milford, MA) overnight at 4°C. Sera were serially diluted in RPMI containing 15%FBS and reacted with the coated antigens for 2 hrs at 37°C. Alkaline phosphatase-conjugated anti-mouse IgG (1:1000; Sigma-Aldrich, St Louis, MO) was used as a secondary Ab, while p-nitrophenyl phosphate (Sigma-Aldrich, Saint Louis, MO) or PhosphaGlow™ AP substrate (KPL,Gaithersburg, MA) were used as substrates. The optical density was read on a microplate reader (MRX Revelation DYNEX) at 405nm, and the chemiluminescence was read on a VICTOR3 instrument (Perkin-Elmer). To detect Ab response to the human mAbs or the control antigen (keyhole limpet hemocyanin: KLH), ELISA plates were coated with the mAbs or KLH (1 μg/ml), and reacted with diluted sera as described above.
To determine the Ig subtypes of gp120-specific Abs in the sera, Microfluor 2 Black plates (Thermo, Milford, MA) were coated overnight at 4°C with 1μg/ml sheep polyclonal anti-C5 Ab (Cliniqa, Fallbrook, CA). Gp120LAI (1μg/ml) was then added and incubated 2 hrs at 37°C. After washing, serially diluted sera were reacted for 2 hrs at 37°C. Rabbit Abs against mouse IgA, IgG, IgG1, IgG2a, or IgG2b (Zymed, San Francisco, CA) were then added, and alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma-Aldrich, Saint Louis, MO) was used as a secondary Ab, along with PhosphaGlow™ AP substrate (KPL,Gaithersburg, MA).
To detect the presence of Abs to the CD4bs in the immune sera, gp120LAI captured by sheep anti-C5 Ab (Cliniqa, Fallbrook, CA) (1μg/ml) was incubated with serially diluted sera in the presence or absence of sCD4 (1 μg/ml). Alkaline phosphatase-conjugated anti-mouse IgG (Sigma-Aldrich, Saint Louis, MO) was then used along with p-nitrophenyl phosphate substrate (Sigma-Aldrich, Saint Louis, MO). In parallel control experiments, we also measured the reduction of anti-CD4bs mAb 654D (1 to 0.03 μg/ml) binding to gp120 in the presence of sCD4 (data not shown).
Virus neutralization assay
Serum neutralization activity was determined in a single-cycle infectivity assay using HIV-1 isolates produced in PHA-stimulated PBMCs and the TZM-bl target cells (Choudhry et al., 2007). Viruses (200 TCID50) were incubated for 1 h at 37°C in 96 well plates with diluted sera. In some experiments, the diluted sera were pre-incubated with the V3LAI/HXB2 peptide or an irrelevant peptide control (180 μg/ml). Freshly trypsinized TZM-bl cells were added (104/well) to the virus-serum mixture in the presence of DEAE (diethylaminoethyl) (Sigma-Aldrich, Saint Louis, MO) and Indinavir (1μM). After 48 hrs, Bright-Glo (Promega, Madison, WI) reagent was added, and luminescence intensity was determined. Two anti-V3 mAbs known to have neutralizing activity (694/98D and 447-52D) were also included in this assay as controls.
Statistical analysis
Statistical analyses were performed using one-way ANOVA with Dunnett’s multiple comparison test for each of the concentrations tested in Fig. 1A or two-way ANOVA with Bonferroni’s post-test for Fig.1B and Fig 7B. For two-group comparisons in Fig.2C and Fig 5 unpaired t test (one-tail) was performed. These analyses were done with GraphPad Prism 5.
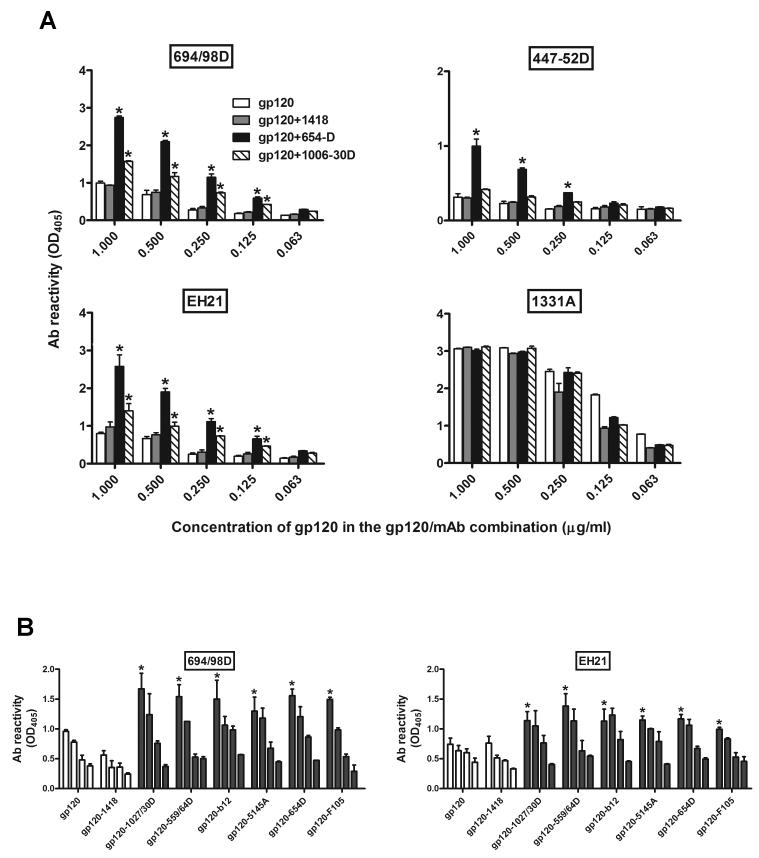
Fig. 1.
Reactivity of mAbs to V3, C1, and C5 with gp120/mAb complexes. (A) gp120 or gp120/mAb complexes were captured on the ELISA plates by polyclonal anti-C5 Abs and reacted with biotinylated mAbs specific for V3 (694/98D and 447/52D) or C1 (EH21). Alternatively, gp120 or the complexes were directly coated onto ELISA plates and reacted with biotinylated mAbs specific for C5 (1331A). The gp120/mAb complexes were prepared with 1 μg/ml of recombinant gp120LAI and 2 μg/ml of mAbs to the CD4bs (654D) or C2 (1006-30D) and serially diluted by 2 fold. Gp120 mixed with a control mAb (1418) was also included as a control. The x-axis shows the gp120 concentrations in each of the gp120/mAb mixtures. Relative binding of the biotinylated mAbs was determined using alkaline phosphatase-conjugated streptavidin. Means and standard deviations were calculated from duplicate wells. Data from one of five repeated experiments are shown. *, p<0.05 as compared with gp120 alone. (B) Different gp120/CD4bs mAb complexes or uncomplexed gp120 (gp120 and gp120+1418) were captured on the ELISA plates by polyclonal anti-C5 Abs and reacted with biotinylated mAbs specific for V3 (694/98D) or C1 (EH21). The gp120/mAb complexes were prepared as described above. The bars in each set represent the serial dilutions of the gp120/mAb mixtures as described above. *, p<0.05 as compared to gp120 alone at the first 2-3 dilutions tested, except for gp120-F105 which is significant only at the first dilution.
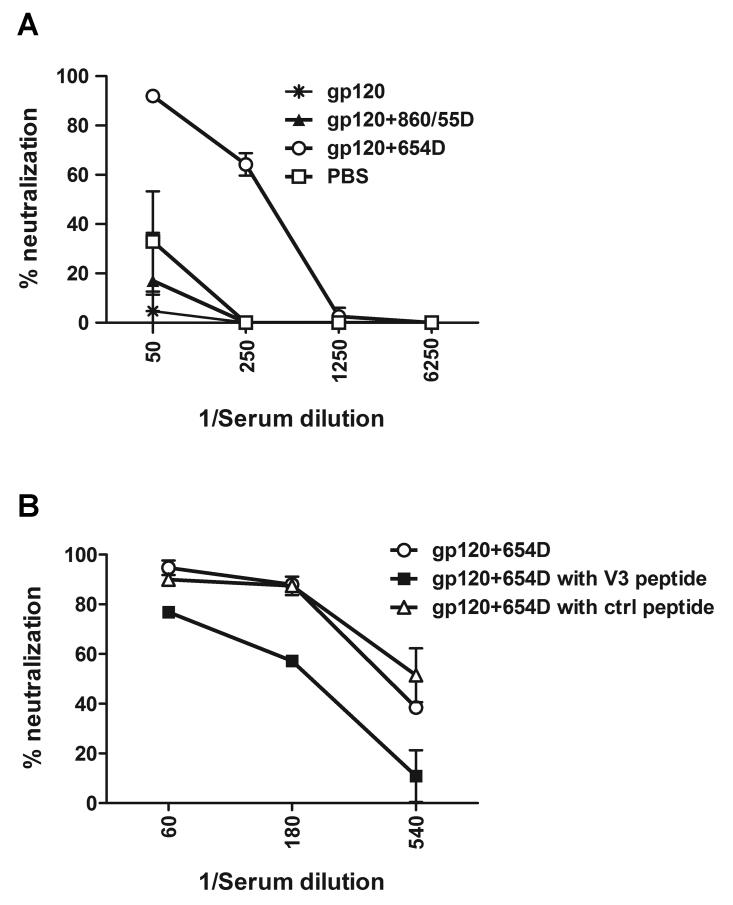
Fig. 7.
Neutralization of HIV-1LAI by sera from mice immunized with gp120 or gp120/mAb complexes. (A) Sera collected after the final immunization were tested for neutralizing activity in the single round infection assay with TZM-bl cells and HIV-1LAI. Mice were immunized with gp120LAI alone, gp120LAI mixed with CD4bs mAb 654D, or control mAb (860-55D), or no antigen (PBS) in the presence of RIBI adjuvant. (B) Treatment of serum from gp120+654D-immunized mice with V3 peptide or a control peptide. Serially diluted serum was pre-incubated with V3 peptide or control peptide (180 μg/ml) or left untreated, and tested in the neutralization assay as described in the Materials and Methods. Virus infectivity in cells treated with virus in the presence of pooled normal mouse sera was assessed in each assay and considered as background (0% neutralization). Data from one of two repeat experiments are shown.
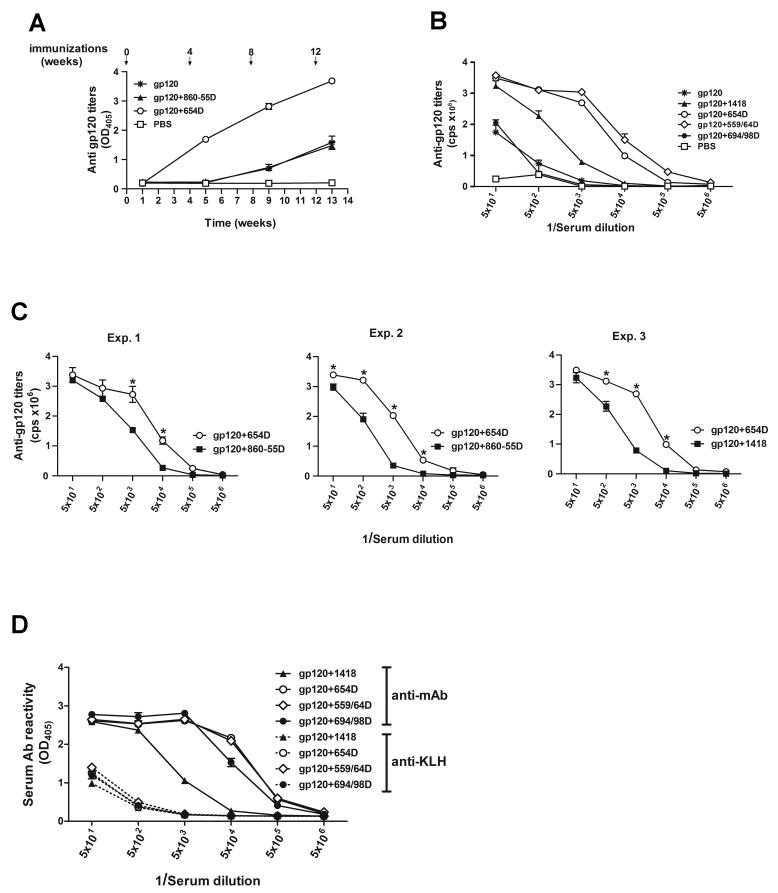
Fig. 2.
Reactivity of serum Abs from mice immunized with rgp120 or gp120-Ab complex against recombinant gp120. (A) The kinetics of serum Ab responses induced in BALB/c mice immunized with gp120 alone, gp120 complexed with the anti-CD4bs mAb 654D, gp120 mixed with an irrelevant anti-parvovirus mAb 860-55D, or no antigen (PBS) in the presence of RIBI adjuvant. Sera were pooled from each group of mice and tested at a dilution of 1:320. (B) Anti-gp120 Ab titers in sera from 1 week after final immunization. BALB/c mice were immunized with gp120 or gp120 combined with mAbs to CD4bs (654-D and 559/64D), V3 (694/98D), parvovirus (1418), or with PBS. (C) Comparison of anti-gp120 titers in pooled sera from 3 different immunization experiments.*, p<0.05 at the designated serum dilutions. (D) Serum Ab titers to human mAbs used to form the immune complexes. Reactivities of sera to the respective mAbs are shown as solid lines, while reactivities to keyhole limpet hemocyanin (KLH), used as a negative control, are shown as dotted lines. For B, C and D, pooled sera were diluted 10 fold starting from 1:50. ELISA was performed using recombinant gp120LAI, human mAbs, or KLH coated directly on the plates and alkaline phosphatase-conjugated anti-mouse IgG as a secondary antibody. Alkaline phosphatase substrates p-nitrophenyl phosphate (OD405) or PhosphaGlow chemiluminescent (cps) were used. Means and standard deviations were derived from duplicate wells.
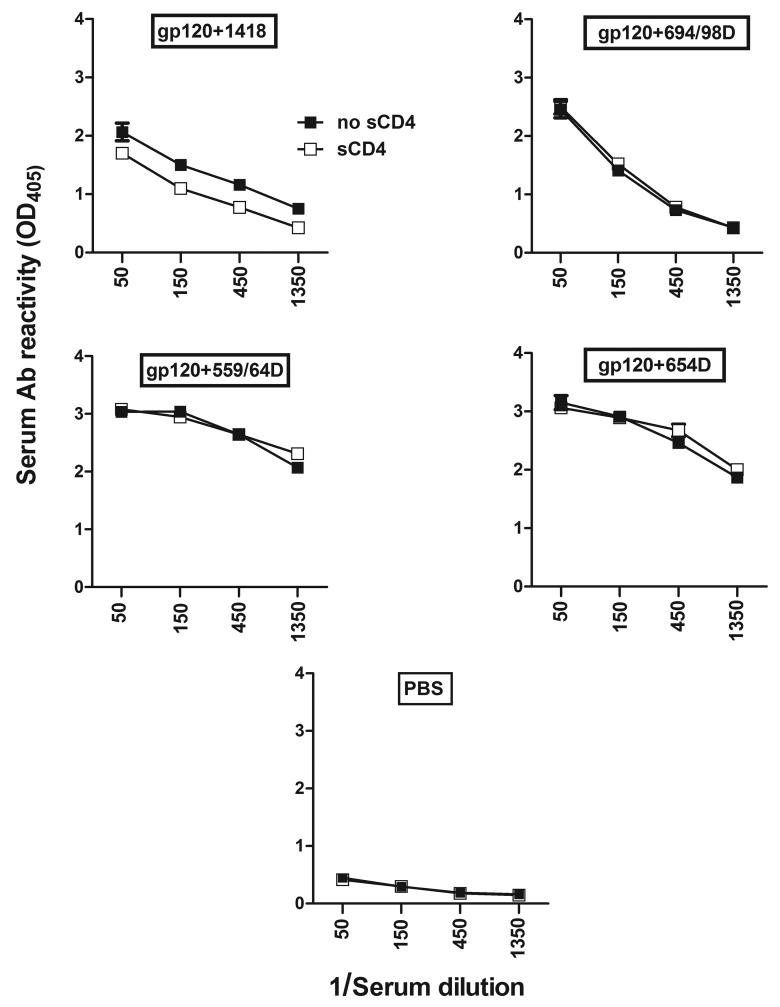
Fig. 5.
Detection of Abs to the CD4-binding site of gp120 in sera of mice immunized with the gp120/Ab complexes. Serum Abs to the CD4bs was measured by ELISA by comparing serum reactivity to gp120 in the presence or absence of sCD4. Sera were collected after the last immunization from mice immunized with gp120 combined with mAbs to CD4bs (654-D and 559/64D), mAb to V3 (694/98D), or control mAb (1418), or no antigen (PBS). These sera were serially diluted and reacted with gp120 captured on the wells in the presence or absence of sCD4 (1 μg/ml). Means and standard deviations from duplicate wells are shown. There is no statistical significant difference between Ab reactivities in the presence versus absence of sCD4.
RESULTS
The binding of anti-CD4bs mAbs to gp120 enhances exposure of the V3 and C1 regions of gp120
Previous studies have reported that CD4 binding to gp120 can increase accessibility of the V3 loop to proteases as well as to anti-V3 mAbs (Mbah et al., 2001; Sattentau and Moore, 1991; Sattentau et al., 1993). To assess if the binding of anti-CD4bs mAbs to gp120 could also induce structural changes that increase the exposure of specific Ab epitopes on gp120, we tested the reactivity of different biotinylated mAbs with the gp120/anti-CD4bs mAb complex (gp120+654D) as compared to the gp120/anti-C2 mAb complex (gp120+1006-30D), uncomplexed gp120 mixed with an irrelevant mAb 1418, or gp120 alone (Fig. 1A). The results showed that a significantly higher level of anti-V3 mAbs (694/98D and 447-52D) bound to gp120 complexed with anti-CD4bs mAb 654D, as compared to gp120 alone (p<0.05) or to other gp120/mAb combinations (p<0.05). The enhanced binding was observed at the different gp120+654D concentrations tested. Similarly, we observed enhanced binding of anti-C1 mAb EH21 to gp120+ 654D, as compared to gp120 alone (p<0.05) and the other gp120/mAb combinations (p<0.05). The enhanced binding was seen when the gp120/mAb complexes were captured with anti-C5 Ab (Fig. 1A) or directly coated onto the ELISA wells (data not shown). In contrast, no enhancement was seen with the binding of anti-C5 mAb 1331A (p>0.05), indicating that only specific regions of gp120 were affected by the anti-CD4bs mAb. These results are consistent with previous studies showing that the anti-CD4bs mAbs mainly enhanced the binding of mAbs to V3 and V2, but not to C5 (Moore and Sodroski, 1996). We subsequently tested six different anti-CD4bs mAbs for the capacity to increase the exposure of V3 and C1. As shown in Fig. 1B, all six anti-CD4bs mAbs increased mAb binding to the V3 and C1 regions of gp120 (p<0.05). These results indicate that the binding of anti-CD4bs mAbs induces conformational changes in gp120, such that the V3 and C1 regions react better with Abs. In addition to the gp120/anti-CD4bs mAb complexes, gp120 complexed with anti-C2 mAb 1006-30D also had enhanced reactivity with anti-V3 mAb 694/98D and anti-C1 mAb EH21 (p<0.05), albeit at lower levels. However, the reactivity of this gp120/anti-C2 complex with anti-V3 mAb 447 was not altered (p>0.05), suggesting the differential effects of anti-CD4bs vs. anti-C2 mAbs on V3 reactivity. The reasons for the observed differences between anti-CD4bs and anti-C2 mAbs are not known, but are not likely due to differences in the mAb affinity forgp120, since the relative affinities of the anti-CD4bs and anti-C2 mAbs tested in this study were comparable, with the half maximal binding values ranging from 0.003 to 0.02 μg/ml.
Immunization with gp120/anti CD4bs mAb complexes elicits higher titers of gp120-specific Ab response
To evaluate the effects of anti-CD4bs mAbs on Ab response to gp120 in vivo, BALB/c mice were immunized i.p. 4 times with gp120LAI complexed with 654-D, a human mAb to the CD4bs. For comparison, we also immunized mice with gp120LAI alone, gp120 mixed with an irrelevant anti-parvovirus mAb 860-55D, or saline. We determined the kinetics of anti-gp120 Ab production by testing sera collected a week after each injection for Ab reactivity with the homologous gp120LAI protein in ELISA. The data showed that mice immunized with gp120, gp120+ 860-55D, or gp120+ 654D produced serum Ab specific for gp120. However, immunization with gp120+ 654D elicited higher and faster gp120-specific Ab responses than immunization with gp120 or gp120+ 860-55D (Fig. 2A). Mice that received gp120 alone or gp120 mixed with the irrelevant mAb started to show detectable anti-gp120 Abs only after 3 injections, while mice immunized with gp120+ 654D had already relatively high amounts of Abs after the second injection. No gp120-specific serum Abs were ever detected in mice immunized with adjuvant only.
To assess whether the enhancing effects could also be mediated by other mAbs, we immunized mice with gp120 complexed with two different anti-CD4bs mAbs 654D or 559/64D, gp120 complexed with anti-V3 mAb 694/98D, gp120 mixed with an irrelevant anti-parvovirus mAb 1418, gp120 alone, or no antigen. Sera collected at one week after the final immunization were tested in ELISA for the relative titers of anti-gp120 Abs induced in the different groups of mice. Immunization with gp120 complexed with the anti-CD4bs mAbs 654D or 559/64D induced higher titers of anti-gp120 Abs than immunization with gp120 alone or gp120 mixed with an irrelevant mAb (Fig. 2B). However, immunization with gp120 complexed with the anti-V3 mAb 694/98D did not have the same enhancing effect. Mice immunized with adjuvant alone did not have any detectable anti-gp120 Ab titers (Fig. 2B), and none of the sera tested were reactive with bovine serum albumin used as a negative control (data not shown). The enhancing effect of anti-CD4bs mAb 654D was consistently observed in different animals immunized in three separate experiments (p<0.05) (Fig. 2C). For comparison, we tested Ab response to the human mAbs used to form the immune complexes and observed that mice immunized with the gp120/mAb complexes (gp120+654D, gp120+559/64D, and gp120+694/98D) all generated Ab response to the respective mAbs and the titers were higher than that observed in mice immunized with the uncomplexed gp120+1418 mixture (Fig. 2D). Collectively, the results shown in Figs. 1 and 2 indicate that the binding of anti-CD4bs mAbs to gp120 influence Ab reactivity and Ab response elicited against this antigen.
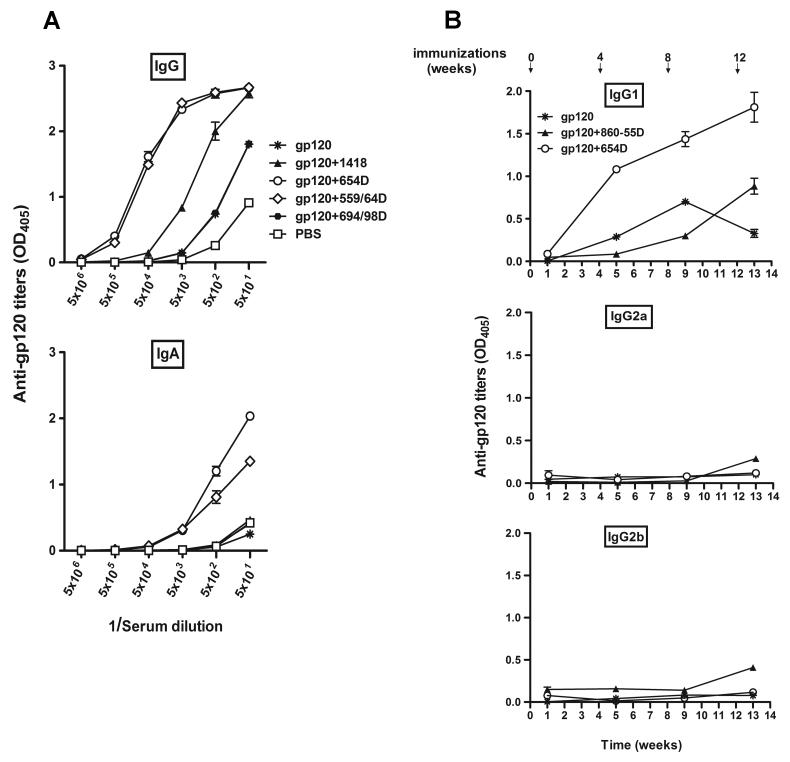
Immunization with gp120/anti CD4bs Ab complexes elicits gp120-specific IgG1 and IgA
To determine the Ig isotypes elicited by immunization with gp120/anti-CD4bs mAbs complexes, we tested sera obtained after the final immunization for gp120-specific IgG and IgA. Mice immunized with gp120+654D or gp120+559/64D showed 100-1000x higher titers of gp120-specific IgG (end point titers of 5×106) as compared to the other groups of mice (Fig. 3A). These mice also produced high titers of gp120-specific IgA (end point titer of 5×104), while the other groups of mice did not (Fig. 3A). In a subsequent experiment, we evaluated the IgG subclasses induced following immunization with gp120+654D as compared to gp120 alone or gp120 mixed with an irrelevant mAb 860-55D. Sera from one week after each immunization were tested for anti-gp120 IgG1, IgG2a, and IgG2b. Mice receiving gp120+654D produced higher levels of gp120-specific IgG1 as compared to the other groups (Fig. 3B), indicating that Th2-like responses were induced by the gp120/mAb complex (Stevens et al., 1988). Similar results were obtained with sera from mice immunized with gp120+559/64D (data not shown).
Fig. 3.
Ig classes induced in mice immunized with gp120 in the presence or absence of mAbs. Mice were immunized with gp120 alone, gp120 complexed with different anti-gp120 mAbs, or PBS. For comparison, mice were also immunized with gp120 combined with anti-parvovirus mAbs (1418 or 860-55D). The results obtained with sera from two different immunization experiments are shown in A and B. (A) Sera were collected a week after the final boost, serially diluted, and tested for titers of gp120-specific IgG and IgA. (B) For detection of gp120-specific IgG1, IgG2a, and IgG2b, sera collected one week after each immunization were used and tested at a dilution of 1:640. Gp120LAI was captured on ELISA plates coated with sheep anti-C5 antibody and reacted with diluted mouse sera. Rabbit antibodies against different mouse Ig subtypes and subclasses and alkaline-phosphatase-conjugated anti-rabbit IgG were used for detection along with alkaline phosphatase substrates p-nitrophenyl phosphate (OD405) or PhosphaGlow chemiluminescent. Means and standard deviations were derived from duplicate wells.
Immunization with gp120/anti CD4bs Ab complexes elicits higher anti-V3 Ab response
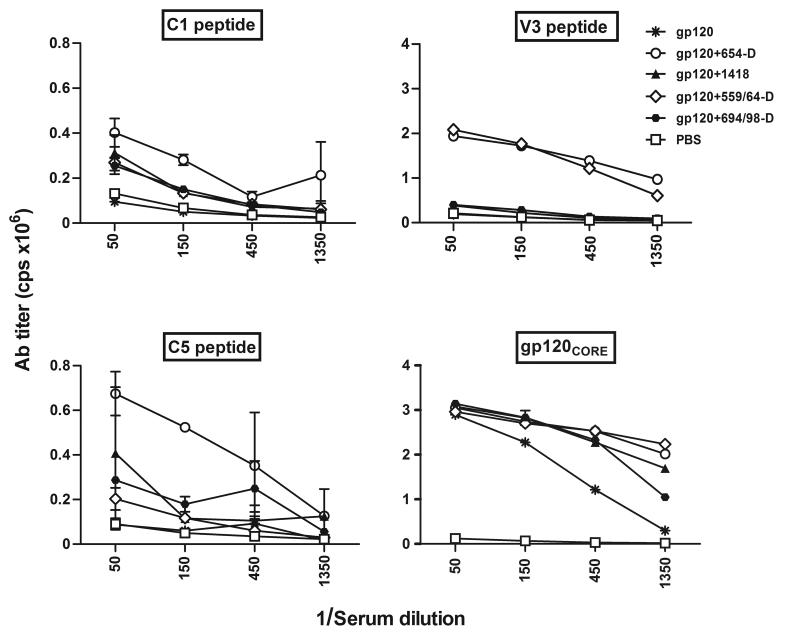
We next asked if certain regions of gp120 were preferentially targeted by Abs elicited in mice immunized with gp120/anti-CD4bs mAbs complexes. To address this question, sera obtained after the final immunization were tested by ELISA for their reactivity with gp120 peptides representing the C1, C5 and V3 regions and with a gp120 core protein lacking C1, C5, V1/V2, and V3 (Fig. 4). Enhanced reactivity against the V3 peptide was observed with sera from mice immunized with gp120 complexed with anti-CD4bs mAbs 654D and 559/64D as compared with the other groups. In fact, high levels of anti-V3 Abs were detected only in mice receiving gp120+654D or gp120+559/64D, while sera from the other groups had no significant reactivity above that seen in control mice. In contrast, reactivities against C1 and C5 peptides were similar or only slightly higher in sera from mice immunized with gp120+654D or gp120+599/64D. All groups of mice also had comparable serum Ab reactivity with the gp120 core. These results demonstrate that immunization with gp120/anti-CD4bs mAb complexes enhanced the Ab response to V3, while still eliciting comparable Ab titers to other gp120 regions.
Fig. 4.
Reactivity of serum Abs from mice immunized with rgp120 or gp120-mAb complexes with V3, C1, C5, and gp120 core. Sera collected from the final bleed were tested by ELISA using peptides representing the V3, C1 and C5 regions of gp120 and against core gp120 protein lacking V1/V2, V3, and the N and C termini. Peptides or core gp120 were coated on the ELISA plates and reacted with serially diluted sera. Mouse Ab binding was assessed by alkaline-phosphatase-conjugated secondary antibody to mouse IgG. Note the differences in the scales of y-axis for the right and left graphs.
Since Abs are detected against the core gp120 bearing the CD4bs, we next checked the sera for the presence of Abs against the CD4bs by comparing the serum Ab binding to gp120 in the presence or absence of sCD4 (Fig. 5). The results demonstrate that immunization with gp120 mixed with the irrelevant mAb 1418 induced production of Abs to the CD4bs, as indicated by the reduced Ab reactivities in the presence of sCD4, although the difference in reactivities in the presence versus absence of sCD4 was not statistically significant. In contrast, none of the other groups elicited Abs that competed with sCD4 in the binding to gp120, suggesting that Abs to the CD4bs were not induced to significant levels. At this point, the epitopes in the gp120 core recognized by the sera of mice immunized with the different gp120/mAb complexes remain unknown. It is also unclear as to why the gp120/anti-V3 mAb complex did not generate anti-CD4bs Abs. Further studies are needed to investigate this issue, but a previous study reported that some degrees of inhibition was observed when anti-CD4bs mAbs b12 and F91 were reacted with certain gp120/anti-V3 mAb complexes (Moore and Sodroski, 1996)
Abs elicited by immunization with gp120/anti-CD4bs mAb complexes react preferentially with the homologous V3 peptide
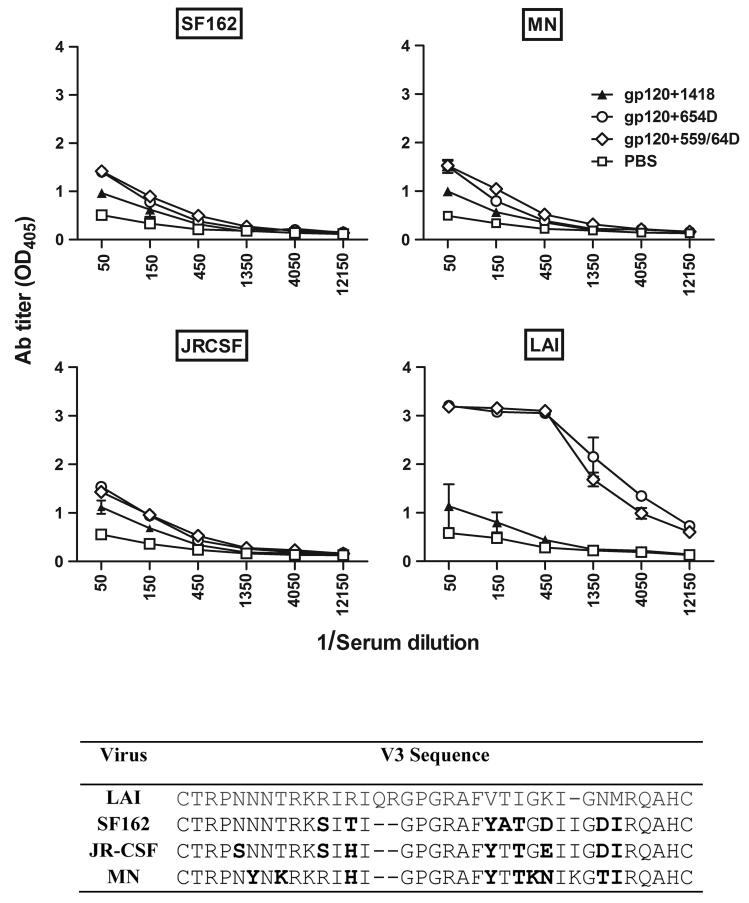
To assess if the anti-V3 Abs elicited in mice immunized with the gp120/anti-CD4bs mAb complexes were able to recognize peptides representing V3 from different HIV-1 strains, sera collected after the last immunization were serially diluted and tested by ELISA for their reactivity to different V3 peptides with sequences from SF162, MN, JR-CSF, and the homologous LAI (Fig 6). The reactivity of sera from mice receiving gp120 mixed with the irrelevant mAb 1418 was also tested for comparison. All groups of mice receiving different gp120/mAb combinations had serum reactivity with each of the four V3 peptides above the level observed with the control mouse sera. However, sera from mice immunized with gp120/mAbs complexes (gp120+654D and gp120+559/64D) had much higher reactivity with the homologous V3 peptide than with the heterologous V3 peptides. In contrast, sera from mice immunized with gp120+1418 had similar, albeit much lower, reactivity to homologous and heterologous V3 peptides. These results indicate that immunization with gp120/anti-CD4bs mAb complexes generates high levels of anti-V3 Abs that preferentially react with the homologous V3, although these complexes can also induce low levels of cross-reactive anti-V3 Abs.
Fig. 6.
Serum reactivity of mice immunized with gp120 or gp120/mAb complexes with V3 peptides of heterologous strains. Sera from the final bleed were tested by ELISA for reactivity with different V3 peptides of heterologous strains SF162, MN, JR-CSF as compared to the homologous V3LAI peptide. Differences in the V3 sequences of these viruses are shown. V3 peptides were coated on the ELISA plates and reacted with serially diluted sera. Serum Ab binding was detected using an alkaline-phosphatase-conjugated secondary antibody to mouse IgG.
Serum Abs elicited by immunization with gp120/anti-CD4bs mAb complexes potently neutralize homologous, but not heterologous HIV-1 isolates
Next we assessed the neutralizing activity of sera from mice immunized with gp120 complexed with the anti-CD4bs mAbs (mAb 654D), gp120 mixed with the irrelevant mAbs (mAb 860-55D), or gp120 alone. Neutralization assays with single-round infection of different HIV-1 strains were performed in TZM-bl cells (Choudhry et al., 2007). Serial dilutions of immune sera obtained after the final immunization were initially tested against HIV-1LAI expressing envelope homologous to the gp120 immunogen used for immunization (Fig. 7). Strong neutralizing activity was found in the sera of mice immunized with the gp120/anti-CD4bs mAbs complex (gp120+654D). At a 1:50 dilution, sera from this group were able to neutralize HIV-1LAI almost completely, and significant neutralizing activity was still present at a dilution of 1:250. In contrast, sera from the other groups (gp120 and gp120+ 860-55D) showed no neutralizing activity above that of control sera from mice receiving adjuvant alone. To assess whether the neutralizing activity observed in the serum from gp120+654D-immunized mice was mediated by anti-V3 Abs, the serum was treated with V3 peptide or an irrelevant control peptide prior to use in the neutralization assay (Fig. 7B). Treatment with V3 peptide, but not with the control peptide, significantly reduced the serum neutralizing activity (p<0.05 at each of the 3 serum dilutions tested), indicating that anti-V3 Abs play a major role in neutralization against this virus, albeit Abs directed to other yet undefined epitopes may also be present and contribute to the neutralization observed in the serum.
We subsequently tested the ability of sera from mice immunized with gp120/anti-CD4bs complexes (gp120+654D and gp120+559/64D) to neutralize other HIV-1 isolates known to have different degrees of sensitivity to Ab-mediated neutralization, i.e. SF162 (subtype B), BX08 (subtype B), JRFL (subtype B) and DJ263 (CRF02_AG) (Table 1). For comparison, we also tested sera from mice immunized with gp120 complexed with the anti-V3 mAb (gp120+694/98D), gp120 combined with an irrelevant control Ab (1418), or no antigen. While sera from mice immunized with gp120+ 654D or gp120+559/64D potently neutralize HIV-1LAI, these same sera had little or no neutralization activity against the other viruses tested. Mice immunized with gp120+694/98D showed weak or no neutralizing serum activity against all five viruses, including the homologous LAI. Sera from mice immunized with gp120+1418 had no neutralizing activity, similar to the control group receiving no antigen. Taken together, these results indicate that the binding of anti-CD4bs mAb to gp120 enhances the immunogenicity of this antigen such that significantly higher levels of anti-gp120 Abs are generated upon immunization with the gp120/anti-CD4bs mAb complexes than with uncomplexed gp120 or gp120 combined with other mAbs. Notably, the high levels of Abs elicited by these complexes are directed to the V3 region and display potent virus-neutralizing activity, at least against the homologous HIV-1LAI.
TABLE 1.
Neutralization of different HIV-1 isolates by sera from mice immunized with different gp120/mAb combinations
| % Neutralization of HIV-1 isolates | |||||
|---|---|---|---|---|---|
| LAI | JRFL | DJ263 | BX08 | SF162 | |
| Mice immunized with a | |||||
| gp120+1418 | <c | < | < | < | < |
| gp120+654D | 83 | 35 | 23 | < | 30 |
| gp120+559/64D | 90 | < | 21 | < | 29 |
| gp120+694/98D | 26 | 46 | 27 | 19 | < |
| PBS | < | < | < | < | < |
| MAbs to V3b | |||||
| 694/98D | NDd | 33 | < | ND | 82 |
| 447/52D | ND | < | < | ND | 71 |
All mouse sera were tested at 1:50 dilution
Anti-V3 mAbs were tested at 1μg/ml
Virus neutralization of <19%
Not done
DISCUSSION
This study investigates the capacity of anti-CD4bs Abs forming immune complexes with the envelope glycoprotein gp120 in augmenting Ab responses against HIV-1. The data demonstrate that the interaction of anti-CD4bs mAbs with gp120 altered the antigenic and immunogenic properties of this antigen. When BALB/c mice were immunized with the gp120/anti-CD4bs mAb complexes, serum anti-gp120 Ab responses were generated with faster kinetics and to higher titers. This finding is in agreement with very early reports showing the ability of immune complexes to enhance Ab responses due to their deposition on FDCs that can retain antigens for long period of time and stimulate B cell proliferation and germinal center formation following T cell-dependent or complement-dependent CD40 activation (Gaspal et al., 2006). Immunization with gp120 mixed with an irrelevant control mAb also induced higher anti-gp120 Ab titers as compared to immunization with gp120 alone, indicating that IgG may have an Fc-mediated adjuvant activity as described earlier in the literature (Dawe, Myers, and Segre, 1970; Heyman, 1990; Wernersson, Kleinau, and Heyman, 2000; Wiersma, Coulie, and Heyman, 1989). It should be noted, however, gp120/anti-CD4bs mAb complexes displayed a unique property in that these complexes enhanced Ab response against the V3 region of gp120, without diminishing Ab response to the other gp120 regions. Indeed, high levels of serum anti-V3 Abs were detected in animals immunized with these complexes, but not in animals immunized with uncomplexed gp120, while all groups of animals generated comparable Ab titers to the gp120 core. Animals immunized with the gp120/anti-V3 mAb complex also elicited a poor anti-V3 Ab response, presumably because of the shielding of this epitope by the mAb. These results correspond with findings reported by us and others that anti-V3 mAbs reacted better with gp120 bound by anti-CD4bs mAbs than with gp120 alone (Fig. 1 and (Pinter, Honnen, and Tilley, 1993)). These findings are also consistent with data demonstrating that the interaction of CD4 with soluble gp120 or native envelope on HIV virions resulted in increased reactivity of anti-V3 mAbs (Mbah et al., 2001; Sattentau and Moore, 1991; Sattentau et al., 1993). A few other anti-gp120 mAbs directed to V2, C1, or C2 have also been shown to enhance the binding of some anti-V3 mAbs (Fig. 1 and (Moore and Sodroski, 1996)), but whether immunization with these other gp120/mAb complexes induces more robust Ab responses to V3 remains to be determined. The enhanced binding of anti-V3 mAbs suggests that the V3 region is more exposed on these complexes. Alternatively, these mAbs may restrain V3 flexibility and make it adopt a conformation that is better recognized by Abs.
The V3 region is known to have highly variable sequences but it also maintains a conserved structure required for its interaction with the chemokine receptors (Zolla-Pazner, 2005). Abs generated to this immunogenic site also displayed different binding and neutralizing activities, as exemplified by the two anti-V3 mAbs tested here for reactivity with the gp120/anti-CD4bs mAb complexes, 694/98D and 447-52D. MAb 694/98D preferentially binds to V3LAI and neutralizes HIV-1LAI most potently, but it also is effective against a few other neutralization-sensitive strains of HIV-1. On the other hand, mAb 447-52D is one of the anti-V3 mAbs known to neutralize diverse primary HIV-1 isolates, particularly among the subtype B viruses. Both mAbs displayed enhanced reactivity with the gp120/anti-CD4bs mAb complexes (Fig. 1), indicating that the two distinct, albeit overlapping, V3 epitopes are accessible on the immune complexes. Nevertheless, sera from mice immunized with the gp120/anti-CD4bs mAb complexes did not display broad cross-reactivity like mAb 447-52D (Fig. 6 and Table 1). The sera showed enhanced binding to the V3LAI peptide, but not to other V3 peptides. The sera also had potent anti-V3 neutralizing activity specific fort the homologous HIV-1LAI isolate, and failed to neutralize heterologous viruses, including the highly neutralization-sensitive SF162. These data indicate that although the gp120/anti-CD4bs mAb complexes elicit the production of V3-specific neutralizing Abs, the Abs generated are skewed toward V3 epitopes that are not shared among different HIV-1 isolates. It should be noted that this study tested immune complexes made with gp120LAI that has a distinctive V3 sequence due to a two-amino acid insertion (Fig. 6). Further studies are necessary to determine whether highly strain-specific Abs are similarly generated in response to immune complexes made of gp120 that contains a more representative V3 sequence. Nevertheless, other factors such as the repertoire of mouse Ig with relatively short CDR H3 loops and the particular anti-CD4bs mAb tested may also account for the exquisite specificity for the serum Abs from the gp120/654 immunized mice. In addition, more boosts and longer resting periods between injections may be necessary to elicit more broadly reactive neutralizing Abs, since data from a previous immunization study using recombinant gp120 proteins indicate that neutralizing Abs against heterologous viruses are generated only after the sixth and seven injections following a resting period (Haigwood et al., 1992).
Anti-CD4bs Abs induce gp120 to undergo conformational changes, as indicated by enhanced Ab reactivity to V3 and C1 on the gp120/anti-CD4bs mAb complexes (Fig. 1). However, only V3 immunogenicity was markedly enhanced when gp120/anti-CD4bs complexes were used as immunogens (Fig.4). The C1 region in the N-terminus of gp120 did not appear to be as immunogenic as V3 for BALB/c mice. Indeed, the levels of anti-C1 Abs detected in the sera after immunization with gp120 or gp120/mAb combinations were relatively low, as compared to the Ab levels against V3, whole gp120 or gp120 core. Whether a similar repertoire of Abs is elicited by the gp120/anti-CD4bs mAb complexes in other mouse strains and in other species is not yet known; however, V3 has been documented to be highly immunogenic in different species including humans. CD4 binding to gp120 also enhances V3 reactivity (Mbah et al., 2001; Sattentau and Moore, 1991; Sattentau et al., 1993), but previous immunization experiments utilizing CD4-envelope complexes did not determine if such complexes also generated high levels of V3-specific Ab responses (Denisova et al., 1996; Fouts et al., 2002; He, D’Agostino, and Pinter, 2003; Kang et al., 1994). We should note, however, that unlike CD4, the binding of anti-CD4bs Abs to gp120 does not trigger formation of the chemokine-receptor binding site. Anti-CD4bs Abs actually prevent gp120 from binding the chemokine receptor and from reacting with mAbs specific for the chemokine-receptor binding site ((Raja et al., 2003) and Hioe et al., unpublished data). Hence, it is unlikely that Abs to the chemokine-receptor site (anti-CD4i) would be produced in response to the gp120/anti-CD4bs mAb complexes. This is supported by our observation that gp120 reactivity of serum Abs from mice immunized with the immune complexes was not enhanced by soluble CD4 (Fig. 5). Nevertheless, a previous immunization study using gp120 complexed with mAb A32, which specifically induces the binding of chemokine-receptor binding site, also did not appear to induce cross-reactive Abs against this conserved region on gp120 (Liao et al., 2004). Hence, it is of interest to evaluate in future studies as to which gp120/mAb combinations will alter not only antigenicity but also immunogenicity of the gp120 antigen.
It is reasonable to expect that Abs to the highly conserved CD4bs should be able prevent the interaction of diverse isolates of HIV-1 with their common CD4 receptor and provide some protection for the infected hosts. However, except for recombinant IgG1 b12, the majority of anti-CD4bs mAbs identified so far have poor or no neutralizing activities against most HIV-1 primary isolates (Hioe et al., 1997; Pantophlet et al., 2003). Moreover, higher titers of anti-CD4bs Abs are actually found in HIV+ subjects with rapid disease progression than in slow progressors or long-term non-progressors (Chien et al., 2004a; Chien et al., 2002). Our previous studies also demonstrate that the binding of anti-CD4bs Abs to gp120 interferes with gp120 antigen processing and down-modulates class II MHC-restricted T cell responses to gp120 (Chien et al., 2004a; Chien et al., 2004b). These inhibitory activities are exhibited specifically by high-affinity anti-CD4bs mAbs, and not by mAbs to other gp120 epitopes examined so far (Tuen et al., 2005). These effects have been initially detected in vitro on the responses of human gp120-specific CD4 T cell lines, and are consistent with recent in vivo observations showing the suppression of gp120-specific lymphoproliferation in mice receiving gp120/anti-CD4bs mAb complexes (Visciano et al. manuscript in preparation). The data presented in this paper also raise another possibility that anti-CD4bs Abs elicited during HIV infection would enhance Ab responses to gp120, but the Abs have a narrow breath of neutralization activity. Hence, the generation of high titers of anti-CD4bs Abs, as observed in HIV+ rapid progressors prior to any clinical signs of AIDS, may not be beneficial to the host.
This study provides the first evidence that the use of immune complexes can augment and direct Ab responses to a specific neutralizing epitope on HIV-1 envelope gp120. Although the Abs induced by gp120/anti-CD4bs mAb complexes tested here have restricted virus-neutralizing activities, further studies utilizing this approach may lead to identification of optimal gp120/mAb complexes and immunization protocols that will elicit not only higher levels of anti-gp120 Abs but also Abs against cross-reactive neutralizing epitopes. The vaccination strategy that focuses Ab responses toward neutralizing epitopes on HIV envelope glycoproteins will be valuable for the pursuit of effective Ab-based vaccines against HIV.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Jennifer Fuller and Susan Zolla-Pazner for reviewing and editing the manuscript, as well as Sandra Cohen and Kathy Revesz for assistance with neutralization assays. The work was supported by a Merit Review Award and the Research Enhancement Award Program of the US Department of Veterans Affairs, the New York University Center for AIDS Research Immunology Core (AI-27742), and by NIH grant AI-48371.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alber DG, Killington RA, Stokes A. Solid matrix-antibody-antigen complexes incorporating equine herpesvirus 1 glycoproteins C and D elicit anti-viral immune responses in BALB/c (H-2K(d)) and C3H (H-2K(k)) mice. Vaccine. 2000;19(78):895–901. doi: 10.1016/s0264-410x(00)00222-x. [DOI] [PubMed] [Google Scholar]
- Bandres JC, Wang QF, O’Leary J, Baleaux F, Amara A, Hoxie JA, Zolla-Pazner S, Gorny MK. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J Virol. 1998;72(3):2500–4. doi: 10.1128/jvi.72.3.2500-2504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouige P, Iscaki S, Cosson A, Pillot J. Molecular analysis of the modulatory factors of the response to HBsAg in mice as an approach to HBV vaccine enhancement. FEMS Immunol Med Microbiol. 1996;13(1):71–9. doi: 10.1111/j.1574-695X.1996.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Brady LJ, van Tilburg ML, Alford CE, McArthur WP. Monoclonal antibody-mediated modulation of the humoral immune response against mucosally applied Streptococcus mutans. Infect Immun. 2000;68(4):1796–805. doi: 10.1128/iai.68.4.1796-1805.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien PC, Jr., Chen D, Chen PD, Tuen M, Cohen S, Migueles SA, Connors M, Rosenberg E, Malhotra U, Gonzalez C, Hioe CE. HIV-1-infected patients with envelope-specific lymphoproliferation or long-term nonprogression lack antibodies suppressing glycoprotein 120 antigen presentation. J Infect Dis. 2004a;189(5):852–61. doi: 10.1086/380133. [DOI] [PubMed] [Google Scholar]
- Chien PC, Jr., Cohen S, Kleeberger C, Giorgi J, Phair J, Zolla-Pazner S, Hioe CE. High levels of antibodies to the CD4 binding domain of human immunodeficiency virus type 1 glycoprotein 120 are associated with faster disease progression. J Infect Dis. 2002;186(2):205–13. doi: 10.1086/341297. [DOI] [PubMed] [Google Scholar]
- Chien PC, Jr., Cohen S, Tuen M, Arthos J, Chen PD, Patel S, Hioe CE. Human immunodeficiency virus type 1 evades T-helper responses by exploiting antibodies that suppress antigen processing. J Virol. 2004b;78(14):7645–52. doi: 10.1128/JVI.78.14.7645-7652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry V, Zhang MY, Sidorov IA, Louis JM, Harris I, Dimitrov AS, Bouma P, Cham F, Choudhary A, Rybak SM, Fouts T, Montefiori DC, Broder CC, Quinnan GV, Jr., Dimitrov DS. Cross-reactive HIV-1 neutralizing monoclonal antibodies selected by screening of an immune human phage library against an envelope glycoprotein (gp140) isolated from a patient (R2) with broadly HIV-1 neutralizing antibodies. Virology. 2007;363(1):79–90. doi: 10.1016/j.virol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe DL, Myers WL, Segre D. Enhancement of the immune response in rabbits by administration of immunoglobulin-G. Immunology. 1970;18(6):897–907. [PMC free article] [PubMed] [Google Scholar]
- Denisova G, Stern B, Raviv D, Zwickel J, Smorodinsky NI, Gershoni JM. Humoral immune response to immunocomplexed HIV envelope glycoprotein 120. AIDS Res Hum Retroviruses. 1996;12(10):901–9. doi: 10.1089/aid.1996.12.901. [DOI] [PubMed] [Google Scholar]
- Fouts T, Godfrey K, Bobb K, Montefiori D, Hanson CV, Kalyanaraman VS, DeVico A, Pal R. Crosslinked HIV-1 envelope-CD4 receptor complexes elicit broadly cross-reactive neutralizing antibodies in rhesus macaques. Proc Natl Acad Sci U S A. 2002;99(18):11842–7. doi: 10.1073/pnas.182412199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspal FM, McConnell FM, Kim MY, Gray D, Kosco-Vilbois MH, Raykundalia CR, Botto M, Lane PJ. The generation of thymus-independent germinal centers depends on CD40 but not on CD154, the T cell-derived CD40-ligand. Eur J Immunol. 2006;36(7):1665–73. doi: 10.1002/eji.200535339. [DOI] [PubMed] [Google Scholar]
- Gigler A, Dorsch S, Hemauer A, Williams C, Kim S, Young NS, Zolla-Pazner S, Wolf H, Gorny MK, Modrow S. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J Virol. 1999;73(3):1974–9. doi: 10.1128/jvi.73.3.1974-1979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Conley AJ, Karwowska S, Buchbinder A, Xu JY, Emini EA, Koenig S, Zolla-Pazner S. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66(12):7538–42. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Xu JY, Karwowska S, Buchbinder A, Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993;150(2):635–43. [PubMed] [Google Scholar]
- Haigwood NL, Nara PL, Brooks E, Van Nest GA, Ott G, Higgins KW, Dunlop N, Scandella CJ, Eichberg JW, Steimer KS. Native but not denatured recombinant human immunodeficiency virus type 1 gp120 generates broad-spectrum neutralizing antibodies in baboons. J Virol. 1992;66(1):172–82. doi: 10.1128/jvi.66.1.172-182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308(5730):1906–8. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- He Y, D’Agostino P, Pinter A. Analysis of the immunogenic properties of a single-chain polypeptide analogue of the HIV-1 gp120-CD4 complex in transgenic mice that produce human immunoglobulins. Vaccine. 2003;21(2730):4421–9. doi: 10.1016/s0264-410x(03)00451-1. [DOI] [PubMed] [Google Scholar]
- Heyman B. The immune complex: possible ways of regulating the antibody response. Immunol Today. 1990;11(9):310–3. doi: 10.1016/0167-5699(90)90126-t. [DOI] [PubMed] [Google Scholar]
- Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol. 2000;18:709–37. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- Hioe CE, Xu S, Chigurupati P, Burda S, Williams C, Gorny MK, Zolla-Pazner S. Neutralization of HIV-1 primary isolates by polyclonal and monoclonal human antibodies. Int Immunol. 1997;9(9):1281–90. doi: 10.1093/intimm/9.9.1281. [DOI] [PubMed] [Google Scholar]
- Hochleitner EO, Gorny MK, Zolla-Pazner S, Tomer KB. Mass spectrometric characterization of a discontinuous epitope of the HIV envelope protein HIV-gp120 recognized by the human monoclonal antibody 1331A. J Immunol. 2000;164(8):4156–61. doi: 10.4049/jimmunol.164.8.4156. [DOI] [PubMed] [Google Scholar]
- Ivan J, Velhner M, Ursu K, German P, Mato T, Dren CN, Meszaros J. Delayed vaccine virus replication in chickens vaccinated subcutaneously with an immune complex infectious bursal disease vaccine: quantification of vaccine virus by real-time polymerase chain reaction. Can J Vet Res. 2005;69(2):135–42. [PMC free article] [PubMed] [Google Scholar]
- Jeffs SA, Gorny MK, Williams C, Revesz K, Volsky B, Burda S, Wang XH, Bandres J, Zolla-Pazner S, Holmes H. Characterization of human monoclonal antibodies selected with a hypervariable loop-deleted recombinant HIV-1(IIIB) gp120. Immunol Lett. 2001;79(3):209–13. doi: 10.1016/s0165-2478(01)00289-9. [DOI] [PubMed] [Google Scholar]
- Jemmerson R, Paterson Y. Mapping epitopes on a protein antigen by the proteolysis of antigen-antibody complexes. Science. 1986;232(4753):1001–4. doi: 10.1126/science.2422757. [DOI] [PubMed] [Google Scholar]
- Kang CY, Hariharan K, Nara PL, Sodroski J, Moore JP. Immunization with a soluble CD4-gp120 complex preferentially induces neutralizing anti-human immunodeficiency virus type 1 antibodies directed to conformation-dependent epitopes of gp120. J Virol. 1994;68(9):5854–62. doi: 10.1128/jvi.68.9.5854-5862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwowska S, Gorny MK, Buchbinder A, Gianakakos V, Williams C, Fuerst T, Zolla-Pazner S. Production of human monoclonal antibodies specific for conformational and linear non-V3 epitopes of gp120. AIDS Res Hum Retroviruses. 1992;8(6):1099–106. doi: 10.1089/aid.1992.8.1099. [DOI] [PubMed] [Google Scholar]
- Kessler JA, 2nd, McKenna PM, Emini EA, Chan CP, Patel MD, Gupta SK, Mark GE, 3rd, Barbas CF, 3rd, Burton DR, Conley AJ. Recombinant human monoclonal antibody IgG1b12 neutralizes diverse human immunodeficiency virus type 1 primary isolates. AIDS Res Hum Retroviruses. 1997;13(7):575–82. doi: 10.1089/aid.1997.13.575. [DOI] [PubMed] [Google Scholar]
- Kumar R. Virus enhancement following infection with antibody-coated infectious bursal disease virus (IBDV) in chickens. Indian J Exp Biol. 2001;39(12):1314–7. [PubMed] [Google Scholar]
- Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, Katinger H, Parren PW, Robinson J, Van Ryk D, Wang L, Burton DR, Freire E, Wyatt R, Sodroski J, Hendrickson WA, Arthos J. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420(6916):678–82. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- Liao HX, Alam SM, Mascola JR, Robinson J, Ma B, Montefiori DC, Rhein M, Sutherland LL, Scearce R, Haynes BF. Immunogenicity of constrained monoclonal antibody A32-human immunodeficiency virus (HIV) Env gp120 complexes compared to that of recombinant HIV type 1 gp120 envelope glycoproteins. J Virol. 2004;78(10):5270–8. doi: 10.1128/JVI.78.10.5270-5278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbah HA, Burda S, Gorny MK, Williams C, Revesz K, Zolla-Pazner S, Nyambi PN. Effect of soluble CD4 on exposure of epitopes on primary, intact, native human immunodeficiency virus type 1 virions of different genetic clades. J Virol. 2001;75(16):7785–8. doi: 10.1128/JVI.75.16.7785-7788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskie MJ, Wen YM, Di Q, Davis HL. Immunization against hepatitis B virus by mucosal administration of antigen-antibody complexes. Viral Immunol. 1998;11(4):245–52. doi: 10.1089/vim.1998.11.245. [DOI] [PubMed] [Google Scholar]
- Moore JP, McCutchan FE, Poon SW, Mascola J, Liu J, Cao Y, Ho DD. Exploration of antigenic variation in gp120 from clades A through F of human immunodeficiency virus type 1 by using monoclonal antibodies. J Virol. 1994;68(12):8350–64. doi: 10.1128/jvi.68.12.8350-8364.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70(3):1863–72. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyambi PN, Mbah HA, Burda S, Williams C, Gorny MK, Nadas A, Zolla-Pazner S. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J Virol. 2000;74(15):7096–107. doi: 10.1128/jvi.74.15.7096-7107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oli MW, Rhodin N, McArthur WP, Brady LJ. Redirecting the humoral immune response against Streptococcus mutans antigen P1 with monoclonal antibodies. Infect Immun. 2004;72(12):6951–60. doi: 10.1128/IAI.72.12.6951-6960.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantophlet R, Ollmann Saphire E, Poignard P, Parren PW, Wilson IA, Burton DR. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J Virol. 2003;77(1):642–58. doi: 10.1128/JVI.77.1.642-658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A, Honnen WJ, Tilley SA. Conformational changes affecting the V3 and CD4-binding domains of human immunodeficiency virus type 1 gp120 associated with env processing and with binding of ligands to these sites. J Virol. 1993;67(9):5692–7. doi: 10.1128/jvi.67.9.5692-5697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MR, Hideshima T, Cannon T, Mukherjee M, Mayer KH, Byrn RA. An IgG human monoclonal antibody that reacts with HIV-1/GP120, inhibits virus binding to cells, and neutralizes infection. J Immunol. 1991;146(12):4325–32. [PubMed] [Google Scholar]
- Raja A, Venturi M, Kwong P, Sodroski J. CD4 binding site antibodies inhibit human immunodeficiency virus gp120 envelope glycoprotein interaction with CCR5. J Virol. 2003;77(1):713–8. doi: 10.1128/JVI.77.1.713-718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roic B, Cajavec S, Ergotic N, Lipej Z, Madic J, Lojkic M, Pokric B. Immune complex-based vaccine for pig protection against parvovirus. J Vet Med B Infect Dis Vet Public Health. 2006;53(1):17–23. doi: 10.1111/j.1439-0450.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- Sattentau QJ, Moore JP. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991;174(2):407–15. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau QJ, Moore JP, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67(12):7383–93. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334(6179):255–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- Thali M, Furman C, Ho DD, Robinson J, Tilley S, Pinter A, Sodroski J. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol. 1992;66(9):5635–41. doi: 10.1128/jvi.66.9.5635-5641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuen M, Visciano ML, Chien PC, Jr., Cohen S, Chen PD, Robinson J, He Y, Pinter A, Gorny MK, Hioe CE. Characterization of antibodies that inhibit HIV gp120 antigen processing and presentation. Eur J Immunol. 2005;35(9):2541–51. doi: 10.1002/eji.200425859. [DOI] [PubMed] [Google Scholar]
- Wen YM, Qu D, Zhou SH. Antigen-antibody complex as therapeutic vaccine for viral hepatitis B. Int Rev Immunol. 1999;18(3):251–8. doi: 10.3109/08830189909043028. [DOI] [PubMed] [Google Scholar]
- Wernersson S, Kleinau S, Heyman B. Immune complex-mediated enhancement of antibody responses without induction of delayed-type hypersensitivity. Scand J Immunol. 2000;52(6):563–9. doi: 10.1046/j.1365-3083.2000.00813.x. [DOI] [PubMed] [Google Scholar]
- Wiersma EJ, Coulie PG, Heyman B. Dual immunoregulatory effects of monoclonal IgG-antibodies: suppression and enhancement of the antibody response. Scand J Immunol. 1989;29(4):439–48. doi: 10.1111/j.1365-3083.1989.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson RA, Piscitelli C, Teintze M, Cavacini LA, Posner MR, Lawrence CM. Structure of the Fab fragment of F105, a broadly reactive anti-human immunodeficiency virus (HIV) antibody that recognizes the CD4 binding site of HIV type 1 gp120. J Virol. 2005;79(20):13060–9. doi: 10.1128/JVI.79.20.13060-13069.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang SH, Kwong PD, Gupta R, Rizzuto CD, Casper DJ, Wyatt R, Wang L, Hendrickson WA, Doyle ML, Sodroski J. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol. 2002;76(19):9888–99. doi: 10.1128/JVI.76.19.9888-9899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Xu L, Dey B, Hessell AJ, Van Ryk D, Xiang SH, Yang X, Zhang MY, Zwick MB, Arthos J, Burton DR, Dimitrov DS, Sodroski J, Wyatt R, Nabel GJ, Kwong PD. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445(7129):732–7. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S. Improving on nature: focusing the immune response on the V3 loop. Hum Antibodies. 2005;14(34):69–72. [PubMed] [Google Scholar]